Abstract
Background
Ursolic acid is a promising anticancer agent. The current study aims to evaluate the single- and multiple-dose pharmacokinetics (PK) as well as the safety of ursolic acid nanoliposomes (UANL) in healthy volunteers and in patients with advanced solid tumors.
Methods
Twenty-four healthy volunteers in the single-dose PK study were divided into three different groups, which received 37, 74, and 98 mg/m2 of UANL. Eight patients in the multiple-dose PK study were administered with 74 mg/m2 of UANL daily for 14 days. The UA plasma concentrations were determined using ultra-performance liquid chromatograph-tandem mass spectrometry.
Results
The plasma concentration profiles of all subjects were characterized by a biexponential decline after infusion. The mean peak plasma concentration (Cmax) increased linearly as a function of the dose (r = 0.999). The mean area under the plasma concentration-time curve (AUC) from 0 to 16 hours also increased proportionally with dose escalation (r = 0.998). However, the clearance was constant over the specific dose interval. In the multiple-dose PK study, the trough and average concentrations remained low. The mean AUC, half-life, Cmax, time to Cmax, and the volume of distribution on the first day were similar to those on the last day. All subjects tolerated the treatments well. Most UANL-associated adverse events varied from mild to moderate.
Conclusions
UANL exhibits relatively linear PK behavior with dose levels from 37 mg/m2 to 98 mg/m2. No drug accumulation was observed with repeated doses of UANL. The intravenous infusion of UANL was well tolerated by healthy volunteers and patients with advanced tumors.
Introduction
Ursolic acid (UA; 3β-hydroxy-urs-12-en-28-oic acid) is a naturally derived pentacyclic triterpene acid that is widely present in food, medicinal herbs, and other plants.Citation1 Its extensive pharmacological effects include hepatoprotective, antioxidant, anti-inflammatory, antiviral, and cytotoxic activities. In recent years, UA has become a major focus in cancer research because of its activities at various stages of tumor development and its low toxicity. Although the concrete mechanisms of its anticancer effects are poorly understood, several studies have found that UA can inhibit proliferation and induce apoptosis of many tumor cell lines.Citation2 UA-induced apoptosis occurs through multiple pathways such as the inhibition of DNA replication,Citation3 induction of Ca2+ release,Citation4 activation of caspasesCitation5,Citation6 and c-Jun N-terminal kinase,Citation7,Citation8 phosphorylation of glycogen synthase kinase 3-β, downregulation of antiapoptotic genes,Citation9 inhibition of cyclooxygenase-2 and inducible nitric oxide synthase,Citation10,Citation11 suppression of matrixmetallopeptidase-9,Citation12 and the inhibition of protein tyrosine kinase,Citation13 phosphatidylinositol-3-kinase,Citation14 single transducer and activator of transcription 3,Citation15 adenosine 5′-monophosphate-activated protein kinase,Citation16 and nuclear factor κ-light-chain-enhancer of activated B cellsCitation17 pathways. Furthermore, UA can inhibit the differentiation, angiogenesis, invasion, and metastasis of tumor cells as well as interfere with numerous enzymes such as those directly involved with DNA synthesis and repair.Citation2,Citation18–Citation20
However, because of its poor solubility in water, the oral administration of UA often causes its low bioavailability.Citation21 The literature has limited data on the mechanism of absorption and pharmacokinetics (PKs) of UA. A previous study using a Caco-2 cell monolayer model suggested that the main mechanism involved in the absorption of UA was passive diffusion and P-glycoprotein transporter mediated active transport. Liao et al performed a PK study of UA in rats after administration of the traditional Chinese medicinal preparation, Lu-Ying extract.Citation22 The results showed that the absorption of UA was rapid, with the peak concentration occurring 1 hour after oral administration of Lu-Ying extract. Although the oral administration dose of Lu-Ying extract contained UA 80.32 mg/kg, its concentrations in rat plasma were extremely low. Recently, a PK study of UA in rats showed that the time to peak concentration of UA in plasma was about 30 minutes, indicating that UA was rapidly absorbed. The half-life of UA in plasma was less than 1 hour, indicating that UA was rapidly eliminated.Citation23 Thus, the therapeutic development of UA was limited by its low bioavailability and the enhancement of UA solubility is a real challenge for its development. The nanocarrier-mediated drug delivery system has been used to improve the therapeutic activity and safety of drugs for years. The nanoliposomal formulation is a promising approach for poorly water-soluble drugs.Citation24 Thus, UA nanoliposomes (UANL) were developed for the first time in the People’s Republic of China. The intravenous (IV) administration of UANL is considered to improve bioavailability because the nanoparticles bypass the stomach. To date, a wide range of preclinical studies in China have been completed (Li, unpublished data, 2005). These studies revealed that the nanoliposome-encapsulated UA inhibited the growth of various human cancer cells and nude mice xenografts. A further unpublished study has shown that UANL induces minimal toxic effects even with long-term application. Tissue distribution experiments in mice suggested that UANL first enters the stomach and intestines, and then its concentrations decline rapidly. Conversely, the hepatic UA concentration increased rapidly, and exceeded the UA concentrations in the stomach and intestines at 4 hours after IV injection. The results demonstrated that UANL delivers UA to the liver, where it accumulates. Consequently, the drug disposition behavior changes in vivo, and the toxic and side effects of UA on other tissues is decreased (unpublished data). The antitumor activities of UA and the minimal toxic effects that were observed in preclinical studies promoted the human clinical trials of UANL. Thus, a PK study on UANL was started by the Tianjin Medical University Cancer Institute and Hospital.
To date, no PK studies of UANL in humans have been reported. This study is the first report of a PK investigation of UANL in humans. Thus, this study aimed to characterize the PK profiles of UANL in healthy Chinese volunteers and in Chinese patients with advanced solid tumors, as well as to monitor the UANL toxicities after IV administration.
Patients and methods
Selection of healthy volunteers and patients
This present study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Tianjin Cancer Hospital. All subjects were informed about the study program and signed informed consent forms before participating in the study. The healthy volunteers in the single-dose PK study were chosen based on the following criteria: aged 18 years to 75 years; with normal organ function throughout the body; no drug administration for 2 weeks prior to participation in the study; good living habits; willingness to consume no cigarettes or alcohol during the entire treatment period; and good compliance with the study program requirements. Patients with histologically confirmed solid tumors that had proven intractable to standard therapy or had received no standard therapy were eligible for the multiple-dose PK study. One inclusion criterion for the multiple-dose PK study is that the patients had not undergone any operation, chemotherapy, biotherapy, or radiotherapy for 4 weeks before participating in the study. Other inclusion criteria included: aged 18 years to 75 years; Eastern Cooperative Oncology Group (ECOG) status of 0 to 2; survival expectancy of over 3 months; adequate bone marrow function, as defined by a white blood cell count of ≥4.0 × 109/L, an absolute neutrophil count of ≥2.0 × 109/L, a platelet count of ≥100.0 × 109/L, and a hemoglobin level of ≥100.0 g/L; adequate renal function, as defined by serum creatinine ≤ 1.5 times the upper limit of normal (ULN); adequate hepatic function, as defined by alanine aminotransferase (ALT) level ≤ 1.5 × ULN, aspartate aminotransferase (AST) level ≤ 1.5 × ULN, and a normal total bilirubin level; and no obvious cardiac, pulmonary, or psychiatric disorders. Patients with an active infection and serious brain tumors or metastases were excluded from the study. All patients with probable chances of pregnancy were required to use adequate contraception during the entire study.
Study design
Injectable UANL was supplied by Liyuanheng Pharmaceutical Co, Ltd (Wuhan, China). The UANL was manufactured as a freeze-dried powder, with 3 mg of UANL in each vial. The present study has an open-label, dose-escalation study design. Based on previous pharmacological, toxicological, and tolerability data,Citation25 the initial UANL dose for the single-dose PK study was 37 mg/m2. The dosage was sequentially increased to 74 and 98 mg/m2 in the other corresponding dose-assigned groups. Twenty-four patients in the single-dose PK study received 37, 74, and 98 mg/m2 of UANL via a 4-hour IV infusion. Eight patients who were enrolled in the multiple-dose PK study received 74 mg/m2 of UANL daily for 2 consecutive weeks.
PK sample collection
Single-dose PK 4 mL blood samples were collected at the following time points: before treatment, at 0.5, 1, and 2 hours during the infusion, at the end of the 4-hour infusion, and finally, at 5, 15, and 30 minutes as well as at 1, 1.5, 2, 3, 4, 6, 8, and 12 hours after the infusion.
On the 1st and 14th days of the multiple-dose PK study, 4 mL blood samples were collected before treatment, at 30 minutes as well as at 1 and 2 hours during the infusion, at the end of the 4-hour infusion, and finally, at 5, 15, and 30 minutes as well as at 1, 1.5, 2, 3, 4, 6, 8, and 12 hours after the infusion. On the 6th, 7th, and 8th days of the study, 4 mL blood specimens were collected before treatment and at the end of the 4-hour infusion. The samples of plasma were prepared by centrifugation (10 minutes at 600× g), and were subsequently stored at −80°C until further analysis.
Analytical assays
Ursolic acid (>99.5% purity) and glycyrrhetinic acid (internal standard, >99.5% purity) were obtained from the National Institute for Food and Drug Control (Beijing, China). High-performance liquid chromatography (HPLC)-grade acetonitrile and methanol were purchased from Fisher Scientific Inc (Fairlawn, NJ, USA). HPLC-grade ethyl acetate was purchased from Concord Technology Co, Ltd (Tianjin, China). HPLC-grade water was prepared using a Milli-Q system (EMD Millipore, Billerica, MA, USA). Analytical grade ammonium formate was purchased from Runjie Chemical Reagent Co, Ltd (Shanghai, China).
The chromatographic system consisted of an ACQUITY™ ultra-performance liquid chromatography (UPLC) system coupled to an ACQUITY™ triple quadrupole mass spectrometry system and a BEH C8 column (100 mm × 2.1 mm; inner diameter, 1.7 μm; Waters Corp, Milford, MA, USA). Data were processed using the MassLynx version 4.1 software (Waters Corp).
The determination of UA in human plasma was performed by UPLC/tandem mass spectrometry (MS/MS) method as previously described.Citation26 The lower limit of quantitation (LLOQ) for UANL was 10 ng/mL, and the assays were linear from 10 ng/mL to 5000 ng/mL. The mean extraction recovery of UANL was 73.2%, and the matrix ion suppression ranged from −11.4% to −5.6%. The intra- and interday precisions were less than 7.0% and 7.2%, respectively. The accuracy of UANL was within ±2.0%.
PK data analysis and statistical analysis
The plasma concentration vs time data were analyzed using noncompartmental methods. The DAS 2.1 PK analysis system (Anhui, China) was used to assess the PK parameters. The peak plasma concentration (Cmax) and the time-to-peak plasma concentration (Tmax) were obtained via the experimental observations. The elimination half-life (t1/2) was calculated as 0.693/λz (λz is the slope of the terminal phase). The area under the curve (AUC) of the plasma concentration versus time from zero to infinity (AUC0–∞) was equivalent to the sum of the areas from time T0 to the time of the last measured concentration. Its value was calculated using the linear trapezoidal method (until Cmax), the log-trapezoidal method (until the last measurable concentration), and the extrapolated area. The extrapolated area was determined by dividing the final measured concentration by the slope of the terminal log-linear phase. Trough values on the 6th, 7th, and 8th days were averaged for each dose level. All statistical tests were two-tailed, and significance was set at the 0.05 level. Differences in the mean values of the physical examinations and in the PK parameters among the three groups were compared by analyses of variance or the Kruskal–Wallis test using the Statistical Package for the Social Sciences (SPSS) software (version 16.0; IBM, Armonk, NY, USA). The t-test or Wilcoxon’s test were used to investigate the differences between the two groups.
Results
Subject characteristics
Thirty-two eligible and consenting subjects were recruited for this study. Twenty-four healthy volunteers were enrolled in the single-dose PK study, whereas there were eight patients in the multiple-dose study. The characteristics of the healthy volunteers and patients are shown in . All healthy subjects had normal results for the physical and laboratory examinations. No statistically significant differences were found in the age, height, weight, body surface area, and body mass index among the three healthy groups. In the multiple-dose PK study, the mean age of the eight patients was 49 years. There were six male and two female patients, all of whom had ECOG performance status score of 0 to 1. Four patients had non-Hodgkin’s lymphoma, two had hepatoma, one had Hodgkin’s lymphoma, and one had gastric cancer.
Table 1 Summary of healthy volunteer and patient characteristics
Single-dose PK study in healthy volunteers
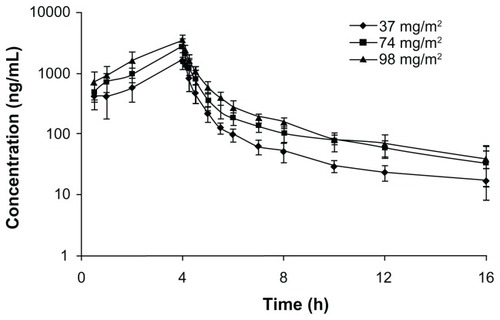
The total UA plasma concentration-versus-time profiles of the healthy volunteers who received 37 to 98 mg/m2 of UANL are shown in . The plasma concentration profiles of all subjects were characterized by a biexponential decline after infusion. The major PK parameters at each dose are summarized in . The respective mean Cmax for the subjects in the UANL groups were 1835, 2865, and 3457 ng/mL, in order of increasing UANL dose. The respective mean AUC0–16 h were 4203, 7175, and 9696 ng · hour/mL. The mean Cmax increased linearly as a function of dose (r = 0.999). Similarly, the mean area under the plasma concentration-time curve from 0 to 16 hours (designated as AUC0–16h) increased proportionally with dose escalation (r = 0.998). However, the mean t1/2, volume of distribution (Vd) and clearance (CL) were not significantly different among the three doses (P > 0.05), suggesting that clearance was constant over that specific dose interval. These results show that UANL displays linear PKs after IV administration. No correlation was found between the observed PK profile (in terms of the AUC) and the patient characteristics (age, height, weight, body mass index, or body surface area).
Table 2 Main pharmacokinetic parameters after single intravenous doses of ursolic acid nanoliposomes in healthy volunteers
Multiple-dose PK study in patients with advanced solid tumors
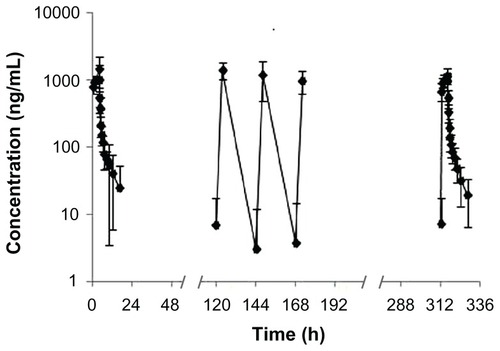
The mean plasma concentration versus time plot for the eight patients after receiving 74 mg/m2 of UANL during the 14-day period via continuous IV infusion is illustrated in . The UANL PK parameters on the first day and the last day for the patients are summarized in . The mean steady-state Cmax, trough concentration (Cmin), and average concentration (Cav) were 1211, 7.31, and 196 ng/mL, respectively. No accumulation was observed with repeated doses of UANL. Comparison of the PK parameters at 74 mg/m2 between the healthy volunteers and patients showed that Tmax, t1/2, and Vd were not significantly different between the two subject groups (P > 0.05). However, the mean AUC, Cmax, and CL were significantly lower in the patients than in the healthy volunteers (P < 0.05).
Table 3 Main pharmacokinetic parameters at day 1 and day 14 after multiple doses of ursolic acid nanoliposomes in patients with advanced solid tumors
Safety
The National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0) was used to grade adverse events (AEs). presents a summary of the toxicities associated with the administration of UANL in healthy volunteers and patients. The IV infusions of UANL were well tolerated by the healthy volunteers and the patients with advanced tumors. All subjects were evaluated for tolerability. No AE was observed in patients who receiving 37 mg/m2 of UANL. The incidence of UANL-associated AEs did not show an increasing trend after the provision of the larger doses. Nausea, diarrhea, and abdominal distention were the common AEs that were observed. Most UANL-associated AEs were either grade 1 or 2. Only one patient developed grade 3 AEs in the form of elevated AST and ALT levels with diarrhea at the same time after receiving 74 mg/m2 of UANL.
Table 4 Numbers of subjects with ursolic acid nanoliposome-associated adverse events
Discussion
Medicinal plants are often investigated as sources of new drugs for treating cancer. UA is one of the most promising chemopreventive agents for cancer.Citation27 The water solubility of UA is limited, which consequently limits its bioavailability in the body. The nanoliposome-encapsulation of UA was expected to increase its bioavailability and therapeutic efficacy. The formulated nanoliposomes prepared in this study were relatively uniform in size. The UANL average diameter was 200 nm, and the average entrapment efficiency determined by dialysis method was 95%. Conventional UA was found to be mainly distributed in tissues with extensive vascular supply such as the lung, spleen, and liver at 1 hour after oral administration.Citation23 However, based on the unpublished preclinical studies on nanoliposomal UA in rats, UANL probably had a lower Vd and higher hepatic drug concentration over conventional UA. These UANL characteristics may contribute towards eliminating its drug-related side effects and nonspecific drug accumulation.
In the present study, we determined the total UA concentration in human plasma by UPLC-MS/MS.Citation26 The LLOQ under the present conditions was 10 ng/mL. The results indicated that the method was specific, sensitive, and convenient for the determination of the UA concentration in biological samples. Subsequently, we investigated the PKs and safety of UANL in healthy volunteers and patients with advanced solid tumors. Following a single dose via IV administration, the total UA plasma concentration of all subjects followed a biexponential decline. After the end of the IV infusion, the UA plasma concentration rapidly decreased to a concentration that was approximately ten times less than the peak concentration within 2 hours. The PK characteristics of UANL were linear and dose-proportional in the range of 37 mg/m2 to 98 mg/m2. These results showed that the plasma concentration of the total UA decreased with constant clearance. The preclinical studies of UANL in rats showed a similar trend of the PK profiles (Li, unpublished data, 2005). In the multiple-dose PK study, the steady-state Cmin and Cav remained low. The mean AUC, t1/2, Cmax, Tmax, and Vd on the first day were similar to those on the last day, thereby indicating that the PKs of UANL had no apparent changes after repeated administration. These data showed that no drug accumulation was observed with continuous administration of UANL. The mean AUC, Cmax, and CL in the patients on the first day were significantly lower than those in the healthy volunteer group. Several possible reasons may account for this difference. First, the small sample sizes in the present study may contribute to this large difference. Then, a difference in the hepatic functional reserve of the healthy volunteers and patients may explain their different levels of response to the UANL. Whether this difference can be ascribed to the hepatic metabolic enzymes and/or other catabolic enzymes is unclear.
All subjects in this study tolerated the treatments well. Most UANL-associated AEs varied from mild to moderate and were not dose-related. Only one healthy volunteer developed grade 3 AEs, such as AST and ALT elevation as well as diarrhea at the same time, after receiving 74 mg/m2 of UANL. The most frequently observed AEs included abdominal distention, nausea, and diarrhea. The UANL side effects after the 14-day continuous infusion included grade 1 skin pruritus, arthralgia, and triglyceride elevation. A previous tolerability study in humans had similar results, thereby suggesting that UANL had minimal toxic effects.Citation25 The dose-limiting toxicity of UANL was hepatotoxicity. Thus, particular attention should be given to any clinical and laboratory evidence of hepatotoxicity in future clinical studies of UANL. Correlation analyses were conducted to determine if a relationship could be established between the observed PK profiles and UANL toxicities. No correlation was found, possibly because of the small sample size.
Conclusion
For the first time, UANL was developed in the People’s Republic of China. This study is the first report of a PK investigation of UANL in humans. In the present study, UANL is safe and presents apparent linear PK behavior for dose levels within the range of 37 mg/m2 to 98 mg/m2. No drug accumulation was observed with repeated UANL administration even with 14 days of continuous IV infusion. The IV infusion of UANL was well tolerated by healthy volunteers and patients with advanced tumors. Further Phase II studies of UANL should be conducted to extensively observe its clinical efficacy and safety.
Acknowledgments
The authors are grateful to Dr Jing Ping from Department of Project Development, Liyuanheng Pharmaceutical Co, Ltd, China (current address: Department of Pharmacy, Asian Heart Hospital, China), for data acquisition and quality control checks.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuJPharmacology of oleanolic acid and ursolic acidJ Ethnopharmacol199549257688847885
- SultanaNClinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural productJ Enzyme Inhib Med Chem201126561664221417964
- KimDKBaekJHKangCMApoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replicationInt J Cancer200087562963610925354
- BaekJHLeeYSKangCMIntracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cellsInt J Cancer19977357257289398053
- ChoiYHBaekJHYooMAInduction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cellsInt J Oncol200017356557110938399
- HarmandPODuvalRDelageCUrsolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cellsInt J Cancer2005114111115523687
- XavierCPRLimaCFPedroDFNUrsolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cellsJ Nutr Biochem7262012 [Epub ahead of print.]
- ZhangYKongCZengYUrsolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitroMol Carcinog201049437438520146252
- KassiESourlingasTGSpiliotakiMUrsolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cellsCancer Invest200927772373319440893
- SubbaramaiahKMichaluartPSpornMBUrsolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cellsCancer Res20006092399240410811116
- SuhNHondaTFinlayHJNovel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophagesCancer Res19985847177239485026
- ChaHJParkMTChungHYUrsolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cellsOncogene19981667717789488041
- HollosyFMeszarosGBokonyiGCytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cellsAnticancer Res2000206B4563457011205305
- WuBWangXChiZ-FUrsolic acid-induced apoptosis in K562 cells involving upregulation of PTEN gene expression and inactivation of the PI3K/Akt pathwayArch Pharm Res201235354354822477202
- PathakAKBhutaniMNairASUrsolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cellsMol Cancer Res20075994395517855663
- ZhengQ-YJinF-SYaoCUrsolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cellsBiochem Biophys Res Commun2012419474174722387548
- ShishodiaSMajumdarSBanerjeeSUrsolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1Cancer Res200363154375438312907607
- KanjoormanaMKuttanGAntiangiogenic activity of ursolic acidIntegr Cancer Ther20109222423520462855
- NovotnyLVachalkovaABiggsDUrsolic acid: an anti-tumorigenic and chemopreventive activityMinireview Neoplasma2001484241246
- OvesnaZVachalkovaAHorvathovaKPentacyclic triterpenoic acids: new chemoprotective compoundsMinireview Neoplasma2004515327333
- LiuJOleanolic acid and ursolic acid: research perspectivesJ Ethnopharmacol20051001–2929415994040
- LiaoQYangWJiaYLC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extractYakugaku Zasshi2005125650951515930819
- ChenQLuoSZhangYDevelopment of a liquid chromatography–mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution studyAnal Bioanal Chem201139982877288421249342
- Merisko-LiversidgeELiversidgeGGCooperERNanosizing: a formulation approach for poorly-water-soluble compoundsEur J Pharm Sci200318211312012594003
- ZhaoCCQianZZWangHQA phase I clinical trial of ursolic acid nanoliposome for evaluating its single-dose to lerance and safetyChinese Journal of New Drugs2011202148151
- XiaYWeiGSiDQuantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic studyJ Chromatogr B Analyt Technol Biomed Life Sci20118792219224
- HsuYLKuoPLLinCCProliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cellsLife Sci200475192303231615350828