?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this study was to prepare a suitable formulation for dermal delivery of diflucortolone valerate (DFV) that would maintain the localization in skin layers without any penetration and to optimize efficiency of DFV. Drug-loaded lecithin/chitosan nanoparticles with high entrapment efficiency (86.8%), were successfully prepared by ionic interaction technique. Sustained release of DFV was achieved without any initial burst release. Nanoparticles were also incorporated into chitosan gel at different ratios for preparing a more suitable formulation for topical drug delivery with adequate viscosity. In ex-vivo permeation studies, nanoparticles increased the accumulation of DFV especially in the stratum corneum + epidermis of rat skin without any significant permeation. Retention of DFV from nanoparticle in chitosan gel formulation (0.01%) was twofold higher than commercial cream, although it contained ten times less DFV. Nanoparticles in gel formulations produced significantly higher edema inhibition in rats compared with commercial cream in in-vivo studies. Skin blanching assay using a chromameter showed vasoconstriction similar to that of the commercial product. There were no barrier function changes upon application of nanoparticles. In-vitro and in-vivo results demonstrated that lecithin/chitosan nanoparticles in chitosan gel may be a promising carrier for dermal delivery of DFV in various skin disorders.
Introduction
Steroids are a class of drugs that are commonly used in the treatment of many diseases. Topical corticosteroids, first experienced in clinical practice in the early 1950s, are widely used for skin disorders that require steroid therapy for anti-inflammatory and immunosuppressive purposes; yet the adverse effects of steroids significantly limit their usage since long-term treatment is required in such diseases.Citation1,Citation2 Due to skin atrophy, steroid acne, hypopigmentation, transepidermal water loss (TEWL) increase, epidermal thinning, and allergic contact dermatitis that may arise during treatment, the actual dose must be precisely adjusted so that the ratio of benefit/risk should be clearly identified.Citation3
The physicochemical characteristics of the drug, the type of vehicle, and the physiological conditions of the skin can significantly affect percutaneous absorption.Citation4,Citation5 An important point to consider in the treatment of skin disorders with steroids is the permeation of these molecules to systemic circulation through the skin layers. Permeation of topical drugs through the skin, leading to uptake into the systemic circulation, is generally not desired and may lead to systemic side effects of topical treatments. As an example, topical corticosteroids, besides their well known local side effects, may have systemic adverse effects such as hypothalamic–pituitary–adrenal axis suppression, Cushing’s syndrome, femoral head osteonecrosis, and cataracts.Citation6,Citation7 Several formulation approaches have been developed for topical administration of steroids, such as creams, ointments, lotions, micelles, submicron emulsions, liposomes, solid lipid and polymeric nanoparticles, which include conventional and novel delivery systems. These formulations have been proposed to increase percutaneous absorption of therapeutic agents as well as reducing the damage to the skin barrier function.Citation8–Citation11 A desired formulation for this purpose should ideally be able to pass through the stratum corneum (SC) and be able to maintain therapeutically relevant concentrations in the epidermis/dermis, without high serum levels and systemic exposure.
Colloidal drug-carrier systems such as nanoparticles, micelles, and liposomes can be classified as the most promising drug-delivery systems for targeting steroids to the skin layers, especially to the viable epidermis layer where anti-inflammatory reactions take place. At this point, a significant example for this perspective are the nanoparticles which may alter the flux, provide drug deposition, localization, and even selectively permeabilize the SC.Citation12,Citation13 As a result of these properties, nanotechnology systems may allow consecutive and long-term administration of steroidal drugs to the target tissue and improve the pharmacokinetics by optimizing the necessary dose for treatment.
Lecithin is a natural lipid mixture of phospholipids and biocompatible excipient and is frequently used for the preparation of various nanoparticulate delivery systems.Citation14 Chitosan, poly(β-(1,4)-2-amino-2-deoxy-D-glucose), is one of the major polymers used in pharmaceutical dosage forms. Chitosan is obtained by the alkaline deacetylation of chitin, which exists in the natural structure of crustacean exoskeletons. The outstanding properties that makes chitosan so popular in the pharmaceutical field are its being nontoxic and potentially safe, its biodegradability, and its biocompatibility.Citation15 In the literature, nanoparticles have been obtained from the supramolecular self-organizing interaction of negatively charged lipid material lecithin and the positively charged polysaccharide chitosan, without preliminary vesicle formation.Citation16–Citation18
Diflucortolone valerate (DFV) is a potent corticosteroidal molecule which is commonly used in the treatment of skin disorders. According to the corticosteroidal classification system, DFV is classified as a high potent steroid for the available formulations of ointments and creams. However depending on its potency, many side effects are observed with respect to the other members of the steroid drugs.Citation19
Effectiveness of topical corticosteroids is related to their vasoconstrictive, anti-inflammatory, immunosuppressive, and antiproliferative effects in the treatment of psoriasis and atopic dermatitis.Citation20,Citation21 The skin blanching assay has been used in many studies depending on the direct correlation between corticosteroid-induced skin blanching and vasoconstriction. The efficacies of various formulations have been compared with each other as an indicator of the potency of the drug or efficacy of the delivery vehicle. Although a visual parameter, the quantification by chromameter for the exact determination of degree of skin blanching is a new combination technique, which is also used in this study.Citation22
Therefore, this study was constructed on two major goals: one was to develop nanoscale formulations of DFV using lecithin/chitosan nanoparticles; and the second was to optimize its efficacy by designing a formulation which can be used safely with minimized potential adverse effects. The optimized nanoparticles were then incorporated into chitosan gels at different ratios in order to enhance the permeation and retention of DFV in skin, in comparison with commercial cream and chitosan gel formulations containing 0.1% DFV. For this purpose, in-vitro release, ex-vivo permeation, anti-inflammatory activity, skin blanching assay, and TEWL measurements were investigated throughout this study.
Materials and methods
Materials
DFV was kindly gifted from Roche, Turkey. Lecithin (Lipoid S45) and chitosan (Chitoclear FG, specifications: molecular weight [MW] 140 kDa, deacetylation degree 95%, viscosity 93 cP for 1% w/v solution in 1% v/v acetic acid) were purchased from Lipoid AG (Ludwigshafen, Germany) and Primex (Haugesund, Norway), respectively. Medium MW chitosan (specifications: MW 190–310 kDa, deacetylation degree 75%–85%, viscosity 200–800 cP for 1% w/v solution in 1% v/v acetic acid) for the preparation of gel formulation was obtained from Sigma-Aldrich (St Louis, MO, USA). Polyethylene glycol 400 (PEG-400) was provided by Merck KGaA (Darmstadt, Germany). The commercial product of DFV (Temetex® cream) was used as the control formulation. All other chemicals were of analytical grade.
Preparation of nanoparticles
The nanoparticles of lecithin/chitosan were prepared according to the method previously reported by Sonvico et al,Citation17 via direct injection of soybean lecithin alcoholic solution into aqueous chitosan solution. In this method, the nanoparticles were obtained from the supramolecular self-organizing interaction of negatively charged lipid material lecithin and the positively charged polysaccharide chitosan, without preliminary vesicle formation. Briefly, two different types of nanoparticle formulations were prepared. The only difference between these two formulations was the existence of isopropyl myristate (IPM) in the formulation, keeping all the other steps the same. For this purpose, 4 mL of ethanolic solution containing lecithin (2.5% w/v), drug (0.1%), and IPM (2%) was prepared. Under mechanical stirring, this solution was rapidly injected (rate of 40 mL/min) into 46 mL of chitosan solution, which was prepared by dilution of 0.5 mL of 1% chitosan solution in 0.275 N HCl. In the colloidal suspension, lecithin/chitosan was in the ratio of 20:1 (w/w). The same procedure was employed for the formation of blank nanoparticles either with or without IPM.
Characterization of nanoparticles
Size and zeta potential measurements of nanoparticles
Particle size and zeta potential of the nanoparticle formulations were measured by dynamic laser light scattering method with Malvern NanoZS (Malvern Instruments, Malvern, UK) at 25°C. For these measurements, material and dispersant refractive indexes were used as 1.365 and 1.330, respectively (viscosity 0.8872 cP; and dielectric constant 78.547). Results were expressed in terms of mean ± standard error values after six consecutive measurements.
Entrapment efficiency
Drug-loaded nanoparticles were centrifuged at 3000 × g for 10 minutes, the sediment was dissolved in ethanol, and the amount of the possible existing DFV, which was precipitated in the preparation process, was assayed. Then, the suspension was ultra-centrifuged at 150000 × g for 2 hours to separate the free drug from encapsulated drug. The clear fluid under the nanoparticle layer was recovered and assayed for dissolved DFV. The amount of DFV in the samples was quantified by high performance liquid chromatography (HPLC) (Agilent 1100, Germany) system using a reverse phase column (C18; 250 mm × 4.6 mm), acetonitrile/water (55:45) as mobile phase with a flow rate of 1.5 mL/min. The ultraviolet detection wavelength was set at 240 nm. The entrapment efficiency and drug-loading capacity values were calculated using Equationequations (1)(1) and Equation(2)
(2) below:
Morphology of nanoparticles
The morphology of nanoparticles was examined by Libra 120 transmission electron microscope (TEM) (Carl Zeiss, Oberkochen, Germany). The sample was stained with 2% w/v uranyl acetate solution for 20 seconds, immobilized on a copper grid coated with Formvar®, and dried for viewing by TEM.
Differential scanning calorimetry (DSC) analysis
DSC measurements of pure drug, blank nanoparticles, physical mixture of drug and blank nanoparticles and drug-loaded nanoparticles were carried out using Perkin Elmer, DSC8000. Measurements were performed at a flow rate of 50 mL/min and evaluated within a range of −50 to 300°C.
In-vitro drug release
The in-vitro release of DFV from the nanoparticles was carried out using dialysis membrane method. Nanoparticle suspension equivalent to 1 mg drug entrapped was transferred in dialysis bags with a molecular mass cutoff of 12,000 Da. The bags were suspended in 20 mL of ethanol: phosphate buffer saline (PBS) (pH 7.2) at the ratio of 3:7 (v/v) at 37°C ± 0.5°C in a shaking water bath at 100 rpm. At predetermined time intervals, aliquots were withdrawn, and the amount of drug was quantified by the abovementioned HPLC method.
Preparation of chitosan gel formulations
Blank chitosan gel in water was prepared by dissolving 2 g of chitosan in 100 mL of acetic acid 1.5% w/v water solution. To prepare the chitosan gel containing pure DFV, the drug was dissolved at a concentration of 10% (w/w) in PEG-400, and afterwards it was added into the chitosan gel with continuous stirring until a homogenous dispersion was obtained. The final concentration of DFV was 0.1% (w/w). Nanoparticle dispersions with and without DFV were incorporated into the blank chitosan gel at three different ratios: 1:1, 1:3, and 1:9 (nanoparticle: gel ratio by weight).
Characterization of chitosan gel formulations
pH measurements
Direct measurements of formulations were made using a digital pH-meter (Model pHI 71; Beckman Coulter, Brea, CA, USA).
Rheological analysis
The rheological properties of formulations were characterized by using a HAAKE rheometer (HAAKE MARS Modulars Advanced Rheometer Systems; Thermo Fisher Scientific, Waltham, MA, USA) in flow mode and in conjunction with parallel steel plate geometry (35 mm, gap 0.3 mm). Oscillatory analysis of each formulation under examination was performed after determination of its linear viscoelastic region, and frequency sweep analysis was performed over the frequency range of 0.1–10.0 Hz following application of a constant stress. Each measurement was repeated three times, and then the storage modulus (G′) and loss modulus (G′) were determined using HAAKE RheoWin software (Thermo Fisher Scientific).
Mechanical characterization of gel formulations
Mechanical properties (hardness, adhesiveness, cohesiveness, compressibility, and elasticity) of the formulations were determined using a texture analyzer (TA.XTplus; Stable Micro Systems, Godalming, Surrey, UK) equipped with 5 kg of load cell in texture profile analysis mode. Beakers were filled with the formulation and the analytical probe (Perspex, 10 mm/150 mm; diameter/length) was compressed twice into each sample to a depth of 15 mm at a rate of 2 mm/s. A delay period of 15 seconds was allowed between the end of the first and the beginning of the second compression. All analyses were performed at least in triplicate at ambient temperature and fresh sample used in each case. Data collection and calculation were performed using the Texture Exponent 3.0.5.0 software package of the instrument. From the resultant force–time plot, mechanical parameters were defined.Citation23
Stability of formulations
All formulations were stored at 25°C and 60% relative humidity (TK 252 Test Cabinet; Nüve, Ankara, Turkey) in capped glass vials for a period of 3 months. Samples were withdrawn at scheduled time points (10, 30, 60, and 90 days) and analyzed in terms of all characterization properties.
Skin permeation experiments
The ex-vivo permeation experiments of DFV from nanoparticles, gel formulations, and commercial formulation were performed using Franz-type diffusion cells across rat abdominal skin. Receptor compartment (5 mL) was composed of a mixture of ethyl alcohol: PBS at pH 7.2 in the ratio of 3:7 (v/v). This compartment was constantly kept at 37°C ± 0.5°C throughout the experiments. The available diffusion area between donor and receptor compartments was 0.63 cm2. Infinite dose regimen was applied in all experiments. Samples of 300 μL were withdrawn from the receptor compartment at specified time intervals and immediately replaced with the same volume of fresh solution to maintain sink conditions.
At the end of the 6-hour permeation experiments, the excess amount of formulation was removed and SC+epidermis layers were separated from dermis with heat application. The drug was extracted from skin layers using acetonitrile/water (60:40) mixture under sonication for 15 minutes. The amount of DFV in the permeation medium and accumulated in the skin layer sample was determined by the HPLC method. Skin concentration of DFV was calculated by normalizing the amount of drug recovered in the SC+epidermis and dermis by the weight of tissues and expressed as μg of drug per mg of tissue. All experiments were done in six replicates.
The enhancement ratio was also calculated, to demonstrate the accumulation capacity of lecithin/chitosan nanoparticles for DFV, by dividing the amount accumulated with the test formulation (Q-test) by the amount accumulated with the commercial cream (Q-cream).
In-vivo studies
Male albino Wistar rats weighing 180–220 g were used for the in-vivo studies. The animals were housed in standard environmental conditions and fed with standard rodent diet with water ad libitum. The experimental protocol was approved by the Local Animal Ethical Committee of Dokuz Eylul University (Approval No 12/2009). In-vivo studies were carried out with commercial cream, selected nanoparticle in gel formulation of DFV and chitosan gel. Their anti-inflammatory, skin blanching activities and TEWL measurements were performed.
Anti-inflammatory activity studies
The formulations were evaluated for their anti-inflammatory activity using a carrageenan-induced rat paw edema model.Citation24 Inflammation was introduced in rats under anesthesia by using 100 μL of a 1% (w/v) λ-carrageenan (Sigma-Aldrich) solution in saline. The solution was injected into the plantar surface of the left hind paw of the rats. The contra-lateral paw received the same volume of sterile saline. To evaluate the topical anti-inflammatory activity of the formulations, four groups of animals (n = 6) with carrageenan-induced paw edema were examined. An initial group of rats was used as a control (untreated), and they received only the carrageenan solution. Formulations were applied to the plantar surface (0.125 mg/cm2) of the hind paw of the rats by gentle rubbing with the formulation 50 times with the index finger for each treatment, 1 hour before induction of inflammation. The increase in paw volume (edema) was measured using digital water plethysmometer (PV-01; SINGA Corporation, Taipei, Taiwan) at 0, 1, 2, 3, 4, and 5 hours after carrageenan administration. The percentage of paw thickness increase from time zero was calculated for each group.
Skin-blanching assay
After shaving the abdominal skin of anesthetized rats, formulations were applied and left on the skin for a period of 1 hour. The degree of blanching was quantified at different time intervals before and after application of the formulations with a Minolta chromameter (CR-400; Konica Minolta, Ramsey, NJ, USA). The instrument was calibrated using the white calibration plate immediately before the study. Baseline readings (zero time) were taken at the untreated control site prior to the application of the formulations. At each observation time, the recorded L-, a- and b-values for each treated and untreated sites were corrected, by subtracting the baseline (time zero) values to yield baseline corrected values.Citation25
TEWL measurement
TEWL measurement was performed with the anesthetized rats by Tewameter (Courage+Khazaka Electronic GmbH, Cologne, Germany) equipped with a TEWL probe. All measurements were taken before and after application of the formulations for 2 minutes by keeping the probe over rat abdominal skin after the removal of the hair in a certain area of 2 cm × 2 cm. All data were expressed in g/m2h after measurements of six replicates.Citation26
Statistical analysis
Statistical data analyses were performed on all data using one-way analysis of variance followed by a multiparametric Tukey’s post-hoc test, with P < 0.05 set as the minimal level of significance.
Results and discussion
Preparation and characterization of nanoparticles
It was previously shown that spherical nanoparticles can be formed by using lecithin and chitosan depending on the electrostatic interaction between the polycationic chitosan and negatively charged soybean lecithin in which lecithin molecules form the core and chitosan molecules form the hydrophilic shell layer to protect the inner structure.Citation17,Citation18 Therefore, DFV-loaded homogenous lecithin/chitosan nanoparticles were successfully prepared using direct injection method with small particle size distribution (polydispersity index < 0.2) in this study. As shown in , nanoparticles with high positive surface charge were attributed to the presence of chitosan chains on the particle surface (41.4 ± 1.60 mV).
Table 1 Physicochemical properties of nanoparticles
The linearity of the calibration curve for DFV has been established within the concentration range of 0.5–12.5 μg/mL, with a correlation coefficient of R2 = 0.9997. Compared with nanoparticles prepared without IPM, significantly higher entrapment efficiency and DFV loading were obtained with nanoparticles including IPM (P < 0.05). The entrapment efficiency and the drug loading of lecithin/chitosan nanoparticles ranged from 53.2% to 86.8% and from 6.3% to 10.3%, respectively. This result was in accordance with previously published data.Citation16 It was considered that DFV, due to its lipophilic structure, was located in the lipid core of lecithin/chitosan nanoparticles whose lipid composition has been modified by the presence of IPM. Thus, IPM existence in nanoparticle formulation enhanced the entrapment efficiency, and as a result, further experiments were performed with the nanoparticle formulations containing IPM (both blank and DFV-loaded nanoparticles).
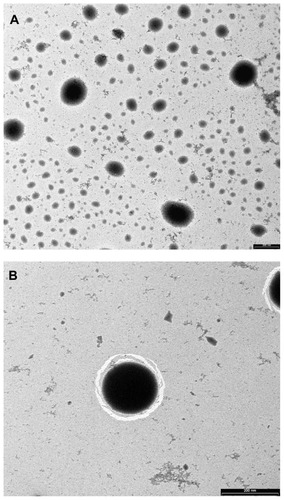
TEM images of nanoparticles showed spherical particles (), and the particles were characterized () by a contrasted corona (chitosan) surrounding a lipidic core (lecithin+IPM) in which the drug was reasonably dissolved. A good correlation was obtained in the particle size as observed by both zetasizer and TEM.
Figure 1 Transmission electron microscopy images of diflucortolone valerate–loaded nanoparticles at magnification ratio of 33,000× (A), at magnification ratio of 71,000× (B).
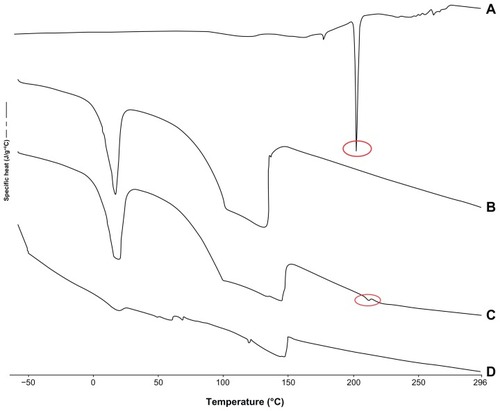
Furthermore, DSC studies were performed, and DFV-loaded nanoparticles showed no melting peak, indicating the absence of crystallinity; whereas DFV in pure form exhibited a melting peak at 207°C, indicating crystalline nature of the drug (). The results of the DSC experiments confirmed the loading of DFV into lecithin/chitosan nanoparticles.
Figure 2 Differential scanning calorimetry thermograms of pure DFV (A), blank nanoparticles (B), physical mixture of blank nanoparticles and DFV (C), and drug-loaded nanoparticles (D).
Abbreviation: DFV, diflucortolone valerate.
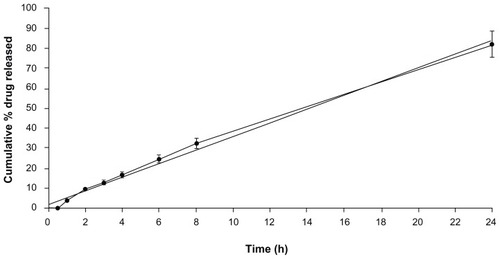
The in-vitro release of DFV from the lecithin/chitosan nanoparticles was investigated using a dialysis bag.Citation27 This method has been used to distinguish the differences between formulations that affect the release behavior of DFV. shows the in-vitro release profile of DFV from nanoparticles. A sustained drug release over a period of 24 hours was observed without any initial burst release, confirming the strong interaction between lecithin and chitosan.Citation14 DSC analysis also confirmed that DFV was amorphously distributed within the lipidic matrix and did not present on the surface, which might be the probable reason for no initial burst release of drug during in-vitro release experiments. The cumulative release data were fitted into different release models: zero order, first order, Higuchi’s square root plot, and Hixson Crowell cube root plot. Zero order release pattern was best fitted with release profile of the formulation with r2 of 0.996.
Stability studies were performed with prepared nanoparticle dispersions. After 3 months of storage, blank and DFV-loaded nanoparticle suspensions, which were kept in glass-capped vials at 25°C and 60% relative humidity, showed good stability of the colloidal suspension, without evidence of aggregation or precipitation. No significant changes were obtained in the physicochemical characterization parameters (P > 0.05) ().
Table 2 Stability results of the nanoparticle formulations
Preparation and characterization of gels
To design a more suitable formulation for topical delivery, aqueous chitosan gels were prepared and nanoparticle suspensions were incorporated in these gels at three different ratios (nanoparticle/gel 1:1, 1:3, and 1:9). The most significant property that forms an advantage for these gel formulations is maintaining the formulation duration over the skin for a certain period of time, therefore increasing the efficiency.
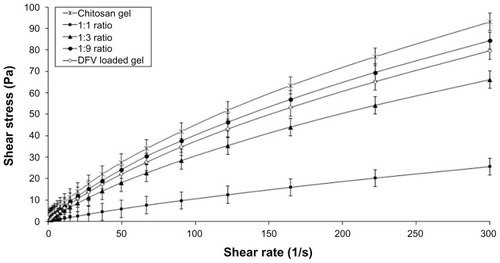
The pH values of all gel formulations were found to be acceptable and similar to skin pH (average of 4.80 ± 0.12), as the formulation is intended for topical use. The rheological properties of semisolid vehicles are important as they have the potential to affect the sensory feeling of formulations.Citation28 Similarly, these rheological properties are also important, in order to predict the effects of the formulations and improve their quality and stability. shows the rheograms of prepared gel formulations obtained by plotting the applied shear rate as a function of the shear stress. As it can be seen in the rheograms, the formulations showed non-Newtonian behavior in which the viscosity decreases with increasing shear rate.Citation3 All formulations exhibited shear-thinning behavior that is a desired property in topical preparations because it facilitates the application on the skin surface. In addition to that, the viscosity values of gels decreased with increasing amount of DFV nanoparticles in formulations, as expected. These results are in accordance with the literature findings.Citation29 Similarly, a study by Mucha et alCitation30 reported an increase in the shear stress and viscosity of chitosan solutions with increasing chitosan concentration also due to the increase in entanglement among the macromolecular chains.
Figure 4 Flow rheograms of chitosan gel formulations – shear rate versus shear stress (n = 3).
Abbreviations: DFV, diflucortolone valerate; Pa, pascal.
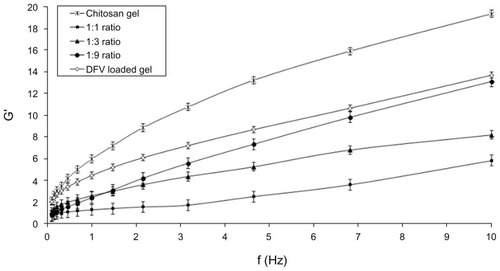
The possible interaction between a nanocarrier and a vehicle system should possibly affect the viscoelastic properties of the topical formulation. In accordance with that, the movement capability of the nanocarrier in the vehicle should also be influenced by these interactions, if any are present.Citation28 Therefore, the change in rheological properties of the gel formulations in response to nanoparticle addition was measured using a constant strain in the linear viscoelastic regions for the formulations. In this study, oscillatory stress sweep test was performed to determine the linear viscoelastic regimen for all formulations. The constant stress was found as 0.06 Pa for frequency sweep analysis, which was performed to determine the viscoelastic performance of formulations. The storage (elastic) modulus (G′) and loss (viscous) modulus (G′) were measured for gel formulations.Citation31,Citation32 The storage modulus as a function of frequency for chitosan hydrogels of varying nanoparticle concentrations are presented in . Altering the concentration of nanoparticles dramatically changed the viscoelastic properties of the gel. Reduction of the percentage of nanoparticle in the gel from 50% to 10% increased the G′ values. The enhancement of the elastic modulus with the chitosan concentration has been attributed to the presence of more polymer chains responsible for more entangled junctions.Citation28
Figure 5 The elastic modulus plotted versus frequency of formulations (n = 3).
Abbreviation: DFV, diflucortolone valerate; G′, elastic (storage) modulus.
Mechanical properties of gels for topical delivery are important for the maximum benefit of the patient from the formulation; these being responsible for the correct dosage transfer to the target site and easy application on the substrate. Texture profile analysis defines the mechanical parameters in terms of hardness, adhesiveness, cohesiveness, compressibility, and elasticity. The mechanical properties of formulations are shown in . The hardness and compressibility of the gels were raised when the viscosity of the gel formulations increased. The highest compressibility (0.028 ± 0.004 N · mm) was obtained with 1:9 ratio. All gel formulations showed high cohesiveness (>0.857 N · mm), and they also had an acceptable elasticity value compared with previous literature findings.Citation33 In this study, the adhesiveness of gel decrement was affected by added nanoparticle ratio. All the formulations were found easily spreadable and nondripping in nature.
Table 3 Mechanical properties of formulations
The stability of chitosan gels containing lecithin/chitosan nanoparticles in terms of drug content and gel stability was investigated during 3 months. No significant change was observed for the pH, viscosity, and drug content values of the formulations (P > 0.05). No phase separation was detected, which indicated that chitosan gel containing DFV nanoparticles had good physical stability.
Skin permeation experiments
It is well known that vehicles used in topical formulations can greatly influence the rate and extent of drug permeation into and across the skin, which clearly modifies the potency of the topical corticosteroid formulation. The nanoparticles tended to suppress the penetration (transdermal delivery) through the skin while enhancing permeation (dermal delivery) of the drug into the upper skin layers. For this purpose, in order to assess the advantages of this carrier-based formulation in topical administration, the accumulation and permeation across rat skin were compared with chitosan gel and commercially available cream of DFV.
In the permeation experiments, two parameters were obtained: (1) the rate of permeation (flux) of the drug through the skin into the receptor fluid; and (2) the concentration of the drug in the skin (SC+epidermis and dermis).Citation34 Permeation rates were calculated by taking samples from the receptor phase at various time points, while the skin concentration reported here refers to the 6-hour time point at the end of the experiment.
During the permeation studies, DFV was not found in the receptor medium with any preparation at the end of 6 hours. This indicated that the activity of the drug presented in the skin did not produce a significant transdermal transport through the rat skin throughout the experiment. Therefore, the drug accumulated in the skin sample layers was investigated. The amounts of DFV accumulated in the skin layers was normalized by the weight of tissue and expressed as μg DFV per mg of tissue. presents the amount of DFV found in the SC+epidermis and dermis.
Figure 6 Amount of DFV accumulated from formulations in the SC+epidermis (dark bars) and dermis (light bars) (n = 6).
Note: *Denotes significantly different from commercial cream and chitosan gel (P < 0.05).
Abbreviations: DFV, diflucortolone valerate; SC, stratum corneum.
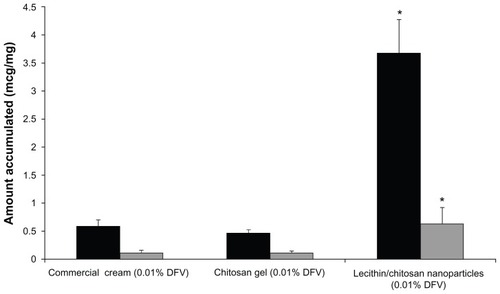
The direct application of the nanoparticle colloidal suspension on skin surface was performed to show the vehicle effect. It produced a significantly higher amount of DFV in the SC+epidermis and dermis in comparison with the tested dosage forms (P < 0.05). The enhancement ratio of the nanoparticle dispersion versus commercial cream was calculated to demonstrate the accumulating capacity of lecithin/chitosan nanoparticles. The results indicated that these nanoparticles showed 6.33- and 5.79-fold higher retention compared with commercial cream for DFV in SC+epidermis and dermis, respectively. The statistical difference between the nanoparticle dispersion and the semisolid formulation group in the accumulation of DFV in the SC+epidermis could be due to the unique characteristics of the prepared nanoparticles, as well as their positive charge and large surface area, ensuring an excellent contact surface between the vehicles and skin over the entire application area and promoting permeation and accumulation of drug in the skin.Citation18
Since epidermal lipids are found in high amounts within the penetration barrier, lipid carriers attach themselves to the skin surface and allow lipid exchange between the outermost layers of the skin and enhance the penetration of active substances. As expected, the penetration enhancer effect of lecithin/chitosan nanoparticles was determined both in SC+epidermis and dermis. Overall, it can be concluded that lecithin, the main component of our system, was responsible for the enhancer effect.
Moreover, the accumulation studies of DFV from nanoparticle colloidal suspension incorporated in chitosan gels at different ratios (1:9, 1:3, and 1:1, DFV concentrations of 0.01%, 0.025%, and 0.05% w/w, respectively) were realized. The presence of nanoparticles in chitosan gels produced lower skin retention of DFV compared with the undiluted form of nanoparticles. The result disclosed that the increased amount of nanoparticles in chitosan gel did not significantly affect the skin retention of DFV (P > 0.05) (). Therefore, it can be assumed that the concentration of nanoparticles in chitosan gels does not seem to play a determinant role.
Figure 7 Accumulation of DFV from nanoparticles in chitosan gels as a function of drug concentration in the SC+epidermis (dark bars) and dermis (light bars) (n = 6).
Abbreviations: DFV, diflucortolone valerate; SC, stratum corneum.
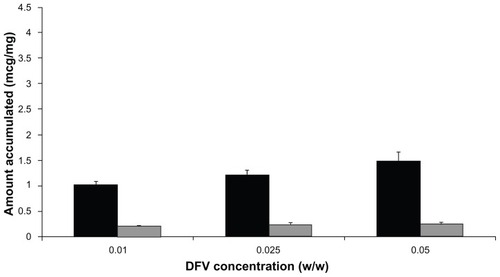
It was found that gel formulation prepared at 1:9 ratio increased the retention of DFV in the skin layers when compared with commercial cream. It is a remarkable point to consider that although the concentration of DFV was 10 times higher in the commercial cream formulation with respect to the gel; the retention of DFV in the skin layers was significantly higher with gel formulations (P < 0.05).
In-vivo studies
Anti-inflammatory activity studies
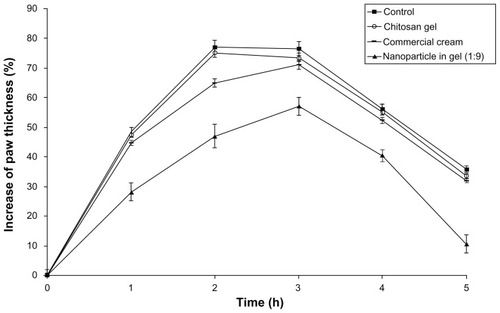
The anti-inflammatory activity of the formulations was evaluated by hind-paw edema test using plethysmometer, which is the most convenient, rapid, and accurate assessment technique, providing the measurement of changes in paw volume by water displacement.Citation24 In this study, the progress of the paw edema test was in accordance with the previous findings existing in the literature.Citation35 Induction of acute inflammation in control rats resulted in a prominent increase in paw thickness throughout the first hour after intraplantar injection of carrageenan and reached a peak of inflammation after 3 hours (). From the statistical analysis, it was found that gel formulations containing DFV nanoparticles had a significant anti-inflammatory effect (P < 0.05) compared with commercial and control groups. Overall, according to these results, nanoparticle in gel (1:9) formulation can be considered promising topical formulations with better anti-inflammatory activity than commercial cream for effective therapy.
Skin blanching assay
In the specific case of steroids, the skin blanching (or vasoconstriction) response is a useful technique, which provides information on potency of the drug or efficacy of the delivery vehicle. The assay is also useful for the comparison of the topical availability of corticosteroid formulations containing the same concentration of the same corticosteroid.Citation22,Citation36 In many studies, the vasoconstriction assay employed visual evaluation of the degree of blanching by trained observers.Citation37,Citation38
In this study, a chromameter was utilized to quantify and compare the vasoconstrictor effect of DFV. Chromameters are compact portable instruments used for the assessment of surface color based on the tristimulus analysis of a reflected xenon light pulse. The major advantage of this device is to minimize the relativity factor depending on the human eye.Citation39 Moreover, this method is noninvasive and involves only one investigator, instead of several trained judges for visual scoring. In principle, the chromameter records three-dimensional color reflectance including “L”, “a” and “b”. L represents luminance, which is the relative brightness value, with 0 meaning totally black and 100 meaning pure white. The balance between red (100)/green (−100) and yellow (100)/blue (−100) is represented by “a” and “b”, respectively.Citation40 Blanching effect that expected to be formed after administration of formulation on male albino Wistar rat skin was measured with the chromameter, and the results were correlated to the results of ex-vivo permeation studies. The difference in color (Δa) between the gel-treated site and the untreated site as the control were described.
Studies on corticosteroids indicated that drug content in the SC+epidermis could be related to the skin-blanching response and that drug concentration may be used to estimate formulation efficiency. SC drug concentration could be affected by different factors, among which application conditions (application time, vehicle composition, drug concentration in the vehicle, anatomical site) may play an important role.Citation41 Therefore, drug content in the SC+epidermis was determined and correlated with the observed skin blanching effect.
Various studies, using a vasoconstrictor assay, have shown that huge differences exist between generic and original formulations containing the same glucocorticoid at the same concentration in different vehicles.Citation42 The amount of drug needed for the same blanching response differs according to the steroid solubility in the vehicle. However, the same thermodynamic activity of the drug in the vehicle leads to the same pharmacodynamic response, provided that the formulation does not change either the solubility of the drug in the SC or its diffusivity across the SC. In one study, the skin blanching response of betamethasone 17-benzoate increases proportionally with the concentration of the dissolved and diffusible drug, reaching the maximal response when the concentration equals the solubility of the drug in the vehicle. The blanching response from suspensions of the drug is independent of the betamethasone 17-benzoate concentration.Citation43
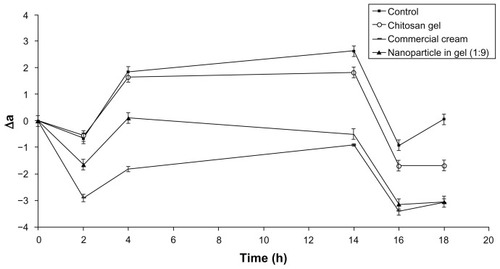
Montenegro et alCitation41 assessed the effect of application time on skin blanching response and SC concentration after topical application of 0.1% betamethasone-17-valerate cream. Their study suggested that the duration of application should be carefully chosen to assess drug topical bioavailability, since after long application times, skin blanching response may not be related to drug concentration in the SC. Schafer-Korting et alCitation9 suggested that drug levels in the skin layers are reported to parallel dermal availability and to be related to the skin-blanching response of topical glucocorticoids. Their comparative studies indicated a correlation of in-vitro penetration and in-vivo skin blanching data. In this present study, it was known that lecithin/chitosan nanoparticles dramatically increased the skin accumulation of DFV; skin blanching results were found in accordance with these skin permeation studies. As shown in , when compared with the value of color difference (Δa) between the control and DFV including formulations of treated site, skin blanching effect was observed. There was no significant difference between the chitosan gel and control group, as was expected (P > 0.05). Commercial formulation showed the maximum vasoconstrictor effect at the initial time points (2 and 4 hours); but in the last three measurement time points there was no significant difference with the gel formulations containing DFV-loaded nanoparticles (P > 0.05). This possibly depends on the fast penetration of DFV from commercial formulations towards the vascular endothelium structure. However, within the time course, the DFV from gel formulations showed similar vasoconstriction activity even though they contain 1/10 of DFV with respect to commercial formulation. The maximum blanching response was obtained at 16 hours after application of the formulations, similar to previously reported corticosteroid skin blanching studies.Citation37,Citation41 The similar results between tested gel formulations and commercially available formulation suggest that these gel formulations could also be a good candidate for topical application since they show no penetration.Citation25
TEWL measurements
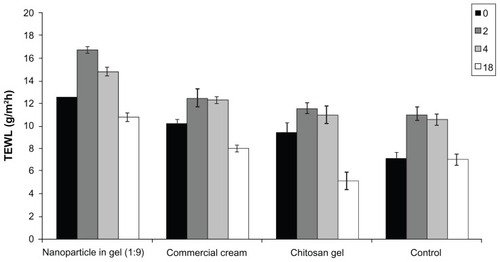
TEWL measurements, a noninvasive technique commonly used to assess the barrier function of the SC, were also performed.Citation26 Prolonged glucocorticosteroid therapy increases basal TEWL, indicating an effect on permeability barrier function. This change is commonly associated with the reduction of lipid content, SC thickness, and number of lamellar bodies.Citation43,Citation44 In this study, after TEWL measurements showed that there was no significant difference (P > 0.05) from 12.53 ± 0.68 g/h · m2 to 10.76 ± 0.45 g/h · m2 before and after treatment with nanoparticle in gel, which further suggested that barrier function of the skin (see ).
Conclusion
Topical steroid delivery without significant permeation is a challenging issue in treatment of skin disorders. In this study, by formulating lecithin/chitosan nanoparticles, sustained release of DFV was achieved up to 24 hours. In addition, improved accumulation of the DFV in SC+epidermis was successfully accomplished even incorporating these nanoparticles into chitosan gel formulation, which provided a more suitable dosage form for topical drug delivery. Stable chitosan gels containing these DFV-loaded nanoparticles (1:9) showed adequate drug content, compatible pH with skin, and sufficient rheological and mechanical properties for topical application. Although the amount of DFV in commercial cream formulation was 10 times higher than the gel including nanoparticles, better results were obtained with nanoparticle in gel formulation by means of ex-vivo permeation and in-vivo studies. This is a remarkable point for the reduction of the side effects of DFV. The formulation produced significantly higher anti-inflammatory effect when compared with commercial cream as a result of pharmacodynamic studies in rats (P < 0.05). Furthermore, change of skin blanching as a result of vasoconstrictor effect of this formulation, which had no penetration rates, was similar to the commercial product. The system also did not cause any changes in the barrier function of the skin, as confirmed by TEWL measurements. This clearly indicates that chitosan gel, which includes lecithin/chitosan nanoparticles, could be classified as a promising vehicle for the dermal delivery of DFV in various skin disorders.
The formed eczema intensity will be evaluated by both scoring methods and histopathologically for further studies.
Acknowledgments
This work was supported by The Scientific and Technological Research Council of Turkey (Project number: 109S433).
Disclosure
The authors report no conflicts of interest in this work.
References
- BrazziniBPimpinelliNNew and established topical corticosteroids in dermatology: clinical pharmacology and therapeutic useAm J Clin Dermatol200231475811817968
- PatelHKBarotBSParejiyaPBShelatPKShuklaATopical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studiesColloids Surf B Biointerfaces2013102869423000677
- FontanaMCRezerJFCoradiniKLealDBBeckRCImproved efficacy in the treatment of contact dermatitis in rats by a dermatological nanomedicine containing clobetasol propionateEur J Pharm Biopharm201179224124921605671
- FangJYWuPCHuangYBTsaiYHIn vitro permeation study of capsaicin and its synthetic derivatives from ointment bases using various skin typesInt J Pharm1995126119128
- SenyigitTPadulaCOzerOSantiPDifferent approaches for improving skin accumulation of topical corticosteroidsInt J Pharm200938015516019635541
- FisherDAAdverse effects of topical corticosteroid useWest J Med199516221231267794369
- LevinCMaibachHITopical corticosteroid-induced adrenocortical insufficiency: clinical implicationsAm J Clin Dermatol20023314114711978135
- GilletAComperePLecomteFLiposome surface charge influence on skin penetration behaviourInt J Pharm20114111–222323121458550
- Schafer-KortingMMehnertWKortingHCLipid nanoparticles for improved topical application of drugs for skin diseasesAdv Drug Deliv Rev20075942744317544165
- SenyigitTOzcanIOzerOInnovative topical formulations for treatment of dermatitisRecent Pat Inflamm Allergy Drug Discov20126318620122827752
- GokceEHKorkmazEDelleraESandriGBonferoniMCOzerOResveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applicationsInt J Nanomedicine201271841185022605933
- ProwTWGriceJELinLLNanoparticles and microparticles for skin drug deliveryAdv Drug Deliv Rev20116347049121315122
- RajanRVasudevanDTEffect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazoleJ Adv Pharm Technol Res20123211211622837959
- HafnerALovrićJPepićIFilipović-GrčićJLecithin/chitosan nanoparticles for transdermal delivery of melatoninJ Microencapsul201128880781522117177
- OzcanISenyigitTGökceEHOzerOCurrent status of chitosan on dermal/transdermal drug delivery systemsDavisSPChitosan: Manufacture, Properties and UsageNew YorkNOVA Science Publishers Inc2010449484
- SenyigitTSonvicoFBarbieriSOzerOSantiPColomboPLecithin-chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skinJ Control Release2010142336837319932722
- SonvicoFCagnaniARossiAFormation of self-organized nanoparticles by lecithin/chitosan ionic interactionInt J Pharm2006324677316973314
- TanQLiuWGuoCZhaiGPreparation and evaluation of quercetin- loaded lecithin-chitosan nanoparticles for topical deliveryInt J Nanomedicine201161621163021904452
- HornEJDommSKatzHILebwohlMMrowietzUKragballeKTopical corticosteroids in psoriasis: strategies for improving safetyJ Eur Acad Dermatol Venereol201024211912420175860
- ColemanGLKanferIHaighJMComparative blanching activities of proprietary, diflucortolone valerate topical preparationsDermatologica19781564224230342295
- HaighJMKanferIAssessment of topical corticosteroid preparations: the human skin blanching assayInt J Pharm198419245262
- SchwarbFPSmithEWHaighJMSurberCAnalysis of chromameter results obtained from corticosteroid-induced skin blanching assay: comparison of visual and chromameter dataEur J Pharm Biopharm19994726126710382110
- JonesDSLawlorMSWoolfsonADExamination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-comaleic anhydride) and poly(vinylpyrrolidone): rheological and mathematical interpretation of textural parametersJ Pharm Sci20029192090210112210055
- MorrisCJCarrageenan-induced paw edema in the rat and mouse from: methods in molecular biologyWinyardPGWilloughbyDAInflammation ProtocolsTotowaHumana Press Inc2003115121
- WuPCChangJSHuangYBChaiCYTsaiYHEvaluation of percutaneous absorption and skin irritation of ketoprofen through rat skin: in vitro and in vivo studyInt J Pharm200122222523511427353
- BarelAOClarysPStudy of the stratum corneum barrier function by transepidermal water woss measurements: comparison between two commercial instruments: Evaporimeter® and Tewameter®Skin Pharmacol1995841861957488395
- JainSMittalAAmitJKEnhanced topical delivery of cyclosporine-A using PLGA nanoparticles as carrierCurr Nanosci201174524530
- ModdaresiMBrownMBZhaoYTamburicSJonesSAThe role of vehicle–nanoparticle interactions in topical drug deliveryInt J Pharm201040017618220727392
- El-HefianEAYahayaAHRheological study of chitosan and its blends: an overviewMaejo Int J Sci Technol201042210220
- MuchaMRheological characteristics of semi-dilute chitosan solutionsMacromol Chem Phys1997198471484
- HernándezRZamora-MoraVSibaja-BallesteroMVega-BaudritJLópezDMijangosCInfluence of iron oxide nanoparticles on the rheological properties of hybrid chitosan ferrogelsJ Colloid Interface Sci2009339535919699487
- José MouraMMargarida FigueiredoMHelena GilMRheological study of genipin cross-linked chitosan hydrogelsBiomacromolecules200783823382918004810
- JonesDSWoolfsonADBrownAFTextural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymersInt J Pharm1997151223233
- BillichAAschauerHAszódiAStuetzAPercutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimusInt J Pharm2004269293514698574
- SenyigitTTekmenISonmezUSantiPOzerODeoxycholate hydrogels of betamethasone-17-valerate intended for topical use: in vitro and in vivo evaluationInt J Pharm201140312312921047547
- MugglestoneCJMarizSLaneMEThe development and registration of topical pharmaceuticalsInt J Pharm2012435222622486961
- BachMLippoldBCInfluence of penetration enhancers on the blanching intensity of betamethasone 17-benzoateInt J Pharm199816897108
- HerkenneCAlbertiINaikAIn vivo methods for the assessment of topical drug bioavailabilityPharm Res20082518710317985216
- HaighJMMeyerESmithEWKanferIThe human skin blanching assay for in vivo topical corticosteroid assessment II. Subject- and observer-dependent variation in blanching responsesInt J Pharm1997152185192
- PershingLKBakhtianSPonceletGCorlettJLShahVPComparison of skin stripping, in vitro release, and skin blanching response methods to measure dose response and similarity of triamcinolone acetonide cream strengths from two manufactured sourcesJ Pharm Sci2002911312132311977107
- MontenegroLAdemolaJIBoninaFPMaibachHIEffect of application time of betamethasone-17-valerate 0.1% cream on skin blanching and stratum corneum drug concentrationInt J Pharm19961405160
- WoodfordRBarryBWBioavailability and activity of betamethasone 17-benzoate in gel and cream formulations: comparison with proprietary topical corticosteroid preparations in the vasoconstrictor assayCurr Ther Res19741643383454208350
- LamaudLLambreyBSchallaWSchaeferHCorrelation between transepidermal water loss and penetration of drugsJ Invest Dermatol198482556563
- WesterRCMaibachHIHuman percutaneous absorption and transepidermal water loss (TEWL) correlationBronaughRLMaibachHIPercutaneous AbsorptionFloridaTaylor and Francis, Boca Raton2005509520