Abstract
Nanosilver particles (NSPs), are among the most attractive nanomaterials, and have been widely used in a range of biomedical applications, including diagnosis, treatment, drug delivery, medical device coating, and for personal health care. With the increasing application of NSPs in medical contexts, it is becoming necessary for a better understanding of the mechanisms of NSPs’ biological interactions and their potential toxicity. In this review, we first introduce the synthesis routes of NSPs, including physical, chemical, and biological or green synthesis. Then the unique physiochemical properties of NSPs, such as antibacterial, antifungal, antiviral, and anti-inflammatory activity, are discussed in detail. Further, some recent applications of NSPs in prevention, diagnosis, and treatment in medical fields are described. Finally, potential toxicology considerations of NSPs, both in vitro and in vivo, are also addressed.
Introduction
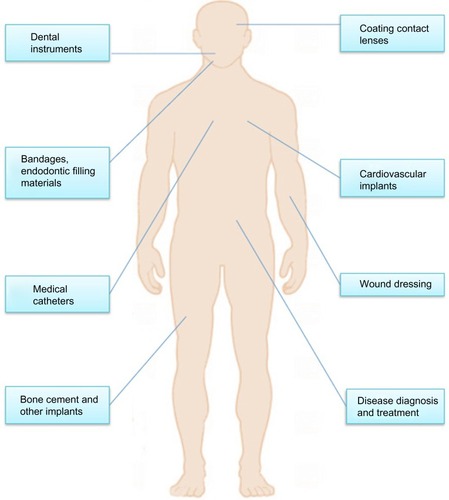
Nanosilver particles (NSPs) generally present at 1 to 100 nm in size in at least one dimension.Citation1–Citation4 As particle size decreases, the surface area-to-volume ratio of NSPs increases dramatically, which leads to significant changes in their physical, chemical, and biological properties. NSPs have been among the most commonly used nano-materials in our health care system for hundreds of years. Recently, NSPs have become of intense interest in biomedical applications (), because of their antibacterial, antifungal, antiviral, and anti-inflammatory activity.Citation5,Citation6
NSPs have been widely used for diagnosis,Citation7 treatment,Citation8 drug delivery,Citation9 medical device coating,Citation10 wound dressings,Citation11 medical textiles,Citation12 and contraceptive devices.Citation13 As the use of nanosilver products is continually increasing, a better understanding of nanosilver biological interactions and their toxicity becomes necessary. This review critically discusses NSP synthesis methods, properties, and current and emerging medical NSP applications. Finally, recent advances concerning NSP potential toxicity will also be described.
NSP synthesis
Different synthetic NSP routes lead to variable sizes, shapes, morphology, and even stability. Generally, these methods can be classified into three broad categories: physical, chemical, and biological (or green) synthesis.
Physical synthesis
Evaporation/condensation and laser ablation are the main physical techniques for deriving nanosilver from metal samples. The evaporation/condensation technique uses a furnace tube under atmospheric pressure to produce NSPs; however, conventional furnace tubes have several drawbacks, such as high energy consumption, and require a long time to achieve thermal stability. Jung et al used a small ceramic heater with a local heating area, thus the evaporated vapor could cool at a suitable rate and a high concentration of nanosilver could be obtained.Citation14 Laser synthesis employs the laser ablation of metals in solution without chemical reagents, which leads to pure nanosilver colloids.Citation15 The concentration and morphology of nanosilver are affected by laser fluence and the number of laser shots. Greater laser fluence and amount of time, lead to larger particle size and higher particle concentration.Citation16 Recently, Tien et al reported a novel arc-discharge method of producing silver suspension in pure water without any surfactants or stabilizers.Citation17 In their research, silver wires were utilized as positive and negative electrodes and etched in pure water. During discharge, the surface layer of the silver wires was evaporated and condensed in the water, thus stable and well-dispersed NSPs of 20–30 nm in size were obtained.Citation17
Chemical synthesis
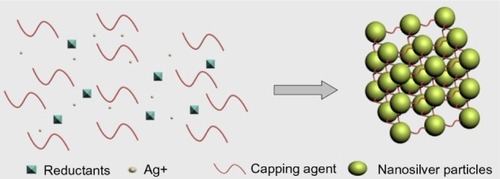
Chemical reduction is the most frequent method of nanosilver synthesis, and uses silver salt, reductants, and a stabilizer or capping agents as three main components to control NSP growth (). Among these, silver nitrate is a silver salt that is often used for NSPs, due to its low cost and chemical stability compared to the other available silver salts.Citation17 The reductants include borohydride,Citation18 citrate,Citation19 ascorbate,Citation20 and hydrogen gas.Citation11
Borohydride is a strong reducing agent that can result in small particles with a faster reduction rate, because borohydride can also act as an NSP stabilizer and avoid aggregation of NSPs during its decomposition.Citation11 It is hard to obtain high concentrations of NSPs because of their aggregative instability. Using a stabilizer in preparation is a common strategy. The stabilizers include surfactants and ligands or polymers that contain functional groups such as polyvinylpyrrolidone, poly(ethylene glycol), poly(methacrylic acid), poly(methyl methacrylate), and others. Furthermore, temperature-sensitive polymers such as poly(N-isopropylacrylamide) and collagen can also serve as stabilizers, and nanosilver capped by those chemicals allows for novel thermal switching applications.Citation1
NSPs can also be synthesized in a two-phase water-organic system. This method produces uniform and controllable nanoparticles. In this system, metal precursor and reducing agent are separated in two phases, thus the rate of interaction can be controlled by the intensity of interphase transport between aqueous and oil phases; however, large amounts of surfactant and organic solvent may contaminate the surface of formed NSPs, and the removal of surfactant and organic solvent is also time-consuming and expensive.
Biological synthesis
Biosynthesis (green synthesis) of nanosilver has received extensive attention due to the growing need for environmentally friendly synthesis methods that use eco-friendly reducing and capping agents, such as protein;Citation21 peptides;Citation22 carbohydrate;Citation23 various species of bacteria,Citation24 fungi,Citation25 and yeast;Citation26 and algae and plants.Citation27 For example, Naik et al synthesized NSPs of 60–150 nm in size using silver-binding peptides identified from a combinatorial phage-display peptide library. The peptides were placed in an aqueous solution of 0.1 mM silver nitrate for 24–48 hours at room temperature.Citation21 Thomas et al developed an economical, fascicled, and in situ approach to prepare large-scale chitosan–nanosilver (400 nm) films using chitosan as a chelating and stabilizing agent; the films demonstrated excellent antibacterial action against Escherichia coli and Bacillus.Citation28 Sintubin et al reviewed different biological synthesis methods using microorganisms or plants for nanosilver synthesis.Citation26
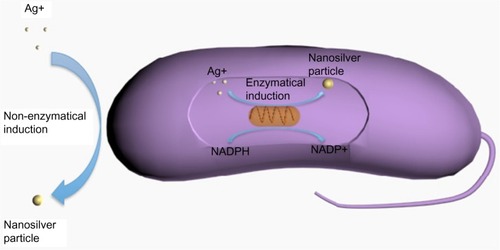
In biological synthesis, as the reducing agents and stabilizers are molecules produced by protein, carbohydrate, bacteria, fungi, yeasts, algae, or plants, organic solvents and toxic reagents are avoided. The possible mechanism of biological synthesis includes enzymatic and non-enzymatic reduction (). Nicotinamide adenine dinucleotide phosphate-dependent reductase can produce NSPs by enzymatic reduction; however, the enzymatic reduction rate is often slow (between 24 and 120 hours).Citation29 The non-enzymatic reduction of silver is similar to chemical reduction, but the reducing and stabilizing agents are microorganisms or plants. Non-enzymatic reduction is usually fast, often completed within a few minutes, and can handle extreme parameters, such as high pH or high temperature, that accelerate the synthesis.Citation24
Figure 3 Biological (or green) synthesis of nanosilver particles.
Abbreviations: NADP+, nicotinamide adenine dinucleotide phosphate (oxidized form); NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); Ag, silver.
The main advantage of biogenic synthesis over other methods is that the green synthesis avoids organic solvents and toxic reagents. Thus, biosynthesized NSPs are more stable than those that are chemically produced, and they can remain stable over a long period of time.Citation30 In addition, biological synthesis makes it possible to produce NSPs under a nontoxic silver nitrate concentration because microbial cells can continue to multiply;Citation31 however, the biosynthesis drawback is that the purification process may lead to pathogenic bacteria and the potential bacteria may cause contamination, which should be a reason for exercising caution in medical application.Citation26
NSP performance
Antibacterial properties
NSPs have a broad antibacterial effect on a range of Gram-negative and Gram-positive bacteria and antibiotic-resistant bacteria strains.Citation32 Antimicrobial efficacy of NSPs depends on their size and concentration. Normally, a high concentration leads to more effective antimicrobial activity, while particles of small sizes can kill bacteria at a lower concentration. Apart from size and concentration, shape also influences the antimicrobial efficiency of NSPs. Sadeghi et al investigated the antimicrobial activity of different nanosilver shapes, which included silver nanoplates, silver nanorods, and silver nanoparticles, on Staphylococcus aureus and E. coli. They found that silver nanoplates had the best antimicrobial activity.Citation33 It has also been reported that NSPs combined with various antibiotics have better antimicrobial effects than NSPs or antibiotics alone. Li et al, for example, found a greater antibacterial effect on E. coli when amoxicillin and silver nanoparticles were combined than when they were applied separately.Citation34
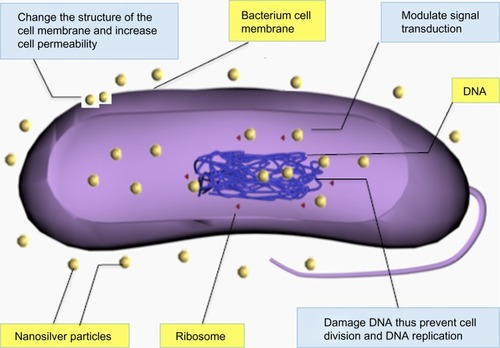
Although the antimicrobial effect of nanosilver has been widely studied, the exact mechanism of NSPs is still elusive. It is widely accepted that NSPs can anchor to and subsequently penetrate the bacterial cell wall, thereby causing structural change of the cell membrane and increasing cell permeability, leading to cell death ().Citation35 The formation of free radicals and subsequent free radical-induced membrane damage is another potential mechanism, which has been investigated by Kim et al.Citation32 It has also been found that NSPs can release silver ions and interact with the thiol groups of many vital enzymes and phosphorus-containing bases, thus inhibiting some functions in cells, such as preventing cell division and DNA replication.Citation36 In addition, NSPs may modulate signal transduction through changing the phosphotyrosine profile of bacterial peptides for the potential antibacterial mechanism ().Citation37
Antifungal properties
Nanosilver is an effective antifungal agent against a broad spectrum of common fungi. Kim et al investigated NSP antifungal properties on a total of 44 strains of six fungal species, and found that NSPs can inhibit the growth of Candida albicans, Candida glabrata, Candida parapsilosis, Candida krusei, and Trichophyton mentagrophytes effectively.Citation38 Nasrollahi et alCitation39 and Kim et alCitation40 observed that NSPs can disrupt cellular membrane and inhibit the normal budding process; however, the exact mechanisms of action of nanosilver against fungi are still not clear.
Antiviral properties
NSPs are also an antiviral agent against HIV-1,Citation41 hepatitis B virus,Citation42 respiratory syncytial virus,Citation43 herpes simplex virus type 1,Citation44 and monkeypox virus.Citation45 It has been observed that NSPs have higher antiviral activity than silver ions, due to species difference as they dissolve to release Ag0 (atomic) and Ag+ (ionic) clusters, whereas silver salts release Ag+ only.Citation46 Lara found that the anti-HIV mechanism of nanosilver is based on the inhibition of the initial stages of the HIV-1 cycle.Citation47 NSPs can bind to glycoprotein (gp)120, thus inhibit cluster of differentiation (CD) 4-dependent binding, fusion, and infectivity. They act as an effective virucidal agent to block HIV-1 cell-free and cell-associated infection. Furthermore, NSPs inhibit post-entry stages of the HIV-1 life cycle.Citation47 Although the mechanism underlying their viral-inhibitory activity is not yet fully understood, NSPs could be considered to be a broad-spectrum agent against a variety of viral strains and are not prone to developing resistance.
Anti-inflammatory properties
NSPs show anti-inflammatory properties in both animal models and in clinic. For example, in the swine model with contact dermatitis induced by topically applying 1,2-dinitrochlorobenzene, nanosilvers altered the expression of proinflammatory cytokines transforming growth factor-β and tumor necrosis factor-α.Citation48 Shin and Ye found that NSPs attenuated nasal symptoms in allergic rhinitis mice and inhibited OVA-specific immunoglobulin E, IL-4, and interleukin-10, and that inflammatory cell infiltration and goblet cell hyperplasia were inhibited by nanosilvers.Citation49 In a human clinical study, wound dressing containing NSPs promoted the healing of chronic leg ulcers by not only reducing bacteria numbers in the wound bed, but by decreasing inflammatory response as well.Citation8 NSPs’ ability to reduce cytokine release and matrix metalloproteinases,Citation8,Citation50 decrease lymphocyte and mast cell infiltration,Citation48 and induce apoptosis in inflammatory cellsCitation8,Citation49 may explain their anti-inflammatory mechanisms.
Medical NSP applications
Wound dressings
Robert Burrell developed the world’s first commercially available nanosilver product (Acticoat™; Smith and Nephew, London, UK) to treat various wounds in clinic, including burns, chronic ulcers, toxic epidermal necrolysis, and pemphigus.Citation51 Huang et al observed that NSP-loaded wound dressings significantly reduced the healing time by an average of 3.35 days and increased bacterial clearance from infected wounds compared to silver sulfadiazine, with no adverse effects;Citation52 however, Chen et al showed that nanosilver-loaded wound dressings could enhance healing in superficial burn wounds but made no difference in deep burn wounds, compared with 1% silver sulfadiazine.Citation53 This suggests that NSPs accelerate reepithelialization but not angiogenesis.
Currently, new dressings are being fabricated with the aim of increasing antibacterial efficacy and promotion of wound healing. For example, Lu et al developed a wound dressing composed of NSPs and chitosan, and found that it significantly increased wound healing during treatment of deep partial-thickness wounds and inhibited infection, as well as diminished the risk of silver absorption, compared with 1% silver sulfadiazine dressings.Citation54
Cardiovascular implants
The first cardiovascular medical device containing silver in clinic was a prosthetic silicone heart valve coated with silver element, which was designed to prevent bacterial infection on the silicone valve and to reduce inflammation response;Citation55 however, metal silver may cause hypersensitivity, inhibits normal fibroblast function, and leads to paravalvular leakage in patients.Citation56 NSPs are safe and nontoxic in medical devices, unlike metal silver. Therefore, Andara et al synthesized a new nanocomposite with NSPs and diamond-like carbon as a surface coating for heart valves and stents, and found that the surface of the nanocomposite showed antithrombogenic and antibacterial properties.Citation57 In addition, Ghanbari et alCitation58 and Fu et alCitation59 also constructed antibacterial multilayer films containing NSPs, and investigated their antibacterial, mechanical, and hemodynamic properties in vitro for use in cardiovascular implant coating.
Catheters
Much research has been conducted to investigate NSPs as antibacterial materials for coating catheters, including central venous catheters and neurosurgical catheters. Silverline (Spiegelberg GmbH and Co. KG, Hamburg, Germany) and ON-Q Silver Soaker™ (I-Flow Corporation, CA, USA) are two commercially available medical catheters containing NSPs to prevent catheter-associated infections.Citation51 Medical catheters are prone to bacterial infection, which can rapidly spread to the wound and its surrounding, and lead to serious complications. Because of their superior antibacterial properties and lack of observed toxicity, NSPs can decrease the incidence of bacterial infection and complications after surgery, thus they have been widely accepted for use in medical catheters. Andara et al found that plastic catheter tubes coated with nanosilver could inhibit bacterium growth in vitro for at least 72 hours, with no significant toxicity, in an animal model.Citation57 In a pilot clinical study, 19 patients who received a nanosilver catheter did not show catheter-associated ventriculitis, and all cerebrospinal fluid cultures were negative, while five patients were positive for catheter-associated ventriculitis in the control group (20 patients).Citation60
Bone cement
Alt et al evaluated antibacterial activity of plain poly(methyl methacrylate) bone cement loaded with different NSP concentrations in vitro, and found that bone cement-loaded 1% nanosilver completely inhibited the proliferation of Staphylococcus epidermidis, methicillin-resistant S. epidermidis, and methicillin-resistant S. aureus, with no significant difference between the nanosilver bone cement and the nontoxic control group in quantitative and qualitative cytotoxicity tests.Citation61 NSPs were also added to ultra-high-molecular-weight polyethylene for fabricating inserts for total joint replacement components, and it was found that NSPs drastically reduced the wear and tear of the polymer.Citation62
Dental materials
NSPs also have applications in dental instruments and bandages. Yoshida et al showed that a resin composite incorporated with NSP-containing materials had a long-term inhibitory effect against Streptococcus mutans.Citation63 Yamamoto et al also showed that a resin composite containing silver ion-implanted fillers released silver ions with antibacterial effects on oral streptococci.Citation64 In addition, Magalhães et al showed that incorporating NSPs in endodontic filling materials provided a significantly enhanced anti-bactericidal effect against Streptococcus milleri, S. aureus, and Enterococcus faecalis.Citation65 NSPs in dental adhesives are also very effective against streptococci without affecting the adhesive mechanical properties, thus enabling their use in orthodontic treatments.Citation66
Biodiagnosis
NSPs can be used for bio-diagnosis, where plasmonic properties of NSPs strongly depend on size, shape, and dielectric medium that surrounds it.Citation67 Zhou et al developed a silver nanoparticle array biosensor for clinical detection of serum p53 in head and neck squamous cell carcinoma.Citation68 NSPs are also employed to produce dual-imaging/therapy-immunotargeted nanoshells to locate cancer cells and can absorb light and selectively destroy targeted cancer cells through photothermal therapy.Citation69 In addition, NSPs can detect the interaction between amyloid β-derived diffusible ligands (ADDL) and the anti-ADDL antibody, which are related to the development of Alzheimer’s disease;Citation70 however, silver is easily oxidized and forms plasmonically unattractive compounds such as halides in biological solutions, which deteriorates the plasmonic performance of NSPs.Citation18
Other medical applications
NSPs have applications in the diagnosis and treatment of cancer,Citation71 and are drug carriers that can deliver therapeutic agents,Citation72 which are used in eye care for coating contact lenses.Citation17 In addition, the use of nanosilver in combination with vanadium oxide in battery cell components is one example of advanced silver nanotechnology improving battery performance in next-generation active implantable medical devices.Citation73
NSP toxicity
NSPs may have potential toxicities at some concentrations and can cause various health problems if used improperly. Thus, it is necessary to address the biosafety of NSPs in human health.
In vitro toxicity
NSPs have been reported to be cytotoxic to several types of cells, including human peripheral blood mononuclear cells,Citation74 human alveolar epithelial cell line (A549)Citation75, murine and human alveolar macrophage cell line,Citation76 neuroendocrine cells,Citation77 rat liver cell line,Citation78 and mouse germline cells.Citation79 Alt et al, however, found that bone cement containing 1.0% nanosilver did not lead to significant cytotoxicity in mouse fibroblasts (L929) and human osteoblast cell line.Citation61 Although the details of the toxic mechanism are unclear, it suggests that NSPs are ionized in the cells, which leads to activate ion channels and changes the permeability of the cell membrane to both potassium and sodium,Citation80 interaction with mitochondria,Citation81 and induction of the apoptosis pathway via the production of reactive oxygen species,Citation82 which leads to cell death.
In vivo toxicity
Chen and Schluesener have reviewed biodistribution, organ accumulation, degradation, possible adverse effects, and toxicity associated with the medical use of nanosilver.Citation13 Respiratory tract, gastrointestinal tract, skin, and female genital tract are the main entry portals of nanosilver into the human body through direct substance exchange with the environment. Additionally, systemic administration is also a potential route of entry, since colloidal silver nanoparticles have been exploited for diagnostic imaging or therapeutic purposes. Inhalation and instillation experiments in rats showed that low concentration, but detectable, ultrafine silver (14.6±1.0 nm) appeared in the lung and was subsequently distributed to the blood and other organs, such as heart, liver, kidney, and even brain.Citation83 In a recent oral toxicity study of rats, Kim et al also found that silver nanoparticles accumulated in blood, liver, lungs, kidneys, stomach, testes, and brain, but NSPs showed no significant genotoxicity after oral administration of silver nanoparticles of 60 nm average size for 28 days at different doses.Citation84 Lee et al showed that NSPs less than 12 nm in size affected early development of fish embryos, caused chromosomal aberrations and DNA damage, and induced proliferation arrest in cell lines of zebrafish;Citation85 however, Lansdown found that silver was not a cause of neurotoxic damage, even though silver deposits have been identified in the region of cutaneous nerves,Citation86 and Ji et al found that NSPs did not affect respiratory system in a 28-day in vivo study.Citation87
Animal and human studies indicate that it is difficult to remove silver completely once it has been deposited in the body; however, nanosilver can be excreted through the hair, urine, and feces.Citation88 There is no consensus on nanosilver’s toxicity to humans, and most toxicity investigations of silver nanoparticles are based on in vitro cellular experiments and relatively short-term animal experiments.
Conclusion
NSPs represent a prominent nanoproduct and are already widely used in medical applications, including wound dressing, diagnosis, and pharmacological treatment. Since the shape, size, and composition of NSPs can have significant effects on their function and possible risks to human health, extensive research is needed to fully understand their synthesis, characterization, and possible toxicity. In this review, we first gave an overview of NSP synthesis, then reviewed applications of NSPs in the field of biomedicine. Finally, possible toxicology was discussed.
There is a limited number of well-controlled studies on the potential toxicities of nanosilver, though these studies tend to suggest that NSPs can induce toxicity in living beings. It should be noted that in vitro conditions are drastically different from in vivo conditions; however, longer-term studies and assessment of NSP toxicity must be conducted so that NSP exposure does not exceed toxic levels.
Acknowledgments
This work was supported by International Science and Technology Cooperation Program of China (S2013ZR0398), Chongqing Basic Scientific Research Grant (cstc2013jcyjC80001), Chongqing Agriculture Development Grant (14408, 12402), the NSERC Discovery Grant, Manitoba Health Research Council, Dr Moorehouse Fellowship, Manitoba Institute of Child Health and China 863 Project (Grant 2012AA020504).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenJOuyangJKongJZhongWXingMMPhoto-cross-linked and pH-sensitive biodegradable micelles for doxorubicin deliveryACS Appl Mater Interfaces2013583108311723530535
- MohamedAXingMMNanomaterials and nanotechnology for skin tissue engineeringInt J Burns Trauma201221294122928165
- TianYChenJZahtabiFKeijzerRXingMNanomedicine as an innovative therapeutic strategy for pediatric lung diseasesPediatric Pulmonol2013481110981111
- XingMZhongWXuXThomsonDAdhesion force studies of nanofibers and nanoparticlesLangmuir20102614118091181420552953
- El-BadawyAFeldhakeDVenkatapathyRState of the Science Literature Review: Everything Nanosilver and MoreWashington, DCUS Environmental Protection Agency2010
- ZhongWXingMMMaibachHINanofibrous materials for wound careCutan Ocul Toxicol201029314315220518622
- UchiharaTSilver diagnosis in neuropathology: principles, practice and revised interpretationActa Neuropathol2007113548349917401570
- SibbaldRGContreras-RuizJCouttsPFierhellerMRothmanAWooKBacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcersAdv Skin Wound Care2007201054955817906429
- SkirtachAGMuñoz JavierAKreftOLaser-induced release of encapsulated materials inside living cellsAngew Chem Int Ed Engl200645284612461716791887
- GalianoKPleiferCEngelhardtKSilver segregation and bacterial growth of intraventricular catheters impregnated with silver nanoparticles in cerebrospinal fluid drainagesNeurol Res200830328528717767809
- MooreKA new silver dressing for wounds with delayed healingWounds UK2006227078
- VigneshwaranNKatheAAVaradarajanPVNachaneRPBalasubramanyaRHFunctional finishing of cotton fabrics using silver nanoparticlesJ Nanosci Nanotechnol2007761893189717654961
- ChenXSchluesenerHJNanosilver: a nanoproduct in medical applicationToxicol Lett2008176111218022772
- JungJHOhHCNohHSJiJHKimSSMetal nanoparticle generation using a small ceramic heater with a local heating areaJ Aerosol Sci2006371216621670
- TsujiTIryoKWatanabeNTsujiMPreparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle sizeAppl Surf Sci20022021–28085
- AbidJPWarkAWBrevetPFGiraultHHPreparation of silver nanoparticles in solution from a silver salt by laser irradiationChem Commun (Camb)2002779279312119726
- TienDCLiaoCYHuangJCNovel technique for preparing a nano-silver water suspension by the arc-discharge methodReviews on Advanced Materials Science200818750756
- EvanoffDDJrChumanovGSynthesis and optical properties of silver nanoparticles and arraysChemphyschem2005671221123115942971
- PyatenkoAYamaguchiMSuzukiMSynthesis of spherical silver nanoparticles with controllable sizes in aqueous solutionsJ Phys Chem C20071112279107917
- Blanco-AndujarCTungLDThanhNTKSynthesis of nanoparticles for biomedical applicationsAnnual Reports Section “A” (Inorganic Chemistry)2010106553568
- NaikRRStringerSJAgarwalGJonesSEStoneMOBiomimetic synthesis and patterning of silver nanoparticlesNat Mater20021316917212618805
- NamKTLeeYJKraulandEMKottmannSTBelcherAMPeptide-mediated reduction of silver ions on engineered biological scaffoldsACS Nano2008271480148619206318
- AnishaBSBiswasRChennazhiKPJayakumarRChitosan- hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic woundsInt J Biol Macromol20136231032024060281
- SintubinLDe WindtWDickJLactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticlesAppl Microbiol Biotechnol200984474174919488750
- BalajiDSBasavarajaSDeshpandeRMaheshDBPrabhakarBKVenkataramanAExtracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungusColloids Surf B Biointerfaces2009681889218995994
- SintubinLVerstraeteWBoonNBiologically produced nanosilver: current state and future perspectivesBiotechnol Bioeng2012109102422243622674445
- ShankarSSAhmadASastryMGeranium leaf assisted biosynthesis of silver nanoparticlesBiotechnol Prog20031961627163114656132
- ThomasVYallapuMMSreedharBBajpaiSKFabrication, characterization of chitosan/nanosilver film and its potential antibacterial applicationJ Biomater Sci Polym Ed200920142129214419874682
- Anil KumarSAbyanehMKGosaviSWNitrate reductase-mediated synthesis of silver nanoparticles from AgNO3Biotechnol Lett200729343944517237973
- SaifuddinNWongCWYasumiraAANRapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiationE-Journal of Chemistry2009616170
- MukherjeePAhmadAMandalDFungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesisNano Lett2001110515519
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomedicine2007319510117379174
- SadeghiBGarmaroudiFSHashemiMComparison of the antibacterial activity on the nanosilver shapes: nanoparticles, nanorods and nanoplatesAdv Powder Technol20122312226
- LiPLiJWuCWuQLiJSynergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticlesNanotechnology200516919121917
- SondiISalopek-SondiBSilver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteriaJ Colloid Interface Sci2004275117718215158396
- MatsumuraYYoshikataKKunisakiSTsuchidoTMode of bactericidal action of silver zeolite and its comparison with that of silver nitrateAppl Environ Microbiol20036974278428112839814
- ShrivastavaSBeraTRoyASinghGRamachandraraoPDashDCharacterization of enhanced antibacterial effects of novel silver nanoparticlesNanotechnology20071822225103
- KimKJSungWSMoonSKChoiJSKimJGLeeDGAntifungal effect of silver nanoparticles on dermatophytesJ Microbiol Biotechnol20081881482148418756112
- NasrollahiAPourshamsianKMansourkiaeePAntifungal activity of silver nanoparticles on some of fungiInternational Journal of Nano Dimension201113233239
- KimKJSungWSSuhBKAntifungal activity and mode of action of silver nano-particles on Candida albicansBiometals200922223524218769871
- SunRWChenRChungNPHoCMLinCLCheCMSilver nano-particles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cellsChem Commun (Camb)2005405059506116220170
- LuLSunRWChenRSilver nanoparticles inhibit hepatitis B virus replicationAntivir Ther200813225326218505176
- TaylorPLOmotosoOWiskelJBMitlinDBurrellREImpact of heat on nanocrystalline silver dressings. Part II: physical propertiesBiomaterials200526357230724016005958
- Baram-PintoDShuklaSPerkasNGedankenASaridRInhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonateBioconjug Chem20092081497150221141805
- RogersJVParkinsonCVChoiYWSpeshockJLHussainSMA preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formationNanoscale Res Lett200834129133
- TaylorPLUssherALBurrellREImpact of heat on nanocrystalline silver dressings. Part I: chemical and biological propertiesBiomaterials200526357221722916005512
- LaraHHAyala-NuñezNVIxtepan-TurrentLRodriguez-PadillaCMode of antiviral action of silver nanoparticles against HIV-1J Nanobiotechnology20108120145735
- NadwornyPLWangJTredgetEEBurrellREAnti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis modelNanomedicine20084324125118550449
- ShinSHYeMKThe effect of nano-silver on allergic rhinitis model in miceClin Exp Otorhinolaryngol20125422222723205228
- CastilloPMHerreraJLFernandez-MontesinosRTiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by Toll-like receptor ligandsNanomedicine (Lond)20083562763518834270
- ChaloupkaKMalamYSeifalianAMNanosilver as a new generation of nanoproduct in biomedical applicationsTrends Biotechnol2010281158058820724010
- HuangYLiXLiaoZA randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysisBurns200733216116617175106
- ChenJHanCMLinXWTangZJSuSJ[Effect of silver nanoparticle dressing on second degree burn wound]Zhonghua Wai Ke Za Zhi20064415052 Chinese16620649
- LuSGaoWGuHYConstruction, application and biosafety of silver nanocrystalline chitosan wound dressingBurns200834562362818226459
- GrunkemeierGLJinRYStarrAProsthetic heart valves: Objective Performance Criteria versus randomized clinical trialAnn Thorac Surg200682377678016928482
- JamiesonWRFradetGJAbelJGSeven-year results with the St Jude Medical Silzone mechanical prosthesisJ Thorac Cardiovasc Surg2009137511091115 e219379975
- AndaraMAgarwalAScholvinDHemocompatibility of diamondlike carbon–metal composite thin filmsDiam Relat Mater20061511–1219411948
- GhanbariHViatgeHKidaneAGBurriesciGTavakoliMSeifalianAMPolymeric heart valves: new materials, emerging hopesTrends Biotechnol200927635936719406497
- FuJJiJFanDShenJConstruction of antibacterial multilayer films containing nanosilver via layer-by-layer assembly of heparin and chitosan-silver ions complexJ Biomed Mater Res A200679366567416832825
- LacknerPBeerRBroessnerGEfficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalusNeurocrit Care20088336036518320144
- AltVBechertTSteinrückePAn in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cementBiomaterials200425184383439115046929
- MorleyKSWebbPBTokarevaNVSynthesis and characterisation of advanced UHMWPE/silver nanocomposites for biomedical applicationsEur Polym J2007432307314
- YoshidaKTanagawaMMatsumotoSYamadaTAtsutaMAntibacterial activity of resin composites with silver-containing materialsEur J Oral Sci1999107429029610467945
- YamamotoKOhashiSAonoMKokuboTYamadaIYamauchiJAntibacterial activity of silver ions implanted in SiO2 filler on oral streptococciDent Mater19961242272299002839
- MagalhãesAPRSantosLBLopesLGNanosilver application in dental cementsISRN Nanotechnology2012201216
- AhnSJLeeSJKookJKLimBSExperimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticlesDent Mater200925220621318632145
- HaesAJVan DuyneRPA nanoscale optical blosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticlesJ Am Chem Soc200212435105961060412197762
- ZhouWMaYYangHDingYLuoXA label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinomaInt J Nanomedicine2011638138621468351
- LooCLoweryAHalasNWestJDrezekRImmunotargeted nanoshells for integrated cancer imaging and therapyNano Lett20055470971115826113
- HaesAJHallWPChangLKleinWLVan DuyneRPA localized surface plasmon resonance biosensor: first steps toward an assay for Alzheimer’s diseaseNano Lett20044610291034
- LiuJZhaoYGuoQTAT-modified nanosilver for combating multidrug-resistant cancerBiomaterials201233266155616122682937
- SkirtachAGAntipovAAShchukinDGSukhorukovGBRemote activation of capsules containing Ag nanoparticles and IR dye by laser lightLangmuir200420176988699215301477
- EtheridgeMLCampbellSAErdmanAGHaynesCLWolfSMMcCulloughJThe big picture on nanomedicine: the state of investigational and approved nanomedicine productsNanomedicine20139111422684017
- ShinSHYeMKKimHSKangHSThe effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cellsInt Immunopharmacol20077131813181817996693
- ParkSLeeYKJungMCellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cellsInhal Toxicol200719Suppl 1596517886052
- SotoKGarzaKMMurrLECytotoxic effects of aggregated nanomaterialsActa Biomater20073335135817275430
- HussainSMJavorinaAKSchrandAMDuhartHMAliSFSchlagerJJThe interaction of manganese nanoparticles with PC-12 cells induces dopamine depletionToxicol Sci200692245646316714391
- HussainSMHessKLGearhartJMGeissKTSchlagerJJIn vitro toxicity of nanoparticles in BRL 3A rat liver cellsToxicol In Vitro200519797598316125895
- McAuliffeMEPerryMJAre nanoparticles potential male reproductive toxicants? A literature reviewNanotoxicology200713204210
- KoneBCKaletaMGullansSRSilver ion (Ag+)-induced increases in cell membrane K+ and Na+ permeability in the renal proximal tubule: reversal by thiol reagentsJ Membr Biol1988102111192456393
- CarlsonCHussainSMSchrandAMUnique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen speciesJ Phys Chem B200811243136081361918831567
- HsinYHChenCFHuangSShihTSLaiPSChuehPJThe apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cellsToxicol Lett2008179313013918547751
- TakenakaSKargERothCPulmonary and systemic distribution of inhaled ultrafine silver particles in ratsEnviron Health Perspect2001109Suppl 454755111544161
- KimYSKimJSChoHSTwenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley ratsInhal Toxicol200820657558318444010
- LeeKJNallathambyPDBrowningLMOsgoodCJXuXHIn vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryosACS Nano20071213314319122772
- LansdownABCritical observations on the neurotoxicity of silverCrit Rev Toxicol200737323725017453933
- JiJHJungJHKimSSTwenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley ratsInhal Toxicol2007191085787117687717
- DiVincenzoGDGiordanoCJSchrieverLSBiologic monitoring of workers exposed to silverInt Arch Occup Environ Health19855632072154066049