Abstract
Nanomaterials are the subject of intense research, focused on their synthesis, modification, and biomedical applications. Increased nanomaterial production and their wide range of applications imply a higher risk of human and environmental exposure. Unfortunately, neither environmental effects nor toxicity of nanomaterials to organisms are fully understood. Cost-effective, rapid toxicity assays requiring minimal amounts of materials are needed to establish both their biomedical potential and environmental safety standards. Drosophila exemplifies an efficient and cost-effective model organism with a vast repertoire of in vivo tools and techniques, all with high-throughput scalability and screening feasibility throughout its life cycle. Here we report tissue specific nanomaterial assessment through direct microtransfer into target tissues. We tested several nanomaterials with potential biomedical applications such as single-wall carbon nanotubes, multiwall carbon nanotubes, silver, gold, titanium dioxide, and iron oxide nanoparticles. Assessment of nanomaterial toxicity was conducted by evaluating progression through developmental morphological milestones in Drosophila. This cost-effective assessment method is amenable to high-throughput screening.
Introduction
Nanomaterials have been the subject of intense research focused on their synthesis, modification, and applications.Citation1–Citation3 During the last few years, nanoparticles have become important tools, with an expectation that they will have a considerable effect in the biomedical sciences, and are attractive for applications including imaging agents, gene and drug delivery vehicles, in vivo and in vitro biosensors, and nanoscale thermal therapies.Citation4–Citation9 Nanoparticles can also be found in some everyday use products and in agricultural applications.Citation10,Citation11 Among the wide variety of nanomaterials possible, some of the most commonly used are single (SWCNTs) and multiwalled carbon nanotubes (MWCNTs), metallic silver and gold nanoparticles, oxides such as titanium dioxide and iron oxide (IOs), and semiconductor quantum dots.
It stands to reason that the increase in nanomaterial production associated with their wide range of applications implies a higher risk for human and environmental exposure.Citation12 To date, neither environmental effects nor toxicity of nanomaterials to organisms are fully understood. Toxicity assessments can provide the necessary information to establish adverse effects a substance may have in an organism at the cellular, tissue, and organ levels. Unfortunately, cost-effective toxicity assessments have not been developed in tandem with the fast-growing field of nanomaterials synthesis. To reach a consensus and establish clear and specific regulations for human and environmental safety, one needs to follow a product-focused, science-based approach.Citation13,Citation14 Product-specific assessments will yield conclusions based on the specific properties of the nanomaterial (size, surface modification, concentration, and exposure route), instead of broad generalizations.
The primary advantage of in vitro assessments is their reductionist approach. Unfortunately, this limits the scope of the research to specific homogeneous cell types and may not be relevant to biological events in an otherwise more complex functional organism.Citation15–Citation18 Although cell cultures can include multiple cell types mimicking in vivo situations,Citation19,Citation20 in vitro assessments are not able to simulate the microenvironment present in a complex organism, including, but not limited to, three-dimensional gradients of molecular cues, exposure routes, dosing, and lack of physical barriers intrinsic to tissues and organs.
In vivo assessments are performed using whole organisms in which spatial organization is unaltered. The most common in vivo nanotoxicity assessments use rodents as model organisms, but other model organisms such as Caenorhabditis elegans, Danio reiro, and Drosophila are gaining popularity.Citation21–Citation26 Rodents, being mammals, can be used to study complicated processes underlying normal human development, diseases, and behavior. Using rats or mice as model organisms allows scientists to mimic possible exposure routes that occur in humans, such as inhalation,Citation27 dermal exposure,Citation28 and injections.Citation29 In addition, organ biodistribution and dose equivalencies such as minimum lethal dose and median lethal dose can be directly extrapolated.Citation29 Rats have recently lost favor as animal models in some fields. One reason is that genetic manipulations are limited in this model organism because its genome does not tolerate the insertion of foreign DNA to the extent of other organisms like the mouse, C. elegans, or Drosophila.Citation30
C. elegans and Drosophila possess many of the same advantages but differ mostly on the degree of tractability and accessibility that can be used in experimental manipulation. Drosophila presents the possibility of assessing the six principal exposure routes: intravenous, dermal, subcutaneous, inhalation, intraperitoneal, and oral.Citation31 However, these different exposure routes have not been fully assessed, and oral ingestion is the most widely employed exposure route in nanotoxicity research using Drosophila.Citation26,Citation32–Citation34
Drosophila exemplifies an efficient and cost-effective model organism with a vast repertoire of tools and techniques, all with high-throughput scalability and screening feasibility,Citation35–Citation37 throughout its life cycle.Citation26 A female can lay as many as 3,000 eggs in her lifetime,Citation38 providing a constant supply of individuals in every stage of development. Furthermore, as a result of Drosophila’s small size, the amount of nanomaterial required for in vivo testing is in the nanogram range.Citation26,Citation32 This is orders of magnitude smaller than what is required for testing in other model systems, such as C. elegansCitation21,Citation22 and zebrafish.Citation23,Citation24 Drosophila’s single-cell resolution, together with its neuromuscular system consisting of a series of segmental repeats in a well-known pattern,Citation39 allows for accessible, simple, and precise identification of developmental stages from morphological and molecular perspectives. In addition, single identifiable cells can be tracked throughout the entire embryonic development, thanks to the existence of a clear cuticle during the embryonic and larval stages. This in turn allows the study of developmental effects of nanomaterials in a specific area or system of interest.Citation40
Methods
Drosophila embryos
Canton S wild-type Drosophila melanogaster embryos kept at 25°C and 60% relative humidity were dechorionated using a 50% hypochlorite wash solution followed by staging according to Campos-Ortega and Hartenstein.Citation41 Briefly, stage 15 embryos were selected and placed with their dorsoventral axis parallel to the 0.5 cm2 coverslip in which they were fixed and then covered with a drop of halocarbon oil series 700 (Halocarbon Products Corp, River Edge, NJ, USA). These steps were performed using an Olympus MVX10 MacroView (Center Valley, PA, USA).
Nanomaterials
Silver, titanium dioxide (TiO2), and gold nanoparticles and carbon nanotubes were obtained commercially. The silver (Ag) nanoparticles (MKnano, Mississauga, ON, Canada) had a diameter smaller than 90 nm and purity of 99.9%. Gold (Au) nanoparticles (Sigma-Aldrich, St Louis, MO, USA) had a diameter smaller than 150 nm and purity of 99.9%. The TiO2 nanoparticles (Degussa P25; Evonik Industries, Piscataway Township, NJ, USA) had a diameter smaller than 20 nm and purity of 99.9%. The SWCNT (Cheap Tubes, Inc., Brattleboro, VT, USA) had an outer diameter of 1 to 2 nm, an inner diameter of 0.8 to 1.6 nm, length of 5 to 30 μm, a surface area (SA) of 407 m2/g, and purity higher than 90 wt%. The MWCNT (Cheap Tubes, Inc.) had an outer diameter smaller than 8 nm, an inner diameter of 2 to 5 nm, length of 10 to 30 μm, an SA of 500 m2/g, and purity higher than 95 wt%.
IO nanoparticles were synthesized by the coprecipitationCitation42 or thermal decomposition methodCitation43 and coated with carboxymethyl dextran (CMDx), using an amine silane as a grafting agent.Citation44,Citation45 The methods for obtaining these nanoparticles have been published by our group, along with detailed characterization of their colloidal properties.Citation44–Citation46 We refer to CMDx-coated IO obtained by coprecipitation as Cop-IO. The primary particle diameter determined by transmission electron microscopy (JEOL 1200 EX; Jeol Ltd, Tokyo, Japan) was 12±2 nm, and the hydrodynamic diameter, determined by dynamic light scattering (BI-90Plus; Brookhaven Instruments, Holtsville, NY, USA), was 77±5 nm. We have previously shown that particles obtained by these methods consist of small aggregates of primary nanoparticles coated with a CMDx shell.Citation45 We refer to CMDx-coated IO obtained by thermal decomposition as thermo-IO. The primary particle diameter, determined by transmission electron microscopy, was 12±1 nm, and the hydrodynamic diameter, determined by dynamic light scattering, was 38±5 nm. We have previously shown that particles obtained by these methods consist of single IO primary particles coated with a CMDx shell.Citation44
Tissue-specific nanomaterial microtransfer in Drosophila
We used the Sutter’s Xenoworks micromanipulator and digital microinjector System (Sutter Instruments, Novato, CA, USA) in conjunction with TransferTip-R microcapillary needles (Eppendorf, Hamburg, Germany) mounted on a fully motorized Olympus IX81 inverted microscope (Olympus) to deliver nanomaterials. We used a modified pulsed-flow approach with regulated injection pressures, allowing for greater control and consistency of delivered sample. An essential aspect of our approach is the application of the smallest amount of pressure possible once inside the living embryo to ensure delivery of nanomaterials with minimal disruption of cell membranes. We refer to this approach as microtransferring of nanomaterials, and it results in the presentation of selected nanomaterials at cell/tissue interfaces. Taking advantage of the multisegmented body plan in Drosophila, we consistently delivered nanomaterials in the abdominal segments 5/6 intersegmental boundary by following an anterior trajectory after entry through the posterior embryonic axis.
As bare nanomaterials, such as Ag, Au, and TiO2 nanoparticles, and single-walled SWCNT and MWCNT have tendencies to sediment in water, and the colloidal stability of TiO2 nanoparticles has been shown to improve when suspended in a solution of 10% fetal bovine serum or 1% human serum albumin,Citation47 we decided to suspend our nanomaterials in 10% bovine serum albumin solution (BSA 10%). This resulted in nanomaterial suspensions with minimized tendencies to clog fine needle tips. Our control groups consisted of embryos subject to ultrapure H2O and BSA 10% microtransfers. IO nanoparticles were suspended in ultrapure H2O; all other nanomaterials were suspended in BSA 10%. After microtransfer procedures, each embryo was allowed to recover at 25°C and 60% relative humidity (RH). Mortality assessments were recorded 48 hours after the procedure. Mortality determinations took into consideration any embryonic development past stage 15 ().
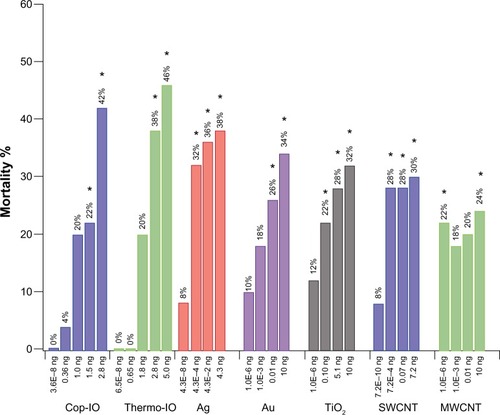
Figure 1 Overall mortality of Drosophila embryos after microtransfer of nanomaterials.
Notes: Overall mortality (OM) is the sum of the three mortality-scoring criteria. OM values are normalized against the OM of the corresponding diluent (nanomaterial OM–diluent OM). Treatments with IO nanoparticles are normalized against H2O, and Ag, Au, TiO2, SWCNT, and MWCNT are normalized against BSA 10%. For each condition tested, n=50. *Statistical relevance was established through Fisher’s exact test (P<0.005).
Abbreviations: IO, iron oxide; SWCNT, single-wall carbon nanotube; MWCNT, multiwall carbon nanotube; BSA, bovine serum albumin; Ag, silver; Au, gold; TiO2, titanium dioxide; H2O, water; Cop, coprecipitation; Thermo, thermal decomposition.
Microtransferred volume quantification
To determine the amount of nanoparticles delivered using the microtransferring technique, we measured volume displacements once we reached the desired delivery location inside the developing embryo. We performed these measurements using our high spatiotemporal resolution imaging and manipulation system and estimated that our system is capable of consistently delivering an average volume of 0.00145 μL per five pulsed microtransfers, and later estimated delivery concentrations and total nanoparticles per embryos. To estimate the volume of the microtransfer, an initial marking was drawn around the outer surface of the needle, and the nanoparticle solution was loaded as close as possible to this marking without surpassing it. Without disconnecting the microneedle from the micropipette holder or pressure tubing of the microinjector, the micropipette holder was placed in a horizontal position over the objective, and an image was acquired before microtransfer (xo) and after every five microtransfers (xn). Using the Volocity 6.3 image acquisition software (PerkinElmer, Waltham, MA, USA), the lengths from the meniscus to the tape were determined. The microtransferred volume was determined using the cylinder volume formula (V=πr2 h), where r is the internal radius of the microneedle and h is the measured length determined from the acquired images. The equation can be written as V=πr2(x1−x2)/n. The concentrations of the microtransfer solutions for each nanoparticle are summarized in .
Table 1 Nanomaterial delivery amounts and dosage quantifications
We established extrapolation of delivered doses based on body SA from Drosophila embryos to humans. Body SA comparison for dose extrapolation is the method suggested by the US Food and Drug Administration for clinical trials.Citation48 Body SA of a Drosophila embryo was calculated through a simple formula based on a prolate spheroid, a body equivalent ellipsoid by Reading and FreemanCitation49: SA =4πac, c = H/2, H = height =500 μm, a = minor axis =75 um, SA =2.36×10−7 m2. Human SA was calculated by averaging the values obtained from five of the main equations for body SA of a human,Citation50–Citation53 resulting in SA =1.74 m2. A conversion factor between embryo and human SA was calculated (human SA/embryo SA). The amount of nanomaterials per human dosage was calculated by applying the conversion factor to the amount of nanomaterials per embryo dosage. The equivalent microtransferred volume in a human was also established by applying the conversion factor to microinjected volume in an embryo. Dosage (μg/m2) = amount of nanomaterials per microtransfer/SA. Using the conversion factor, the equivalent microtransferred volume was calculated as 10.7 mL, which represents only 0.018% of the volume of an average human ().
Statistical analysis
Note that n=50 for all conditions tested. The Shapiro–Wilk test showed that our data did not exhibit a normal distribution. Therefore, and to establish the significance of our data set, we performed a nonparametric analysis, using Fisher’s exact test (α=0.05).
Results
Tissue-specific nanotoxicity assessment
Tissue-specific nanomaterial assessment was conducted through direct microtransfer of nanomaterials into target tissues, which yields quantifiable mortality results based on simple developmental morphological milestones in Drosophila. This assessment takes full advantage of the single identifiable cell nature of the Drosophila system, and instead of employing the commonly used microinjection techniques,Citation54 microtransferring resulted in a more gentle and constant release of nanomaterials to the desired location, with no disruption of target tissues. Thus, potential damage to cells caused by accelerated, high-pressure pulsed injections was minimized by direct microtransfer of small amounts of nanomaterials.
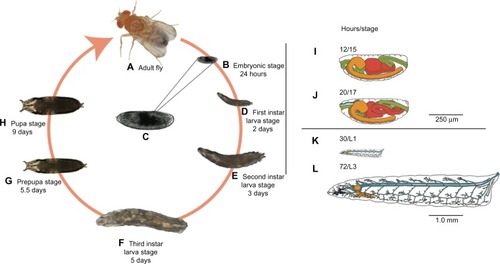
We used stage 15 embryos (), which have roughly completed 50% of their development, because eggshell membranes are fully developed and dorsal closure is completed.Citation38 These morphological features serve as recognizable landmarks for stage identification and provide important structural integrity.
Figure 2 Drosophila life cycle.
Notes: All stages of the Drosophila life cycle are readily accessible and amenable to manipulation with a variety of basic to high-end tools and techniques. Imaging techniques can be applied in every stage of development (A–H), thanks to the clear cuticle during embryonic (B and C) and larval (D–F) stages. Here we use stage 15 embryos (C), which correspond to roughly 50% of completed embryonic development. Under ideal growing conditions, this stage is reached approximately 12 hours after egg laying and features a developing central nervous system (orange), digestive tract (green and red), and many other systems (not shown) with development underway (I). In stage 15, the midgut has one compartment that divides into two distinct compartments as the embryo progresses to stage 16. We used this feature as an indication of initial survival after nanoparticle delivery and used morphological features characteristic of later developmental stages (J–L) for mortality determinations. For a detailed review of these morphological features, please see Campos-Ortega and Hartenstein.Citation41 Note that time points in the figure correspond to time elapsed from egg laying to the end of a particular developmental stage.
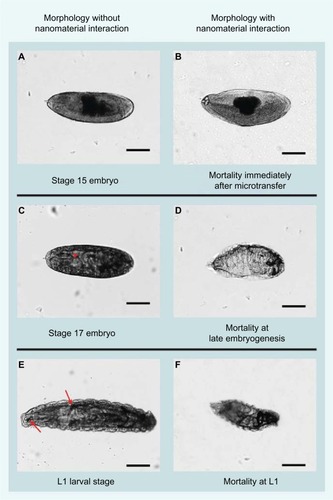
Developmental effects were assessed 48 hours after microtransfer in terms of overall mortality (OM) and identification of specific developmental stages, in which each embryo was found dead. After multiple preliminary trials, the following trends were chosen as scoring criteria for the quantification of mortality at specific stages of development: number of dead embryos that did not progress past developmental stage 15 (we surmise these embryos died as a result of the delivery procedure), number of dead embryos at late embryogenesis (developmental stages 16 and 17), and number of dead larva (). The data obtained through this quantification were analyzed two different ways: by overall mortality, which is the sum of all the scoring criteria, and by scoring criteria with highest mortality. For comparison purposes of the latter, we analyzed the shift in scoring criteria with highest mortality from one concentration to another, as this comparison yields suggestions on stability of the nanomaterial and treatment acuteness.
Figure 3 Comparative morphology between nanoparticle-treated and untreated Drosophila embryos.
Notes: Untreated stage 15 embryo (A) is used as reference to determine mortality of embryos that did not progress past stage 15 after delivery of nanomaterials (B). During late embryogenesis (C), rhythmic muscle contractions and a gas-filled tracheal system (arrowhead) are prominent developmental hallmarks. We used the absence of muscle contractions in the presence of the gas-filled tracheal system to determine (D) survival after initial nanoparticle delivery and failure to progress to the first instar (L1) wandering larval stages (E). Mortality at the L1 stage (F) was characterized by a fully developed tracheal system and mouth hooks by fully developed L1 development but failed to progress to later developmental stages. These individuals showed a developed tracheal system and mouth hooks (arrows in E), but no locomotion and no visceral muscle contractions. Scale bars =140 μm.
We tested eight nanomaterials at different concentrations: SWCNTs, MWCNTs, Ag, Au, and TiO2, and IO nanoparticles synthesized by coprecipitation coated with 3-Aminopropyltriethoxysilane (APS) and carboxymethyldextran (Cop-IO) and synthesized by thermo-decomposition coated with CMDx (Thermo-IO).
We employed predicted environmental concentrations (PEC) in water calculated by Muellerand and NowackCitation12 for TiO2, Ag and SWCNT/MWCNT as our lowest concentrations. PEC values were originally determined by a substance flow analysis from the products to the environment.Citation12 There are no data available on the PEC for Au,Citation55 and in addition, the PEC for iron has not been calculated, as it is such an abundant element in the environment. Therefore, we decided to conduct our trials with the lowest employed concentrations at orders of magnitude similar to those established for TiO2, Ag, and SWCNT/MWCNT.
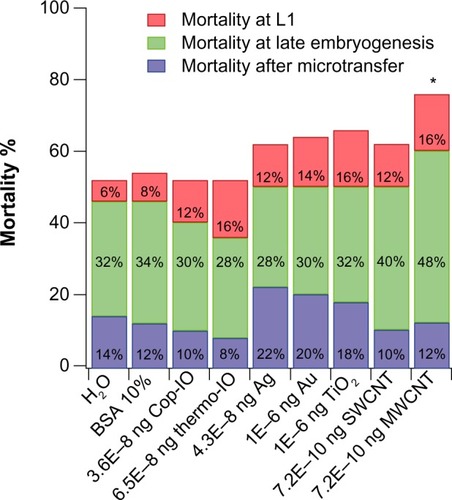
Effects of PECs
Of the nanomaterials tested at the PEC, only MWCNT treatment showed statistically relevant effects in Drosophila embryo viability compared with the respective control. This suggests that a possible threshold of minimal toxic dose could be established by determining the maximum allowable concentration to be permitted in the environment (). None of the IO nanoparticles had statistically relevant effects in Drosophila embryo viability when treated at the lowest concentration, suggesting that if the environmental concentration were to be of a similar order of magnitude as that used for the other nanomaterials, there would not be a statistically relevant mortality effect ().
Figure 4 Mortality of Drosophila embryos after microtransfer of nanomaterials at predicted environmental concentrations.
Notes: The effects of the nanomaterials were compared with the effects caused by microtransferring the liquid in which these were diluted. Treatments with IO nanoparticles are compared with treatment with H2O (grey background), and Ag, Au, TiO2, SWCNT, and MWCNT are compared with BSA 10% (white background). For each condition tested, n=50. *Statistical relevance was established through Fisher’s exact test (P<0.005).
Abbreviations: BSA, bovine serum albumin; IO, iron oxide; SWCNT, single-wall carbon nanotube; MWCNT, multiwall carbon nanotube; Ag, silver; Au, gold; TiO2, titanium dioxide; H2O, water; Cop, coprecipitation; Thermo, thermal decomposition.
The two highest microtransferred amounts of Cop-IO nanoparticles, 1.5 and 2.8 ng, had statistically relevant effects in Drosophila embryo viability with normalized overall mortality percentages of 22% and 42%, respectively (). In the case of Cop-IO microtransfer, the shift in scoring criteria with highest mortality from late embryogenesis to immediately after microtransfer occurs from the third to the fourth amount (1.0–1.5 ng). This suggests that the biocompatibility and stabilizing properties of CMDx are having a favorable effect in shifting the toxic effect to higher concentrations.
As with Cop-IO nanoparticles, Thermo-IO treatment presents statistically relevant effects in Drosophila embryo viability only at the second highest microtransferred amounts (2.8 and 5.0 ng), with normalized overall mortality of 38% and 46%, respectively (). Even though the two highest concentrations of Thermo-IO-CMDx present higher overall mortality than the two highest concentrations of Cop-IO-APS-CMDx, the shift in highest mortality from late embryogenesis to immediately after microtransfer occurs from the fourth to the fifth microtransferred amount (2.8–5.0 ng). This suggests that nanoparticles synthesized by thermo-decomposition lead to slightly higher overall mortality, but nanoparticles synthesized by coprecipitation present a more acute effect, as the individuals die faster at lower concentrations.
For Ag nanoparticles, all concentrations higher than the PEC (ie, 4.30E−04, 0.04, and 4.3 ng) present statistically relevant effects in Drosophila embryo viability, with normalized overall mortality of 32%, 36%, and 38%, respectively (). Furthermore, treatment with Ag nanoparticles shows a shift in scoring criteria, with highest mortality from late embryogenesis to immediately after microtransfer, from the first (PEC) to the second amount (4.30E−08 to 4.30E−04 ng). This suggests that treatment with Ag nanoparticles elicits an acute toxic effect and that Ag nanoparticles have a low effective dose.
For Au nanoparticles, the two highest microtransferred amounts (ie, 0.10 and 10 ng) had statistically relevant effects in Drosophila embryo viability, with normalized overall mortality of 26% and 34%, respectively (). Also, treatment with Au nanoparticles shows a shift in scoring criteria, with highest mortality from late embryogenesis to L1 and then to immediately after microtransfer.
For TiO2 nanoparticles, the three highest microtransferred amounts (ie, 0.10, 5.1, and 10 ng) had statistically relevant effects in Drosophila embryo viability, with normalized overall mortality of 22%, 28%, and 32%, respectively (). As with treatment with Au nanoparticles, TiO2 nanoparticles show a shift in scoring criteria, with highest mortality from late embryogenesis to L1, and then to immediately after microtransfer. Therefore, as TiO2 and Au nanoparticles were administered at the same concentrations, TiO2 elicits a more acute toxic effect and has a lower effective dose than Au nanoparticles. This is most likely caused by the oxidative stress induced by reactive oxygen species produced by TiO2.Citation56–Citation58
For SWCNTs, the three highest microtransferred amounts (ie, 7.2E−04, 0.07, and 7.2 ng) had statistically relevant effects in Drosophila embryo viability, with normalized overall mortality of 28%, 28%, and 30%, respectively (). Furthermore, treatment with SWCNT shows a shift in scoring criteria with highest mortality from late embryogenesis to L1, from the fourth to the fifth microtransferred amount. These results suggest that SWCNTs affect Drosophila embryos similar to Au and TiO2, where embryo mortality is delayed by a shift in scoring criteria with highest mortality from late embryogenesis to L1, and then it shifts back. In contrast, MWCNTs had statistically relevant effects in Drosophila embryo viability only at the lowest (PEC) and the highest microtransferred amounts (7.2E–10 and 7.2 ng), with normalized overall mortality of 22% and 24%, respectively. Contrary to the rest of the nanomaterials, treatment with MWCNTs does not show a clear shift in scoring criteria with higher mortality. MWCNTs only show a slight shift from late embryogenesis to immediately after microtransfer, at the second microtransferred amount, but at the third amount, the shift reverts back to late embryogenesis. Overall mortality results of SWCNT and MWCNT are consistent with what other researchers have found,Citation59–Citation63 and SWCNTs showed higher toxicity than MWCNTs.
The results for MWCNT are puzzling because they show statistically relevant mortality only at the lowest and highest doses. CNTs have a tendency to form agglomerates,Citation64 and there is ongoing debate about whether or not the degree of agglomeration affects CNT toxicity.Citation17,Citation64 With the current methodology, the specific toxic effects cannot be identified, but they can be deduced. Toxicity could be a result of chemical interactions between the biological environment and the nanomaterial or as a result of a physical obstruction. It is possible that as the concentration in the microtransferred solution increases, so does the size of the clusters. An increase in cluster size will diminish the possibility for dispersion, as well as the SA-to-volume ratio, of the nanomaterial. Large enough clusters can be encysted if dispersion is halted and a decrease in SA-to-volume ratio can decrease the amount of free terminals available for interactions with the biological environment. Either case can explain a decrease in mortality after an increase in concentration. Mortality can again increase once a saturation threshold has been surpassed because with an increase in concentration, both the possibilities of agglomeration and the presence of free unclustered nanotubes increase. This could explain not only the effects of CNT but also the effects of Ag, Au, and TiO2 nanoparticle treatment in which the mortality occurs earlier after a first increase in concentration and is delayed after a second increase in concentration.
Discussion
Interaction of nanoparticles with living organisms to determine toxicity effects and safety considerations must be understood. Drosophila is emerging as a suitable organism for the study of toxicity of several nanomaterials. Nanotoxicity assessment studies have been previously conducted. Most of these studies use oral ingestion routes during third instar larvalCitation32,Citation65–Citation68 and adult stagesCitation33,Citation67,Citation69–Citation74 to assess nanotoxicologic effects of several nanomaterials. Unfortunately, and because of the relatively small amounts of food intake during these stages, it is very difficult to accurately estimate actual amounts of ingested food. In addition, it is possible that nanomaterials in Drosophila food may change its composition. Food composition plays an important part in Drosophila’s feeding behaviors,Citation75–Citation79 and thus, is an important factor to consider when using oral administration routes. In addition, several recent studies addressed the effect of silver nanoparticle toxicity, using oral ingestion as their administration routes, during third instar larvaCitation32,Citation68 and adult stages.Citation33,Citation69–Citation71,Citation73,Citation74 If we consider that nanosilver has strong antimicrobial and antifungal properties,Citation80,Citation81 together with the fact that Drosophila feeds mostly on microorganisms, particularly yeast, it is then possible to speculate that we may have confounding mortality effects arising from the unfavorable feeding conditions. Ingestion represents an important administration route, but more accurate screening tools are required.Citation67 To avoid the potential pitfalls of this and other indirect methods, we chose a direct microtransfer approach. This ensures accurate exposure to the nanomaterials under consideration in specific tissues and at known concentrations in the nanogram range, thus allowing for more accurate assessment of toxicity, which is of utmost importance when determining safety exposure margins.
Our assay consists of a uniform methodology that allows for overall mortality quantification, which can be normalized against a control trial of the solution in which the nanomaterials were suspended. This assessment also includes a novel and simple methodology for volume quantification that allows for dosage extrapolation. The controls also account for the mortality caused by the mechanical damage of needle puncturing that precedes microtransfer, leading to results that are independent of human manipulation and that are, consequently, more reproducible. Because of the small amounts of nanomaterials required and the relatively short life cycle of Drosophila, we were able to use a high number of replicates (n=50) for each condition tested. This high-resolution assessment allows not only for a general evaluation of embryonic viability but also for the identification of specific stage of mortality. In turn, this can render information in terms of minimal toxic dose, acuteness of toxic effect, maximum allowable concentration in the environment, and stability of surface modification as a function of how delayed the toxic effects elicited by nanomaterials are.
The toxicity assessment of IO, Ag, Au, and TiO2 nanoparticles, SWCNTs, and MWCNTs yielded important information on their intrinsic and relative toxicity. The results on mortality at predicted environmental concentrations can help establish future safety regulations in terms of maximum allowable concentrations in the environment, particularly for MWCNTs. Methods such as those described here can be applied to systematic studies aiming to modify nanomaterial physicochemical properties to minimize their adverse effect on organisms in the environment. Furthermore, our assessment can be further developed to establish more specific molecular interactions linked to the toxicity of specific tissues or organs.
Drosophila allows us to register morphological changes throughout development, and as future work, this methodology could be adapted to other stages of development. The nanomaterials could be traced across the life cycle in the surviving embryos, especially if fluorescently tagged nanomaterials are employed. Other tools such as transgenic flies with fluorescent markers against caspase 3; lactate dehydrogenase, to identify necrotic tissue; detection of intact lysosomes, and detection of reactive oxygen species, to assess stress response, can be integrated as mortality markers. This way, more-specific conclusions could be reached and specific organ and/or system toxic effects could be assessed (ie, neurotoxicity). As a validated model for human diseases, Drosophila also presents the possibility of simultaneously assessing effects on viability and nanomaterial applications in the treatment or understanding of human diseases. Finally, Drosophila’s cost-effectiveness, requiring nanomaterial amounts in the nanogram ranges, increases the possibility of this assessment being conducted as a high-throughput assay.
Conclusion
The current rate at which new nanomaterial compositions, morphologies, and synthesis routes are developed far outpaces the rate at which their in vivo toxicity can be tested using traditional mammalian animal models. We have developed a cost-effective, tissue-specific nanomaterial toxicity assay using direct microtransfer of nanomaterials to embryos of Drosophila melanogaster. Monitoring progression through simple development morphological milestones allows for overall mortality quantification and identification of specific stages of mortality in only 48 hours. The described methods are systematic and general enough to be employed in the assessment of other nanomaterials. Because of the small amounts of nanomaterials needed per embryo, and because of the short life cycle of Drosophila, the reported method lends itself for large numbers of replicates. Furthermore, given the wide array of molecular tools available for manipulation of Drosophila and its widespread use in a variety of disease models, the direct microtransfer technique described here could also enable application of Drosophila for in vivo testing of nanomaterial efficacy in a variety of biomedical applications.
Acknowledgments
This material is based on work supported by, or in part by, the US Army Research Laboratory and the US Army Research Office under contract/grant number W911NF-09-1-0219 to FAC-M and CR, which supported AH and SV-A, as well as US National Science Foundation grant NSF-IOS-0818243, which supported SV-A. Carlos Marti, Rosa Martínez, and Geidy Acevedo-Méndez helped in embryo collection and preparation techniques. Finally, the National Institute of Health’s RISE-2-BEST Program (grant 1R25GM088023) also supported the work of SV-A.
Disclosure
FAC-M is currently the AAAS Roger Revelle Fellow in Global Stewardship. The other authors have no conflicts of interest to disclose in respect of this work.
References
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- KarousisNTagmatarchisNTasisDCurrent progress on the chemical modification of carbon nanotubesChem Rev201011095366539720545303
- WangZMaLGold nanoparticle probesCoordination Chem Rev200925311–1216071618
- BoniniMBertiDBaglioniPNanostructures for magnetically triggered release of drugs and biomoleculesCurr Opinion Colloid Interface Sci2013185459467
- ChoKWangXNieSChenZGShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res20081451310131618316549
- DeMGhoshPSRotelloVMApplications of nanoparticles in biologyAdv Mater2008202242254241
- MartinCRKohliPThe emerging field of nanotube biotechnologyNat Rev Drug Discov200321293712509757
- MurphyCJGoleAMStoneJWGold nanoparticles in biology: beyond toxicity to cellular imagingAcc Chem Res200841121721173018712884
- PankhurstQAThanhNTKJonesSKDobsonJProgress in applications of magnetic nanoparticles in biomedicineJ Physics D: Appl Physics20094222224001
- Kalpana SastryRAnshulSRaoNHNanotechnology in food processing sector-An assessment of emerging trendsJ Food Sci Technol201350583184124425990
- KhotLRSankaranSMajaJMEhsaniRSchusterEWApplications of nanomaterials in agricultural production and crop protection: A reviewCrop Protection201235C6470
- MuellerNCNowackBExposure modeling of engineered nanoparticles in the environmentEnviron Sci Technol200842124447445318605569
- HamburgMAScience and regulation. FDA’s approach to regulation of products of nanotechnologyScience2012336607929930022517845
- HoldenJPSunsteinCRSiddiquiIAPolicy principles for the US decision-making concerning regulation and oversight of applications of nanotechnology and nanomaterials Memorandum for the Heads of Executive Departments and AgenciesWashington, DCExecutive Office of the President2011
- KrollAPillukatMHHahnDSchnekenburgerJCurrent in vitro methods in nanoparticle risk assessment: limitations and challengesEur J Pharm Biopharm200972237037718775492
- StoneVJohnstonHSchinsRPDevelopment of in vitro systems for nanotoxicology: methodological considerationsCrit Rev Toxicol200397613626
- LewinskiNColvinVDrezekRCytotoxicity of nanoparticlesSmall200841264918165959
- RogersEJHsiehSFOrgantiNSchmidtDBelloDA high throughput in vitro analytical approach to screen for oxidative stress potential exerted by nanomaterials using a biologically relevant matrix: human blood serumToxicol In Vitro20082261639164718593597
- Rothen-RutishauserBMKiamaSGGehrPA three-dimensional cellular model of the human respiratory tract to study the interaction with particlesAm J Respir Cell Mol Biol200532428128915640437
- JepsonMAClarkMAStudying M cells and their role in infectionTrends Microbiol1998693593659778729
- PluskotaAHorzowskiEBossingerOvon MikeczAIn Caenorhabditis elegans nanoparticle-bio-interactions become transparent: silica-nanoparticles induce reproductive senescencePLoS One200948e662219672302
- MohanNChenCSHsiehHHWuYCChangHCIn vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegansNano Lett20101093692369920677785
- UsenkoCYHarperSLTanguayRLIn vivo evaluation of carbon fullerene toxicity using embryonic zebrafishCarbon N Y20074591891189818670586
- AsharaniPVLian WuYGongZValiyaveettilSToxicity of silver nanoparticles in zebrafish modelsNanotechnology2008192525510221828644
- FakoVEFurgesonDYZebrafish as a correlative and predictive model for assessing biomaterial nanotoxicityAdv Drug Deliv Rev200961647848619389433
- LiuXVinsonDAbtDHurtRHRandDMDifferential toxicity of carbon nanomaterials in Drosophila: larval dietary uptake is benign, but adult exposure causes locomotor impairment and mortalityEnviron Sci Technol200943166357636319746737
- SungJHJiJHParkJDSubchronic inhalation toxicity of silver nanoparticlesToxicol Sci2009108245246119033393
- WuJLiuWXueCToxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposureToxicol Lett200919111819501137
- LiuTLiLTengXSingle and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed miceBiomaterials20113261657166821093905
- HunterPThe paradox of model organisms. The use of model organisms in research will continue despite their shortcomingsEMBO Rep20089871772018670440
- FischerHCChanWCNanotoxicity: the growing need for in vivo studyCurr Opin Biotechnol200718656557118160274
- AhamedMPosgaiRGoreyTJNielsenMHussainSMRoweJJSilver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogasterToxicol Appl Pharmacol2010242326326919874832
- PosgaiRCipolla-McCullochCBMurphyKRHussainSMRoweJJNielsenMGDifferential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: size, coatings and antioxidants matterChemosphere2011851344221733543
- BarandehFNguyenPLKumarROrganically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivoPLoS One20171e29424
- Carrero-MartínezFChibaACell adhesion molecules at the Drosophila neuromuscular junctionUmemoriHHortschMThe Sticky SynapseNew YorkSpringer20091138
- TweedieSAshburnerMFallsKFlyBase ConsortiumFlyBase: enhancing Drosophila Gene Ontology annotationsNucleic Acids Res200937Database issueD555D55918948289
- DasTCaganRDrosophila as a novel therapeutic discovery tool for thyroid cancerThyroid201020768969520578898
- AshburnerMGolicKHawleyRSDrosophila: A Laboratory HandbookCold Spring Harbor, NYCold Spring Harbor Laboratory Press2004
- HoangBChibaASingle-cell analysis of Drosophila larval neuromuscular synapsesDev Biol20012291557011133154
- MargaritisLHKafatosFCPetriWHThe eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshellJ Cell Sci1980431356774986
- Campos-OrtegaJAHartensteinVThe Embryonic Development of Drosophila melanogasterBerlinSpringer-Verlag1985
- BeeAMassartRNeveuSSynthesis of very fine maghemite particlesJ Magnetism Magnetic Mater1995149169
- ParkJAnKHwangYUltra-large-scale syntheses of monodisperse nanocrystalsNat Mater200431289189515568032
- BarreraCHerreraAZayasYRinaldiCSurface modification of magnetite nanoparticles for biomedical applicationsJ Magnetism Magnetic Mater20093211013971399
- HerreraAPBarreraCRinaldiCSynthesis and functionalization of magnetite nanoparticles with aminopropylsilane and carboxymethyldextranJ Mater Chem20081836503654
- CreixellMHerreraAPLatorre-EstevesMThe effect of grafting method on the colloidal stability and in vitro cytotoxicity of carboxymethyl dextran coated magnetic nanoparticlesJ Mater Chem20102085398547
- AllouniZECimpanMRHølPJSkodvinTGjerdetNRAgglomeration and sedimentation of TiO2 nanoparticles in cell culture mediumColloids Surf B Biointerfaces2009681838718980834
- Guidance for IndustryEstimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers2005 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078932.pdfAccessed August 21, 2011
- ReadingBDFreemanBSimple formula for the surface area of the body and a simple model for anthropometryClin Anat200518212613015696524
- Du BoisDDu BoisEFA formula to estimate the approximate surface area if height and weight be known. 1916Nutrition1989553033112520314
- GehanEAGeorgeSLEstimation of human body surface area from height and weightCancer Chemother Rep19705442252355527019
- HaycockGBSchwartzGJWisotskyDHGeometric method for measuring body surface area: a height-weight formula validated in infants, children, and adultsJ Pediatr19789316266650346
- MostellerRDSimplified calculation of body-surface areaN Engl J Med19873171710983657876
- KiehartDPCrawfordJMMontagueRACollection, dechorionation, and preparation of Drosophila embryos for quantitative microinjectionCSH Protoc20072007pdb.prot471721357063
- JonerEJHartnikTAmundsenCENanoparticles and the EnvironmenEnvironmental Fate and Ecotoxicity of Engineered NanoparticlesÅs, NorwayBioforsk, Norwegian Pollution Control Authority2008
- HussainSMHessKLGearhartJMGeissKTSchlagerJJIn vitro toxicity of nanoparticles in BRL 3A rat liver cellsToxicol In Vitro200519797598316125895
- JinCTangYFanXYIn vivo evaluation of the interaction between titanium dioxide nanoparticle and rat liver DNAToxicol Ind Health201329323524423443408
- LongTCSalehNTiltonRDLowryGVVeronesiBTitanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicityEnviron Sci Technol200640144346435216903269
- JiaGWangHYanLCytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullereneEnviron Sci Technol20053951378138315787380
- LamCWJamesJTMcCluskeyRArepalliSHunterRLA review of carbon nanotube toxicity and assessment of potential occupational and environmental health risksCrit Rev Toxicol200636318921716686422
- ShvedovaAACastranovaVKisinERExposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cellsJ Toxicol Environ Health A200366201909192614514433
- SmartSKCassadyAILuGQMartinDJThe biocompatibility of carbon nanotubesCarbon200644610341047
- WarheitDBLaurenceBRReedKLRoachDHReynoldsGAWebbTRComparative pulmonary toxicity assessment of single-wall carbon nanotubes in ratsToxicol Sci200477111712514514968
- WickPManserPLimbachLKThe degree and kind of agglomeration affect carbon nanotube cytotoxicityToxicol Lett2007168212113117169512
- PandeyAChandraSChauhanLKNarayanGChowdhuriDKCellular internalization and stress response of ingested amorphous silica nanoparticles in the midgut of Drosophila melanogasterBiochim Biophys Acta2013183012256226623046978
- DemirETurnaFValesGKayaBCreusAMarcosRIn vivo genotoxicity assessment of titanium, zirconium and aluminium nanoparticles, and their microparticulated forms, in DrosophilaChemosphere201393102304231024095613
- VecchioGGaleoneABrunettiVMutagenic effects of gold nanoparticles induce aberrant phenotypes in Drosophila melanogasterNanomedicine2012811722094122
- GorthDJRandDMWebsterTJSilver nanoparticle toxicity in Drosophila: size does matterInt J Nanomedicine2016343350
- DasSDebnathNPatraPDattaAGoswamiANanoparticles influence on expression of cell cycle related genes in Drosophila: a microarray-based toxicogenomics studyToxicol Environ Chem2012945952957
- VecchioGGaleoneAMalvindiMACingolaniRPompaPPRanking the in vivo toxicity of nanomaterials in Drosophila melanogasterJ Nanoparticle Res20131591936
- PhilbrookNAWinnLMAfroozARSalehNBWalkerVKThe effect of TiO(2) and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 miceToxicol Appl Pharmacol2011257342943622005274
- PompaPPVecchioGGaleoneAIn Vivo toxicity assessment of gold nanoparticles in Drosophila melanogasterNano Research201144405412
- KeyCSReavesDTurnerFBangJJImpacts of silver nanoparticle ingestion on pigmentation and developmental progression in DrosophilaAtlas J Biol201115261
- PanacekAPrucekRSafarovaDAcute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogasterEnviron Sci Technol201145114974497921553866
- WuQWenTLeeGParkJHCaiHNShenPDevelopmental control of foraging and social behavior by the Drosophila neuropeptide Y-like systemNeuron200339114716112848939
- WuQZhangYXuJShenPRegulation of hunger-driven behaviors by neural ribosomal S6 kinase in DrosophilaProc Natl Acad Sci U S A200510237132891329416150727
- WuQZhaoZShenPRegulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systemsNat Neurosci20058101350135516172603
- KaunKRChakaborty-ChatterjeeMSokolowskiMBNatural variation in plasticity of glucose homeostasis and food intakeJ Exp Biol2008211Pt 193160316618805815
- LingoPRZhaoZShenPCo-regulation of cold-resistant food acquisition by insulin- and neuropeptide Y-like systems in Drosophila melanogasterNeuroscience2007148237137417658221
- JohnstonHJHutchisonGChristensenFMPetersSHankinSStoneVA review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicityCrit Rev Toxicol201040432834620128631
- LubickNNanosilver toxicity: ions, nanoparticles – or both?Environ Sci Technol20084223861719192768

