?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ultrasound-mediated drug delivery is a novel technique for enhancing the penetration of drugs into diseased tissue beds noninvasively. By encapsulating drugs into microsized and nanosized liposomes, the therapeutic can be shielded from degradation within the vasculature until delivery to a target site by ultrasound exposure. Traditional in vitro or ex vivo techniques to quantify this delivery profile include optical approaches, cell culture, and electrophysiology. Here, we demonstrate an approach to characterize the degree of nitric oxide (NO) delivery to porcine carotid tissue by direct measurement of ex vivo vascular tone. An ex vivo perfusion model was adapted to assess ultrasound-mediated delivery of NO. This potent vasodilator was coencapsulated with inert octafluoropropane gas to produce acoustically active bubble liposomes. Porcine carotid arteries were excised post mortem and mounted in a physiologic buffer solution. Vascular tone was assessed in real time by coupling the artery to an isometric force transducer. NO-loaded bubble liposomes were infused into the lumen of the artery, which was exposed to 1 MHz pulsed ultrasound at a peak-to-peak acoustic pressure amplitude of 0.34 MPa. Acoustic cavitation emissions were monitored passively. Changes in vascular tone were measured and compared with control and sham NO bubble liposome exposures. Our results demonstrate that ultrasound-triggered NO release from bubble liposomes induces potent vasorelaxation within porcine carotid arteries (maximal relaxation 31%±8%), which was significantly stronger than vasorelaxation due to NO release from bubble liposomes in the absence of ultrasound (maximal relaxation 7%±3%), and comparable with relaxation due to 12 μM sodium nitroprusside infusions (maximal relaxation 32%±3%). This approach is a valuable mechanistic tool for assessing the extent of drug release and delivery to the vasculature caused by ultrasound.
Introduction
Ultrasound-mediated drug delivery
Ultrasound has been investigated as a method for triggering enhanced drug delivery within the human vasculature. This technique is broadly appealing, given the potential of ultrasound to control drug delivery spatially and temporally in a noninvasive manner. Ultrasound-mediated drug delivery (UMDD) has been demonstrated in a number of tissue beds, including the blood–brain barrier, cardiac tissue, prostate, and large arteries.
Acoustic cavitation is a physical mechanism that is hypothesized to mediate UMDD.Citation1–Citation4 Cavitation refers to nonlinear bubble activity that occurs within the vasculature upon ultrasound exposure and can exert mechanical stress on nearby cells and junctions. Mechanical stress can trigger the reduction of barriers to drug delivery, such as endothelial tight junctions or phospholipid membranes, via transient permeabilization. Cavitation can be nucleated at moderate peak-to-peak acoustic pressure amplitudes (<1 MPa) by ultrasound contrast agents.Citation5–Citation8
Nitric oxide (NO) is a molecule that plays a mechanistic role in UMDD. A potent vasodilating gas, NO is involved in the regulation of paracellular and transcellular transport pathways,Citation1,Citation9 and is implicated as a regulatory promoter of hyperpermeability.Citation3,Citation10 Attenuation of NO production in the etiology of progressionCitation5,Citation11 of atherosclerosis and diabetic vascular diseaseCitation1,Citation2,Citation12–Citation14 further highlights the need for novel therapeutic NO modulation and delivery strategies.
Drug delivery quantification
Strategies to study ultrasound-mediated drug release and delivery in vitro and ex vivo have involved optical and electrophysiological techniques.Citation3,Citation4,Citation15–Citation19 Optical techniques, such as fluorescenceCitation16,Citation20,Citation21 or luminescence,Citation18,Citation22,Citation23 take advantage of the native optical properties of the therapeutic or conjugation of tracer molecules. Electrophysiological approaches, such as voltage-clamp techniques,Citation19,Citation24,Citation25 directly assess the changes in membrane potential provoked during UMDD, but often require isolated cells cultured in vitro, where cellular processes can vary from in vivo conditions.Citation26–Citation28 In vivo animal models of UMDD provide relevant bioeffect information, but are costly and subject to considerable intersubject variability. The ability to detect and monitor the response of intact, isolated vascular tissue in real time would constitute a significant advancement in the study of UMDD.
Isolated tissue bath perfusion systems have been used extensively to characterize contractility changes induced by a therapeuticCitation20,Citation29–Citation33 in a variety of muscular tissue beds, including gastric,Citation1,Citation2,Citation12–Citation14 peripheral vascular,Citation3,Citation4,Citation15–Citation19 and cardiovascularCitation16,Citation20,Citation21 beds. In these systems, dose-dependent changes in active muscular tension can be characterized in response to vasorelaxing agents such as bradykinin,Citation18,Citation22,Citation23 sodium nitroprusside,Citation19,Citation24,Citation25 nitroglycerin,Citation26–Citation28 and NO.Citation20,Citation29–Citation33 Adaptation of the isolated tissue bath model to drug delivery studies could provide relevant, real-time quantitative data on the drug release and delivery profiles triggered by ultrasound. We hypothesize that these isolated tissue bath systems can be used to characterize NO-mediated changes in carotid vascular tone upon ultrasound exposure.
Materials and methods
Tissue bath system
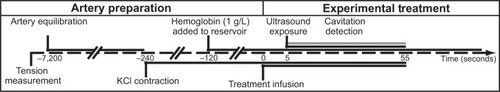
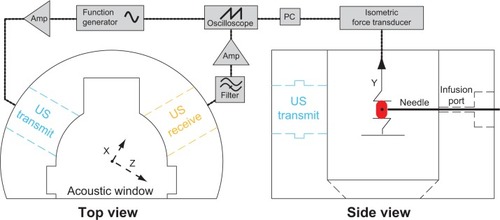
Due to their similarity to human arteries, ease of excision, and robust post mortem viability,Citation31 porcine carotid arteries were chosen as the model artery to study NO delivery. Carotid arteries were obtained post mortem from young Yorkshire pigs (~20 kg) according to a protocol approved by the Institutional Animal Care and Use Committee at the Cincinnati Department of Veterans Affairs Medical Center (Animal Welfare Assurance Number A3446-01). Seven pigs were initially anesthetized with intravenous ketamine 30 mg/kg, followed by pentobarbital 35 mg/kg for deep surgical anesthesia. Prior to artery excision, the animals were euthanized with saturated KCl solution (~[KCl]in vivo 0.18 M). Segments of porcine carotid tissue were harvested immediately following sacrifice and stored in ice-cold, oxygenated Krebs–Henseleit buffer (KHB, NaCl 115.9 mM; KCl 5.4 mM; MgSO4-7H2O 1.2 mM; NaHCO3 25 mM; D-glucose 11.1 mM; NaH2PO4 0.5 mM) until use within 18 hours of excision from the animal. Previous investigatorsCitation32 have suggested that arteries remain viable for up to 3 days if obtained and handled in this manner. The tissue was dissected free of loose adventitia and connective tissue, and segmented into rings. These rings were cut to a length between 3.95 mM and 4.05 mM; the mean wet mass after blotting was 20.5 mg with a standard deviation of 2.6 mg. Each segment was mounted on two rigid, stainless-steel wires. The bottom wire was fixed to the tissue bath, and the top wire was coupled to an isometric force transducer (Radnoti LLC, Monrovia, CA, USA) for measurement of arterial tension. The electronic configuration is depicted in . The tension signal was sampled digitally at 20 Hz (LabChart 6; AD Instruments, Colorado Springs, CO, USA) and saved to a PC for post-processing. The carotid segment was submerged in a custom reservoir filled with KHB maintained at physiologic temperature with continuous bubbling of a 95% O2/5% CO2 gas mixture to maintain physiologic pH (range 7.35–7.45).
Figure 1 Electronic configuration of the ultrasound tissue bath system.
Notes: Two submersible ultrasound transducers are coupled to the reservoir: a transmit transducer (1 MHz, 3% duty cycle, blue) focuses on the lumen of the artery, while a second receive transducer (7 MHz, orange) detects cavitation emissions from nitric oxide-loaded bubble liposomes perfused within the vessel lumen (red) via a 26-gauge blunt injection needle.
Abbreviation: US, ultrasound.
Following mounting of the artery and submersion in physiologic KHB, healthy arteries underwent a brief contraction and relaxation cycle.Citation32 Artery rings not exhibiting this behavior were deemed unfit for experimentation and discarded. Prior to treatment, the arterial ring was pre-stretched to relieve the passive elastic component of arterial tension in a manner similar to that described by Herlihy and Murphy.Citation32 Briefly, the artery was stretched incrementally by translating the force transducer with a micropositioning stage (Radnoti LLC). To equilibrate the artery within physiologic buffer, the artery was alternately contracted and relaxed by serial infusions of KHB and KHB containing additional KCl, substituted in an equimolar fashion for NaCl. KCl was chosen as the optimal contractile agent in this biomedical engineering study due to its ability to induce rapid smooth muscle constriction consistently across carotid arteries excised from different pigs of the same breed. Each artery was contracted initially to steady state using KHB containing 50 mM KCl after each increment. Following a KHB wash, this process was repeated until a maximum KCl contraction was achieved. Healthy arteries consistently responded to precontraction with 50 mM KCl-doped KHB; however, the extent of precontraction was variable (range 200–330 mN). The basal ring tension that produced a maximum contraction tension was determined to be 75 mN. Thus, subsequent rings were manually prestretched to this basal tension upon mounting.
Bubble liposomes
Bubble liposomes were manufactured according to the method described by Endo-Takahashi et alCitation34 for bubble liposomes. This liposomal formulation consisted of dipalmitoylphosphatidylcholine (DPPC), N-[1-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium (DOTAP), polyethylene glycol (PEG) 2000, and PEG 750 in a 79:15:3:3 molar ratio. After manufacturing, 1 mL (1 mg lipid) of the liposomal emulsion was pipetted into glass vials, which were evacuated of headspace air with a laboratory vacuum and stored at 4°C until use. Immediately prior to use, the vials were warmed to room temperature and 2.5 mL of gas was injected into the vial headspace through a rubber septum. For NO-loaded bubble liposomes (NOBLs), this gas consisted of a 50:50 volume ratio of NO gas (Sigma-Aldrich, St Louis, MO, USA) and octafluoropropane (C3F8, or OFP) gas (Specialty Gases of America, Toledo, OH, USA). To assess the effect of cavitation alone (without NO) on vasorelaxation, C3F8 bubble liposomes were also manufactured by injecting 2.5 mL of C3F8. Following this step, the vial was mechanically agitated by shaking vigorously for 45 seconds (Vialmix®, Lantheus Medical Imaging, North Billerica, MA, USA). This agitation step heated the emulsion slightly, so the vial was allowed to equilibrate to room temperature. Immediately prior to experimental use, 500 μL of the liposomal emulsion was diluted into oxygenated (pO2: 722 mmHg) room temperature KHB to a final concentration of 0.05 mg lipid/mL for infusion into the system.
NOBL characterization
A sample of the NOBL suspension was diluted (1:1000; v/v) into room temperature, aerated phosphate-buffered saline (Sigma-Aldrich) and the size distribution was measured using an impedance-based particle sizer (Multisizer 4, 30 μm aperture; Beckman Coulter, Brea, CA, USA). Each measurement analyzed 100 μL of the diluted sample through a 30 μm aperture over 30 seconds. Each measurement produced a number density histogram, corrected for the dilution, with bins logarithmically spaced between 0.6 μm and 18 μm. This histogram was transformed into a volume-weighted distribution for further analysis. The results from three measurements, each using a fresh vial of NOBLs, were averaged to produce a final volume-weighted size distribution.
The frequency-dependent attenuation coefficient, α(f) in dB/cm, was determined using a broadband substitution technique,Citation35 the components of which were described previously in Raymond et al.Citation36 Briefly, NOBLs were diluted (1:500) into a reservoir containing aerated phosphate-buffered saline, stirred, and allowed to flow by gravity into a sample chamber with acoustically transparent polycarbonate film windows (CLINIcell 25, Mabio, Tourcoing, France). The reservoir, sample chamber, and transducers were mounted in a test tank filled with distilled water maintained at 37°C±0.5°C using a circulating water bath (Neslab EX, Newington, NH, USA). A pair of broadband transducers (PI-20, Olympus NDT, Waltham, MA, USA) was used to acquire the through-transmission spectrum over the frequency range of 1–30 MHz (31 KPa peak negative pulse pressure; 33 dB dynamic range). The attenuation spectrum was computed from the received amplitude spectra in the absence (diluent alone) and presence of the bubble liposomes, respectively. Acoustic attenuation measurements were made in triplicate and a separate vial of NOBLs was used for each measurement.
Hemoglobin
The short half-life (<1 second) of NO in the presence of hemoglobin and oxygen in vivo limits the spatial extent over which NO can be effective as a signaling molecule.Citation37 In order to mimic the in vivo milieu more closely and to quench nonencapsulated NO, 1 g/L hemoglobin (porcine, Sigma-Aldrich) was added to the reservoir prior to each treatment. We observed that hemoglobin concentrations greater than 1 g/L produced a negligible decrease in vasorelaxation during NOBL + KHB infusions and occasionally caused vasospasmic contractions. Thus, 1 g/L hemoglobin was used in all subsequent treatments.
Ultrasound exposure and cavitation detection
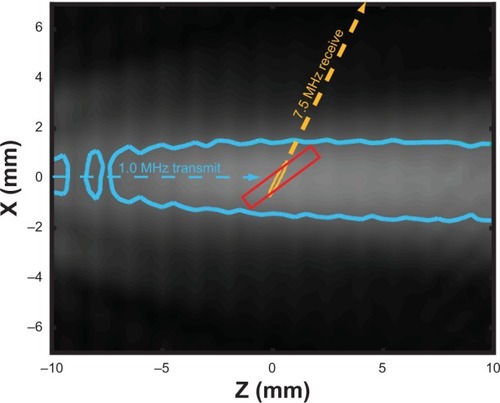
To reveal the mechanism of ultrasound-mediated drug release and delivery of NO to vascular tissue, two ultrasound transducers were coupled to the tissue bath reservoir. One transducer was used to deliver pulsed ultrasound to nucleate cavitation from the liposomes and a second transducer was used to monitor the acoustic emissions passively for evidence of acoustic cavitation. The experimental configuration is depicted in . Prior to each experiment, a calibrated 1 MHz therapy transducer (Olympus Panametrics, Waltham, MA, USA) was aligned confocally with a 7.5 MHz passive cavitation detector (PCD; Olympus Panametrics) using an ultrasonic pulser receiver (5077PR; Olympus NDT). The confocal point was positioned in the axial center of the carotid ring using a three-axis translation stage (Newport 423, Irvine, CA, USA). This geometry was chosen to ensure that the ultrasound therapy field encompassed the entire carotid artery, while cavitation emissions were detected primarily from the lumen. During experimental treatment, a function generator (Agilent Technologies, Santa Clara, CA, USA) supplied a sinusoidal signal (30 cycles, 100 Hz pulse repetition frequency) that was amplified (750A250; Amplifier Research, Souderton, PA, USA) and used to drive the 1 MHz therapy transducer. Acoustic scattering within the tissue bath reservoir affected the beam profile slightly. Therefore, an in situ pressure field calibration was performed in the absence of the artery ring and wires ().
Figure 2 In situ ultrasound beam profiles of the 1 MHz and 7.5 MHz transducers.
Notes: The –3 dB contours of the transmit field (blue) and receive sensitivity (orange) are depicted, along with the anticipated location of the artery (red, box).
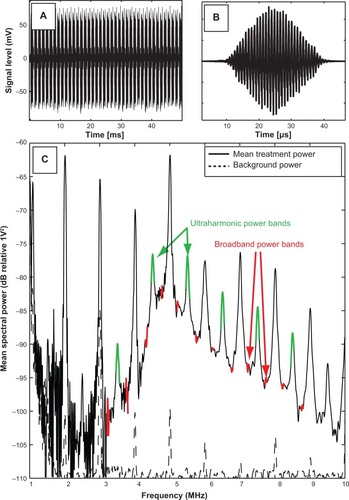
During ultrasound exposure, acoustic emissions were detected by the PCD, high-pass filtered (1 MHz high-pass, #07766 TTE, Los Angeles, CA, USA), amplified (10×, Model 5185, Signal Recovery, Oak Ridge, TN, USA), and sampled at 20 MHz with an oscilloscope (LeCroy Model LT372, Chestnut Ridge, NY, USA). Sequences of 128 voltage time traces were saved to a PC for post-processing (). To enable cavitation detection spaced more consistently in time, one of every four pulses was saved. The traces were analyzed using a custom MatLab (The Mathworks Inc, Natick, MA, USA) script to extract spectral information associated with specific modes of bubble activity. A window (Blackman window, MatLab) was applied to each scattered pulse () and transformed into the frequency domain. Each frequency spectrum was scaled to account for the energy lost due to windowing. A frequency domain comb filter was applied to the power spectrum () to extract the ultraharmonic (100 kHz bands at odd multiples of 500 kHz) and broadband (50 kHz bands between harmonic and ultraharmonic frequencies) components of the spectrum. The energy contained in the ultraharmonic and broadband signals was associated with stable and inertial cavitation, respectively.Citation38,Citation39 This process is depicted graphically in .
Figure 3 Graphical representation of the signal processing technique used to assess cavitation emissions detected by the single element passive cavitation detector.
Notes: (A) Scattered pulses detected by the passive cavitation detector were filtered (1 MHz low pass filter), amplified (10×), digitized, and saved to a PC in sequence mode. Individual pulses were windowed in the time domain (B), and then transformed into the frequency domain. Ultraharmonic and broadband components of the power spectrum (C) were quantified, and served as indicators of stable and inertial cavitation, respectively.
Ultrasound parameters were chosen to promote stable cavitation nucleated by the NOBLs within the lumen of the arterial ring over the 55-second treatment. A 30-cycle therapy pulse was chosen to minimize standing waves in the reservoir. To determine the optimal acoustic pressure to promote stable cavitation, a dose-escalation approach was employed. An artery was mounted within the reservoir filled with oxygenated KHB containing 1 g/L hemoglobin. NOBL infusions (0.2 mL/min) with concurrent ultrasound exposure and cavitation detection were performed serially. Between each treatment, the reservoir was flushed with fresh hemoglobin-doped KHB and the acoustic pressure increased incrementally. This process was repeated at peak-to-peak acoustic pressure amplitudes ranging from 0 MPa to 0.38 MPa. The peak-to-peak acoustic pressure amplitude that promoted maximal ultraharmonic energy over the 50-second ultrasound exposure (0.34 MPa) was chosen for all subsequent exposures.
Treatment
During treatment, the lumen of each artery was infused for 55 seconds with randomized combinations of buffer alone, buffer with NOBLs, or buffer with 12 μM sodium nitroprusside (Sigma-Aldrich) using a 24-gauge blunt hypodermic needle connected to a syringe pump (KD Scientific, #23; Canning Vale, Australia). After 5 seconds of infusion, ultrasound or sham exposure and cavitation detection commenced. This experimental procedure is diagrammed in .
Tension analysis
Arterial ring tension was analyzed using a custom MatLab script. Each tension curve was normalized to the maximum tension produced by 50 mM KCl KHB using EquationEquation 1(1) ,
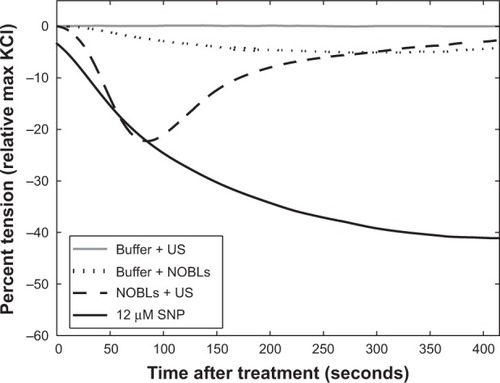
Figure 5 Representative tension reduction (relative to the maximum KCl contraction tension) for buffer + US, buffer + NOBLs, NOBLs + US (peak-to-peak acoustic pressure amplitude 0.34 MPa), and control agonist sodium nitroprusside. Each treatment’s minimum tension (maximal relaxation) was used for grouped analysis.
Abbreviations: SNP, sodium nitroprusside; NOBLs, nitric-oxide loaded bubble liposomes; US, ultrasound.
Statistical analyses
In general, the mean and standard deviation of each data set was reported. Statistical analyses were performed using MatLab (Statistical Toolbox). Normal distributions were confirmed using Lilliefors test, with a threshold P-value of 0.05. Differences in means between treatment groups were analyzed using a one-way unbalanced analysis of variance. P-values less than 0.05 were considered to be statistically significant. Subsequently, pairwise comparisons with a Bonferroni correction were performed to compare across individual treatments to minimize the family-wise error rate for multiple comparisons.
Linear regression was performed to test for a correlation between maximal relaxation and (a) wet tissue weight, (b) 35 mM KCl tension, and (c) cavitation energy. A one-way unbalanced analysis of variance was used to test for significant correlations. For these tests, the P-value, f-statistic (Fstat), and degrees of freedom (df) are reported. Spearman’s rank correlation coefficients (ρ) were computed to test for monotonic statistical relationships. The null hypothesis of no correlation was tested to produce a P-value; values below 0.05 were considered to be statistically significant.
Results
Liposome characterization
As depicted in , NOBLs had a broad, bimodal volume-weighted size distribution, ranging in diameter from approximately 1.5 μm to 15 μm. The peak volume density occurred at a diameter of 2.5 μm. A separate population of particles existed at 11 μm, diminishing in diameter above 15 μm.
Figure 6 (A) Size distribution (n=3) of the NOBLs, weighted by volume (black) and number density (gray).
Notes: The presence of particles >4 μM (by volume) agrees with Endo-Takahashi et al.Citation34 Dots indicate one standard deviation. (B) Acoustic attenuation as a function of frequency, as determined using the system described in Raymond et al.Citation36
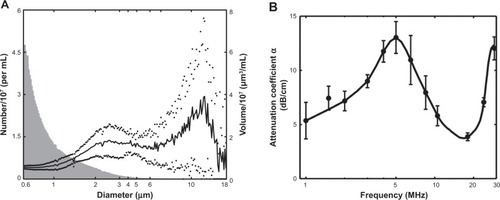
shows the measured attenuation coefficient as a function of frequency for NOBLs (n=3). NOBLs attenuated ultrasound across the 33 dB bandwidth of the system (1–30 MHz). A strong, broad resonance peak (α =13.0 dB/cm) was observed at 5 MHz. A second resonance peak (α =12.3 dB/cm) was observed near 28 MHz. NOBLs attenuated weakly between these two frequencies.
Cavitation
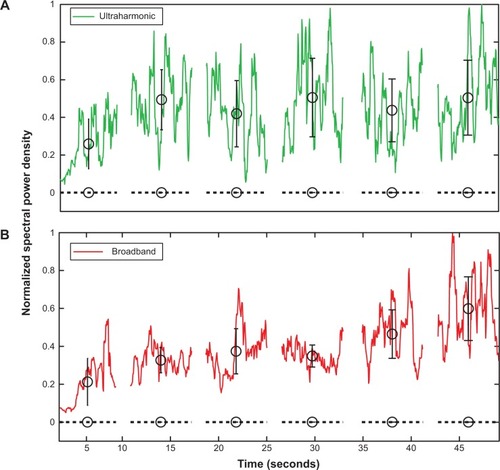
In general, ultraharmonic and broadband acoustic emissions within the arterial lumen persisted throughout the 50-second exposures in all eight ultrasound-treated arteries. show representative traces of the ultraharmonic and broadband acoustic power detected by the PCD as a function of time. A representative spectrum, averaged over the 55-second treatment, is depicted in . Ultraharmonic energy was typically strong, yet variable, during the first few seconds of ultrasound exposure, and remained steady for the duration of the 50-second exposure. Within the NOBL + ultrasound treatment group, no correlation was observed between maximal relaxation and the average spectral emissions (ultraharmonic, ρ=−0.45, P=0.21; harmonic, ρ=−0.46, P=0.25; broadband, ρ=−0.50, P=0.21).
Figure 7 Spectral analysis of scattered acoustic signal from NOBLs.
Notes: Averaged spectrum of detected cavitation emissions across a 50-second ultrasound exposure during NOBL infusion into the lumen of a carotid artery (solid), compared to spectral emission from sham ultrasound exposures (buffer + ultrasound alone, dotted). (A) Ultraharmonic (eg, 3f/2, 5f/2.) and (B) broadband frequency components of the transmitted fundamental frequency (f, 1 MHz) were consistently observed, indicating strong, persistent nonlinear bubble activity. Black circles indicate the mean of the local data group, bars indicate ± one standard deviation.
Abbreviation: NOBLs, nitric oxide-loaded bubble liposomes.
Vasorelaxation
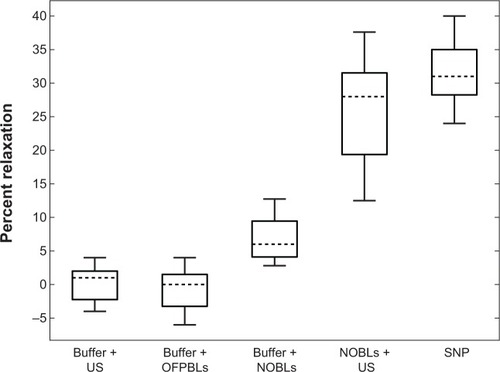
Seven carotid rings each from seven separate pigs were exposed to each experimental treatment, as shown in . Carotid rings relaxed in response to treatments in a characteristic manner, ie, a sharp tension decrease followed by steady restoration to the tension level induced by 35 mM KCl precontraction. Neither the order of treatment nor the time post-excision from the animal (ρ=−0.45, P=0.21) resulted in an apparent trend in maximal relaxation. Relaxation after 12 μM sodium nitroprusside infusions was significantly stronger (32%±5%) than NOBL infusions (P<0.01, Fstat =104.15, df =13) and sham control treatments, which consisted of buffer and NOBLs (7%±3%), buffer and ultrasound (1%±2%), or C3F8 bubble liposomes and ultrasound exposure (2%±1%). Arterial rings exposed to NOBLs + ultrasound experienced strong maximal relaxation (31%±8%) that was significantly greater than buffer and NOBLs (P<0.001, Fstat =29.01, df =13) and statistically identical to treatment with 12 μM sodium nitroprusside (P=0.18, Fstat =2.06, df =13). Across all experimental samples, no correlation was observed between the maximal relaxation and precontraction tension (P=0.82, Fstat =0.05, df =21) or wet tissue weight (P=0.33, Fstat =0.98, df =21).
Figure 8 Carotid artery vasorelaxation as a function of treatment.
Notes: Data means are indicated by horizontal dotted lines and boxes indicate the 25th and 75th percentiles. The range of adjacent data is indicated by error bars. Seven arterial rings from seven pigs were used for all treatments. Statistically significant differences in data means (P<0.05) were observed between all data sets except (A) buffer + US/buffer + OFPBL and (B) NOBLs + US/SNP.
Abbreviations: SNP, 12 μM sodium nitroprusside; US, ultrasound; NOBLs, nitric-oxide loaded bubble liposomes; OFPBL, octafluoropropane-loaded bubble liposomes.
Discussion
Liposome characterization
The characteristics of NOBLs can be compared directly with previously reported data on C3F8-loaded bubble liposomes manufactured using similar methods. Endo-Takahashi et alCitation34 reported a volume-weighted size distribution peak at 749 nm with a significant volume-weighted population greater than 4 μm. The bubble liposomes of Endo-Takahashi et alCitation34 containing only C3F8, are smaller than the NOBLs described in this study (). NOBLs have a bimodal volume-weighted size distribution with a primary peak at 2.5 μm and variable, secondary peak at 11 μm. This size distribution has implications for clinical translation: nanosized NOBLs are small enough to pass through the pulmonary capillary bedCitation41 yet large enough to permit strong cavitation nucleation from 1 MHz ultrasound exposure.Citation7 In future studies, subpopulations of the bimodal distribution could be isolated by differential centrifugationCitation42 to avoid undesired retention in micron-sized capillaries. However, these techniques present a trade-off between frequency-dependent ultrasound response and drug-loading capability, which are both highly dependent on particle size.Citation43
The size discrepancy between the primary peaks could be explained by the use of a NO/C3F8 blend in this study, compared with C3F8 alone used in the report by Endo-Takahashi et al.Citation34 NO is more soluble in lipid than in aqueous solution,Citation44 yet reacts more readily with oxygen in these environments. NO is further soluble in perfluorocarbon, demonstrated by prolonged release profiles in an in vivo hamster model.Citation45 In this study, efforts were made to ensure that the procedure used to manufacture NO/octafluoropropane-loaded liposomes mimicked closely the procedure outlined by Endo-Takahashi et al.Citation34 Kim et al describe an alternative method to encapsulate NO within echogenic liposomes which minimizes oxidation and degradation of NO prior to experimental use.Citation46 Future work should implement these procedures and tailor the gas and lipid composition in NOBL formulations to achieve an optimal release profile and size distribution to improve therapeutic benefit.
Using this liposomal formulation, the pharmacokinetics of NOBLs in vivo are unclear. The peak number density of NOBLs occurred at 600 nm, which was the lower limit of size detection used here. Particles in this size range, capable of retention within tumor vasculature,Citation47 can effectively attenuate ultrasound at theragnostic frequencies.Citation48 Further, DOTAP, a cationic lipid, likely conferred an affinity to the anionic endothelial glycocalyx.Citation49 This strong affinity for the vascular walls would decrease circulation time in vivo,Citation50 possibly necessitating local infusion, which would limit the applicability of NOBLs to tissues downstream from traditional intra-arterial access sites. Enhanced targeting and localization near the endothelium could also be achieved ultrasonically by use of acoustic pulses to optimize radiation forceCitation51 and acoustic streaming.Citation52 The presence of long chain (PEG 2000) and short chain (PEG 500) polyethylene glycol has been used in liposomal formulations to mitigate potential immunogenicity and antigenicity, thus prolonging circulation time.Citation53 In our model, bubble liposomes were injected directly into the lumen of the arterial ring, where they were exposed to 30 cycle pulses of 1 MHz ultrasound at a peak-to-peak acoustic pressure amplitude of 0.34 MPa. Hemoglobin at in vivo concentrations would completely quench NO produced within the lumen of a blood vessel, thus necessitating NO delivery in close proximity to the endothelium.Citation15,Citation54 Future studies investigating ultrasound-mediated NO release from liposomes should consider these issues.
Ultrasound exposure and cavitation
The data presented here demonstrate that strong relaxation by NO-loaded liposomes can be enhanced by ultrasound exposure at acoustic pressures lower than previously described.Citation46,Citation55 In this study, vascular relaxation was triggered from NOBLs with pulsed ultrasound at a peak-to-peak acoustic pressure amplitude of 0.34 MPa. Despite the detection of strong cavitation at this acoustic pressure, the precise mechanism of NO release and delivery remains unclear. Smith et al describe acoustically driven diffusion at moderate acoustic pressures,Citation56,Citation57 and other investigatorsCitation7,Citation58,Citation59 have postulated that the lipid shell of microbubbles must first be ruptured before cavitation nucleation can occur, liberating encapsulated gas during rapid fragmentation. At a 0.34 MPa pressure exposure, it is likely that NO was released from the bubble liposomes gradually over a number of acoustic cycles. Radhakrishnan et alCitation60 have detected loss of echogenicity from contrast agents at acoustic pressures below the stable and inertial cavitation thresholds. In their experiments using Definity® and echogenic liposomes, the onset of stable and inertial cavitation was concomitant with an 80% loss of echogenicity. Thus, the release of NO from bubble liposomes could also occur at acoustic peak-to-peak pressures lower than the 0.34 MPa utilized in the present study. The minimum pressure amplitude that triggers the delivery of NO to vascular tissue warrants further elucidation.
In this study, C3F8 stabilized NO within the bubble liposomes prior to activation by ultrasound. The different properties of these gases (eg, molecular size, solubility) could promote a differential diffusion profile during acoustically driven oscillation. Upon membrane distention during volumetric bubble expansion, NO would likely dissolve more readily in the surrounding aqueous medium due to its high solubility. Also, after liberation of NO, the residual C3F8 bubble activity could promote microstreaming and enhanced convection and penetration into nearby vascular tissue. The diffusivity of NO, roughly 3,300 μm2 per second in muscular tissue,Citation24,Citation54,Citation61 is high enough to support a linear diffusion distance of roughly 250 μm (approximate radial depth of porcine carotid smooth muscle cellsCitation11,Citation62) over several seconds. NOBL infusions with ultrasound exposure consistently caused sharp decreases in vascular tension, typically occurring in the order of tens of seconds, followed by a steady tension restoration period, which lasted several minutes ().
Vasorelaxation
Arterial segments relaxed significantly more when treated with NOBL infusions in the presence of pulsed ultrasound, compared with infusions with NOBLs alone (). The ability of ultrasound to promote the delivery of encapsulated NO to vascular tissue agrees with observations by previous investigators.Citation46,Citation63,Citation64 Kim et alCitation46 using NO-loaded echogenic liposomes, demonstrated increased vasodilation of rabbit carotids after exposure to 5.7 MHz color Doppler ultrasound at a peak negative acoustic pressure of 0.35 MPa. Huang et al used a mixture of NO and argon gas to encapsulate NO and trigger effective release to porcine carotid tissue.Citation55 In this study, the release of NO from the liposome into the surrounding medium was decreased by roughly 65% using the argon/NO mixture. At lower peak-to-peak pressure amplitudes (<0.40 MPa), other encapsulated bioactive gases, such as xenon, have been shown to exhibit enhanced release and delivery profiles with ultrasound.Citation16,Citation56,Citation65 Other studies have demonstrated the feasibility of encapsulating NO or NO-yielding molecules to prevent physiologic degradation.Citation58,Citation66 For example, McKinlay et al demonstrated strong vasorelaxation of porcine coronary rings incubated with NO bound within a porous organic metal framework.Citation58 Consequently, future studies should focus on elucidating the mechanisms of NO release from NOBLs, so that efficient exposure protocols can be developed to translate ultrasound-mediated NO delivery into the clinic.
System development
Although the ultrasound tissue bath system is capable of providing real-time feedback on NO delivery to vascular tissue, it has a few limitations. Here, a volatile anesthetic was used to sedate the animals prior to carotid excision, which can reduce endothelium function significantly by inhibiting endothelial production of NO.Citation12,Citation24 Cavitation near the vascular endothelium during UMDDCitation11,Citation14,Citation67 is hypothesized to liberate endothelial NO synthase from membrane-bound proteins, such as caveolin-1.Citation46,Citation63,Citation64 Increased cytosolic endothelial NO synthase results in increased endothelium-derived NO,Citation10,Citation68 an effect likely absent in our model. Future studies should employ sedatives that do not adversely affect endothelial response, or should use abattoir-derived vascular tissue.
During arterial precontraction, porcine hemoglobin was diluted into the reservoir to a concentration of 1 g/L. At this concentration, arteries consistently re-established equilibrium contraction tension following NO-induced vasorelaxation. This was likely due to the quenching of excess NO by hemoglobin, a reaction well documented in vivo.Citation16,Citation40,Citation65 Because free hemoglobin quenches NO a thousand times faster than hemoglobin within intact erythrocytes,Citation60,Citation69 the presence of free hemoglobin in the bath likely resulted in a weaker vasorelaxation than expected in vivo in the absence of hemolysis.
At higher hemoglobin concentrations (>40 g/L) or in whole blood (120 g/LCitation31,Citation40), arteries routinely experienced periodic contractions that prevented restoration of tension to equilibrium. This effect was likely indicative of vasospasm, which has been documented in cerebral arteries as a result of subarachnoid hemorrhage.Citation12 While this effect was a limitation in our model, Fathi et alCitation67 describe a potential role of NO delivery to treat cerebral tissue following subarachnoid hemorrhage. Also, using intact erythrocytes, Kim et al describe a technique to release NO from loaded echo-genic liposomes within the rabbit carotid artery to reduce ischemic neurologic deficits.Citation46 Intact, fresh erythrocytes also ensure the presence of viable oxyhemoglobin to bind NO efficiently.Citation68 Future applications of this ultrasound tissue bath model should implement hemoglobin in the form of intact erythrocytes to ease the transition to possible clinical applications, such as localized ultrasound-mediated delivery of NO for arresting development of atheroma,Citation1,Citation4,Citation13 induction of vascular hyperpermeability,Citation1,Citation3 or treatment of vasospasm after subarachnoid hemorrhage.Citation68
The system described here also implemented an infusion of treatment combinations directly to the lumen of the vessel. This approach, while likely difficult to replicate in vivo, ensured steady replenishment of drugs and bubbles during experimental treatment, and permitted simple adaptation of a previously established tissue perfusion model. An infusion of 12 μM sodium nitroprusside, directly infused into the lumen of the artery, likely only remained in the arterial lumen for a few seconds before being dispersed throughout the large reservoir filled with Krebs buffer. Together, these changes may have resulted in a much lower amount of sodium nitroprusside, and its product NO, delivered to the vascular rings compared with preparations using sodium nitroprusside in traditional tissue bath systems.
Smooth muscle relaxation
In vivo, NO-mediated modulation of smooth muscle tone is well described by two biochemical pathways: (a) stimulation of guanosine 3′:5′ cyclic monophosphate (cGMP) and adenosine 3′:5′ cyclic monophosphate and (b) direct modulation of calcium-activated, potassium channel (K+Ca) permeability.Citation40 Bolotina et al describe the effect of exogenous NO on cGMP and K+Ca. These authors observed that cGMP-mediated vasorelaxations from exposure to NO were transient in nature and concentration-dependent, as determined by quantifying maximal relaxation,Citation69 which is the metric used here. According to Tare et al the K+Ca pathway can affect the temporal nature of NO-mediated vasorelaxation, an interesting future application of this novel system. Accordingly, these authors integrated vasorelaxation over time to quantify integrated relaxation.Citation40 Due to the potential for sensitivity to the K+Ca-dependent pathway of NO-mediated vasorelaxation, integrated relaxation could be considered as a potential metric in future applications of this system, and may assist in understanding the temporal nature of ultrasound-mediated NO release from bubble liposomes.
Conclusion
The ultrasound tissue bath system described here demonstrates a novel, effective technique to characterize ultrasound-mediated delivery of a bioactive gas to vascular tissue. Ultrasound tissue bath systems can be used to monitor UMDD in real time. The data presented demonstrate that NO can be released from bubble liposomes with 1 MHz pulsed ultrasound exposure and deposited into vascular tissue. NO penetration into tissue causes potent vasorelaxation, which manifests as a change in isometric vascular tension. Future studies using this technique may focus on monitoring the delivery of other bioactive agents that cause vasorelaxation, such as proteins, cells, or nucleic material.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 HL074002) and a pilot grant from the University of Cincinnati Cardiovascular Center of Excellence. The authors gratefully acknowledge Shane Duffield (University of Cincinnati; Biomedical Engineering) for his assistance with size distribution measurements and the technical staff at the National Center for Physical Acoustics (University of Mississippi, Oxford, MS, USA) for manufacturing the custom tissue bath reservoir for ultrasound studies. The authors would also like to thank Dr Neil Weintraub (Medical College of Georgia) for useful discussions about vascular biology and implementation of the tissue bath system.
Disclosure
The authors report no conflicts of interest in this work.
References
- VykhodtsevaNMcDannoldNHynynenKProgress and problems in the application of focused ultrasound for blood–brain barrier disruptionUltrasonics200848427929618511095
- LaingSTKimHKopechekJAUltrasound-mediated delivery of echogenic immunoliposomes to porcine vascular smooth muscle cells in vivoJ Liposome Res201020216016719842795
- MukherjeeDWongJGriffinBTen-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administrationJ Am Coll Cardiol20003561678168610807476
- WuJNyborgWLUltrasound, cavitation bubbles and their interaction with cellsAdv Drug Deliv Rev200860101103111618468716
- LiTLiuGLiJMechanisms of prostate permeability triggered by microbubble-mediated acoustic cavitationCell Biochem Biophys201264214715322722876
- McDannoldNVykhodtsevaNHynynenKTargeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activityPhys Med Biol200651479380716467579
- BaderKBHollandCKGauging the likelihood of stable cavitation from ultrasound contrast agentsPhys Med Biol201358112714423221109
- DengCXXuQApfelRHollandCKIn vitro measurements of inertial cavitation thresholds in human bloodUltrasound Med Biol19962279399488923712
- KomarovaYMalikABRegulation of endothelial permeability via paracellular and transcellular transport pathwaysAnnu Rev Physiol201072146349320148685
- DuránWNBeuveAVSánchezFANitric oxide, S-nitrosation, and endothelial permeabilityIUBMB Life2013651081982624078390
- DavignonJRole of endothelial dysfunction in atherosclerosisCirculation200410923 Suppl 1
- MacdonaldRLWeirBKA review of hemoglobin and the pathogenesis of cerebral vasospasmStroke19912289719821866764
- JamesANRyanJPParkmanHPEffects of the selective serotonin reuptake inhibitor, fluoxetine, on regional gastric contractilityNeurogastroenterol Motil2005171768215670267
- SenaCMPereiraAMSeiçaREndothelial dysfunction – a major mediator of diabetic vascular diseaseBiochim Biophys Acta20131832122216223123994612
- VanhouttePMEndothelium-dependent contractions in arteries and veinsBlood Vessels19872431411443109528
- PhillipsLCKlibanovALBowlesDKRagostaMHossackJAWamhoffBRFocused in vivo delivery of plasmid DNA to the porcine vascular wall via intravascular ultrasound destruction of microbubblesJ Vasc Res201047327027419923850
- PhillipsLCKlibanovALWamhoffBRHossackJAIntravascular ultrasound detection and delivery of molecularly targeted microbubbles for gene deliveryIEEE Trans Ferroelectr Freq Control201259715961601
- MylonopoulouEBazán-PeregrinoMArvanitisCDCoussiosCCA non-exothermic cell-embedding tissue-mimicking material for studies of ultrasound-induced hyperthermia and drug releaseInt J Hyperthermia201329213314423406389
- DengCXSielingFPanHCuiJUltrasound-induced cell membrane porosityUltrasound Med Biol200430451952615121254
- WeintraubNLJoshiSNBranchCAStephensonAHSpragueRSLonigroAJRelaxation of porcine coronary artery to bradykinin. Role of arachidonic acidHypertension1994236 Pt 29769818206638
- DoinikovAABouakazATheoretical investigation of shear stress generated by a contrast microbubble on the cell membrane as a mechanism for sonoporationJ Acoust Soc Am201012811120649196
- IharaEHiranoKDerkachDNNishimuraJNawataHKanaideHThe mechanism of bradykinin-induced endothelium-dependent contraction and relaxation in the porcine interlobar renal arteryBr J Pharmacol2000129594395210696094
- SheikovNMcDannoldNSharmaSHynynenKEffect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endotheliumUltrasound Med Biol20083471093110418378064
- NakamuraKTerasakoKTodaHMechanisms of inhibition of endothelium-dependent relaxation by halothane, isoflurane, and sevofluraneCan J Anaesth19944143403468004742
- FanZLiuHMayerMDengCXSpatiotemporally controlled single cell sonoporationProc Natl Acad Sci U S A201210941164861649123012425
- KleschyovALDoes nitric oxide mediate the vasodilator activity of nitroglycerin?Circ Res2003939e104e11214551241
- SuttonJTHaworthKJPyne-GeithmanGHollandCKUltrasound-mediated drug delivery for cardiovascular diseaseExpert Opin Drug Deliv201310557359223448121
- ParkJFanZDengCXEffects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cellsJ Biomech201144116416920863503
- JiaLFurchgottRFInhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factorJ Pharmacol Exp Ther199326713713788229764
- ArcherSLHuangJMHamplVNelsonDPShultzPJWeirEKNitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinaseProc Natl Acad Sci U S A19949116758375877519783
- PondWGMersmannHJBiology of the Domestic PigIthaca, NY, USACornell University Press2001
- HerlihyJTMurphyRALength-tension relationship of smooth muscle of the hog carotid arteryCirc Res19733332752834746719
- TunstallRRShuklaPGrazul-BilskaASunCO’RourkeSTMT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteriesJ Pharmacol Exp Ther2010336112713320959363
- Endo-TakahashiYNegishiYKatoYSuzukiRMaruyamaKAramakiYEfficient siRNA delivery using novel siRNA-loaded bubble liposomes and ultrasoundInt J Pharm20124221–250450922119963
- AIUM Technical Standards CommitteeMethods for Specifying Acoustic Properties of Tissue Mimicking Phantoms and ObjectsLaurel, MD, USAAmerican Institute of Ultrasound in Medicine1995
- RaymondJLHaworthKJBaderBKBroadband attenuation measurements of phospholipid-shelled ultrasound contrast agentsUltrasound Med Biol201440241042124262056
- ThomasDDLiuXKantrowSPLancasterJRThe biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2Proc Natl Acad Sci U S A200198135536011134509
- HitchcockKEIvancevichNMHaworthKJUltrasound-enhanced rt-PA thrombolysis in an ex vivo porcine carotid artery modelUltrasound Med Biol20113781240125121723448
- DattaSCoussiosC-CAmmiAYMastTDde Courten-MyersGMHollandCKUltrasound-enhanced thrombolysis using Definity as a cavitation nucleation agentUltrasound Med Biol20083491421143318378380
- TareMParkingtonHCColemanHAEDHF, NO and a prostanoid: hyperpolarization-dependent and -independent relaxation in guinea-pig arteriesBr J Pharmacol2000130360561810821789
- RedenbachDMEnglishDHoggJCThe nature of leukocyte shape changes in the pulmonary capillariesAm J Physiol19972734 Pt 1L733L7409357847
- FeshitanJAChenCCKwanJJBordenMAMicrobubble size isolation by differential centrifugationJ Colloid Interface Sci2009329231632418950786
- MartinKHDaytonPACurrent status and prospects for microbubbles in ultrasound theranosticsWiley Interdiscp Rev Nanomed Nanobiotechnol201354329345
- LiuXMillerMJJoshiMSThomasDDLancasterJRAccelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranesProc Natl Acad Sci U S A1998955217521799482858
- OrtizDCabralesPBriceñoJCTransport of nitric oxide by perfluorocarbon emulsionBiotechnol Prog20132961565157223966236
- KimHBrittonGLPengTHollandCKMcphersonDDHuangS-LNitric oxide-loaded echogenic liposomes for treatment of vasospasm following subarachnoid hemorrhageInt J Nanomedicine2014915516524379666
- YuanFDellianMFukumuraDVascular permeability in a human tumor xenograft: molecular size dependence and cutoff sizeCancer Res19955517375237567641188
- CasciaroSConversanoFRagusaAOptimal enhancement configuration of silica nanoparticles for ultrasound imaging and automatic detection at conventional diagnostic frequenciesInvest Radiol2010451171572420562708
- Van TeeffelenJWBrandsJStroesESVinkHEndothelial glycocalyx: sweet shield of blood vesselsTrends Cardiovasc Med200717310110517418372
- HoEARamsayEGinjMCharacterization of cationic liposome formulations designed to exhibit extended plasma residence times and tumor vasculature targeting propertiesJ Pharm Sci20109962839285320091826
- DaytonPKlibanovABrandenburgerGFerraraKAcoustic radiation force in vivo: A mechanism to assist targeting of microbubblesUltrasound Med Biol19992581195120110576262
- WuJDuGAcoustic streaming generated by a focused Gaussian beam and finite amplitude toneburstsUltrasound Med Biol19931921671768516962
- JokerstJVLobovkinaTZareRNGambhirSSNanoparticle PEGylation for imaging and therapyNanomedicine20116471572821718180
- LancasterJRA tutorial on the diffusibility and reactivity of free nitric oxideNitric Oxide19971118309701041
- HuangSLKeePHKimHNitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasiaJ Am Coll Cardiol200954765265919660697
- BrittonGLKimHKeePHIn vivo therapeutic gas delivery for neuroprotection with echogenic liposomesCirculation2010122161578158720921443
- SmithDABPorterTMMartinezJDestruction thresholds of echogenic liposomes with clinical diagnostic ultrasoundUltrasound Med Biol200733579780917412486
- McKinlayACXiaoBWraggDSWheatleyPSMegsonILMorrisREExceptional behavior over the whole adsorption-storage-delivery cycle for NO in porous metal organic frameworksJ Am Chem Soc200813031104401044418627150
- StrideESaffariNMicrobubble ultrasound contrast agents: a reviewProc Inst Mech Eng H2003217642944714702981
- RadhakrishnanKBaderKBHaworthKJRelationship between cavitation and loss of echogenicity from ultrasound contrast agentsPhys Med Biol201358186541656324002637
- MalinskiTTahaZGrunfeldSPattonSKapturczakMTomboulianPDiffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensorsBiochem Biophys Res Commun19931933107610828323533
- GrovesPHBanningAPPennyWJLewisMJCheadleHANewbyACKinetics of smooth muscle cell proliferation and intimal thickening in a pig carotid model of balloon injuryAtherosclerosis1995117183968546758
- SheikovNMcDannoldNVykhodtsevaNJoleszFHynynenKCellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubblesUltrasound Med Biol200430797998915313330
- DengJHuangQWangFThe role of caveolin-1 in blood– brain barrier disruption induced by focused ultrasound combined with microbubblesJ Mol Neurosci201146367768721861133
- OwusuBYStapleyRPatelRPNitric oxide formation versus scavenging: the red blood cell balancing actJ Physiol (Lond)2012590204993500022687616
- DonadeeCRaatNJHKaniasTNitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesionCirculation2011124446547621747051
- FathiARBakhtianKDPlutaRMThe role of nitric oxide donors in treating cerebral vasospasm after subarachnoid hemorrhageActa Neurochir Suppl2011110Pt 1939721116922
- BeckmanJSKoppenolWHNitric oxide, superoxide, and peroxynitrite: the good, the bad, and uglyAm J Physiol19962715 Pt 1C1424C14378944624
- BolotinaVMNajibiSPalacinoJJPaganoPJCohenRANitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscleNature199436864748508537512692