Abstract
Chronic kidney disease-mineral bone disorder is frequent in patients with renal failure. It is characterized by abnormalities in mineral and bone metabolism with resulting hyperphosphatemia, low serum vitamin D, secondary hyperparathyroidism, altered bone morphology and strength, higher risk of bone fractures, and development of vascular or other soft tissue calcifications. Besides the recommendation to reduce phosphorus dietary intake, many drugs are currently available for the treatment of calcium/phosphate imbalance. Among them, phosphate binders represent a milestone. Calcium-based binders (calcium carbonate, calcium acetate) are effective in lowering serum phosphate, but their use has been associated with an increased risk of hypercalcemia and calcifications. Calcium-free binders (sevelamer hydrochloride, sevelamer carbonate, and lanthanum carbonate) are equally or slightly less effective than calcium-containing compounds. They would not induce an increase in calcium levels but may have relevant side effects, including gastrointestinal symptoms for sevelamer and risk of tissue accumulation for lanthanum. Accordingly, new phosphate binders are under investigation and some of them have already been approved. A promising option is sucroferric oxyhydroxide (Velphoro®, PA21), an iron-based phosphate binder consisting of a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches. The present review is focused on pharmacology, mode of action, and pharmacokinetics of sucroferric oxyhydroxide, with a discussion on comparative efficacy, safety, and tolerability studies of this drug in chronic kidney disease and patient perspectives such as quality of life, satisfaction, and acceptability. Sucroferric oxyhydroxide has proven to be as effective as sevelamer in reducing phosphatemia with a similar safety profile and lower pill burden. Experimental and clinical studies have documented a minimal percentage of iron absorption without inducing toxicity. In conclusion, the overall benefit–risk balance of sucroferric oxyhydroxide is deemed to be positive, and this new drug may therefore represent a good alternative to traditional phosphate binders for the treatment of hyperphosphatemia in dialysis patients.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction to the epidemiology and management issues in CKD
Chronic kidney disease (CKD), defined by the presence of kidney damage and/or reduced function for a period greater than 3 months, is an increasingly relevant public health problem all over the world. According to a very recent systematic analysis, in 2010 the global prevalence of CKD in adults was 10.4% in men and 11.8% in women, with lower values in more developed countries and higher values in low- and middle-income countries.Citation1
The incidence of the disease rises with age and it is expected to further increase with gradual population aging.Citation2 Other major risk factors for reduced kidney function include: hyperuricemia, proteinuria, urinary malignancies, anemia, stroke, arterial hypertension, presence of renal cysts, female sex, smoking, and coronary artery disease.Citation3–Citation5
Whereas in the past CKD was primarily a consequence of glomerulonephritis and interstitial nephritis, presently the leading causes of renal failure are diabetes mellitus and hypertension.Citation6,Citation7 In particular, it is estimated that the prevalence of diabetic CKD stage 5 in European countries will increase 3.2% per year up to 2025.Citation8
CKD shows a natural tendency to evolve toward end-stage renal disease, although this occurs with different times depending on the underlying etiology, and patients are burdened with a greater risk of comorbidities and mortality than general population, especially due to poor cardiovascular outcomes. Indeed, CKD patients frequently develop left ventricular hypertrophy, hypertension, valvular heart disease, left ventricular systolic failure, diastolic failure, arrhythmias, accelerated atherosclerosis progression, ischemic artery disease, and sudden cardiac death.Citation9–Citation13
Some therapeutic strategies, such as the blockage of renin-angiotensin-aldosterone system, allow to slow down but not to fully halt CKD progression. For this reason, other potential treatments are currently under investigation but conclusive results are still lacking.Citation14 Conversely, nephrologists are provided with validated medications to well enough control CKD complications including anemia, metabolic acidosis, hyperkalemia, and calcium/phosphate imbalance. Even so, researchers are ever looking for new treatment options in order to improve therapeutic effectiveness and solve important concerns regarding some adverse effects induced by the currently used drugs. An example is recombinant human erythropoietin that in the 80s definitely revolutionized the treatment of anemia, previously requiring frequent blood transfusions.Citation15 Despite the obvious benefits for the management of anemia due to CKD, some safety issues secondary to erythropoietin administration are still open and not easily resolvable. They are especially related to the risk for cancer progression, since erythropoietin acts as a cell growth factor, and to the potential unfavorable cardio- and cerebrovascular outcomes because of the increase in hematocrit, blood pressure, and thrombopoiesis.Citation15–Citation18
Focusing on calcium/phosphate metabolism, patients with progressive renal function impairment commonly develop the so-called CKD-mineral bone disorder (CKD-MBD), a syndrome characterized by abnormalities in mineral and bone metabolism with resulting altered bone morphology and strength, higher risk of bone fractures, and formation of vascular or other soft tissue calcifications. From a biochemical point of view calcium, phosphorus, parathyroid hormone, and vitamin D serum levels are often outside the physiological range.Citation19,Citation20
A significant role in the pathophysiology of CKD-MBD is also played by klotho and fibroblast growth factor 23 (FGF23). Klotho is a FGF23 tissue coreceptor and tends to decrease as renal function declines.Citation21 This probably represents the first alteration to appear and also the factor responsible for progressive peripheral resistance to FGF23,Citation22 a phosphaturic hormone produced by osteoblasts and osteocytes. As a result, FGF23 serum levels rise. At first, this is a compensatory mechanism aimed at inducing phosphaturia; however, with time FGF23 increase turns out to contribute to CKD-MBD pathogenesis because it inhibits 1α-hydroxylase activity at the renal level so reducing 1,25-dihydroxyvitamin D3 expression. The consequences are the following: secondary hyperparathyroidism, altered phosphorus and calcium serum levels, increased bone remodeling, vascular calcifications, and further FGF23 production.Citation23 Moreover, high FGF23 is an important risk factor for cardiovascular disease since it has been associated with left ventricular hypertrophy, activation of the renin-angiotensin system, increased sodium absorption in the renal distal tubules with subsequent sodium retention and volume expansion, increased markers of inflammation, and oxidative stress.Citation24
The control of serum phosphate and FGF23 levels in CKD patients is therefore essential to preserve bone mineral content, prevent hyperparathyroidism, and reduce cardiovascular risk. It is noteworthy that not only food but also several drugs are a source of absorbable phosphate.Citation25
Outline of current and emerging treatment options for CKD with reference to phosphate binders
Besides the recommendation to reduce the dietary intake of phosphorus, many drugs are currently available for the treatment of CKD-MBD; they include vitamin D sterols (calcitriol, cholecalciferol, ergocalciferol), active vitamin D analogs (paricalcitol, doxercalciferol, alfacalcidol), calcimimetics (cinacalcet), and phosphate binders. According to the purposes of this review, attention will be focused on the last class of medications.
Their mechanism of action is based on the binding of dietary phosphate within the gastrointestinal lumen to prevent its absorption. Depending on the presence or absence of calcium in their molecular structure, phosphate binders can be classified as calcium-based and calcium-free.Citation23,Citation26
Calcium-containing compounds (calcium carbonate, calcium acetate) have proven to be efficient in lowering serum phosphate concentrations. However, an increased risk of hypercalcemia, positive calcium balance, and development of vascular calcifications has been observed in patients receiving these drugs.Citation27
Calcium-free binders (sevelamer hydrochloride, sevelamer carbonate, lanthanum carbonate) are equally or slightly less effective than calcium-based binders and their use does not seem to be associated with high calcium levels: this would reduce the risk for vascular calcifications.Citation28–Citation31 Nevertheless, calcium-free phosphate binders are also burdened with clinically significant side effects. Sevelamer carbonate may cause gastrointestinal adverse events including nausea, vomiting, dyspepsia, abdominal pain, and changes in bowel habits.Citation32 Lanthanum carbonate appears to be well tolerated in the short to medium term except for a higher rate of vomiting, but there is the risk of lanthanum accumulation especially in bone and liver. Although some experimental and clinical studies suggest that lanthanum carbonate is fairly safe, large sample and long-term trials are required in order to assess the potential toxicity of lanthanum in the liver.Citation33 Moreover, some authors have recently reported cases of lanthanum deposition in the gastric mucosa associated with peculiar histiocytic lesions, but their clinical significance needs to be clarified.Citation34,Citation35
Beyond the described side effects of both classes of phosphate binders, another relevant issue is the lack of placebo-controlled randomized clinical trials evaluating the impact of these drugs on hard endpoints such as death and cardiovascular outcomes. As a consequence, at present, the treatment of hyperphosphatemia is mostly driven by clinical experience and evaluation of the individual patient.Citation36
Hence, even though numerous drugs are already in use in clinical practice, the “ideal” phosphate binder still does not exist. It should be effective in binding dietary phosphate with low pill burden, minimal gastrointestinal or other untoward effects, and no interaction with other medications; it should be also devoid of safety concerns and inexpensive;Citation37 this is the reason why new phosphate binders have been approved over recent years and others are being investigated for the treatment of phosphate imbalance in CKD.
Among them is bixalomer, an amine functional polymer that was launched in Japan in June 2012 for the treatment of hyperphosphatemia in dialysis patients. Bixalomer seems to effectively reduce phosphatemia with fewer gastrointestinal symptoms compared to sevelamer hydrochloride.Citation38 Currently, approval for the extension of therapy to CKD patients not on dialysis is under evaluation,Citation39 and two postmarketing clinical trials are ongoing to assess the safety and efficacy of long-term use of bixalomer in hemodialysis (NCT01901107) and in peritoneal dialysis patients (NCT01903213).
Another available therapeutic option is RenaGum™. This is a chewing gum formulated with chitosan, a natural linear polysaccharide able to bind salivary phosphate in the mouth and gastrointestinal tract. Actually, it must be regarded as a medical food to be taken together with phosphate binders and not as a replacement for these drugs, but it has proven to efficiently contribute to lower phosphatemia in CKD patients.Citation40–Citation42
A new category of phosphate binders is represented by iron-based compounds. They include drugs still under investigation such as iron–magnesium hydroxycarbonate or SBR759,Citation37 and drugs already available such as ferric citrate hydrate or sucroferric oxyhydroxideCitation43; the last one will be discussed in more detail in the next sections of this review. The presence of iron confers to these drugs the ability to bind phosphorus in the gastrointestinal lumen. Taking iron-based phosphate binders might be accompanied by an increase in serum iron, so patients suffering from important hepatic or gastric diseases or with a history of hemochromatosis or other disorders characterized by iron accumulation have not been included in clinical trials for the potential risk of iron overload. Lastly, non-iron-based binders are also under investigation. An example is Genz-644470, a polymer showing the ability to effectively reduce serum phosphate in hemodialysis patients but with no advantages over sevelamer carbonate.Citation44
Review of pharmacology, mode of action, and pharmacokinetics of iron oxyhydroxide
Iron(III)-oxyhydroxide is the pharmacologically active part of a compound named sucroferric oxyhydroxide (Velphoro®, PA21) and consisting of a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches ().Citation45
Figure 1 Molecular structure of sucroferric oxyhydroxide consisting of a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches.
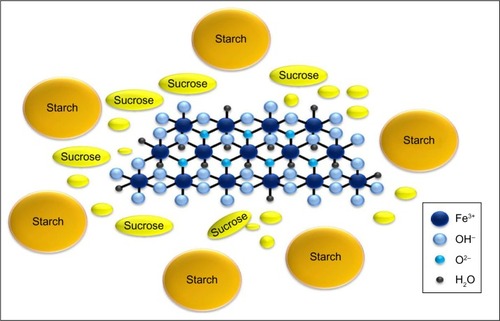
This calcium-free iron-based phosphate binder was approved in the United States by the US Food and Drug Administration (FDA) in November 2013Citation46,Citation47 and in Europe by the European Medicines Agency (EMA) in August 2014Citation48 for the treatment of hyperphosphatemia in CKD patients receiving dialysis.
The final product is a chewable tablet containing 500 mg iron as polynuclear iron(III)-oxyhydroxide, sucrose, and starches, also known as sucroferric oxyhydroxide that is partially water-soluble. Digestion of sucrose results in the formation of glucose and fructose, while starch is digested to glucose and maltose. All obtained products are absorbed. Conversely, polynuclear iron(III)-oxyhydroxide is nearly insoluble and this, although not completely, prevents iron being absorbed by the iron transporters of the duodenum. The function of sucrose molecules is to stabilize the iron, whereas starches help during the process of drug production.Citation49,Citation50
As for all iron-containing binders, the mechanism of action of sucroferric oxyhydroxide depends on the presence of iron. Experimental data suggest that the ability of this compound to bind phosphate is attributable to two different mechanisms. The first one is represented by the adsorption of phosphate to the iron complex. The second mechanism consists in the formation of iron phosphate through a chemical reaction that is favored by low pH values, such as those present within the stomach. In summary, the hypothesis is that the latter process basically occurs in the stomach whilst phosphate adsorption to the iron(III)-oxyhydroxide core happens in the intestinal lumen, both leading to the excretion of bound phosphate in the feces.Citation49
Under experimental conditions reproducing administration of the drug on an empty stomach and full stomach across the physiological pH range, sucroferric oxyhydroxide demonstrated to efficiently bind phosphate at every pH value and release more iron at the lowest pH in the absence of phosphate, whereas the amount of iron freed resulted in being much lower when phosphate was present and minimal with pH ranging from 2.5 to 8.5.Citation45 In more detail, in vitro experiments showed that iron release was equal to 0.3% at higher pH values while it amounted to a maximum of 6.3% at a pH of 1.2–1.5; the last percentage went up to 67% using an artificial gastric juice without phosphate.Citation49
It follows that iron uptake is greater in the fasting state and that, consequently, sucroferric oxyhydroxide must be taken with food.
In animal models treated with (59)Fe-radiolabeled sucroferric oxyhydroxide, iron absorption was proven to be low and long-term therapy only slightly increased tissue iron levels without inducing toxicity.Citation51
With regard to excretion of the drug in preclinical studies, radioactivity was detected only in feces and not in bile or urine.Citation49
A further important point is that CKD patients often have to take many medications throughout the day and this could expose them to the risk of drug interactions. Recent studies involving healthy volunteers have documented a low risk of drug–drug interactions between sucroferric oxyhydroxide and some drugs commonly used in dialysis patients including losartan, furosemide, digoxin, warfarin, and omeprazole. Then, their dosage does not need to be adjusted if sucroferric oxyhydroxide is simultaneously administered.Citation52 However, a potential adsorption of levothyroxine by sucroferric oxyhydroxide has been reported; then, it is recommended that levothyroxine should not be prescribed with this phosphate binder.Citation49,Citation53
Comparative efficacy, safety, and tolerability studies of iron oxyhydroxide in CKD
Over recent years, various clinical studies have been carried out to evaluate efficacy, safety, and tolerability of sucroferric oxyhydroxide compared to other phosphate binders commonly used in clinical practice.
Preliminary data on safety and efficacy of the drug were provided by Phase I studies. Some were conducted to evaluate the risk of drug–drug interactions in healthy volunteers. As mentioned before, the risk turned out to be low with losartan, furosemide, omeprazole, digoxin, and warfarin.Citation52
Another Phase I study recruited patients with stage 3–4 CKD, patients on hemodialysis, and healthy subjects. The aim was to evaluate the effects of 1-week treatment with sucroferric oxyhydroxide 10 g/day on serum phosphate concentrations and the potential risk for iron overload. Therapy induced a significant decrease in phosphatemia and the most common adverse event was mild-to-moderate diarrhea. Moreover, the administration of a single dose of radiolabeled compound demonstrated that iron uptake was low.Citation54
Later, a Phase II, randomized, active-controlled, multi-center, open-label, dose-finding study (NCT00824460) was designed to evaluate efficacy and safety of different dosages of sucroferric oxyhydroxide (1.25, 5.0, 7.5, 10.0, or 12.5 g/day) as compared to sevelamer hydrochloride (4.8 g/day) for 6 weeks in a cohort of 154 hemodialysis patients. Serum phosphate significantly decreased in all groups except that receiving sucroferric oxyhydroxide 1.25 g/day; in particular, the 5 g/day and 7.5 g/day dosages were as efficacious as 4.8 g/day of sevelamer hydrochloride. The adverse events that were more frequently reported included hypophosphatemia and discolored stools for all the sucroferric oxyhydroxide groups, and diarrhea, hypophosphatemia, and hypotension for sevelamer group. Their incidence and discontinuation rate owing to side effects were similar for both drugs.Citation55 These findings were subsequently confirmed by larger studies.
A multicenter, open-label, two-stage, prospective, randomized, parallel-group, active-controlled Phase III study (NCT01324128)Citation56 compared sucroferric oxyhydroxide and sevelamer carbonate in a cohort of hemodialysis and peritoneal dialysis patients with hyperphosphatemia. Subjects were randomized to receive sucroferric oxyhydroxide 1.0–3.0 g/day (n=707) and sevelamer 4.8–14.4 g/day (n=348) according to the following scheme of therapy: an 8-week period for dose titration, 4 weeks without changing the doses, and a 12-week phase of maintenance treatment. After 12 weeks, serum phosphate similarly decreased in the two groups and the efficacy of both treatments was maintained comparable until week 24. It is noteworthy that this noninferiority was obtained with an average per day of 3.1 tablets of sucroferric oxyhydroxide and 8.1 of sevelamer calculated throughout the whole study. Moreover, for an additional 3-week period, 50 patients were treated with maintenance dose of sucroferric oxyhydroxide and 49 patients with a low dose of the same drug (250 mg/day): serum phosphate control was achieved only with the maintenance dose. Nonadherence to therapy was lower for sucroferric oxyhydroxide than sevelamer. At least one adverse event was reported by 83.2% of patients treated with sucroferric oxyhydroxide and 76.1% of subjects receiving sevelamer, and withdrawals due to adverse events were higher in the first group than in the second one. Ferritin levels increased in both groups while transferrin saturation rose in patients treated with sucroferric oxyhydroxide. Such increases occurred early and then stabilized during the study period, suggesting no iron accumulation. Nevertheless, ferritin and transferrin saturation increases were greater in patients receiving sucroferric oxyhydroxide, indicating that minimal iron absorption cannot be excluded. The side effects more often observed in the sucroferric oxyhydroxide group were mild and transient diarrhea, discolored feces, and hyperphosphatemia; patients treated with sevelamer mainly experienced constipation and nausea. However, incidence of severe and serious adverse events and death was similar between the two treatment arms. This study concluded that sucroferric oxyhydroxide lowered serum phosphate in dialysis patients as effectively as sevelamer carbonate but with a lower pill burden and better adherence to therapy.
Afterwards, 644 eligible patients of this trial were enrolled for a 28-week open-label Phase III extension study (NCT01464190)Citation57 during which they continued to receive the same treatment at the same dose as at the end of the first study (n=384 sucroferric oxyhydroxide; n=260 sevelamer). The aim was to examine efficacy and tolerability of sucroferric oxyhydroxide in the long-term. The results confirmed those achieved in the initial study: indeed, sucroferric oxyhydroxide proved to efficiently reduce serum phosphate levels and this was associated with lower pill burden, good tolerability, and no evidence of iron accumulation.
Other clinical trials have been designed to investigate the effects of sucroferric oxyhydroxide in the long-term in hemodialysis (NCT01833494), peritoneal dialysis (NCT01852682), and associated with calcium carbonate in hemodialysis (NCT01850641). They have been completed but no results are yet available.
Patient-focused perspectives such as quality of life and patient satisfaction/acceptability
The clinical studies described in the previous section have revealed that therapy with sucroferric oxyhydroxide shows a safety profile that is similar to that of other calcium-free phosphate binders and requires a lower pill burden to achieve the same efficacy.
High number of pills is associated with poor health-related quality of life in chronic dialysis patients, and traditional phosphate binders represent approximately a half of their daily pill burden.Citation58
As described in the European Medicines Agency Assessment report on Velphoro®,Citation49 the authors of the NCT01324128 Phase III clinical trialCitation56 included evaluation of quality of life (standard SF-36 questionnaire – Version 2.0) and patient preference and satisfaction (Likert scale) among the secondary objectives of the study.
Quality of life scores changed only slightly from baseline and no significant differences were recorded between sucroferric oxyhydroxide group and sevelamer group. Similarly, patient satisfaction for the current phosphate binder did not differ between the two arms of treatment as for number of pills, ease of intake, and overall satisfaction neither at baseline nor over time.
The reason for these results could be that pill burden is a relevant aspect to take into account in the management of chronic diseases because it affects adherence to therapies, but most likely it is not the major element in determining quality of life if compared to other factors, most notably the disease itself and the dialysis treatment in the specific case. However there is no doubt that a low pill burden increases acceptability of and compliance to the treatment. This helps drugs, including phosphate binders, to be effective thereby potentially reducing morbidity and mortality.
Conclusion
At present, nephrologists have the opportunity to treat CKD-MBD by choosing from different drugs that allow controlling hyperphosphatemia in the late stages of renal failure. However, the therapeutic decision is generally based on an individual approach and probably the treatment is started too late compared to the earlier onset of calcium/phosphate imbalance in CKD. Furthermore, the current available drugs may not be as maximally effective as desirable and their use is often associated with high pill burden and side effects. All these factors definitely contribute to the still substantial cardiovascular morbidity and mortality attributable to increased phosphate and FGF23 serum levels in patients with renal impairment. Accordingly, these are also the reasons why researchers are looking for new phosphate binders that are expected to be not only effective but also more tolerated than those already in use in the clinical practice.
Iron-based non-calcium phosphate binders, such as sucroferric oxyhydroxide, may represent an interesting alternative to address and solve the issues described. Although comparative clinical studies with lanthanum carbonate are lacking,Citation43 sucroferric oxyhydroxide has proven to be as effective as sevelamer with a similar rate of undesirable effects and the need for a lower number of pills.
Moreover, sucroferric oxyhydroxide was able to reduce serum phosphate and intact parathyroid hormone concentrations and prevent the development of vascular calcifications in a rat model of chronic renal failure as effectively as lanthanum carbonate and sevelamer carbonate. Sucroferric oxyhydroxide also induced a significant decrease in FGF23 levels.Citation59 Iron itself seems to be involved in the regulation of FGF23 levels. In particular, iron status is an independent negative predictor of plasma FGF23 concentration and iron supplementation is associated with a significant decrease in FGF23 values.Citation60 This is likely due to the role of iron and iron-related pathways in FGF23 synthesis and processing mechanisms.Citation61 As a consequence of this regulatory action and the negative association between iron therapy and FGF23 serum levels documented in dialysis patients,Citation62 iron administration per se and also treatment with iron-based phosphate binders may be helpful in the control of bone metabolism decreasing FGF23 both directly and by reducing phosphate intestinal absorption.
The most important concern arising from the clinical use of sucroferric oxyhydroxide regards the potential iron overload, especially in specific categories of patients. The chemical structure of sucroferric oxyhydroxide prevents iron absorption in the gastrointestinal tract, but experimental studies performed to evaluate pharmacokinetics have shown that a minimal percentage of iron is actually absorbed, even though without inducing toxicity. For this reason, patients with a recent episode of peritonitis, significant gastric or hepatic disease, and history of major gastrointestinal surgery, hemochromatosis, or other disorders with iron accumulation have not been included in clinical trials. It follows that close monitoring of iron homeostasis is mandatory when sucroferric oxyhydroxide is administered to these subjects. On the other hand, CKD patients often show iron deficiencyCitation63 and receive oral or intravenous iron supplementation. Several drug formulations are currently available, each of which is characterized by different iron content per pill (eg, ferrous gluconate: 300 mg, equivalent to 37.5 mg of elemental iron; ferrous sulfate: 329.7 mg, corresponding to 105 mg of elemental iron) and pharmacokinetic profile. Iron absorption occurring with sucroferric oxyhydroxide, which contains 500 mg of iron as polynuclear iron(III)-oxyhydroxide in each tablet, could potentially reduce costs of anemia therapies,Citation64 but this hypothetical advantage should be confirmed in long-term comparative efficacy studies with commonly used iron supplements.
Due to the presence of sucrose, the drug is contraindicated in the rare hereditary cases of fructose intolerance, glucose–galactose malabsorption, or sucrase–isomaltase insufficiency. Furthermore, discolored feces may mask eventual gastrointestinal bleeding and this is another issue that requires attention.Citation49
Another major problem for the nephrologist is when to start treatment of hyperphosphatemia. As hypothesized for traditional phosphate binders,Citation23 also iron-based binders may potentially be more effective in slowing down CKD-MBD progression if started earlier in stage 3–4 CKD. Indeed, phosphate appears not to be the ideal biomarker of calcium/phosphate imbalance since its serum levels are maintained within the normal range for a long time by the compensatory increase in parathyroid hormone and, even first, FGF23. Most likely, FGF23 itself might better drive clinical management of CKD-MBD, but large trials are needed to standardize measurement methods and physiological values of this biomarker.
In conclusion, the overall benefit–risk balance of sucroferric oxyhydroxide is deemed to be positive because of the efficacy, safety profile, and only limited potential iron absorption. This iron-based phosphate binder may therefore represent a new good option for the treatment of hyperphosphatemia in CKD patients on dialysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- MillsKTXuYZhangWA systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010Kidney Int20158895095726221752
- BuemiMLacquanitiABolignanoDDialysis and the elderly: an underestimated problemKidney Blood Press Res200831533033618936550
- HarounMKJaarBGHoffmanSCComstockGWKlagMJCoreshJRisk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, MarylandJ Am Soc Nephrol200314112934294114569104
- LiuWYuFWuYA retrospective analysis of kidney function and risk factors by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in elderly Chinese patientsRen Fail2015371323132826211499
- LacquanitiABolignanoDCampoSMalnutrition in the elderly patient on dialysisRen Fail200931323924519288330
- LowSKSumCFYeohLYPrevalence of chronic kidney disease in adults with type 2 diabetes mellitusAnn Acad Med Singapore201545516417126198322
- ThawornchaisitPDe LoozeFReidCMSeubsmanSATranTTSleighAThai Cohort Study TeamHealth-risk factors and the prevalence of chronic kidney disease: cross-sectional findings from a national cohort of 87 143 Thai open university studentsGlob J Health Sci201575597226156905
- KainzAHronskyMStelVSPrediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025Nephrol Dial Transplant201530Suppl 4iv113iv11826209733
- BabuaCKalyesubulaROkelloEPattern and presentation of cardiac diseases among patients with chronic kidney disease attending a national referral hospital in Uganda: a cross sectional studyBMC Nephrol20151612626238594
- MatsushitaKBallewSHCoreshJInfluence of chronic kidney disease on cardiac structure and functionCurr Hypertens Rep201517958126194332
- PlutaAStróżeckiPKrintusMOdrowąż-SypniewskaGManitiusJLeft ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3Ren Fail20153771105111026156686
- CharytanDMCinelliAZeisbergEMAssociation of circulating angiogenesis inhibitors and asymmetric dimethyl arginine with coronary plaque burdenFibrogenesis Tissue Repair201581326213574
- Franczyk-SkóraBGluba-BrzózkaAWraniczJKBanachMOlszewskiRRyszJSudden cardiac death in CKD patientsInt Urol Nephrol201547697198225962605
- CernaroVTrifiròGLorenzanoGLucisanoSBuemiMSantoroDNew therapeutic strategies under development to halt the progression of renal failureExpert Opin Investig Drugs2014235693709
- CernaroVLacquanitiABuemiALupicaRBuemiMDoes erythropoietin always win?Curr Med Chem201421784985424059228
- SinghAKSzczechLTangKLCHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney diseaseN Engl J Med2006355202085209817108343
- PfefferMABurdmannEAChenCYTREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney diseaseN Engl J Med2009361212019203219880844
- BuemiMCampoSCernaroVDonatoVLacquanitiAErythropoietin and the truths of scienceJ Nephrol201124556456821058263
- MoeSDrüekeTCunninghamJKidney disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO)Kidney Int200669111945195316641930
- NickolasTLJamalSABone kidney interactionsRev Endocr Metab Disord201516215716326156535
- KohNFujimoriTNishiguchiSSeverely reduced production of klotho in human chronic renal failure kidneyBiochem Biophys Res Commun200128041015102011162628
- LarssonTOlausonHUn rapido aggiornamento su FGF23 per il nefrologo clinico. [A brief update on FGF23 for the clinical nephrologist]G Ital Nefrol2014312 Italian
- CernaroVSantoroDLucisanoSNicociaGLacquanitiABuemiMThe future of phosphate binders: a perspective on novel therapeuticsExpert Opin Investig Drugs2014231114591563
- EzumbaIQuarlesLDKovesdyCPFGF23 e cuore. [FGF23 and the heart]G Ital Nefrol2014316 Italian
- SultanaJMusazziUMIngrasciottaYMedication is an additional source of phosphate intake in chronic kidney disease patientsNutr Metab Cardiovasc Dis2015251095996726165250
- SavicaVCalòLAMonardoPSantoroDBellinghieriGPhosphate binders and management of hyperphosphataemia in end-stage renal diseaseNephrol Dial Transplant20062182065206816766546
- LocatelliFDel VecchioLVioloLPontorieroGPhosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profilesExpert Opin Drug Saf201413555156124702470
- BellinghieriGSantoroDSavicaVEmerging drugs for hyperphosphatemiaExpert Opin Emerg Drugs200712335536517874966
- LiuLWangYChenHZhuXZhouLYangYThe effects of non-calcium-based phosphate binders versus calcium-based phosphate binders on cardiovascular calcification and bone remodeling among dialysis patients: a meta-analysis of randomized trialsRen Fail20143681244125225019348
- WangCLiuXZhouYNew conclusions regarding comparison of sevelamer and calcium-based phosphate binders in coronary-artery calcification for dialysis patients: a meta-analysis of randomized controlled trialsPLoS One2015107e013393826230677
- ZhaiCJYangXWSunJWangREfficacy and safety of lanthanum carbonate versus calcium-based phosphate binders in patients with chronic kidney disease: a systematic review and meta-analysisInt Urol Nephrol201547352753525399356
- CoppolinoGLucisanoSRivoliLSevalamer hydrochloride, sevelamer carbonate and lanthanum carbonate: in vitro and in vivo effects on gastric environmentTher Apher Dial201519547147625866250
- ZhangCWenJLiZFanJEfficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic reviewBMC Nephrol20131422624134531
- MakinoMKawaguchiKShimojoHNakamuraHNagasawaMKodamaRExtensive lanthanum deposition in the gastric mucosa: the first histopathological reportPathol Int2015651333725413959
- HaratakeJYasunagaCOotaniAShimajiriSMatsuyamaAHisaokaMPeculiar histiocytic lesions with massive lanthanum deposition in dialysis patients treated with lanthanum carbonateAm J Surg Pathol201539676777125602800
- ZoccaliCMallamaciFCannata-AndíaJPhosphate binders and clinical outcomes in patients with stage 5D chronic kidney diseaseSemin Dial20152858759326278591
- Wu-WongJRMizobuchiMIs there a need for new phosphate binders to treat phosphate imbalance associated with chronic kidney disease?Expert Opin Investig Drugs2014231114651475
- ItoKTakeshimaAShishidoKTreatment of hyperphosphatemia with bixalomer in Japanese patients on long-term hemodialysis with gastrointestinal symptomsTher Apher Dial201418Suppl 2192324975891
- Astellas Pharm IncAstellas Submits Supplemental New Drug Application for Kiklin® Capsules, a Treatment for Hyperphosphatemia, in JapanTokyoAstellas Pharm Inc2015 Available from: https://www.astellas.com/en/corporate/news/pdf/150317_1_Eg.pdfAccessed September 18, 2015
- SavicaVCalòLACaldareraRPhosphate salivary secretion in hemodialysis patients: implications for the treatment of hyperphosphatemiaNephron Physiol20071053p52p5517220638
- SavicaVCalòLAMonardoPSalivary phosphate-binding chewing gum reduces hyperphosphatemia in dialysis patientsJ Am Soc Nephrol200920363964419020004
- BlockGAPerskyMSShamblinBMEffect of salivary phosphate-binding chewing gum on serum phosphate in chronic kidney diseaseNephron Clin Pract20131231–29310123797006
- SchmidHLedererSRNovel iron-containing phosphate binders for treatment of hyperphosphatemiaExpert Opin Pharmacother201516142179219126293683
- MoustafaMLehrnerLAl-SaghirFA randomized, double-blind, placebo-controlled, dose-ranging study using Genz-644470 and sevelamer carbonate in hyperphosphatemic chronic kidney disease patients on hemodialysisInt J Nephrol Renovasc Dis2014714115224748812
- WilhelmMGaillardSRakovVFunkFThe iron-based phosphate binder PA21 has potent phosphate binding capacity and minimal iron release across a physiological pH range in vitroClin Nephrol201481425125824656315
- http://www.velphoro.us [homepage on the Internet]Velphoro® (sucroferric oxyhydroxide) chewable tabletsFresenius Medical Care North America2014 Available from: http://www.velphoro.usAccessed September 20, 2015
- ShahHHHazzanADFishbaneSNovel iron-based phosphate binders in patients with chronic kidney diseaseCurr Opin Nephrol Hypertens201524433033526050119
- Summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002705/WC500175254.pdfAccessed September 20, 2015
- European Medicines AgencyAssessment report: VelphoroLondonEuropean Medicines Agency2014 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002705/WC500175257.pdfAccessed September 20, 2015
- GreigSLPloskerGLSucroferric oxyhydroxide: a review in hyperphosphataemia in chronic kidney disease patients undergoing dialysisDrugs201575553354225761962
- CozzolinoMFunkFRakovVPhanOTeitelbaumIPreclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxideCurr Drug Metab2014151095396525658128
- ChongEKaliaVWillsieSWinklePDrug-drug interactions between sucroferric oxyhydroxide and losartan, furosemide, omeprazole, digoxin and warfarin in healthy subjectsJ Nephrol Epub201444
- LABEL: VELPHORO-ferric oxyhydroxide tablet, chewable [webpage on the Internet]Bethesda, MDUnited States National Library of Medicine Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=237da26c-f38c-4faa-93ad-735e71c9d0c1Accessed September 23, 2015
- GeisserPPhilippEPA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney diseaseClin Nephrol201074141120557860
- WüthrichRPChoncholMCovicAGaillardSChongETumlinJARandomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patientsClin J Am Soc Nephrol20138228028923124782
- FloegeJCovicACKettelerMPA21 Study GroupA phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patientsKidney Int201486363864724646861
- FloegeJCovicACKettelerMLong-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patientsNephrol Dial Transplant20153061037104625691681
- ChiuYWTeitelbaumIMisraMde LeonEMAdzizeTMehrotraRPill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patientsClin J Am Soc Nephrol2009461089109619423571
- PhanOMaillardMMallucheHHStehleJCFunkFBurnierMEffects of sucroferric oxyhydroxide compared to lanthanum carbonate and sevelamer carbonate on phosphate homeostasis and vascular calcifications in a rat model of chronic kidney failureBiomed Res Int2015201551560626221597
- BraithwaiteVPrenticeAMDohertyCPrenticeAFGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation studyInt J Pediatr Endocrinol2012201212723098062
- BhattacharyyaNChongWHGafniRICollinsMTFibroblast growth factor 23: state of the field and future directionsTrends Endocrinol Metab2012231261061822921867
- DegerSMErtenYPasaogluOTThe effects of iron on FGF23-mediated Ca-P metabolism in CKD patientsClin Exp Nephrol201317341642323180041
- BolignanoDCoppolinoGRomeoANeutrophil gelatinase-associated lipocalin (NGAL) reflects iron status in haemodialysis patientsNephrol Dial Transplant200924113398340319549696
- PaiABJangSMWegryznNIron-based phosphate binders – a new element in management of hyperphosphatemiaExpert Opin Drug Metab Toxicol201516113

