Abstract
Loss of estrogen production in women during menopause results in a state of estrogen deficiency which has been associated with multiple problems, including vasomotor symptoms, symptoms of vulvovaginal atrophy, bone loss, and difficulties with sleep, mood, memory, and sexual activity. The only treatment option currently available to address multiple postmenopausal symptoms in women with an intact uterus is estrogen/progestin-containing hormone therapy (HT). Concerns surrounding side effects and published data regarding the association of HT with the increased risk for breast cancer have induced a decrease in the number of women seeking, initiating, and continuing this type of therapy. A combination containing bazedoxifene and conjugated estrogens (BZA/CE) maintains the established benefits of estrogen therapy for treatment of postmenopausal vasomotor symptoms, vulvovaginal atrophy, and osteoporosis, while certain estrogenic effects, such as stimulation of the uterus and breast, are antagonized without the side effects associated with HT. BZA/CE has been evaluated in a series of multicenter, randomized, double-blind, placebo-controlled, and active-controlled Phase III trials known as the Selective estrogens, Menopause, And Response to Therapy (SMART) trials. BZA/CE demonstrated clinically meaningful improvements in vasomotor symptoms, vulvovaginal atrophy, and a protective effect on the skeleton. These clinical benefits were associated with an acceptable safety profile and an improved tolerability compared with HT. BZA/CE showed a favorable safety profile on the breast, endometrium, and ovaries. The incidence of venous thromboembolism was low and the risk does not appear to be any greater than for CE alone or BZA alone or greater than HT. The incidence of coronary heart disease and cerebrovascular accidents were similar to placebo. The overall incidence of cancer (including breast cancer) was low and similar to placebo. The SMART trials demonstrate that BZA/CE is an alternative option for treating non-hysterectomized, symptomatic, postmenopausal women.
Introduction
Loss of ovarian estrogen production in women during menopause results in a state of estrogen deficiencyCitation1 which has been associated with multiple problems, including vasomotor symptoms, symptoms of vulvovaginal atrophy, and difficulties with sleep, mood, memory, and sexual activity.Citation2 During menopause, these symptoms commonly overlap, presenting as a continuum, and may have a negative impact on a woman’s quality of life.Citation3 Menopausal symptoms have also been negatively associated with the ability to work.Citation4 Estrogen deficiency has further been associated with loss of bone mass, often leading to osteoporosis, which is associated with an increased occurrence of skeletal fractures.Citation2 Women who sustain osteoporotic fractures also face a negative impact on their quality of life, often affecting their ability to function and putting them at an increased risk of morbidity and mortality.Citation2,Citation5,Citation6
A variety of therapeutic options are available for the individual symptoms associated with menopause. Estrogen/progestin-containing hormone therapy (HT) is the only currently approved treatment for women with a uterus that holistically addresses the postmenopausal problems of vasomotor symptoms and vulvovaginal atrophy, while maintaining bone mass.Citation3
HT has pharmacologic drawbacks, including vaginal bleeding and breast pain/tenderness, which are the most common reasons for discontinuation of this therapy.Citation7–Citation9 Vaginal bleeding leads to discontinuation of therapy in up to one-third of women and rates of discontinuation can be as high as 68% within the first year of treatment.Citation10 Rates of discontinuation for breast tenderness/pain have been reported to be as high as 40% in some clinical trials.Citation11,Citation12 Progestin-induced irregular vaginal bleeding associated with HT creates distress and often requires additional interventions (ie, transvaginal ultrasonography, endometrial biopsies, and hysteroscopies).Citation13 Increases in breast density associated with progestin-containing HT may lead to mammographic recalls because of difficulty in reading mammogram results. Mammographic recalls also increase anxiety in women, contributing to the decision to discontinue HT.Citation14,Citation15
Women’s concerns about the available therapeutic options for postmenopausal symptoms have resulted in a substantial population who are currently untreated.Citation3 The population of postmenopausal women will grow, given the continual increase in the numbers of women approaching menopause and the extended life expectancy of postmenopausal women.Citation1 Thus, for postmenopausal women with a uterus, new therapeutic alternatives with an improved tolerability and safety profile compared with traditional HT are needed to help alleviate vasomotor symptoms and vulvovaginal atrophy symptoms, and to prevent osteoporosis.
BZA/CE combines bazedoxifene (BZA), a selective estrogen receptor modulator, with conjugated estrogens (CE), and represents a new treatment option in the management of menopausal health for women with a uterus. BZA/CE maintains the established benefits of estrogen therapy for menopausal symptoms of vasomotor symptoms, vulvovaginal atrophy, and prevention of osteoporosis, while preventing the stimulatory estrogenic effects on the uterus and estrogenic/progestogenic effects on breast tissue.
The rationale for development of BZA/CE was that BZA, acting as an estrogen receptor antagonist in uterine tissue, would inhibit the proliferative effects of estrogen on the endometrium in a manner mechanistically distinct from progestins, and therefore reduce the incidence of irregular uterine bleeding.
BZA acts as an estrogen receptor antagonist in breast tissue, so BZA/CE is posited not to induce breast pain or changes in breast density. BZA/CE does not cause morphologic changes in the mammary gland, and it is hypothesized that this is the pharmacologic explanation for the lack of changes in breast pain and density. In vitro and vivo models demonstrate that BZA also inhibits the proliferative stimulatory action of estrogens on breast cancer cells.Citation16
The objective of this review is to present key efficacy and safety findings from the Selective estrogens, Menopause, And Response to Therapy (SMART) trials.Citation17–Citation20
Clinical studies with BZA/CE
BZA/CE has been evaluated in a series of multicenter, randomized, double-blind, placebo-controlled, and active-controlled Phase III investigations known as the SMART trials ().Citation17–Citation20
Table 1 Summary of pivotal SMART trials
The SMART-1 trial (n = 3,397) was conducted at 94 sites in the United States, Europe, and Brazil and enrolled generally healthy, postmenopausal women aged 40–75 years.Citation17 Eligible subjects had to have an intact uterus, a body mass index (BMI) ≤32.2 kg/m2, and normal endometrial biopsy results at screening. SMART-1 evaluated the efficacy of multiple doses of BZA/CE (combinations of BZA 10 mg, 20 mg, and 40 mg with either CE 0.45 mg or CE 0.625 mg) with regard to uterine protection, vasomotor symptoms, vulvovaginal atrophy, and prevention of osteoporosis.Citation17
The one-year interim results from SMART-1 demonstrated that BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg have a low (<1%) incidence of endometrial hyperplasia, while reducing the number and severity of hot flushes, improving symptoms of vulvovaginal atrophy, and preventing bone loss. Therefore, BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg were selected for further evaluation in other SMART trials.Citation17
The SMART-2 trial (n = 318) was conducted at 43 sites in the United States and enrolled healthy, postmenopausal women aged 40–65 years with an intact uterus and a BMI ≤ 34.0 kg/m2. Eligible subjects had to have seven or more moderate to severe hot flushes daily (or at least 50 per week) at screening and be seeking treatment for hot flushes. This study evaluated the effects of BZA 20 mg/CE 0.45 mg and 0.625 mg on vasomotor symptoms compared with the effects of placebo over 12 weeks of treatment. The primary endpoint was change from baseline in the frequency and severity of hot flushes. Secondary endpoints included effects on sleep, quality of life, and satisfaction with treatment.Citation18
The SMART-3 trial (n = 652) was conducted at 66 sites in the United States and enrolled generally healthy, postmenopausal women aged 40–65 years with an intact uterus and a BMI ≤ 34.0 kg/m2. All women had to have a vaginal cytologic smear showing no more than 5% superficial cells, a vaginal pH > 5, and at least one moderate to severe vulvovaginal atrophy symptom at screening. This study examined the effects of BZA 20 mg/CE 0.45 mg and 0.625 mg compared with those of BZA 20 mg alone and placebo on measures of vulvovaginal atrophy over 12 weeks of treatment.Citation19
The SMART-5 trial (n = 1,886) was conducted at 166 sites in the United States, Europe, Latin America, Australia, and New Zealand, and enrolled generally healthy, postmenopausal women aged 40–75 years. Eligible subjects had to have an intact uterus, a BMI ≤ 34 kg/m2, and acceptable endometrial biopsy results at screening. SMART-5 evaluated the efficacy of BZA/CE on uterine protection, prevention of osteoporosis, and effect on breast density.Citation20 This study evaluated the effects of BZA 20 mg/CE 0.45 and 0.625 mg and included CE/medroxyprogesterone acetate (MPA, (Prempro®, Pfizer Pharmaceuticals Inc, New York, NY, USA), BZA, and placebo as comparators.
The results of the one-year SMART-4 study (n = 1,061) are not discussed in this review because a different formulation of BZA/CE was evaluated. Regardless, the results of the SMART-4 study were similar to those of the SMART-1 study.Citation17
Efficacy on hot flushes, health related-quality of life, and sleep
Two clinical trials (SMART-1 and SMART-2) evaluated the efficacy of BZA/CE for the treatment of moderate to severe vasomotor symptoms. In these studies, BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 were associated with clinically meaningful improvements in vasomotor symptoms as compared with placebo, and the efficacy was comparable with that of available hormonal options (HT and estrogen therapy).Citation17,Citation18
In SMART-2, at week 4, BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg were associated with a significant decrease (P < 0.001) in the adjusted mean daily number of moderate to severe hot flushes as compared with placebo. A clinically meaningful reduction of 5–6 hot flushes per day was observed in subjects treated with BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg, respectively, compared with a reduction of three hot flushes in the placebo group. At week 12, BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg were associated with a decrease (P < 0.001) in the adjusted mean daily number of moderate to severe hot flushes, reaching a 74% and a 80% reduction, respectively, representing a clinically meaningful reduction of approximately 7–8 hot flushes per day, compared with a 51% reduction (representing a reduction of approximately five hot flushes per day) in the placebo group ().Citation18
Figure 1 SMART-2 trial: mean daily number of hot flushes with up to 12 weeks of treatment with BZA/CE or placebo for the MITT population using LOCF. Statistical significance (P < 0.01) was achieved for BZA 20 mg/CE 0.45 mg during weeks 3 through 12 compared with placebo. At week 2 through 12, the mean daily number of hot flushes with BZA 20 mg/CE 0.625 mg was statistically significant (P < 0.01) from placebo. *P-value vs placebo <0.001.
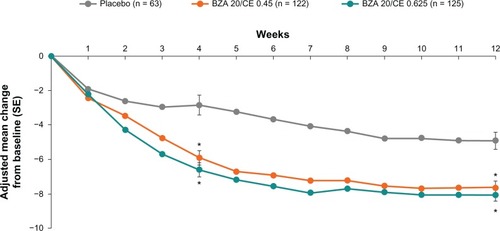
In addition to significant reductions in the number of moderate to severe hot flushes experienced per day, both BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths demonstrated clinically meaningful reductions in the severity of moderate to severe hot flushes compared with subjects who received placebo at week 4 (26% and 29% reduction, respectively) and week 12 (39% to 55% reduction, respectively, P < 0.001, ).Citation18 Similar results were observed in SMART-1, with evidence of efficacy of BZA/CE for the treatment of vasomotor symptoms present through 2 years of therapy.Citation17
Figure 2 SMART-2 trial: mean daily severity score of hot flushes with up to 12 weeks of treatment with BZA/CE or placebo for the MITT population using LOCF. The mean daily severity score of hot flushes was statistically significant (P < 0.001) for both BZA/CE doses during weeks 3 through 12 compared with placebo. The mean daily severity score was calculated by summing the number of mild, moderate, and severe hot flushes multiplied by 1, 2, and 3, respectively, divided by the total number of hot flushes. *P-value vs placebo <0.001.
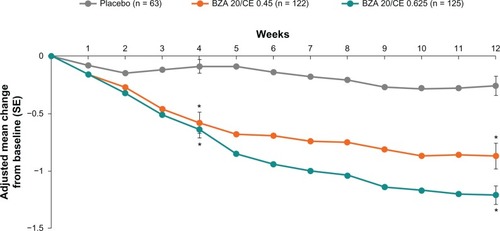
BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 was associated with clinically meaningful improvements in vasomotor symptoms. Comparison with historical data reveals that both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths have an overall comparable efficacy with that of estrogen progestin-containing HT compounds in the relief of moderate to severe hot flushes associated with menopause.Citation21
Both BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups demonstrated efficacy for both the daily number and severity of moderate to severe hot flushes, regardless of racial characteristics, BMI, and years since menopause of the populations evaluated.Citation18
Health-related quality of life, sleep parameters, and patient satisfaction tools were also used to assess patient response to therapy in the SMART-2 study. Clinically significant improvements were reported with BZA 20 mg/CE 0.45 and 0.625 mg at 12 weeks compared with placebo in the vasomotor function domain of the Menopause-Specific Quality of Life questionnaire and significantly greater satisfaction in the ability to control hot flushes during the day and night according to the Menopause Symptoms Treatment Satisfaction Questionnaire.Citation22
A range of components of the Medical Outcomes Study sleep scale also showed significant improvements with BZA/CE versus placebo, including significant decreases in sleep disturbance and significant increases in sleep adequacy.Citation22 Additionally, BZA/CE improved both indices (sleep problem index 1 and 2) included in the Medical Outcomes Study sleep scale (). Statistical mediation modeling demonstrated that BZA/CE affected sleep directly in populations of women with severe vasomotor symptoms, while in less symptomatic women its effect was indirect via improvement in hot flushes.Citation22
Figure 3 SMART-2 trial: mean change from baseline in the Medical Outcomes Study sleep scale at week 12, MITT population using observed case. BZA/CE improved sleep parameters as measured using this scale. *P-value vs placebo <0.001; **P-value vs placebo 0.051; ***P-value vs placebo 0.010.
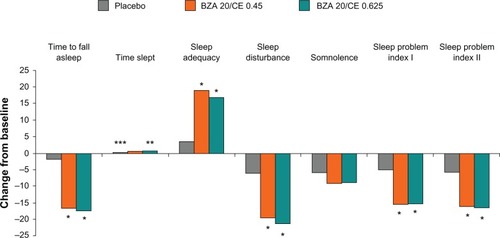
In summary, BZA/CE was associated with a clinically meaningful reduction in vasomotor symptoms, comparable with that observed for HT; moreover, this efficacy was correlated with improvements in sleep and menopause-related quality of life.
Effects on vulvovaginal atrophy
Two studies evaluated the efficacy of BZA/CE on vulvovaginal atrophy, ie, SMART-1 and SMART-3.Citation17,Citation19 In the SMART-3 trial, the efficacy of BZA 20 mg/CE 0.45 and 0.625 mg on vulvovaginal atrophy was evaluated in subjects who had no more than 5% superficial cells at screening on the vaginal smear, a vaginal pH < 5, and presented with a moderate to severe symptom associated with vulvovaginal atrophy.Citation19
BZA 20 mg/CE 0.45 or 0.625 mg showed a significantly greater increase from baseline in the mean proportion of superficial cells compared with placebo (P < 0.001). Both BZA/CE groups also showed a significantly greater reduction from baseline in the mean proportion of parabasal cells (P < 0.001) and a significantly greater increase from baseline in the mean proportion of intermediate cells (P < 0.05) compared with placebo at weeks 4 and 12. Additionally, treatment with BZA 20 mg/CE 0.625 mg resulted in a significantly greater decrease from baseline in vaginal pH (P < 0.001) and a greater improvement in the women’s most bothersome vulvovaginal atrophy symptoms (P < 0.05) compared with placebo ().Citation19
Figure 4 SMART-3 trial: effect of BZA/CE on measurements of vulvovaginal atrophy. (A) Median change from baseline in percentage of vaginal superficial cells at weeks 4 and 12 for treatment with BZA/CE, BZA, or placebo for the MITT population using LOCF. BZA 20 mg/CE 0.625 mg and CE 0.45 mg significantly increased superficial cells at week 4 (P = 0.0034 and P < 0.001) and week 12 (P < 0.001 for both doses) over placebo and BZA using nonparametric analyses. *P-value vs placebo <0.001. (B) Median change from baseline in percentage of vaginal parabasal cells at weeks 4 and 12 for treatment with BZA/CE, BZA, or placebo for the MITT population using LOCF. BZA 20 mg/CE 0.625 mg and CE 0.45 mg significantly decreased superficial cells at week 4 and week 12 (P < 0.001 for both doses) over placebo and BZA using nonparametric analyses. *P-value vs placebo <0.001. (C) Mean change from baseline in vaginal pH at weeks 4 and 12 for treatment with BZA/CE, BZA, or placebo for the MITT population using LOCF. BZA 20 mg/CE 0.625 but not BZA 20 mg/CE 0.45 mg significantly decreased vaginal pH at week 4 and week 12 (P < 0.001) over placebo. *P-value vs placebo <0.001. (D) Percentage of women with MBS data by severity at week 12. MBS significantly improved with BZA 20 mg/CE 0.625 mg compared with placebo and BZA alone at week 12 (P < 0.05). *P-value = 0.048.
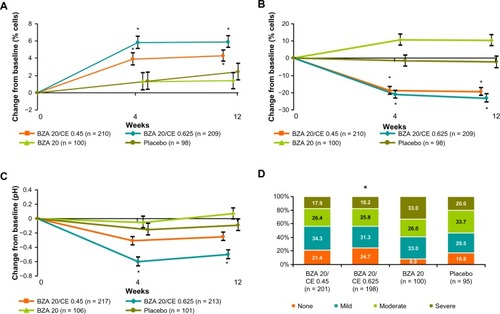
In SMART-1, BZA/CE showed a similar effect on the vaginal epithelium, inducing a greater increase from baseline in the mean proportion of superficial cells compared with placebo (P < 0.001), a greater increase in the mean proportion of intermediate cells (P < 0.001), and a greater decrease in the mean proportion of parabasal cells from baseline compared with placebo (P < 0.001). This maturation of the vaginal epithelium was associated with a statistically significant reduction in the incidence of dyspareunia relative to placebo during weeks 9–12 of therapy (P < 0.001) and in ease of lubrication score from baseline compared with placebo (P < 0.05) on the Arizona Sexual Experiences scale.Citation17,Citation19
Additionally, in SMART-3 (subjects with vulvovaginal atrophy), the Menopause-Specific Quality of Life questionnaire results at week 12 showed significant improvements in sexual function and total scores with both BZA/CE doses versus placebo or BZA 20 mg (P < 0.001). The Menopause Symptoms Treatment Satisfaction Questionnaire results showed that BZA/CE-treated subjects reported significantly greater overall satisfaction with treatment.Citation19
Bone effects
Two clinical trials (SMART-1 and SMART-5) evaluated the safety and efficacy of BZA/CE for the prevention of osteoporosis.Citation17,Citation20 Among subjects ≤5 years since menopause (substudy II of SMART-1 and SMART-5), significant (P < 0.001) increases from baseline to month 12 in lumbar spine bone mineral density (BMD) were demonstrated for both the BZA 20 mg/CE 0.45 mg (1.05% and 0.24% in SMART-1 and SMART-5, respectively) and BZA 20 mg/CE 0.625 mg (1.05% and 0.60%, in SMART-1 and SMART-5, respectively) treatment groups compared with placebo (−1.81% and −1.28%, in SMART-1 and SMART-5, respectively).Citation17,Citation20
In addition, in SMART-1, a statistically significant increase in lumbar spine BMD from baseline to month 24 was observed for both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups compared with a decrease in lumbar spine BMD in the placebo group (P < 0.001). Raloxifene 60 mg was included as an active comparator in SMART-1; among subjects ≤5 years since menopause, both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths demonstrated a statistically significant greater percentage increase in lumbar spine BMD from baseline to months 12 (P < 0.001) and 24 (P = 0.002 and P = 0.001, respectively) compared with raloxifene 60 mg ().Citation17 In SMART-5, BZA 20 mg and CE 0.45 mg/MPA 1.5 mg were included as active comparators. BZA/CE (both doses) showed similar efficacy to CE/MPA and a superior bone effect as compared with BZA 20 mg.Citation20
Figure 5 SMART-1 trial: Adjusted mean percent change in BMD from baseline to months 6, 12, 18, and 24 from the MITT population using LOCF. BZA/CE increased bone mineral density at the lumbar spine as compared with placebo and baseline values (P < 0.01) and raloxifene 60 mg (P < 0.05 BZA/CE). P-value vs placebo ≤0.001 (all BZA/CE groups at 6, 12, 18 and 24 m); P-value versus baseline ≤0.001 (all BZA/CE groups at 6, 12, 18 and 24 m); *P-value versus RAL ≤ 0.05 at 6, 12, 18 and 24 m.
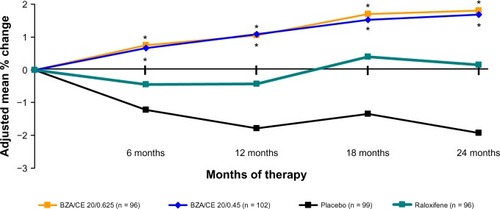
SMART-1 and SMART-5 demonstrated that treatment with BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg effectively prevented loss of total hip BMD, having a comparable effect to that of raloxifene 60 mg and BZA 20 mg at all time points evaluated. In addition, BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg demonstrated a comparable effect to CE 0.45 mg/MPA 1.5 mg in terms of effects on BMD at the total hip.Citation17,Citation20
The results from subpopulation analyses based on the integrated data from SMART-1 and SMART-5 demonstrate that BZA/CE therapy was efficacious regardless of age groups (<60 years versus ≥60 years), BMI, race, and geographic region in improving lumbar spine and total hip BMD.17,20
To evaluate further the effect of treatment on bone metabolism, serum samples for determination of osteocalcin, procollagen type 1 N-propeptide (P1NP), and C-telopeptide were obtained in SMART-1 and SMART-5.Citation17,Citation20
The observed decreases in bone turnover markers were consistent with the observed improvements in BMD. Both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups demonstrated significant decreases from baseline in serum concentrations of C-telopeptide and osteocalcin compared with placebo (P < 0.001) in SMART-1 and SMART-5. In addition, concentrations of P1NP at months 6 and 12 were significantly decreased (P < 0.001) by both doses compared with placebo in SMART-5.Citation17,Citation20
A significantly larger decrease (P < 0.001) in serum concentrations of C-telopeptide and osteocalcin was observed for both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups compared with the raloxifene 60 mg treatment group in SMART-1.Citation17
Similarly, in SMART-5, both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups demonstrated greater decreases in serum osteocalcin and serum C- telopeptide and significant decreases in P1NP compared with the BZA 20 mg treatment group. Moreover, in SMART-5, both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups demonstrated decreases in P1NP and serum osteocalcin that were comparable with the CE 0.45 mg/MPA 1.5 mg treatment group.Citation20
Studies conducted with BZA alone provide important supportive evidence for the long-term efficacy (7-year exposure) of BZA in postmenopausal subjects. Treatment with BZA 20 and BZA 40 mg for 3 years resulted in a statistically significant reduction (P < 0.05) in the incidence of new vertebral fractures compared with placebo which was similar to that observed with raloxifene 60 mg. This positive effect in reduction of new vertebral fractures was observed through 5 and 7 years of treatment.Citation23
Overall, the skeletal effects (changes in lumbar spine and hip BMD and bone turnover markers) observed with BZA/CE therapy were clinically meaningful and greater than for raloxifene and BZA monotherapy and comparable with CE 0.45 mg/MPA 1.5 mg. The increases in BMD and decrease in bone turnover markers observed with BZA/CE as compared with placebo have been associated with a reduction in fracture risk with antiresorptive agents as well as with BZA treatment.
Safety
Endometrial safety
Protection of the endometrium is of major clinical importance for non-hysterectomized postmenopausal women who receive a therapy containing estrogens. Therefore, the tissue selective activity of the selective estrogen receptor modulator component of BZA/CE is key to endometrial protection.Citation16 The incidence of endometrial hyperplasia with BZA/CE was evaluated in SMART-1 and SMART-5. These studies were of sufficient duration to provide evidence that BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg provides appropriate endometrial protection. Both doses of BZA/CE demonstrated an incidence of endometrial hyperplasia <1%, which was comparable with placebo.Citation17,Citation24
Data obtained using transvaginal ultrasound have also shown neutral effects of BZA/CE on the endometrium. BZA/CE were associated with minimal increases in endometrial thickness from baseline (<1 mm) which were similar to placebo.Citation20 More subjects treated with CE/MPA in the SMART-5 trial had an increase in endometrial thickness of >5 mm compared with BZA/CE or placebo.Citation20
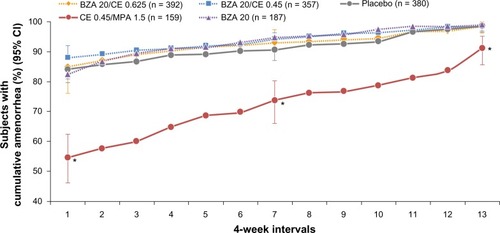
The effects of BZA/CE on endometrial bleeding have also been evaluated in the SMART trials.Citation20,Citation25 In SMART-1 and SMART-5, BZA 20 mg/CE 0.45 and 0.625 mg were associated with high rates of cumulative amenorrhea, similar to placebo. The rates of cumulative amenorrhea over one year were >83%, >87%, and >85% for BZA 20 mg/CE 0.45 mg, BZA 20 mg/CE 0.625 mg, and placebo, respectively, in SMART-1.Citation25 Higher rates of cumulative amenorrhea were observed with BZA 20 mg/CE 0.45 and 0.625 mg (>87% and >84%, respectively) and placebo (>83%) compared with CE/MPA (>54%) in SMART-5 ().Citation20
Figure 6 SMART-5 trial: Percentage (standard error) of subjects with amenorrhea during cycles 1–13 (MITT population) for each treatment group. BZA/CE (both doses), BZA, and placebo demonstrated a similar incidence of cumulative amenorrhea for all cycles. CE/MPA showed a significantly lower amenorrhea rate (P < 0.001 versus all other treatment groups). No difference between any Bazedoxifene/CE group and placebo. *P < 0.001 vs placebo.
Breast safety
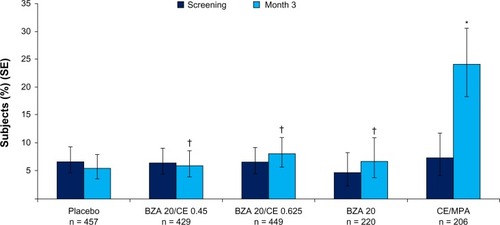
The effect of BZA/CE regimens on the incidence of breast pain/tenderness was evaluated in SMART-1, SMART-2, and SMART-5.Citation17,Citation18,Citation20 In these studies, breast pain/tenderness was measured by daily subject diaries. Both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths demonstrated an incidence of breast pain/tenderness that was not significantly different compared with placebo, raloxifene 60 mg, and BZA 20 mg.Citation17 In SMART-5, the incidence of breast tenderness in subjects treated with BZA/CE was significantly lower than that in subjects treated with CE 0.45 mg/MPA 1.5 mg (P < 0.001, ).Citation20 These results are consistent with the relative incidences of adverse events related to breast pain/tenderness and discontinuation rates due to breast pain/tenderness reported by participants in the SMART trials.Citation17–Citation20
Figure 7 Percentage of women reporting one or more days of breast tenderness at week 12. BZA/CE (both doses), BZA, and placebo demonstrated similar incidences of breast tenderness. CE/MPA showed a significantly higher rates (P < 0.001 versus all other treatment groups). *P < 0.001 vs placebo; †P < 0.001 vs CE/MPA. No difference between any Bazedoxifene/CE group and placebo at 9–12 wks.
In SMART-5, both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg treatment groups demonstrated non-inferiority compared with placebo with regards to mammographic breast density. By contrast, the CE 0.45 mg/MPA 1.5 mg treatment group exhibited a significant increase in breast density compared with the placebo group. BZA/CE treatment did not affect age-related changes in mammographic breast density (ie, natural reduction in breast density throughout the years of menopause). The results demonstrated that, unlike estrogen progestin-containing HT, BZA 20 mg/CE 0.45 mg, and BZA 20 mg/CE 0.625 mg have a neutral (similar to placebo) effect on breast density.Citation20
General safety
Based on the integrated safety results of the SMART trials, the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths are well tolerated and have an acceptable safety profile, supporting use in generally healthy, postmenopausal women with a uterus.Citation17–Citation20
The incidences of treatment-emergent adverse events, serious adverse events, and discontinuations due to adverse events were similar among the treatment groups. The incidence of venous thromboembolism was low, as expected in this relatively healthy population and consistent with what was reported in the Women’s Health Initiative study.Citation26 The risk does not appear to be any greater than for CE alone or BZA alone or greater than for CE/MPA.Citation26,Citation27 The incidences of coronary heart disease and cerebrovascular accidents were similar to those on placebo. The incidence of estrogen-sensitive cancers, including breast cancer, endometrial cancer, and ovarian cancer, was low and similar to placebo.
Clinical laboratory tests demonstrated that the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg doses were associated with a favorable lipid profile (decreases in total cholesterol, low-density lipoprotein, increases in high-density lipoprotein) with the exception of an increase in triglyceride levels.Citation20 Both the BZA 20 mg/CE 0.45 mg and BZA 20 mg/CE 0.625 mg dose strengths were associated with an increase from baseline in serum triglycerides which was similar to that reported for estrogen progestin-containing HT.Citation28 No other clinically significant changes in clinical laboratory tests were noted.
There were no significant differences in the adjusted mean change in body weight with either BZA/CE dose compared with placebo at one year or at the final on-therapy evaluation; this was the case among subjects in the overall population as well as in the two subgroups of women with a BMI <25 kg/m2 or ≥25 kg/m2. The percentages of subjects with body weight changes of potential clinical importance (an increase or decrease from baseline of ≥15% or ≥11 kg) were low and not significantly different between the BZA/CE and placebo groups in the overall population or in the two subgroups of women with a BMI <25 kg/m2 or ≥25 kg/m2.17–20
Changes from baseline in mean systolic blood pressure were less than 2.00 mmHg in the BZA 20 mg/CE 0.45 mg group and BZA 20 mg/CE 0.625 mg group; these changes were similar to those observed for the placebo group. The mean change in systolic blood pressure was comparable with that seen on placebo at all time points evaluated.Citation17–Citation20
Conclusion
Treatment with BZA/CE demonstrated clinically meaningful improvements in vasomotor symptoms and vulvovaginal atrophy as well as a protective effect on the skeleton in postmenopausal women seeking treatment for menopausal symptoms, while protecting the endometrium. BZA/CE also showed significant improvements in tolerability compared with HT, as measured by lower rates of uterine bleeding and breast pain. BZA/CE treatment had an effect on breast density similar to placebo. These clinical benefits were associated with an acceptable safety profile. No apparent increased risk for serious adverse events, such as venous thromboembolism, cardiovascular events, endometrial cancer, or breast cancer, was observed with either dose strength of BZA/CE. BZA/CE is an alternative option for treating non-hysterectomized, symptomatic postmenopausal women.
Disclosure
SM is an employee of Pfizer Inc. JHP was formerly an employee of Wyeth Research, and has consulted for Wyeth/Pfizer, Depomed, BHR Pharma, ASCEND Therapeutics, TherapeuticsMD, Ausio Pharmaceuticals, and Shionogi.
References
- ArcherDFTissue-selective estrogen complexes: a promising option for the comprehensive management of menopausal symptomsDrugs Aging20102753354420583848
- MaartensLWLeusinkGLKnottnerusJAClimacteric complaints in the communityFam Pract20011818919411264270
- LewisVUndertreatment of menopausal symptoms and novel options for comprehensive managementCurr Med Res Opin2009252689269819775194
- GeukesMvan AalstMPNautaMCEThe impact of menopausal symptoms on work abilityMenopause20121927828221997498
- GoldEBSternfeldBKelseyJLRelation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of ageAm J Epidemiol200015246347310981461
- DennersteinLDudleyECHopperJLA prospective population-based study of menopausal symptomsObstet Gynecol20009635135810960625
- Al-AzzawiFHabibaMRegular bleeding on hormone replacement therapy: a myth?Br J Obstet Gynaecol19941016616627947498
- Limouzin-LamotheMAWhat women want from hormone replacement therapy: results of an international surveyEur J Obstet Gynecol Reprod Biol199664SupplS21S248732469
- Pfizer Pharmaceuticals IncPremPro package insert Available from: http://labeling.pfizer.com/showlabeling.aspx?id=133Accessed October 11, 2011
- PerssonIThurfjellEHolmbergLEffect of estrogen and estrogen-progestin replacement regimens on mammographic breast parenchymal densityJ Clin Oncol199715320132079336356
- LeeEInglesSABergVDProgestogen levels, progesterone receptor gene polymorphisms, and mammographic density changes: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density StudyMenopause20121930231022105149
- BrowerVHoming in on mechanisms linking breast density to breast cancer riskJ Natl Cancer Inst201010284384520530764
- KerlikowskeKCookAJBuistDSMBreast cancer risk by breast density, menopause, and postmenopausal hormone therapy useJ Clin Oncol2010283830383720644098
- CrandallCJAragakiAKCauleyJABreast tenderness and breast cancer risk in the estrogen plus progestin and estrogen-alone women’s health initiative clinical trialsBreast Cancer Res Treat201213227528522042371
- HaasJSKaplanCPGerstenbergerEPKerlikowskeKChanges in the use of postmenopausal hormone therapy after the publication of clinical trial resultsAnn Intern Med200414018418814757616
- KommBA new approach to menopausal therapy: the tissue selective estrogen complexReprod Sci20081598499219088368
- LoboRAPinkertonJVGassMLEvaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profileFertil Steril2009921025103819635615
- PinkertonJVUtianWHConstantineGDOlivierSPickarJHRelief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trialMenopause2009161116112419546826
- KaganRWilliamsRSPanKMirkinSPickarJHA randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal womenMenopause20101728128919779382
- PinkertonJVTaylorHPanKChinesAMirkinSBreast parameters with bazedoxifene/conjugated estrogens in randomized, controlled trials of postmenopausal womenMenopause20101712211222
- MacLennanAHBroadbentJLLesterSMooreVOral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushesCochrane Database Syst Rev20044CD00297815495039
- UtianWYuHBobulaJMirkinSOlivierSPickarJHBazedoxifene/conjugated estrogens and quality of life in postmenopausal womenMaturitas20096332933519647382
- de VilliersTJBazedoxifene: a novel selective estrogen receptor modulator for postmenopausal osteoporosisClimacteric20101321021820184423
- PickarJHYehI-TBachmannGSperoffLEndometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapyFertil Steril2009921018102419635613
- ArcherDFLewisVCarrBROlivierSPickarJHBazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal womenFertil Steril2009921039104419635614
- ChlebowskiRTHendrixSLLangerRDInfluence of estrogen plus progestin on breast cancer and mammography in healthy post-menopausal womenJAMA200328932343253
- Pfizer Pharmaceuticals IncPremarin US Package Insert Available from: http://labeling.pfizer.com/showlabeling.aspx?id=131Accessed December 13, 2011
- LoboRABushTCarrBRPickarJHEffects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolismFertil Steril200176132411438314