Abstract
Differences in platelet type between the fetus and the mother can lead to maternal immunization and destruction of the fetal platelets, a condition named fetal and neonatal alloimmune thrombocytopenia (FNAIT). FNAIT is reported to occur in ~1 per 1,000 live born neonates. The major risk is intracranial hemorrhage in the fetus or newborn, which is associated with severe neurological complications or death. Since no countries have yet implemented a screening program to detect pregnancies at risk, the diagnosis is typically established after the birth of a child with symptoms. Reports on broader clinical impact have increased clinical concern and awareness. Along with new treatment options for FNAIT, the debate around antenatal screening to detect pregnancies at risk of FNAIT has been revitalized.
Introduction
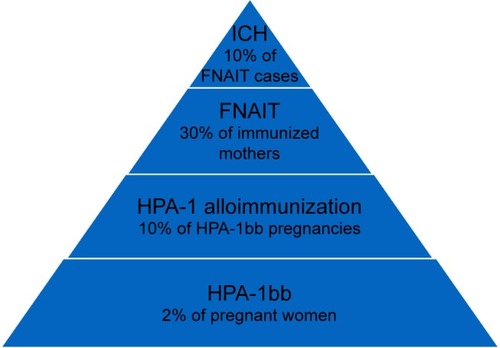
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is not a common pregnancy complication but carries a significant risk of severe fetal and/or neonatal complications and has been recognized as the major cause of primary hemorrhagic morbidity and mortality in fetuses and newborns.Citation1 In neonatal intensive care units, severe thrombocytopenia (platelet count <50×109/L) is reported in 5%–22% of children.Citation2,Citation3 Most of these cases have underlying causes such as prematurity, congenital infections, maternal immune thrombocytopenic purpura, or chronic fetal hypoxia.Citation3,Citation4 However, in otherwise healthy term newborns with isolated severe thrombocytopenia, the most frequent cause is FNAIT.Citation5–Citation7 The condition occurs in ~1 per 1,000 births in Caucasian populations.Citation7
The aim of this review was to give an overview of FNAIT, with a focus on recent developments in its clinical aspects and treatment options.
Pathogenesis
The fetus is semiallogeneic by nature, but generally well tolerated by the maternal immune system. However, some polymorphisms can cause maternal alloimmunization in incompatible pregnancies – resulting in maternal antibodies that target fetal cells for destruction. The clinical manifestations of the conditions depend on the target for the maternal alloantibodies. The most classic immune response in incompatible pregnancies is immune response to RhD antigens on fetal red cells. Red cell alloimmunization can cause hemolytic disease of the fetus or newborn (HDFN). Fetomaternal incompatibility for human neutrophil antigens may induce antibodies targeting neutrophils causing neonatal neutropenia making the newborn susceptible for infections. Incompatibility for human platelet antigens (HPAs) can induce antibodies against fetal platelets, which may lead to FNAIT and hemorrhagic complications. FNAIT is defined as fetal or neonatal thrombocytopenia caused by antibodies targeting alloantigens on fetal platelets (from now on referred to as alloantibodies) because of incompatibility between fetal and maternal platelet antigens.
Maternal antiplatelet immunoglobulins (IgG) are transported across the placenta (mostly IgG1 and IgG3) to the fetal blood system, primarily by the major histocompatibility complex (MHC)-class I-related neonatal Fc receptor.Citation8 These antibodies will bind to the fetal platelets, and the platelets will subsequently be removed from the fetal circulation by phagocytosis.
The HPAs are located on platelet membrane glycoprotein receptors. These glycoproteins play fundamental roles in platelet functions, such as adhesion and aggregation. Most HPAs are based on single-nucleotide polymorphisms resulting in amino acid substitutions localized on the main platelet receptors: integrin αIIbβ3 (GPIIb/IIIa, CD41/CD61: the fibrinogen receptor), the GPIb-IX-V complex (CD42, von Willebrand factor receptor), and the GPIa/IIa complex (α2β1, CD29, the collagen receptor).Citation9,Citation10 HPA-15 is the only exception; this biallelic system is carried by the platelet membrane protein CD109,Citation11 which is a part of the transforming growth factor-β receptor system.Citation12 For a more comprehensive presentation of platelet membrane glycoproteins, the recent review by Zdravic et alCitation13 is suggested.
Platelet-specific antigens were first described in the late 1950s and early 1960s.Citation14,Citation15 To date, 35 different platelet-specific alloantigens have been described as targets for antibodies in FNAIT, of which 12 are grouped in six biallelic systems (HPA-1, -2, -3, -4, -5, and -15; http://www.ebi.ac.uk/ipd/hpa/table1 [accessed September 2016]).
Antigen incompatibility in HPA-1 is found to cause 80%–90% of FNAIT cases in the Caucasian population.Citation16–Citation18 The HPA-1 antigen is located on the integrin β3, defined by a Leu33Pro polymorphism in the PSI domain. Carriers of the Leu33 allelic variant are defined as HPA-1a positive, whereas those who carry homozygous Pro33 alleles are termed HPA-1a negative or HPA-1bb. The classic HPA-1a antigen is a part of the integrin αIIbβ3 complex, also known as the fibrinogen receptor, which is restricted to platelets and megakaryocytes. However, the integrin β3 subunit of the fibrinogen receptor is also part of the integrin αVβ3 complex (vitronectin receptor), which is expressed on other fetal cells, including angiogenic endothelial cells and invasive trophoblasts.Citation19,Citation20
Fetal megakaryocytes – the precursors of platelets – are found in lung and liver as early as in the 12th gestational week. Platelet counts in the healthy fetus are within normal adult range no later than 18th gestational weeks.Citation21,Citation22 Fetal platelet antigens are expressed in normal amounts as early as week 16.Citation20 Maternal IgG alloantibodies from previous pregnancies are detectable in fetal blood from gestational week 6Citation23 and start to increase from early in the second trimester.Citation23,Citation24 Fetal thrombocytopenia caused by maternal alloantibodies may therefore occur very early during pregnancy.
The immune response against HPA-1a is strongly associated with the human MHC class II allele HLA-DRB3*01:01.Citation25–Citation29 In the Norwegian screening study, 90% of HPA-1a immunized women were DRB3*01:01 positive.Citation25 In comparison, 28% of a random population were DRB3*01:01 positive.Citation26 This strong association suggests that CD4 T-cell activation by the DRA/DRB3*01:01 molecule is a determining event in the immune response against HPA-1a. In support of this notion, it has been shown that integrin β3-derived peptides with Leu33 (HPA-1a peptide), but not with Pro33, bind well to the DRA/DRB3*01:01 molecule and thus can be presented by antigen-presenting cells.Citation30,Citation31 Also, HPA-1a-specific DRA/DRB3*01:01-restricted CD4 T cells have been isolated from women who have had a child affected by FNAIT.Citation32,Citation33 Predictably, DRA/DRB3*01:01-restricted HPA-1a-specific CD4 T cells provide essential help to HPA-1a-specific B cells to differentiate to anti-HPA-1a antibody producing plasma cells. Leu 33 has been shown to serve as an anchor residue for stable binding of HPA-1a peptide to the DRA/DRB3*01:01 moleculeCitation31 and is not solvent exposed. T-cell recognition of the allogeneic leu33 residue is therefore indirect.Citation34
Despite large efforts, we still do not completely understand what makes some HPA-1bb women prone to alloimmunization; most are not immunized in connection with an HPA-1 incompatible pregnancy. A recent observation by Li et alCitation35 that infection status in the mother may play a role is interesting and implies that a proinflammatory event trigger the alloimmune response. However, we should also focus on understanding the mechanisms that induce immune tolerance, which could have an important role in preventing most women from becoming alloimmunized.
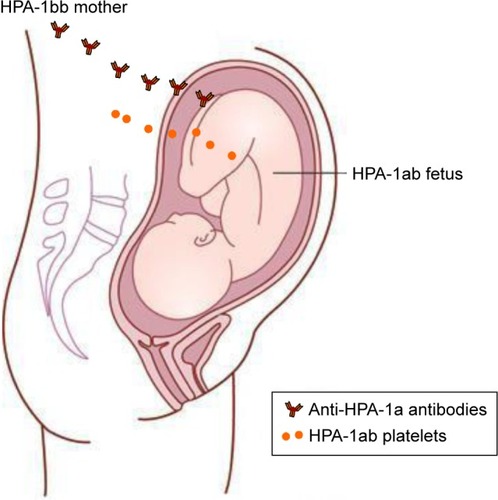
The clinical relevance of different anti-HPA antibodies varies among different ethnic groups. In a Caucasian population, anti-HPA-1a antibodies are by far the most common and clinically relevant cause of FNAIT.Citation17,Citation18,Citation36 Approximately 2% of Caucasian women are HPA-1a negative and at risk of being immunized in connection with an HPA-1 incompatible pregnancy.Citation25,Citation37 The frequency of FNAIT due to anti-HPA-1a antibodies is reported to be 1:1,100 live births.Citation18,Citation25,Citation38 An overview of the pathophysiology of maternal HPA-1 alloimmunization is shown in .
Figure 1 Pathophysiology of maternal HPA-1 alloimmunization.
Abbreviation: HPA, human platelet antigen.
In addition, anti-HPA-5b antibodies cause FNAIT in 7%–16% and anti-HPA-15b in 2%–4% of the cases.Citation16,Citation17,Citation36,Citation39 The African–American population seems to have a lower incidence of anti-HPA-1a-induced FNAIT but have higher risk of alloimmunization to HPA-2 and HPA-5 antigens.Citation40 Among Japanese, HPA-4 and HPA-5 alloimmunizations are most frequent.Citation41,Citation42
The human leukocyte antigen class I (HLA class I) is present on all nucleated cells and platelets in the human body. The genes that encode HLA class I are the most polymorphic in the human genome. Exposure to non-self-HLA can activate the host immune system and lead to the production of alloantibodies. It is well known that anti-HLA class I antibodies can have severe clinical consequences, such as rejection of allograftsCitation43,Citation44 or destruction of transfused platelets.Citation45 Maternal anti-HLA class I antibodies are detected during pregnancy in at least 30% of multigravida.Citation46–Citation49 Although these antibodies have been reported in association with various pregnancy complications, the possible harmful effects on pregnancy are still not clear.Citation50 Numerous reports describe suspected cases of FNAIT with maternal anti-HLA class I antibodies as the only finding and possible explanation of neonatal thrombocytopenia.Citation51–Citation55 It has therefore been suggested that maternal anti-HLA class I antibodies may cause FNAIT, but this is still controversial.Citation46,Citation56
Diagnosis
The diagnosis of FNAIT requires that the fetus/neonate carries a platelet alloantigen that the mother lacks, and to which she has made detectable antibodies.Citation57 The current gold standard for the detection of platelet-specific antibodies is the monoclonal antibody-specific immobilization of platelet antigen (MAIPA) assay,Citation58 a sensitive and specificCitation59,Citation60 capture immunoassay. Quantitation of anti-HPA-1a antibodies is done using a modified MAIPA assay.Citation61 Other HPA alloantibody specificities are normally not quantified. Other techniques are also possible to use. For instance, different Luminex bead-based assays for the detection of anti-HPA-1a antibodies have been tested and are used by some.Citation62 Furthermore, low-avidity anti-HPA-1a antibodies may be detected using surface plasmon resonance technology.Citation63
Cordocentesis has been used in order to identify thrombocytopenic fetuses requiring intrauterine platelet transfusions and also to help decide on antenatal maternal treatment. Because of high risk of procedure-related complications,Citation64 avoidance of invasive procedures is currently recommended.Citation4,Citation65–Citation67
Assays for noninvasive prenatal testing to detect fetal HPA-1a DNA in maternal plasma have been developed and are in use in many research laboratories, but are not yet implemented in routine clinical practice in most countries.Citation68–Citation72 Determination of paternal zygosity may be relevant. If the father is typed and found to be HPA-1aa, the pregnancy will always be HPA-1 incompatible and fetal HPA-1 genotyping is not necessary. Whereas if the father is HPA-1ab, there is a 50% chance of the fetus being HPA-1bb and compatible with the mother, and in this situation knowing the fetal HPA-1 genotype would be clinically helpful.
Clinical presentation and outcome
Neonatal thrombocytopenia
The suspicion of FNAIT is typically raised when a newborn develops widespread skin petechiae shortly after birth and blood tests show severe thrombocytopenia. Most FNAIT cases present with platelet counts well below 50×109/L.Citation25,Citation39,Citation73 Clinical symptoms range from no symptoms to limited or widespread skin petechiae or purpura to symptoms of extra- or intracranial hemorrhage (ICH). Baseline characteristics of HPA-1-induced FNAIT are shown in .
Intracranial hemorrhage
ICH is the major reason for clinical concern in FNAIT. ICH due to FNAIT is reported to occur in 1 of 10,000 births.Citation7 A recent review of prospective screening and intervention studies reported ICH in 7% of severe FNAIT cases (neonatal platelet count <50×109/L).Citation37 Retrospective studies report a frequency of ICH due to FNAIT in 13%–21% of severe cases.Citation17,Citation39,Citation74 The clinical outcome of ICH due to FNAIT is reported to be worse compared to neonatal ICH from other causesCitation75,Citation76 and may be connected to the typical locations of the bleedings in the brain. Severe neurological complications are found in 36%–68% of ICH casesCitation24,Citation26,Citation77,Citation78 and fetal or neonatal death due to ICH is reported in the range of 9%–46%Citation24,Citation57,Citation68,Citation79 when evaluated retrospectively. A large cohort study from the international No Intracranial Hemorrhage (NOICH) registry described 43 cases of ICH caused by FNAIT in depth and found that the majority of bleedings (54%) happened before the third trimester and 67% before 34 gestational weeks.Citation77 Similar results were recently published for a French cohort of ICH cases caused by FNAIT,Citation79 and a recent systematic review on clinical consequences of neonatal alloimmune thrombocytopenia also describes this tendency.Citation7 Results from the NOICH registry further indicated that boys may be more prone to bleeding compared with girls.Citation77
The majority of ICH cases are reported to occur in the first-born child.Citation77 Thus, in most of the reported cases of ICH, antenatal treatment was not given since the risk of FNAIT typically was not known. The recurrence rate of ICH in subsequent pregnancies is reported to be 79%.Citation64 If the index FNAIT child did not have ICH, the risk of ICH in the next pregnancy has been estimated at 7%.Citation64
Intrauterine fetal death (IUFD) is sometimes reported as an FNAIT complication separate from ICH, but there are no data to indicate that the cause of FNAIT-related IUFD should be something else than ICH. Therefore, IUFD will not be discussed separately.
Extracranial hemorrhage
FNAIT may lead to severe bleeding other than ICH in neonates with FNAIT. In the recent overview of extracranial FNAIT hemorrhage by Winkelhorst et al,Citation80 a wide variety of bleeding complications were reported in the 21 cases identified. Gastrointestinal bleedings constituted the majority of cases, followed by pulmonary hemorrhage. Some of these bleedings were life-threatening, and therefore, it is important for clinicians to be aware of possible extracranial bleedings in the fetus or neonate when managing an HPA-alloimmunized pregnancy or caring for a neonate with severe FNAIT.
Miscarriage
Mothers of FNAIT-affected children often report a history of miscarriages, including second trimester miscarriages, suggesting that the risk of miscarriage may be increased.Citation81 In a murine model of FNAIT, it was found that maternal anti-integrin β3 antibodies promoted fetal miscarriage,Citation82,Citation83 and recent data from the same group suggest that anti-integrin β3 antibodies may activate placental natural killer cells leading to placenta damage and miscarriage through antibody dependent cellular cytotoxicity.Citation84
Yet, no human study has specifically addressed whether there is an association between maternal HPA alloimmunization and miscarriage. Given the high prevalence of miscarriage in general and lack of understanding and treatment options for recurrent miscarriage, it is rather surprising that this area of pregnancy outcome for HPA alloimmunization has not received more attention.
Placental function and birth weight
Normal placental function is vital for a successful and uncomplicated pregnancy. Placental development and function are primarily established during the first half of pregnancy, although its growth and maturation continues throughout the pregnancy. Defect placentation is a common denominator for the “great obstetric syndromes” – preeclampsia, intrauterine death, and fetal growth restriction. Growth restricted fetuses/low birth weight newborns have increased risk of disease and death in the newborn period, as well as in adulthood.Citation85–Citation87 How mother and fetus co-exist and “negotiate” to achieve normal placentation is complex and not yet fully understood.
We previously found a strong association between the presence of maternal anti-HPA-1a antibodies and reduced birth weight in boys.Citation88 A similar observation was made in an international multicenter study, showing that 23% of neonates with ICH were below the 10th percentile for birth weight and defined as small for gestational age.Citation77 Recently, we also found a similar association between the presence of maternal anti-HLA class I antibodies and reduced birth weight in cases where the child was thrombocytopenic at birth, and in this work, we also observed concurrently lower placental weight.Citation89
HPA-1 antigens are expressed on the surface of fetal invading trophoblasts as part of the vitronectin receptor, and we know that anti-HPA-1a antibodies can bind the HPA-1a antigen when present on the vitronectin receptor.Citation90,Citation91 The vitronectin receptor promotes cell adhesion and migration of primary cytotrophoblasts.Citation92,Citation93 Recently, it was found that anti-HPA-1a antibody affected adhesion, migration, and invasive capacity of extravillous throphoblasts using a first trimester trophoblast-derived cell line (HTR-8/SVneo), indicating that maternal anti-HPA-1a antibodies could lead to defect placentation, in turn leading to poor fetal growth (Eksteen et al, personal communication, January, 2017). Together with our previous observation that mothers with anti-HPA-1a antibodies give birth to children with lower birth weight, the findings of the present study indicate that anti-HPA-1a antibodies can interfere with placental development. The mechanisms linking FNAIT to placenta/birth weight are currently being explored.
Predictors of clinical outcome
Several studies have demonstrated an association between anti-HPA-1a antibody levels in pregnancy and the severity of neonatal thrombocytopenia.Citation94–Citation96 In a former large prospective screening study in Norway, maternal anti-HPA-1a antibody levels ≥3 IU/mL during pregnancy was found to be highly sensitive (>90%) in predicting severe neonatal thrombocytopenia,Citation96 and this level is currently used as cut-off in the national clinical guidelines to decide on delivery mode and place (www.legeforeningen.no). Some retrospective studies did not find such a relationship.Citation97,Citation98 These discrepant results have been discussed, and it was suggested that quantitation of maternal anti-HPA-1a antibody level by MAIPA can be useful in a screening setting but maybe not in cases where FNAIT was already diagnosed in a previous pregnancy.Citation99 This explanation makes sense; when monitoring a risk pregnancy where a previous child had severe FNAIT, the obstetric history alone has strong predictive value. Furthermore, the anti-HPA-1a antibody level range is typically limited to very high levels in many such cases and statistical models aiming to study antibody level as a continuous variable will therefore fail to demonstrate significant associations.
A retrospective study reported that maternal anti-HPA-1a antibody levels may be used as a prognostic factor of intravenous immunoglobulin (IVIg) therapy success or failure.Citation100 This study was criticized for several weaknesses,Citation101 but the possibility of using anti-HPA-1a antibody levels to decide on antenatal management and delivery is important and deserves more attention. The ongoing Polish FNAIT screening study Prevention of FNAIT in Polish Newborns (PREVFNAIT; http://www.konfliktplytkowy.ihit.waw.pl/en/) will therefore give us valuable data on 1) the effect of antenatal IVIg in a screening setting and 2) whether maternal anti-HPA-1a antibody levels during pregnancy is useful to evaluate a possible IVIg effect.
For decades, the “FNAIT paradigm” has been that maternal anti-HPA-1a antibodies cause destruction of fetal platelets and that it is the low number of platelets in the fetus/newborn that trigger bleeding. Questioning this paradigm, we recently learned that anti-HPA-1a antibodies can bind cerebral endothelium carrying the vitronectin receptor and exert a direct negative effect that may cause ICH, bypassing the role of low platelet number alone as the main trigger for ICH.Citation102 This strengthens the importance of using antibody levels instead of fetal platelet counts as tools in management planning. Following up on this, the most recent FNAIT research reported that in children with ICH caused by FNAIT, anti-HPA-1a antibodies in the mother targeted the vitronectin receptor (αvβ3), whereas maternal sera from FNAIT cases where the fetus/neonate did not have ICH had mainly antibodies binding the fibrinogen receptor (αIIbβ3).Citation103 Therefore, it will be important for future FNAIT research to differentiate between anti-HPA-1a antibodies binding to the fibrinogen receptor on platelets and antibodies binding to both the fibrinogen and the vitronectin receptors. These results need to be confirmed and explored further, but fit well with the current understanding of a direct antibody-mediated effect on vessel wall integrity and trophoblast functions.
Maternal anti-HPA-1a antibody levels in pregnancies where the fetus/neonate has ICH are reported to be very high compared with severe FNAIT cases without detectable ICH.Citation77,Citation79 However, we do not yet have good prospective data to determine whether maternal anti-HPA-1a antibody levels during pregnancy may be useful as an additional predictive factor for the risk of ICH.
The composition and heterogeneity of the terminal sugars of the Fc portion of IgG antibodies can affect the effector functions of the antibody, and certain glycosylation patterns are known to play a role as disease biomarker. Two research groups recently described decreased fucosylation of anti-HPA-1a antibodies causing FNAIT, and that fucosylation may be useful to predict disease severity.Citation104,Citation105
Management
Intravenous immunoglobulin
Previous severe FNAIT, with or without bleeding complications, is currently used clinically to determine the risk of severe FNAIT in subsequent pregnancies, and as such serves as the major basis in antenatal management planning. Weekly IVIg treatment to the mother starting from the second trimester is the first-line therapy of choice in most of the Western countries and is administered when the risk of FNAIT is considered to be high.Citation4,Citation67,Citation106,Citation107 The treatment is considered effective when the neonatal platelet count is increased or ICH avoided in a subsequent pregnancy compared with the previous FNAIT pregnancy, with the underlying assumption that FNAIT gets worse in younger siblings. In a recent Norwegian prospective study, the natural course of FNAIT in several subsequent pregnancies was reported for the first time.Citation108 Our data showed that younger siblings of FNAIT-affected children had unchanged or higher neonatal platelet counts without antenatal treatment in the majority of subsequent pregnancies. Therefore, this study did not support the common opinion that the outcome after HPA-1a alloimmunization is generally worse in the next pregnancy. Thus, increased neonatal platelet count in a subsequent FNAIT pregnancy may not always reflect an antenatal treatment effect. Delbos et al reported that efficacy of IVIg treatment may be dependent on maternal HLA-DRB4*0101 haplotype,Citation79 also suggesting the possible use of several HLA-DR haplotypes as predictive markers of clinical outcome.
In a review of maternal IVIg response, it was commented that despite controversy whether IVIg increases fetal platelet counts, all IVIg studies report identical low frequency of ICH,Citation109 indicating that the possible effect of IVIg may be different from increased platelet count. Results from the NOICH study found that IVIg treatment during the subsequent pregnancy seemed to be protective with regard to ICH in most cases, reducing the ICH recurrence risk from 79% as previously reported to 11%.Citation77 In a murine model of FNAIT, it was demonstrated that treatment with IVIg to immunized mice prevented ICH in the pups,Citation83 and the same group later published data suggesting that IVIg prevented ICH by restoring angiogenesis in the fetal brain.Citation102
Corticosteroids
Some clinicians use systemic corticosteroids alongside IVIg as a means of supporting the action of IVIg. Dexamethasone is not recommended due to risk of oligohydramnios at higher dosesCitation110 and lack of effect at lower doses.Citation111 Prednisone is therefore the recommended choice; however, the potential benefits versus risks deserves further evaluation.Citation112
Cordocentesis
Repeat cordocentesis to measure fetal platelet count followed by intrauterine platelet transfusions in case of severe fetal thrombocytopenia was commonly performed previously, but is nowadays abandoned by many countries due to high risk of procedure-related complications.Citation113,Citation114 Some countries still combine diagnostic fetal blood sampling (FBS) with maternal IVIgs, with or without a second FBS to decide on treatment effect and mode of delivery. However, a complete noninvasive management strategy is advocated by most.Citation65,Citation67,Citation115
Pre-implantation genetic diagnosis
When the mother is HPA-1bb and the father is HPA-1ab, there is a 50% chance that the fetus will be HPA-1a positive. In cases of a previously FNAIT-affected sibling, some women are not eligible for antenatal IVIg treatment due to hypersensitivity. The possibility of performing preimplantation genetic diagnosis for HPA-1 incompatibility in order to select an HPA-1bb embryo for in vitro fertilization (IVF) has been described in a case report.Citation116 This may be a desirable option for couples facing a similar situation.
In vitro fertilization
When the mother is HPA-1bb and the father is HPA-1aa, pre-implantation genetic diagnosis is not helpful, as the fetus will always be HPA-1ab. The use of HPA-1-matched sperm donor is a new procedure. The first woman in Norway to conceive after using an HPA-1-matched sperm donor delivered a healthy boy with normal platelet count in 2014.Citation117 In Spring 2016, the second child was delivered using this treatment strategy, and this pregnancy also underwent without complications and a healthy newborn with normal platelet count was born by caesarean section (Dr Peter Fedorscak, Oslo University Hospital, personal communication, May 2016). Needless to say, more experience with this treatment option is needed before introducing this as part of a general management program. Because of the ethical challenges, we would generally recommend to consider the use of HPA-1-matched sperm donor only for the minority of HPA-1bb women with a history of recurrent severe FNAIT-related complications, where the alternative would be to refrain from further pregnancies.
HPA-1 typing is not routinely performed in connection with the use of IVF. IVF was reported to be involved in four severe cases of anti-HPA-1a-induced FNAIT.Citation118 In three out of the four cases, the fetuses were HPA-1aa homozygous, which could not occur in naturally conceived HPA-1 incompatible pregnancy. In these cases, either the surrogate or mother was HPA-1bb homozygous. The authors speculate that a homozygous HPA-1a fetus express twice as much incompatible antigen on their platelets, inducing a stronger maternal immune response. Alternatively, the severity could be due to antibody binding to all fibrinogen receptors on fetal platelets; only half of the receptors can be bound on platelets in HPA-1ab individuals, leaving platelets partially functional. Anti-HPA-1a antibodies affect HPA-1aa platelet function more than HPA-1ab.Citation90 This finding needs to be confirmed or disproved by others. However, their conclusion that all women should be HPA-1 genotyped before serving as a surrogate mother seems feasible and deserves attention in view of the rapidly increased use of surrogacy.
Mode of delivery
Whether delivery by caesarean section prevents ICH in FNAIT affected neonates is not really known.Citation37,Citation119 Vaginal delivery is advised by some as an option when fetal platelet count is >50×109/L.Citation111 However, this approach requires FBS in order to know the fetal platelet count around the time of delivery. A Dutch pilot study of 32 pregnancies where an older sibling had FNAIT without ICH found that vaginal delivery was not associated with an increased risk of ICH.Citation120 The current policy in the Netherlands is to induce vaginal delivery at 37–38 weeks without FBS first. If the woman has a previous caesarean section, they may sometimes do a FBS before inducing delivery. If the previous child had ICH, delivery is performed by elective caesarean section at 36 weeks (prof Dick Oepkes, Director of the Dutch National Centre for Fetal Therapy, Leiden University Medical Centre in the Netherlands, personal communication, March, 2016). The intervention part of the Norwegian screening study consisted of delivering all HPA-1a alloimmunized women by caesarean section at 36–38 weeks followed by immediate transfusion of HPA-1 compatible platelets if the newborn was severely thrombocytopenic.Citation25 Delivery by caesarean section was one of several interventions in this study, and the isolated effect of delivery mode is therefore difficult to assess. Still, mortality and morbidity were significantly reduced in the screening and intervention population compared with historical controls.Citation29 The current management guideline in Norway recommends delivery by elective caesarean section around 38 weeks if the maternal anti-HPA-1a antibody level is ≥3 IU/mL, irrelevant of previous obstetric history.
Since the vast majority of ICH caused by FNAIT is found to occur before delivery, and little data support the idea that ICH due to FNAIT tend to occur in connection with delivery, it is difficult to argue that the risk of ICH is affected by mode of delivery. The risk of ICH when older siblings did not suffer from ICH is reported to be 7%.Citation57 A larger study sample is therefore needed before we can conclude whether vaginal delivery for this patient group is safe or not. In many places, HPA-1bb platelets are not available in the blood banks on a daily basis. A planned delivery – whether vaginally or by surgery – is therefore important in order to have appropriate platelets available in case of a severely thrombocytopenic newborn.
Postnatal management of the newborn
The majority of FNAIT-affected newborns will not have suffered ICH before delivery. Preventing ICH by increasing platelet count above a certain threshold is therefore considered a neonatal emergency, and prompt correction by platelet transfusion should be done based on clinical suspicion without awaiting laboratory confirmation of the diagnosis.Citation4 However, it is not clear what threshold should be used to trigger platelet transfusion.Citation121,Citation122 Previous reports also suggest that neonates with HPA-5b incompatibility may be at risk of bleeding at higher platelet counts compared with HPA-1 incompatibility.Citation39 It is recommended to give compatible platelet concentrates instead of random donor platelets, due to both larger platelet increment and longer half-life of the transfused platelets.Citation123,Citation124 Random donor platelets may be used when compatible platelets are not available.Citation123 The use of IVIg as treatment to increase neonatal platelet count varies, but is often recommended as supplemental therapy for 1–3 days depending on platelet increment response of transfusions.Citation106,Citation107,Citation122,Citation124 Other management options include corticosteroid therapy, but documentation of effect is poor.Citation125 It is also recommended that all babies with severe FNAIT should have a cranial ultrasound for the detection of ICH.Citation122
In summary, there is consensus on postnatal correction of severe thrombocytopenia, but antenatal treatment management protocols vary. Knowledge gaps on the natural history of FNAIT, together with lack of randomized controlled trials evaluating the effects of different treatment options, are probably a major reason for the struggle to have common management protocols. Still, nobody questions the severity of this disorder. Therefore, there is no doubt that a preventive approach hindering the mother from becoming HPA-1a alloimmunized in the first place would be welcomed by all.
A prophylactic approach to FNAIT
FNAIT is often referred to as the platelet counterpart of HDFN, but traditionally presented with one important exception – time of immunization. Alloimmunization against the RhD antigen mainly occurs in connection with delivery as a result of fetomaternal hemorrhage.Citation126,Citation127 Until recently, it was believed that most immunizations to HPA-1a occurred during the first incompatible pregnancy and not so often in connection with delivery. This assumption was based on retrospective observations. Now, data from several prospective investigations have challenged this belief. In the Norwegian screening study, it was found that >75% of HPA-1a alloimmunizations occurred after delivery, suggesting that delivery may be the immunizing stimulus.Citation96 Similar prospective data were also presented by Turner et alCitation73 and Williamson et al,Citation18 reporting a low frequency of immunizations during first pregnancies (4% and 24%, respectively). Thus, the pathophysiology of FNAIT seems to be more similar to HDFN than previously thought. This recognition has opened the possibility to prevent anti-HPA-1a-induced FNAIT by postnatal administration of HPA-1a antibodies to HPA-1a-negative women delivering an HPA-1a-positive child. This is actually the same strategy that has been used to prevent HDFN with great success during the past 50 years. In order to test this hypothesis, a proof-of-principle study using glycoprotein integrin β3 (GPIIIa)-deficient mice was conducted. Administration of human polyclonal anti-HPA-1a or murine monoclonal anti-HPA-1a (clone SZ21) were both able to suppress the antibody response to transfused human HPA-1a-positive platelets. The induction of antibody-mediated immune suppression was also carried out using murine wild-type platelets transfused to GPIIIa-deficient mice. The antibody response to platelets was significantly reduced in mice receiving anti-GPIIIa in conjunction with platelet transfusion. Breeding experiments further demonstrated that the platelet count in the pups born by the mice that had received prophylaxis after the platelet transfusion was significantly higher compared to controls where the mother did not receive prophylactic treatment. The number of pregnancies with miscarriages and dead pups was also significantly lower when the platelet transfusion was followed by prophylaxis.Citation128 Altogether, these results support the hypothesis that administration of anti-HPA-1a to HPA-1a-negative women after delivery of an HPA-1a-positive child may prevent immunization to HPA-1a, thereby preventing the development of FNAIT in a subsequent pregnancy. The hypothesis to prevent immunization to HPA-1a will be tested in pregnant women in an EU-supported clinical trial (www.profnait.eu).
Another potential strategy to prevent HPA-1a immunization could be to target the antigen-specific T-cell responses in these women. Little is known about how the T-cell response shapes the quality of the subsequent anti-HPA-1a antibody response and whether it could be possible to induce immune tolerance to HPA-1a by oral administration of peptides in an efficient tolerogenic formulation. Such tolerance may depend on induction of tolerogenic regulatory T cells or on clonal deletion or induction of clonal anergy.Citation129
Screening
No country has yet started screening of all pregnant women to detect HPA-1a-negative pregnancies, in order to identify women at risk of alloimmunization. The implementation of such a national screening program for FNAIT has been debated in several countries.Citation25,Citation37,Citation107 So far, all countries have turned down the idea because of the lack of prophylaxis or effective treatment modalities. In comparison, RhD typing was introduced in pregnancy screening programs 20 years before prophylaxis was started. Identification and follow-up of RhD-negative pregnancies at risk for anti-D alloimmunization have greatly reduced the risk of morbidity in newborns being exposed. Results from the Norwegian screening study showed that implementation of a screening program for HPA-1a-negative pregnancies could improve clinical outcomeCitation25 and be cost-effective.Citation130 Several other studies have also concluded that a screening and intervention program for maternal HPA-1 alloimmunization is likely to be cost-effective.Citation37,Citation73,Citation131,Citation132 Without antenatal screening, we only detect a minority of cases.Citation133 In a recent assessment of prospective studies on screening for HPA-1a alloimmunization including 176,000 low-risk pregnancies, it was concluded that screening of all pregnancies combined with antenatal treatment may reduce mortality and morbidity associated with FNAIT but that large-scale screening studies are needed to evaluate the effect of currently used interventions.Citation37 Introduction of antenatal screening for FNAIT was considered in relation to the revised World Health Organization screening criteria,Citation134 and it was concluded that the screening criteria were fulfilled.Citation135 The ongoing Polish PREVFNAIT screening study is primarily undertaken to demonstrate the feasibility of implementing antenatal HPA-1 screening as a national routine later. In the Netherlands, the planned HPA screening In Pregnancy study aim to pave the way for the implementation of screening.
Opponents to introducing an antenatal screening program for FNAIT point out that no randomized controlled trials have been conducted to assess the possible clinical and economic benefits of screening and also that there is no consensus on how to treat pregnancies at risk of FNAIT once these pregnancies are identified.Citation4,Citation106 Those in favor of screening state that randomized controlled trials would be unethical to perform and that we will never gain this knowledge without first introducing screening.Citation135,Citation136
In the absence of antenatal screening for the detection of pregnancies at risk, previous obstetric history serves as the main basis for antenatal management protocols.Citation64,Citation119 ICH or severe thrombocytopenia in the previous neonate is considered useful to predict an increased risk of severe FNAIT in subsequent pregnancies. This strategy a priory excludes any management of the first FNAIT-affected pregnancy before the child is born, and it is well documented that severe FNAIT may occur in the first-born child.Citation17,Citation39
Conclusion
Despite large efforts, there are still considerable knowledge gaps on the pathophysiology of FNAIT. This is probably the major reason for the lack of consensus on how to manage pregnancies at risk of FNAIT. New knowledge indicating placenta as a target of maternal anti-HPA-1a antibodies in addition to fetal platelets increases clinical concern. The broader clinical impact of FNAIT together with new treatment opportunities for FNAIT strengthens the need for antenatal screening to detect pregnancies at risk of FNAIT. Without a screening program for the detection of HPA-1a-negative pregnancies, the FNAIT diagnosis is almost always established after birth of a symptomatic child. With the current non-screening policy, we only detect a minority of FNAIT cases. An antibody-mediated prophylaxis to prevent HPA-1 is currently being developed and has the potential to prevent severe mortality and morbidity in newborns from FNAIT. However, it is impossible to offer an antenatal prophylaxis to HPA-1bb pregnant women without knowing who they are. Introduction of antenatal screening programs should therefore be considered.
Disclosure
BS and AH have financial relationships with Prophylix Pharma AS, a small medical company aiming to develop a FNAIT prophylaxis. HT, MTA, and TBS report no conflicts of interest in this work.
References
- BusselJBAlloimmune thrombocytopenia in the fetus and newbornSemin Thromb Hemost200127324525211446658
- CastleVAndrewMKeltonJGironDJohnstonMCarterCFrequency and mechanism of neonatal thrombocytopeniaJ Pediatr19861085 Pt 17497553701523
- StanworthSJClarkePWattsTProspective, observational study of outcomes in neonates with severe thrombocytopeniaPediatrics20091245e826e83419841111
- MurphyMFBusselJBAdvances in the management of alloimmune thrombocytopeniaBr J Haematol200713636637817233844
- BurrowsRFKeltonJGFetal thrombocytopenia and its relation to maternal thrombocytopeniaN Engl J Med1993329146314668413457
- SainioSJarvenpaaALRenlundMRiikonenSTeramoKKekomakiRThrombocytopenia in term infants: a population-based studyObstet Gynecol200095344144610711560
- KamphuisMMParidaansNPPorcelijnLLoprioreEOepkesDIncidence and consequences of neonatal alloimmune thrombocytopenia: a systematic reviewPediatrics2014133471572124590747
- SimisterNEStoryCMHuman placental Fc receptors and the transmission of antibodies from mother to fetusJ Reprod Immunol19973711239501287
- LandauMRosenbergNMolecular insight into human platelet antigens: structural and evolutionary conservation analyses offer new perspective to immunogenic disordersTransfusion201051355856920804530
- RozmanPPlatelet antigens. The role of human platelet alloantigens (HPA) in blood transfusion and transplantationTranspl Immunol2002102–316518112216947
- SchuhACWatkinsNANguyenQA tyrosine703serine polymorphism of CD109 defines the Gov platelet alloantigensBlood20029951692169811861285
- FinnsonKWTamBYLiuKIdentification of CD109 as part of the TGF-beta receptor system in human keratinocytesFASEB J20062091525152716754747
- ZdravicDYougbareIVadaszBFetal and neonatal alloimmune thrombocytopeniaSemin Fetal Neonatal Med2016211192726810319
- ShulmanNRAsterRHPearsonHAHillerMCImmunoreactions involving platelet. VI. Reactions of maternal isoantibodies responsible for neonatal purpura. Differentiation of a second platelet antigen systemJ Clin Invest1962411059106913912392
- Van LoghemJJJDorfmeijerHVan HartMSchreuderFSerological and genetical studies on a platelet antigen (Zw)Vox Sang19594216116913669430
- DavorenACurtisBRAsterRHMcFarlandJGHuman platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopeniaTransfusion2004441220122515265127
- Mueller-EckhardtCKiefelVGrubertA348 cases of suspected neonatal alloimmune thrombocytopeniaLancet1989186343633662563515
- WilliamsonLMHackettGRennieJThe natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screeningBlood199892228022879746765
- ZhouJPopLMGhetieVHypercatabolism of IgG in mice with lupus-like syndromeLupus200514645846616038110
- GruelYBoizardBDaffosFForestierFCaenJWautierJLDetermination of platelet antigens and glycoproteins in the human fetusBlood19866824884922425870
- PahalGSJauniauxEKinnonCThrasherAJRodeckCHNormal development of human fetal hematopoiesis between eight and seventeen weeks’ gestationAm J Obstet Gynecol200018341029103411035358
- Van den HofMCNicolaidesKHPlatelet count in normal, small, and anemic fetusesAm J Obstet Gynecol199016237357392107744
- JauniauxEJurkovicDGulbisBLiesnardCLeesCCampbellSMaterno-fetal immunoglobulin transfer and passive immunity during the first trimester of human pregnancyHum Reprod19951012329733008822462
- SimisterNEPlacental transport of immunoglobulin GVaccine200321243365336912850341
- Kjeldsen-KraghJKillieMKTomterGA screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopeniaBlood2007110383383917429009
- L’AbbeDTremblayLFilionMAlloimmunization to platelet antigen HPA-1a (PIA1) is strongly associated with both HLA-DRB3*0101 and HLA-DQB1*0201Hum Immunol19923421071141358865
- Mueller-EckhardtCMueller-EckhardtGWillen-OhffHImmunogenicity of and immune response to the human platelet antigen Zwa is strongly associated with HLA-B8 and DR3Tissue Antigens198526171763929422
- Reznikoff-EtievantMFMullerJYJulienFPatereauCAn immune response gene linked to MHC in manTissue Antigens19832243123146417831
- ValentinNVergrachtABignonJDHLA-DRw52a is involved in alloimmunization against PL-A1 antigenHum Immunol199027273792298610
- Anani SarabGMossMBarkerRNUrbaniakSJNaturally processed peptides spanning the HPA-1a polymorphism are efficiently generated and displayed from platelet glycoprotein by HLA-DRB3*0101-positive antigen-presenting cellsBlood200911491954195719494351
- WuSMaslankaKGorskiJAn integrin polymorphism that defines reactivity with alloantibodies generates an anchor for MHC class II peptide binding: a model for unidirectional alloimmune responsesJ Immunol19971587322132269120277
- AhlenMTHusebekkAKillieMKSkogenBStugeTBT-cell responses associated with neonatal alloimmune thrombocytopenia: isolation of HPA-1a-specific, HLA-DRB3*0101-restricted CD4+T cellsBlood2009113163838384419136661
- RaymentRKooijTWZhangWEvidence for the specificity for platelet HPA-1a alloepitope and the presenting HLA-DR52a of diverse antigen-specific helper T cell clones from alloimmunized mothersJ Immunol2009183167768619535639
- AhlenMTHHusebekkAKillieILSkogenBStugeTBT cell responses to human platelet antigen-1a involve a unique form of indirect allorecognitionJCI Insight2016114MC5033924
- LiCChenPVadaszBCo-stimulation with LPS or poly I:C markedly enhances the anti-platelet immune response and severity of fetal and neonatal alloimmune thrombocytopeniaThromb Haemost201311061250125824067944
- MandelbaumMKorenDEichelbergerBAuerbachLPanzerSFrequencies of maternal platelet alloantibodies and autoantibodies in suspected fetal/neonatal alloimmune thrombocytopenia, with emphasis on human platelet antigen-15 alloimmunizationVox Sang200589394315938738
- KamphuisMMParidaansNPorcelijnLScreening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic reviewBJOG2010117111335134320618318
- DreyfusMKaplanCVerdyESchlegelNDurand-ZaleskiITcherniaGFrequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working GroupBlood199789440244069192764
- GhevaertCCampbellKWaltonJManagement and outcome of 200 cases of fetomaternal alloimmune thrombocytopeniaTransfusion20074790191017465957
- KimHOJinYKicklerTSBlakemoreKKwonOHBrayPFGene frequencies of the five major human platelet antigens in African American, white, and Korean populationsTransfusion199535108638677570918
- OhtoHMiuraSArigaHIshiiTFujimoriKMoritaSThe natural history of maternal immunization against foetal platelet alloantigensTransfus Med200414639940815569234
- TanakaSOhnokiSShibataHOkuboYYamaguchiHShibataYGene frequencies of human platelet antigens on glycoprotein IIIa in JapaneseTransfusion19963698138178823457
- ZhangQLiangLWGjertsonDWDevelopment of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunctionTransplantation200579559159815753849
- LeePCTerasakiPITakemotoSKAll chronic rejection failures of kidney transplants were preceded by the development of HLA antibodiesTransplantation20027481192119412438971
- NovotnyVMPrevention and management of platelet transfusion refractorinessVox Sanguinis19997611139933848
- KingKEKaoKJBrayPFThe role of HLA antibodies in neonatal thrombocytopenia: a prospective studyTissue Antigens19964732062118740770
- Morin-PapunenLTiilikainenAHartikainen-SorriALMaternal HLA immunization during pregnancy: presence of anti HLA antibodies in half of multigravidous womenMed Biol19846263233256598218
- ReganLBraudePRHillDPA prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancyHum Reprod1991622942982056027
- MassonEVidalCDeschampsMIncidence and risk factors of anti-HLA immunization after pregnancyHum Immunol201374894695123628391
- LashleyEEMeulemanTClaasFHBeneficial or harmful effect of antipaternal human leukocyte antibodies on pregnancy outcome? A systematic review and meta-analysisAm J Reprod Immunol20137028710323496018
- ThudeHSchornerUHelfrichtCLothMMaakBBarzDNeonatal alloimmune thrombocytopenia caused by human leucocyte antigen-B27 antibodyTransfus Med200616214314916623921
- StarcevicMTomicicMMalenicaMZah-MatakovicVNeonatal alloimmune thrombocytopenia caused by anti-HLA-A24 alloantibodiesActa Paediatr201099463063219912145
- MoncharmontPDuboisVObegiCHLA antibodies and neonatal alloimmune thrombocytopeniaActa Haematol2004111421522015153714
- GramatgesMMFaniPNadeauKPereiraSJengMRNeonatal alloimmune thrombocytopenia and neutropenia associated with maternal human leukocyte antigen antibodiesPediatr Blood Cancer2009531979919229975
- SaitoSOtaMKomatsuYSerologic analysis of three cases of neonatal alloimmune thrombocytopenia associated with HLA antibodiesTransfusion200343790891712823751
- TaaningEHLA antibodies and fetomaternal alloimmune thrombocytopenia: myth or meaningful?Transfus Med Rev200014327528010914422
- BerkowitzRLBusselJBMcFarlandJGAlloimmune thrombocytopenia: state of the art 2006Am J Obstet Gynecol200619590791316875656
- KiefelVSantosoSWeisheitMMueller-EckhardtCMonoclonal antibody-specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodiesBlood198770172217262445398
- MetcalfePAllenDChapmanJOuwehandWHInterlaboratory variation in the detection of clinically significant alloantibodies against human platelet alloantigensBr J Haematol19979712042079136966
- MetcalfePAllenDKekomakiRKaplanCde HaasMOuwehandWHAn International Reference Reagent (minimum sensitivity) for the detection of anti-human platelet antigen 1aVox Sang200996214615219076339
- BertrandGJalluVGouetMQuantification of human platelet antigen-1a antibodies with the monoclonal antibody immobilization of platelet antigens procedureTransfusion2005451319132316078919
- PorcelijnLHuiskesEComijs-van OsselenIA new bead-based human platelet antigen antibodies detection assay versus the monoclonal antibody immobilization of platelet antigens assayTransfusion20145461486149224299453
- SocherIAndrei-SelmerCBeinGKrollHSantosoSLow-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technologyTransfusion200949594395219175553
- RadderCMBrandAKanhaiHHWill it ever be possible to balance the risk of intracranial haemorrhage in fetal or neonatal alloimmune thrombocytopenia against the risk of treatment strategies to prevent it?Vox Sang200384431832512757506
- MechoulanAKaplanCMullerJYFetal alloimmune thrombocytopenia: is less invasive antenatal management safe?J Matern Fetal Neonatal Med201124456456720822329
- RadderCMBrandAKanhaiHHA less invasive treatment strategy to prevent intracranial hemorrhage in fetal and neonatal alloimmune thrombocytopeniaAm J Obstet Gynecol2001185368368811568798
- KamphuisMMOepkesDFetal and neonatal alloimmune thrombocytopenia: prenatal interventionsPrenat Diagn201131771271921618560
- Wienzek-LischkaSKrautwurstAFrohnerVNoninvasive fetal genotyping of human platelet antigen-1a using targeted massively parallel sequencingTransfusion2015556 Pt 21538154425873286
- van der SchootCEThurikFFVeldhuisenBde HaasMNoninvasive prenatal blood group and HPA-1a genotyping: the current European experienceTransfusion20135311 Suppl 22834283624004336
- FreixaLNoguesNIbanezMDevelopment and validation of non-invasive approach for fetal HPA-1a genotyping using cell-free fetal DNA present in maternal plasmaVox Sang201099Suppl 222
- SchefferPGde HaasMvan der SchootCENon-invasive fetal blood typing for platelet and red cell antigensVox Sang201099Suppl 211
- Le ToriellecEChenetCKaplanCSafe fetal platelet genotyping: new developmentsTransfusion20135381755176223146000
- TurnerMLBessosHFaggeTProspective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1aTransfusion2005451945195616371049
- SpencerJABurrowsRFFeto-maternal alloimmune thrombocytopenia: a literature review and statistical analysisAust N Z J Obstet Gynaecol200141455511284646
- JocelynLJCasiroOGNeurodevelopmental outcome of term infants with intraventricular hemorrhageAm J Dis Child199214621941971733149
- MaoCGuoJChituwoBMIntraventricular haemorrhage and its prognosis, prevention and treatment in term infantsJ Trop Pediatr199945423724010467837
- TillerHKamphuisMMFlodmarkOFetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: an observational cohort study of 43 cases from an international multicentre registryBMJ Open201333 pii.e002490
- KaplanCDaffosFForestierFMorelMCChesnelNTcherniaGCurrent trends in neonatal alloimmune thrombocytopenia: diagnosis and therapyKaplan-GouetCSchlegelNSalmonCMcGregorJ206th edFranceJohn Libbey Eurotext1991267278
- DelbosFBertrandGCroisilleLAnsart-PirenneHBierlingPKaplanCFetal and neonatal alloimmune thrombocytopenia: predictive factors of intracranial hemorrhageTransfusion20165615966 quiz 5826469867
- WinkelhorstDKamphuisMMde KloetLCZwagingaJJOepkesDLoprioreESevere bleeding complications other than intracranial hemorrhage in neonatal alloimmune thrombocytopenia: a case series and review of the literatureTransfusion20165651230123526996515
- MurphyMFHambleyHNicolaidesKWatersAHSevere fetomaternal alloimmune thrombocytopenia presenting with fetal hydrocephalusPrenat Diagn19961612115211558994253
- LiCPiranSChenPMaternal immune response to fetal platelet GPIbalpha causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapiesJ Clin Invest2011121114537454722019589
- NiHChenPSpringCMA novel murine model of fetal and neonatal alloimmune thrombocytopenia: response to intravenous IgG therapyBlood200610772976298316317099
- Issaka YougbaréW-STHeyuNiProlonged retention and activation of natural killer cells in the placenta cause miscarriage in fetal and neonatal alloimmune thrombocytopeniaEur Symp Granul Platelet Immunobiol20162399928
- BarkerDJThe developmental origins of well-beingPhilos Trans R Soc Lond B Biol Sci200435914491359136615347527
- ClaytonPECianfaraniSCzernichowPJohannssonGRapaportRRogolAManagement of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research SocietyJ Clin Endocrinol Metab200792380481017200164
- HackMFlanneryDJSchluchterMCartarLBorawskiEKleinNOutcomes in young adulthood for very-low-birth-weight infantsN Engl J Med2002346314915711796848
- TillerHKjaerKMHusebekkAPlatelet antibodies and fetal growth: maternal antibodies against fetal platelet antigen 1a are strongly associated with reduced birthweight in boysActa Obstetr Gynecol Scand2011917986
- DahlJHusebekkAAcharyaGMaternal anti-HLA class I antibodies are associated with reduced birth weight in thrombocytopenic neonatesJ Reprod Immunol2016113273426547815
- EksteenMTillerHAverinaMCharacterization of a human platelet antigen-1a-specific monoclonal antibody derived from a B cell from a woman alloimmunized in pregnancyJ Immunol2015194125751576025972474
- VanderpuyeOALabarrereCAMcIntyreJAA vitronectin-receptor-related molecule in human placental brush border membranesBiochem J1991280Pt 19171720617
- ZhouYFisherSJJanatpourMHuman cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion?J Clin Invest1997999213921519151786
- Kabir-SalmaniMShiokawaSAkimotoYAlphavbeta3 integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migrationEndocrinology200314441620163012639947
- BertrandGMartageixCJalluVVitryFKaplanCPredictive value of sequential maternal anti-HPA-1a antibody concentrations for the severity of fetal alloimmune thrombocytopeniaJ Thromb Haemost20064362863716460445
- JaegtvikSHusebekkAAuneBOianPDahlLBSkogenBNeonatal alloimmune thrombocytopenia due to anti-HPA 1a antibodies; the level of maternal antibodies predicts the severity of thrombocytopenia in the newbornBJOG200010769169410826588
- KillieMKHusebekkAKjeldsen-KraghJSkogenBA prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newbornHaematologica200893687087718443267
- BessosHTurnerMUrbaniakSJIs there a relationship between anti-HPA-1a concentration and severity of neonatal alloimmune thrombocytopenia?Immunohematol200521102109
- GhevaertCCampbellKStaffordPHPA-1a antibody potency and bioactivity do not predict severity of fetomaternal alloimmune thrombocytopeniaTransfusion2007471296130517581167
- BessosHKillieMKSeghatchianJSkogenBUrbaniakSJThe relationship of anti-HPA-1a amount to severity of neonatal alloimmune thrombocytopenia – where does it stand?Transfus Apher Sci2009402757819223235
- BertrandGDrameMMartageixCKaplanCPrediction of the fetal status in noninvasive management of alloimmune thrombocytopeniaBlood2011117113209321321239703
- Kjeldsen-KraghJHusebekkAKillieMKSkogenBThe pathophysiology of FNAIT cannot be deduced from highly selected retrospective dataBlood201111892638263921885616
- YougbareILangSYangHMaternal anti-platelet beta3 integrins impair angiogenesis and cause intracranial hemorrhageJ Clin Invest201512541545155625774504
- SantosoSWihadmadyatamiHBakchoulTAntiendothelial alphavbeta3 antibodies are a major cause of intracranial bleeding in fetal/neonatal alloimmune thrombocytopeniaArterioscl Thromb Vasc Biol20163681517152427283740
- SonneveldMENatunenSSainioSGlycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopeniaBr J Haematol2016174231032027017954
- KapurRKustiawanIVestrheimAA prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancyBlood2014123447148024243971
- BusselJDiagnosis and management of the fetus and neonate with alloimmune thrombocytopeniaJ Thromb Haemost20097Suppl 125325719630811
- KanhaiHHPorcelijnLEngelfrietCPManagement of alloimmune thrombocytopeniaVox Sang200793437038518070283
- TillerHHusebekkASkogenBKjeldsen-KraghJKjaerMTrue risk of fetal/neonatal alloimmune thrombocytopenia in subsequent pregnancies: a prospective observational follow-up studyBJOG2016123573874425752647
- VinogradCABusselJBAntenatal treatment of fetal alloimmune thrombocytopenia: a current perspectiveHaematologica201095111807181121037327
- BusselJBBerkowitzRLMcFarlandJGLynchLChitkaraUAntenatal treatment of neonatal alloimmune thrombocytopeniaN Engl J Med198831921137413783141811
- BusselJBBerkowitzRLLynchLAntenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin: a randomized trial of the addition of low-dose steroid to intravenous gamma-globulinAm J Obstet Gynecol199617414141423
- RaymentRBrunskillSJSoothillPWRobertsDJBusselJBMurphyMFAntenatal interventions for fetomaternal alloimmune thrombocytopeniaCochrane Database Syst Rev20115CD004226
- PaidasMJBerkowitzRLLynchLAlloimmune thrombocytopenia: fetal and neonatal losses related to cordocentesisAm J Obstet Gynecol19951722 Pt 14754797856672
- OvertonTGDuncanKRJollyMLetskyEFiskNMSerial aggressive platelet transfusion for fetal alloimmune thrombocytopenia: platelet dynamics and perinatal outcomeAm J Obstet Gynecol2002186482683111967515
- LakkarajaMBerkowitzRLVinogradCAOmission of fetal sampling in treatment of subsequent pregnancies in fetal-neonatal alloimmune thrombocytopeniaAm J Obstet Gynecol20162154471.e1471.e927131591
- AltarescuGEldar-GevaTGrisaru-GranovskySPreimplantation genetic diagnosis for fetal neonatal alloimmune thrombocytopenia due to antihuman platelet antigen maternal antibodiesObstet Gynecol20121192 Pt 133834322270286
- TillerHFedorcsakPSkogenBROld tools revisited give hope – new treatment option for families with a history of severe FNAIT complicationsActa Obstet Gynecol Scand201695448648726669518
- CurtisBRBusselJBManco-JohnsonMJAsterRHMcFarlandJGFetal and neonatal alloimmune thrombocytopenia in pregnancies involving in vitro fertilization: a report of four casesAm J Obstet Gynecol2005192254354715696000
- RaymentRBrunskillSJStanworthSSoothillPWRobertsDJMurphyMFAntenatal interventions for fetomaternal alloimmune thrombocytopeniaCochrane Database Syst Rev20051CD004226
- van denAEOepkesDBrandAKanhaiHHVaginal delivery for fetuses at risk of alloimmune thrombocytopenia?BJOG2006113778178316827760
- BasslerDGreinacherAOkascharoenCA systematic review and survey of the management of unexpected neonatal alloimmune thrombocytopeniaTransfusion2008481929817894790
- RobertsIStanworthSMurrayNAThrombocytopenia in the neonateBlood Rev200822417318618433954
- AllenDVerjeeSReesSMurphyMFRobertsDJPlatelet transfusion in neonatal alloimmune thrombocytopeniaBlood2007109138838917190858
- te PasABLoprioreEvan den AkkerESPostnatal management of fetal and neonatal alloimmune thrombocytopenia: the role of matched platelet transfusion and IVIGEur J Pediatr2007166101057106317177068
- BlanchetteVSJohnsonJRandMThe management of alloimmune neonatal thrombocytopeniaBaillieres Best Pract Res Clin Haematol20001336539011030040
- WoodrowJCClarkeCADonohoeWTPrevention of Rh-haemolytic disease: a third reportBr Med J19651543027928314229706
- ZipurskyAIsraelsLGThe pathogenesis and prevention of Rh immunizationCan Med Assoc J19679721124512574168032
- TillerHKillieMKChenPToward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine modelTransfusion20125271446145722251227
- WeinerHLda CunhaAPQuintanaFWuHOral toleranceImmunol Rev2011241124125921488901
- KillieMKKjeldsen-KraghJHusebekkASkogenBOlsenJAKristiansenISCost-effectiveness of antenatal screening for neonatal alloimmune thrombocytopeniaBJOG200711458859517355359
- Durand-ZaleskiISchlegelNBlum-BoisgardCUzanSDreyfusMKaplanCScreening primiparous women and newborns for fetal/neonatal alloimmune thrombocytopenia: a prospective comparison of effectiveness and costs. Immune Thrombocytopenia Working GroupAm J Perinatol1996134234318960612
- GafniABlanchetteVSScreening for neonatal alloimmune thrombocytopenia: an economic perspectiveCurr Stud Hematol Blood Transfus1988541401473349817
- TillerHKillieMKSkogenBOianPHusebekkANeonatal alloimmune thrombocytopenia in Norway: poor detection rate with non-screening versus a general screening programmeBJOG2009116459459819250370
- AndermannABlancquaertIBeauchampSDeryVRevisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 yearsBull World Health Organ200886431731918438522
- SkogenBKillieMKKjeldsen-KraghJReconsidering fetal and neonatal alloimmune thrombocytopenia with a focus on screening and preventionExpert Rev Hematol20103555956621083473
- Kjeldsen-KraghJHusebekkAKillieMKSkogenBIs it time to include screening for neonatal alloimmune thrombocytopenia in the general antenatal health care programme?Transfus Apher Sci200838318318818499524
- HeierHEBergeLNHervigTImmunisering i svangerskapet. [Immunization during pregnancy]Tidsskr Nor Laegeforen2009129192016201819823209