Abstract
The current “manufacturing paradigm” of transfusion practice has detached transfusion from the clinical environment. As an example, fresh whole blood in large-volume hemorrhage may be superior to whole blood reconstituted from multiple components. Multicomponent apheresis can overcome logistical difficulties in matching patient needs with fresh component availability and can deliver the benefits of fresh whole blood. Because of the different transfusion needs of patients in emerging economies and the vulnerability of these blood systems to emerging infections, fresh whole blood and multicomponent apheresis can better meet patient needs when compared with transplants of the “manufacturing paradigm”. We propose that patient blood management, along with panels of repeat, paid, accredited apheresis and fresh whole-blood donors can be used in emerging economies to support decentralized blood services. This alternative transfusion–medicine paradigm could eventually also be adopted by established economies to focus transfusion medicine on local patient needs and to alleviate the problem of the aging volunteer donor base.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction – blood transfusion’s first paradigm
The inception of blood transfusion lies in the ancient recognition that acute blood loss can be fatal.Citation1 By World War I, developments in blood grouping and preservation allowed the establishment of the first “blood bank”.Citation2 This facilitated the logistics of blood delivery. Blood transfusion continued to develop over a succession of conflicts, stimulating the development of plasma fractionation and the introduction of blood bags.
If transfusion is seen as a series of paradigms,Citation3 the transfusion paradigm by the early 1960s involved the collection and transfusion of whole blood, with a focus on immunologic compatibility. In this “pre-evidence-based medicine” era, transfusion was primarily through whole blood and geared to assist surgery.
The current transfusion paradigm in rich economies
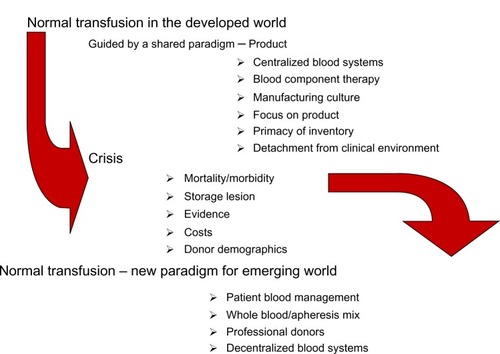
Transfusion may be viewed as a series of paradigms, reflecting changes in technology and clinical practice.Citation4,Citation5 We have previously suggestedCitation6 () that the current transfusion paradigm is a “product” or “manufacturing” paradigm shaped by several determining factors.
Inventory influence
Where blood systems have been delivered through governments, policies of national self-sufficiency in blood-derived therapies have been the norm. The treatment of hemophilia A with plasma products drove these countries to increase blood collection with recovered plasma as the driver. In the US, a plasma industry separate from the blood collection sector ensured that plasma recovered as a by-product of whole blood collection was not a primary shaper of blood collection. Rather, the separation of plasma from red cells was influenced by the development of red cell additive solutions to increase red cell shelf life. In both systems, a primacy on output and inventory became a shaper of transfusion practice.
Regulatory pressures
The blood sector in the rich economies is strongly regulated, primarily because of historical issues relating to blood-borne pathogens. This has contributed to quality management in transfusion activities, with attention focused on product rather than patient. The parameters used to qualify components for transfusion have limited relevance to their clinical efficacy, furthering the detachment of product characteristics from patient needs. Similar issues permeate proposals to extend platelet shelf life.
Transfusion industry
Transfusion in 2013 has become the object of the medical subspecialty of “transfusion medicine”. It is a huge, multibillion dollar industry that delivers products in the developed countries through organizations which, in multisupplier systems such as in the US, compete vigorously for hospital blood needs.Citation7 The blood market in the US alone was estimated at $9.5 billion in 2012.Citation8 summarizes our synthesis of the shapers of the current transfusion paradigm.
Current paradigm – consequences and tensions
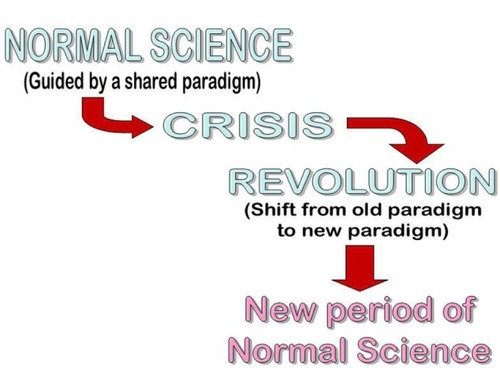
The evolution of transfusion medicine has strengthened greatly the transfusion industry, while contributing to a detachment from the clinical environment. Any research is well integrated into the current paradigm, and would be classified as “normal transfusion” in Kuhnian termsCitation3 (), seldom questioning the tenets of the current paradigm until its replacement by a crisis. Several of these tenets () have converted “blood transfusion” to “stored blood component” transfusion.
The primary thrust of the “inventory” influence has been to maximize the resource through the practical logic of one donation serving many patients. The need to minimize transfusion-associated circulatory overload and transfusion-related acute lung injury (TRALI) is cited in support of the appropriateness of red cell versus whole blood transfusion. However, in massive transfusion, the inventory-driven argument regarding the appropriateness of “one donation–many patients” leads to exposure to many more donors than would occur when transfusing fresh whole blood. Additionally, evidence is lacking as to whether splitting blood donations into multiple components enhances inventory, or whether red cell delivery is physiologically optimal through transfusion of plasma-depleted additive-suspended erythrocytes.
Outcomes in patients experiencing large blood loss
In obstetric hemorrhageCitation9 and trauma,Citation10 morbidity and mortality are improved when transfusing fresh whole blood rather than stored components or reconstituted whole blood.Citation11 Transfusion requirements of patients with acute, severe hemorrhage are thus best met by fresh whole blood with a hemostatically intact profile. Three percent to 8% of trauma patientsCitation10 and 2%–3% of patients with obstetric hemorrhageCitation9 qualify for fresh whole-blood transfusions, but require large amounts of blood, so that 15% of erythrocytes used in the US are for treating injury.Citation12 Like acute, severe hemorrhage, other indications for transfusion may be subject to similar revision of the most appropriate blood product. In cardiac surgery, for example, one unit of fresh whole blood has been reported to be hemostatically equivalent to six platelet units,Citation13 a finding which is likely linked to hemostatic properties additional to platelets, as survival in combat casualties did not differ between different platelet delivery methods.Citation14 Our understanding in this area is hampered by the lack of a definition of what constitutes “fresh blood”. We propose the definition used by the US Defense Medical Training Institute be considered.Citation15 A significant factor in the provision of fresh blood, as defined here, is the possible transmission of viral pathogens, which currently is minimized through screening tests, but may be logistically difficult to achieve in the prescribed time frame. In the military setting, where most of the experience regarding this area has accrued, the relative risk between screened and unscreened blood has been found to be insignificant when using accredited “walk-in” donors,Citation16,Citation17 and is minimized further through rapid tests. As suggested by Spinella et al,Citation16 a comprehensive risk assessment comparing current infectious disease risks to the risk of using stored blood, rather than an unquestioned acceptance of historical concepts, is needed as an aid to decision making.
Blood transfusion in surgery
Transfusion practice in similar established economies is highly variable,Citation18 lacks evidence,Citation19 and is independently associated with adverse events in cancer surgery,Citation20 cardiac surgery,Citation21 and noncardiac surgery;Citation22 although, causality as historically defined remains to be established in most settings.Citation23 The possible harm from stored erythrocytes has focused attention on the loss of nitric oxide during red cell storage,Citation24 leading to proposed new principles for the qualification of transfused erythrocytes, which have been met with reservations from the transfusion community.Citation25 This issue is currently being addressed in a number of randomized controlled trials,Citation26 and continues to yield controversial results.Citation27 Large prospective studiesCitation28 and a recent meta-analysis,Citation29 combined with the demonstrated abnormalities of stored erythrocytes, should suffice to justify a precautionary approach such as has underpinned much of transfusion practice in the past 20 years: based on a Popperian hypothesis, stored erythrocytes are harmful until shown not to be so. Such precautionary ideology continues to guide policy regarding infectious disease risks, which are in many environments, small compared to some of the estimated morbidities from stored red cells. Further specification of what constitutes “storage” in this issue would be helpful in assessing the data. We propose consideration of the large prospective study of Pettilä et al,Citation28 which allows a period of 8 days as the threshold above which red cells are stored with harmful effects on patients. This period is consistent to that found in the most recent review of studies detecting an effect associated with red cell storage period in critically ill patients.Citation30 While the issue is still under review, it may be prudent to direct inventory-driven efforts to continue to improve and prolong red cell storage and develop tools to optimize the available inventories.Citation31
Other components – platelets
Efforts continue to increase the supply of platelets through extending their shelf life. Recent years have seen the introduction of bacterial culture. The US Food and Drug Administration’s (FDA) Blood Products Advisory Committee has recommended that platelets also be subjected to a rapid release test after 4 days of storage,Citation32 while rejecting industry overtures to allow a shelf life of 7 days with a negative test. An inventory-driven approach to improving platelet logistics appears detached from product efficacy and safety matters given that the in vivo properties of platelets stored for 7 versus 5 days deteriorate significantly.Citation33 Clinical implications of platelet storage lesion have been reported from the traumaCitation34 and liver-transplantCitation35 settings.
Because of these risks, interventions aimed at reducing platelet transfusions have included reducing the threshold triggering prophylactic transfusion, administering low-dose platelet transfusions, and administering therapeutic (as opposed to prophylactic) platelet transfusions in specific clinical circumstances,Citation36–Citation39 with due recognition for the need of further studies.Citation40 All these interventions deliver good outcomes if done in the context of intensive patient blood management (PBM).
The continued use of whole blood-derived platelets, which expose a patient to multiple donors to confer the same benefit as a single-donor unit (apheresis), also bears scrutiny. Apheresis platelets can reduce the risk of both transfusion-transmitted infections and TRALI.Citation41,Citation42
The resources currently focused on the maintenance of an inventory may, as with erythrocytes, be better allocated to a more clinically focused strategy involving transfusion of fewer, fresher platelets. As in other areas, definitional problems underlie the concept of fresh platelets. We propose, for comparative purposes, the definition offered by the FDA, ie, platelets drawn, prepared, and transfused on the same day,Citation43 recognizing that practical considerations limit the capacity of any system to deliver such products, but wishing to accentuate the reality that platelet deterioration is progressive from the inception of storage.
Fresh frozen plasma transfusion
Evidence for the use of fresh frozen plasma (FFP) is limited to the supplementation of stored components in massively transfused trauma victimsCitation44 and thrombotic thrombocytopenic purpura.Citation45 Despite this, the widespread use of FFP continues through various poorly-evidenced guidelines. The assumed role of coagulation factors in thawed FFP has prompted studies on the quality of readily thawed refrigerated FFP, which is convenient logistically when addressing emergency transfusion.Citation46 This overlooks the additional vascular protective effects conferred by freshly-thawed FFP,Citation47,Citation48 further exemplifying the product versus patient paradigm.
Plasma supplementation of massively-transfused patients was found to be unnecessary in a study conducted before the introduction of automated plasma removalCitation49 and depletion of the key protein, fibrinogen. FFP’s role in massive transfusion may be addressed through the use of fresh whole blood instead of reconstituted whole blood from several donors. The repeated demonstration of lack of benefit from FFP transfusion should act as further incentive for more trials employing whole blood.
Transfusion in emerging economies: is a transplant the best option?
For the purpose of this discussion we have classified countries as low income (LIC), middle income, and high income (HIC) as defined by the World Bank.Citation50 The World Health Organization (WHO) and donor agencies appear committed to instituting the Western paradigm as the route of choice in the emerging, resource-poor world, emphasizing the need for centralized blood systems, good-manufacturing-practice, and component therapy in sub-Saharan Africa.Citation51 A significant example is the US President’s Emergency Plan for AIDS Relief.Citation52 These donor agencies must take into account qualitative differences in blood usage. The majority of recipients in emerging countries are younger and more likely to be women and children.Citation53 Transfusion most frequently occurs because of acute blood loss from injury and obstetric hemorrhage, which are best treated with fresh whole blood, and for childhood anemia due to malaria.Citation54 The WHO recommends whole blood for malaria,Citation55 in which metabolic acidosis and hypovolemiaCitation56 may be ameliorated by the fresh plasma portion.
The development of transfusion-associated circulatory overload in this patient demographic should not be presumed, because this transfusion complication occurs mostly in elderly patients.Citation57 Similarly, an increase in TRALI is not inevitable, since none was found in otherwise healthy battlefield casualties given whole blood,Citation11 while nonimmune mediated TRALI may be accentuated by stored components.Citation58
The particular profile for blood use in these countries is reflective of their current economies. While we recognize the formidable problems which currently apply to transfusion practice in LIC,Citation54,Citation59 we suggest that an opportunity may exist for the emerging world to bypass the western paradigm. The analogy with telephone systems, where the lack of landlines spurred the development and widespread provision of mobile phone technology, bears reflection.Citation60
A new paradigm – the patient first
PBM identifies a patient at risk for transfusion and formulates a multidisciplinary and multimodal, yet individualized, plan for reducing or eliminating the need for allogeneic transfusion. PBM integrates many hospital departments in a common effort to reduce allogeneic-donor exposures as much as possible, and thereby reduce both the infectious and immunologic risks of transfusion.Citation61,Citation62 Its traction is currently exerted mainly with elective surgical procedures in the orthopedic, cardiac, and transplant areas, which occupy a high proportion of blood use in the rich but not in the emerging economies. PBM has led to sustained decreases in blood usage, with conflicting results in relation to conventional pharmacoeconomics, eg, in pharmacologic stimulation of red cell production.Citation63,Citation64 Pharmacoeconomic analyses demonstrate the lack of conventionally measured cost-effectiveness of most of the blood safety measures in place in the developed world,Citation65 in contrast to many PBM interventions.Citation66 PBM has been embraced by the WHO,Citation67 and it presents an essential component of any emerging transfusion paradigm.
Which products?
As discussed, the primary blood product which currently best satisfies the needs for most aspects of transfusion in LIC is fresh whole blood. The primary impediment to its provision is the qualification of the viral status of donations, which may be particularly impeded in small-throughput operations, as would ensue if collection is decentralized (see below). This could be addressed through the formation of panels of repeat, accredited donors under contract, with a low risk profile assured through appropriate selection and testing, allowing for the use of blood not subject to long-term storage and preceding the completion of viral tests. Rapid testing technology may also be used.
The safety may also eventually be enhanced through pathogen reduction technology, which has been successfully employed to produce hemostatically intact whole blood and is currently under trial.Citation68 External programs for blood systems development in resource-poor economies should monitor the evolution of these emerging technologies.
As the profile of clinical need evolves, other needs will be encountered, including red cell concentrates for the alleviation of normovolemic anemia and platelets for hypoproliferative thrombocytopenia. Standardized protocols for these products and a quality system for their manufacture can be implemented within a hospital environment, without detaching the provision from the clinical interface. Gearing the production more closely to specific patient needs will transition these products from the manufacturing to the medical environment, detaching from mainstream pharmaceutical oversight to hospital accreditation. It will also allow specification of products to match more closely actual clinical needs, rather than antiquated and irrelevant requirements introduced into pharmacopeial type specifications, such as the current and largely irrelevant requirements of FFP and cryoprecipitate, which are specified for hemophilia A treatment. Continuing developments may modify the usage profile for these therapies, while continuing evidence of the storage lesion may make delivery of fresh products important. Further measures to increase safety may include minimizing donor exposures through the use of products obtained through multicomponent apheresis donations.Citation42
Multicomponent-apheresis collection has enhanced capacity to provide inventory flexibility and sufficiencyCitation69 through decentralized collection geared to specific patient needs, while also maximizing the availability of fresh cellular components.Citation70 We recognize that the current high cost of the relevant disposables is a concern, as is its dependence on a regular and reliable electric power supply. We propose that, as infrastructural improvement gains pace in LIC to resolve some of these issues, the cost-effectiveness of multicomponent-apheresis should be considered.Citation71,Citation72 As the LIC evolve toward a transfusion demographic closer to that of HIC, with an aged transfused population of predominantly surgical patientsCitation73 and a predictable requirement for cellular components for hematology–oncology patients, the establishment of a multicomponent apheresis supply, along with the provision of fresh whole blood, would address all transfusion needs.
Blood donation in a new paradigm
The blood donor base in the established economies is being diminished through demographic changes threatening the blood supply.Citation74–Citation76 Concurrently, PBM has seen a decline in blood usage in several HIC environments.Citation77 The current donor population in these HIC environments comprises the “baby-boomer” and “generation X” groups,Citation78 which have different values compared to the “generation Y” and “generation Z” groups from which the donor base will have to be increasingly drawn. These values include those expected to be less conducive to volunteerism.Citation79
The proposal of TomasuloCitation80 to establish panels of committed paid professional donors to ensure safe donors focused on patient outcomes merits consideration. Such a system based on strict contractual arrangements between donor and blood agency may conform better to safety and quality principles. The nonusage of the first donation may be considered, analogous to that applied for paid plasma donors.Citation81 Recalling that apheresis collection may be central to a new paradigm,Citation42 and that the experience of the plasma industry demonstrates that plasmapheresis donors require payment,Citation82 an approach incorporating committed paid apheresis donors may contribute to addressing the problems emerging from the demographic changes outlined above. The experience of plasma collection has shown that the required commitment to regular apheresis cannot be met through unpaid donors, and donor payment is compatible with a safe and regular supply,Citation83–Citation85 although we emphasize that is not an essential feature of a new paradigm as long as patient needs are met. The construction of a panel of committed, healthy repeat donors for the provision of a whole-blood and apheresis-based supply requires more attention devoted to matching donor availability to the clinical needs of patients than has been possible in the operation of traditional blood agencies. The provision of free, long-term medical treatment for mainstream blood donors by the Malaysian blood service, which is a strong supporter of the WHO-sponsored unpaid donor policy, may bear consideration.Citation86 This approach can also form the basis for a new donor recruitment/retention paradigm for apheresis donors. The cost-effectiveness and long-term viability of such a system should be analyzed relative to the current paradigm, and a multifaceted system may be geared to specific patient demographics and needs.
“Catch-all” blood drives driven by inventory considerations rarely generate more than an average of two donations per donor annually. An apheresis-based system can be carefully geared to a planned clinical transfusion program as is possible for much of elective surgery and many medical indications, more appropriately matching to clinical needs as is already done through the provision of human leukocyte antigen-matched apheresis platelets.
The blood center in the new paradigm
In HIC, centralized blood collection and processing centers supplying to remote clinical units are the predominant transfusion system. There are merits in both centralized and decentralized systems in these countries.Citation87,Citation88 In LIC such as sub-Saharan Africa, centralized systems initially introduced by the colonial powers were replaced by decentralized services delivering good transfusion outcomes at lower costs.Citation89–Citation91 Subsequently, economic pressures leading to the need for external aid have resulted in a surge toward centralizing blood systems as a condition of such aid.Citation51 This pressure is often premised on the minimization of human immunodeficiency virus and other infectious disease transmission through such centralization. There is no evidence that centralization in blood services reduced transfusion-transmitted acquired immunodeficiency syndrome, or that it impeded the penetration of West Nile virus and variant Creutzfeldt-Jakob disease in more recent times. The global transfusion landscape continues to be vulnerable to the threat of infectious diseases. Any benefits from centralization are therefore detached from the issue of blood safety. The possible benefits need to be weighed against the increased costs and, in particular, to the inevitable detachment of blood service from patient care, which is a feature of most HIC systems.
The maintenance of adequate inventories must be more inventively geared to patient needs. Expertise in linear programming will assist blood centers to optimize allocation.Citation92 Similarly, a multidisciplinary effort involving the whole range of stakeholders in platelet delivery can reduce outdate ratios as well as decrease platelet shelf life,Citation93 while integrating patient history and engaging knowledgeable staff can limit platelet outdating to 1% of inventory.Citation94
Such techniques will enable inventory management to become subservient to clinical need, rather than, as in the current paradigm, threatening to allow inventory management to dominate and determine clinical need.
The ongoing debate regarding the possible association of mortality and morbidity may require novel approaches linking red cell shelf life to patient category, allowing the limitation of shelf life to 7 days for the penalty of a 3.2% increase in the outdate rate.Citation95 Efforts to introduce these kinds of measures depend entirely on the closer integration of blood delivery and clinical areas, which is difficult to achieve through remote blood centers.
Conclusion
Our purpose in this work was to show how much of what is regarded as infallible dogma in the mainstream transfusion medicine community rests on limited empirical evidence. We have developed concepts in parallel, that have been published elsewhere,Citation6,Citation42 on the application of the thinking of Kuhn and Popper to the field of transfusion medicine. We believe that the concepts we have further developed here may prove useful in understanding the current state of our field and its contribution to patient care in emerging economies. We propose the development of a new transfusion paradigm for the emerging world and its eventual adoption by the rich economies through a process of Kuhnian revolution (). A feature of such a process is the incommensurability between successive paradigms. While the enhanced appreciation of product quality and standardized manufacture of the current paradigm are desirable and retainable features, the detachment from patient-centeredness renders the transition to the new paradigm as dramatic as Kuhn described.Citation96 As patient-centeredness becomes more important in health care, our field should be foremost in adopting its principles.
Acknowledgments
We thank Professor Jean Pierre Allain for guidance and mentorship, particularly on issues of rapid viral testing. Any opinions and conclusions are our own.
Disclosure
The authors report no conflicts of interest in this work.
References
- DzikWHThe James Blundell Award Lecture 2006: transfusion and the treatment of haemorrhage: past, present and futureTransfus Med200717536737417903138
- RobertsonOHTransfusion with preserved red blood cellsBr Med J19181299969169520769077
- StemwedelJanetKuhn: paradigms and normal science [webpage on the Internet]Slideshare2010 Available from: http://www.slideshare.net/docfreeride/kuhn-paradigms-and-normal-scienceAccessed January 5, 2013
- IsbisterJPThe paradigm shift in blood transfusionMed J Aust198814863063083347185
- BlumbergNHealJmTransfusion and the immune system: a paradigm shift in progress?Transfusion199535108798837570922
- FarrugiaAFalsification or paradigm shift? Toward a revision of the common sense of transfusionTransfusion201151121622420723172
- BruceDErie blood bank battles economy, competition [webpage on the Internet]GoErie2010 Available from: http://www.goerie.com/apps/pbcs.dll/article?AID=/20100722/NEWS02/307229932Accessed January 4, 2013
- The blood industry – a market research report (synopsis) [webpage on the Internet]BCC Research2011 Available from: http://www.bccresearch.com/report/the-blood-industry-hlc008g.htmlAccessed January 4, 2013
- AlexanderJMSarodeRMcIntireDDBurnerJDLevenoKJWhole blood in the management of hypovolemia due to obstetric hemorrhageObstet Gynecol200911361320132619461429
- RepineTBPerkinsJGKauvarDSBlackborneLThe use of fresh whole blood in massive transfusionJ Trauma200660Suppl 6S59S6916763483
- SpinellaPCWarm fresh whole blood transfusion for severe hemorrhage: US military and potential civilian applicationsCrit Care Med200836Suppl 7S340S34518594261
- ComoJJDuttonRPScaleaTMEdelmanBBHessJRBlood transfusion rates in the care of acute traumaTransfusion200444680981315157244
- LaveeJMartinowitzUMohrRThe effect of transfusion of fresh whole blood versus platelet concentrates after cardiac operations. A scanning electron microscope study of platelet aggregation on extracellular matrixJ Thorac Cardiovasc Surg19899722042122915556
- PerkinsJGCapAPSpinellaPC31st Combat Support Hospital Research GroupComparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME)Transfusion201151224225220796254
- Defense Medical Readiness Training Institutedmrti.army.mil [homepage on the Internet]Fresh warm whole blood (FWB) – guideline2008 Available from: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&ved=0CDgQFjAC&url=http%3A%2F%2Fwww.dmrti.army.mil%2Fdocuments%2F2008%252005%2520CPG%2520Fresh%2520Warm%2520Whole%2520Blood.doc&ei=i_B4Us37K-iO7Qb1tYGYBw&usg=AFQjCNF8yntbhlFNWW11Sn9rie4TIBa7MAAccessed November 5, 2013
- SpinellaPCPerkinsJGGrathwohlKW31st Combat Support Hospital Research Working GroupRisks associated with fresh whole blood and red blood cell transfusions in a combat support hospitalCrit Care Med200735112576258117828033
- Walk-in blood bank [webpage on the Internet]Australian Government, Department of Defence News2011 Available from: http://www.defence.gov.au/defencenews/stories/2011/may/0503.htmAccessed December 8, 2013
- Use of blood products for elective surgery in 43 European hospitals. The Sanguis Study GroupTransfus Med1994442512687889138
- WilkinsonKLBrunskillSJDoréeCThe clinical effects of red blood cell transfusions: an overview of the randomized controlled trials evidence baseTransfus Med Rev2011252145155. e221345644
- AchesonAGBrookesMJSpahnDREffects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysisAnn Surg2012256223524422791100
- BhaskarBDulhuntyJMullanyDVFraserJFImpact of blood product transfusion on short and long-term survival after cardiac surgery: more evidenceAnn Thorac Surg201294246046722626751
- GlanceLGDickAWMukamelDBAssociation between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgeryAnesthesiology2011114228329221239971
- VamvakasECEstablishing causation in transfusion medicine and related tribulationsTransfus Med Rev2011252818821345640
- BonaventuraJClinical implications of the loss of vasoactive nitric oxide during red blood cell storageProc Natl Acad Sci U S A200710449191651916618048331
- HessJRBiomedical Excellence for Safer Transfusion (BEST) CollaborativeScientific problems in the regulation of red blood cell productsTransfusion20125281827183522229278
- SloanSRSteinerMEStowellCPAssmannSFDelaneyMTriulziDCurrent randomized clinical trials of red cell storage duration and patient outcomesCrit Care Med201240102927 author reply 2927–292822986676
- MiddelburgRAvan de WateringLMGBriëtEvan der BomJGStorage time of red blood cells and mortality of transfusion recipientsTransfus Med Rev2013271364322902231
- PettiläVWestbrookAJNicholADBlood Observational Study Investigators for ANZICS Clinical Trials GroupAge of red blood cells and mortality in the critically illCrit Care2011152R11621496231
- WangDSunJSolomonSBKleinHGNatansonCTransfusion of older stored blood and risk of death: a meta-analysisTransfusion20125261184119522188419
- AubronCNicholACooperDJBellomoRAge of red blood cells and transfusion in critically ill patientsAnn Intensive Care201331223316800
- BlakeJTHardyMDelageGMyhalGDéjà-vu all over again: using simulation to evaluate the impact of shorter shelf life for red blood cells at Héma-QuébecTransfusion20135371544155823145802
- Food and Drug Administration104th Meeting of the Blood Products Advisory CommitteeSilver Spring, MDFood and Drug Administration2012 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM325690.pdfAccessed December 27, 2012
- DumontLJAuBuchonJPWhitleyPSeven-day storage of single-donor platelets: recovery and survival in an autologous transfusion studyTransfusion200242784785412375656
- InabaKBrancoBCRheePImpact of the duration of platelet storage in critically ill trauma patientsJ Trauma201171617661773 discussion 1773–177422182887
- PereboomITde BoerMTHaagsmaEBHendriksHGDLismanTPorteRJPlatelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injuryAnesth Analg200910841083109119299765
- FayedNARoshdyAAKhalilMKMarwanIKTherapeutic rather than prophylactic platelet transfusion policy for severe thrombocytopenia during liver transplantationPlatelets Epub11182013
- CidJLozanoMPlatelet dose for prophylactic platelet transfusionsExpert Rev Hematol20103439740021083030
- HeddleNMCookRJTinmouthASToP Study Investigators of the BEST CollaborativeA randomized controlled trial comparing standard- and low-dose strategies for transfusion of platelets (SToP) to patients with thrombocytopeniaBlood200911371564157319109560
- WandtHSchaefer-EckartKWendelinKStudy Alliance Leukemia. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised studyLancet201238098501309131622877506
- StanworthSJEstcourtLJPowterGTOPPS InvestigatorsA no-prophylaxis platelet-transfusion strategy for hematologic cancersN Engl J Med2013368191771178023656642
- VamvakasECRelative safety of pooled whole blood-derived versus single-donor (apheresis) platelets in the United States: a systematic review of disparate risksTransfusion200949122743275819682339
- VamvakasECReasons for moving toward a patient-centric paradigm of clinical transfusion medicine practiceTransfusion201353488890122882177
- Blood Products Advisory CommitteeNew Standard for Platelet EvaluationOffice for Blood Research and Review2004 Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4057b1_03.pdfAccessed December 4, 2013
- GodierASamamaCMSusenSPlasma/platelets/red blood cell ratio in the management of the bleeding traumatized patient: does it matter?Curr Opin Anaesthesiol201225224224722227445
- BrunskillSJTusoldABenjaminSStanworthSJMurphyMFA systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpuraTransfus Med2007171173517266701
- TholpadyAMonsonJRadovancevicRKleinKBraceyAAnalysis of prolonged storage on coagulation Factor (F)V, FVII, and FVIII in thawed plasma: is it time to extend the expiration date beyond 5 days?Transfusion201353364565022803679
- DuanCCaoYDengXIncreased transforming growth factor β contributes to deterioration of refrigerated fresh frozen plasma’s effects in vitro on endothelial cellsShock2011361545921330944
- PatiSMatijevicNDoursoutMFProtective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thawJ Trauma201069Suppl 1S55S6320622621
- CountsRBHaischCSimonTLMaxwellNGHeimbachDMCarricoCJHemostasis in massively transfused trauma patientsAnn Surg197919019199464685
- How we classify countries [webpage on the Internet]The World Bank2013 Available from: http://data.worldbank.org/about/country-classificationsAccessed December 10, 2013
- AlaFAllainJ-PBatesIExternal financial aid to blood transfusion services in sub-Saharan Africa: a need for reflectionPLoS Med201299e100130922984355
- US Department of Health and Human ServicesTechnical assistance support for the strengthening of blood transfusion services in selected countries under the presidents emergency plan for AIDS relief [webpage on the Internet]2012 Available from: http://www.cdcnpin.org/Display/FundDisplay.asp?FundNbr=4509Accessed December 29, 2012
- World Health OrganizationKey Global Fact and Figures in 2011GenevaWorld Health Organization2012 Available from: http://www.who.int/worldblooddonorday/media/who_blood_safety_factsheet_2011.pdfAccessed December 29, 2012
- MarwahaNWhole blood and component use in resource poor settingsBiologicals2010381687120074978
- World Health OrganizationGuidelines for the Treatment of Malaria2nd edGenevaWorld Health Organization2010 Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdfAccessed December 29, 2012
- MaitlandKPambaANewtonCRLevinMResponse to volume resuscitation in children with severe malariaPediatr Crit Care Med20034442643114525636
- PopovskyMAAudetAMAndrzejewskiCJrTransfusion-associated circulatory overload in orthopedic surgery patients: a multi-institutional studyImmunohematology1996122878915387748
- SillimanCCBoshkovLKMehdizadehkashiZTransfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factorsBlood2003101245446212393667
- MbanyaDBarriers and enablers to introducing comprehensive patient blood management in the hospitalBiologicals201240320520822316645
- AkerJCMbitiIMMobile phones and economic development in AfricaJ Economic Perspectives2010243207232
- HofmannAFarmerSShanderAFive drivers shifting the paradigm from product-focused transfusion practice to patient blood managementOncologist201116Suppl 331121930829
- ShanderAJavidrooziMPerelmanSPuzioTLobelGFrom bloodless surgery to patient blood managementMt Sinai J Med2012791566522238039
- GoodnoughLTShanderACurrent status of pharmacologic therapies in patient blood managementAnesth Analg20131161153423223098
- BeliaevAMMarshallRJGordonMSmithWWindsorJAClinical benefits and cost-effectiveness of allogeneic red-blood-cell transfusion in severe symptomatic anaemiaVox Sang20121031182422150804
- CusterBHochJSCost-effectiveness analysis: what it really means for transfusion medicine decision makingTransfus Med Rev200923111219056030
- DaviesLBrownTJHaynesSPayneKElliottRAMcCollumCCost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic modelHealth Technol Assess20061044iiiivix
- World Health OrganizationGlobal Forum for Blood Safety: Patient Blood Management 14–15 March 2011, Dubai, United Arab EmiratesGenevaWorld Health Organization2011 Available from: http://www.who.int/bloodsafety/events/gfbs_01_pbm_concept_paper.pdfAccessed December 29, 2012
- PidcokeHFMcFaulSJRamasubramanianAKPrimary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over timeTransfusion201353Suppl 1137S149S23301966
- PopovskyMAMulticomponent apheresis blood collection in the United States: current status and future directionsTransfus Apher Sci200532329930415944116
- MatthesGThe potential of multicomponent blood donationLa Transfusione Del Sangue2000454173183
- StraussRGEconomy of platelet transfusions from a hospital perspective: pricing predicates practiceTransfusion200141121617162411778081
- MatthesGAOptions and cost effectiveness of multicomponent blood collectionTransfus Apher Sci200227211512112350046
- SeifriedEKlueterHWeidmannCHow much blood is needed?Vox Sang20111001102121175652
- GreinacherAFendrichKAlpenUHoffmannWImpact of demographic changes on the blood supply: Mecklenburg-West Pomerania as a model region for EuropeTransfusion200747339540117319818
- ZouSMusaviFNotariEPFangCTARCNET Research GroupChanging age distribution of the blood donor population in the United StatesTransfusion200848225125718005327
- KatalinicAPetersEBeskeFPritzkuleitRProjection of morbidity 2030 and 2050: impact for the national health system and blood supplyTransfus Med Hemother201037315515920737019
- WilliamsSCPWhat’s behind the decline in blood transfusions?Standford Medicine20132429
- Millennial generation’s non-negotiables: money, fame and image [webpage on the Internet]New York, NYForbes2012 Available from: http://www.forbes.com/sites/alicegwalton/2012/03/19/millennial-generations-non-negotiables-money-fame-and-image/Accessed January 4, 2013
- Do you give blood? [webpage on the Internet]CNN.com2007 Available from: http://www.cnn.com/HEALTH/blogs/paging.dr.gupta/2007/09/do-you-give-blood.htmlAccessed January 4, 2013
- TomasuloPAn outrageous alternative to volunteerismTransfus Med199994359363
- Plasma Protein Therapeutics AssociationInternational Quality Plasma Program Standard for Qualified Donors2006Annapolis, MDPlasma Protein Therapeutics Association Available from: http://www.pptaglobal.org/safety-quality/standards/iqppAccessed January 4, 2013
- FarrugiaACassarJIs self-sufficiency in haemotherapies a practical or necessary goal?Blood Transfus201311218319223245724
- KretschmerVWeippert-KretschmerMSlonkaJKargerRZeilerTPerspectives of paid whole and plasma donationDev Biol (Basel)200512010111116050162
- ZeilerTKretschmerVSurvey of blood donors on the topic of “reimbursement for blood donors”Infusionsther Transfusionsmed19952211924 German7727959
- BuciunieneIStonienëLBlazevicieneAKazlauskaiteRSkudieneVBlood donors’ motivation and attitude to non-remunerated blood donation in LithuaniaBMC Public Health2006616616792814
- Malaysian Blood ServiceKeistimewaan Penderma Darah (Blood Donor Privileges) Available from: http://www.pdn.gov.my/index.php?option=com_content&view=article&id=91%3Akeistimewaan-penderma-darah&catid=34%3Arandom-posts&Itemid=168&lang=enAccessed January 7, 2013 Malay
- HirschRLBrodheimEBlood distribution systems and the exchange of information between hospital blood banks and regional blood centersVox Sang19814032392447245715
- CardenRDelliFraineJLAn examination of blood center structure and hospital customer satisfaction: what can centralized and decentralized blood centers learn from each other?Health Mark Q2005223214216513599
- SchneiderWHHistory of blood transfusion in sub-saharan AfricaTransfus Med Rev2013271212822981696
- BuggeHFKarlsenNCOydnaEA study of blood transfusion services at a district hospital in MalawiVox Sang20131041374522765350
- JacobsBMercerAFeasibility of hospital-based blood banking: a Tanzanian case studyHealth Policy Plan199914435436210787651
- TettehGAOptimal Allocation of Blood Products [dissertation]Newark, NJNew Jersey Institute of Technology2008 Available from: http://archives.njit.edu/vol01/etd/2000s/2008/njit-etd2008-070/njit-etd2008-070.pdfAccessed January 8, 2013
- FontaineMJChungYTRogersWMImproving platelet supply chains through collaborations between blood centers and transfusion servicesTransfusion200949102040204719538430
- FullerAKUglikKMBraineHGKingKEA comprehensive program to minimize platelet outdatingTransfusion20115171469147621303370
- FontaineMJChungYTErhunFGoodnoughLTAge of blood as a limitation for transfusion: potential impact on blood inventory and availabilityTransfusion201050102233223920497519
- OberheimEHoyningen-HuenePThe incommensurability of scientific theories [webpage on the Internet]The Stanford Encyclopedia of Philosophy2013 Available from: http://plato.stanford.edu/archives/spr2013/entries/incommensurability/Accessed November 25, 2013