Abstract
Human mesenchymal stem cells (MSCs) are considered to be a promising source of cells in regenerative medicine. They have large potential to differentiate into various tissue-specific populations and may be isolated from diverse tissues in desired quantities. As cells of potential autologous origin, they allow recipients to avoid the alloantigen responses. They also have the ability to create immunomodulatory microenvironment, and thus help to minimize organ damage caused by the inflammation and cells activated by the immune system. Our knowledge about the reparative, regenerative, and immunomodulatory properties of MSCs is advancing. At present, there is a very comprehensible idea on how MSCs affect the immune system, particularly in relation to the tissue and organ damage on immunological basis. Hitherto a number of effective mechanisms have been described by which MSCs influence the immune responses. These mechanisms include a secretion of soluble bioactive agents, an induction of regulatory T cells, modulation of tolerogenic dendritic cells, as well as induction of anergy and apoptosis. MSCs are thus able to influence both innate and adaptive immune responses. Soluble factors that are released into local microenvironment with their subsequent paracrine effects are keys to the activation. As a result, activated MSCs contribute to the restoration of damaged tissues or organs through various mechanisms facilitating reparative and regenerative processes as well as through immunomodulation itself and differentiation into the cells of the target tissue.
Introduction
Mesenchymal stem cells (MSCs) were originally described as an adherent population of fibroblast-like cells located in the bone marrow which are capable of osteogenic differentiation.Citation1 Subsequently, it was shown that these multipotent cells can be found in all tissues that are capable of regeneration and are located typically in the perivascular niches as pericytes expressing CD146.Citation2 However, not all MSCs can be considered equivalent to pericytes, nor all pericytes have characteristic MSCs properties.Citation3
Nowadays, MSCs are routinely isolated from tissues such as adipose tissue, peripheral blood, umbilical cord, placental membranes, and many others.Citation4–Citation7 These cells have a remarkable ability to proliferate in vitro; hence, it is possible to harvest quickly the required number of cells either for experimental use or for targeted cell therapies. MSCs play a key role in homeostasis maintenance and maturation of hematopoietic cells in the bone marrow.Citation8
MSCs were initially characterized according to their clonogenic potential represented by the ability to create colony-forming units-fibroblasts (CFU-F). The frequency of CFU-F in the bone marrow is around one cell in 104–105 mononuclear cells.Citation9 The MSCs are characterized by the expression of various surface antigens,Citation10 but none of them seem to be expressed exclusively by MSCs. Therefore, the International Society for Cell Therapy issued guidelines by which MSCs are defined on the basis of the following three criteria:Citation11
MSCs have to be adherent to the culture on plastic under standard tissue culture conditions.
MSCs must express surface markers such as CD44, CD71, CD73, CD90, and CD105 but lack the expressions of CD11b, CD14, CD19, CD34, CD45, CD79α, and co-stimulatory molecules CD80 and CD86. They also have to exhibit low expression levels of major histocompatibility complex (MHC) class I.
MSCs must have the ability to differentiate in vitro into osteoblasts, chondrocytes, and adipocytes.
These main characteristics apply in general to the cultured bone marrow-derived MSCs (BMSCs), but some differences seem to be related to the tissue origin. As various phenotype and function studies revealed, MSCs isolated from different sources are not exactly equivalent and represent a highly heterogeneous populations of cells which are dramatically affected by various extrinsic and intrinsic factors.Citation12 Therefore, safety, efficacy, reproducibility of MSCs production, and compliance with Good Manufacturing Practices criteria must be ensured. Hence, the most concerned questions refer to donor eligibility and screening, various types of isolation/enrichment protocols, open cultivation systems versus bioreactors, usage of different reagents, particularly media and supplements with/without animal or human origin, and controls ensuring safety and traceability of the final product. However, some other important issues such as adequate facility criteria, environmental controls, and cell storage concerns should also be addressed.Citation13,Citation14 Thus, in recent years, many efforts have been made to establish optimized protocols for Good Manufacturing Practices compliant preparation of MSCs, develop tools and define markers for their characterization, and describe and influence their differentiation and immunomodulatory potential in vitro and in vivo.
Typical feature of MSCs is their ability to differentiate into many cell types that are not only of mesenchymal origin. However, the process of differentiation requires action of specific growth factors and chemical mediators.Citation8 Besides these, the process of differentiation is affected by many other factors such as density of cells and their arrangement,Citation15–Citation17 tissue origin from which MSCs are isolated,Citation7 donor age,Citation18 stage of the culture and passage,Citation19–Citation21 variety of electrical and mechanical forces involved,Citation16,Citation22–Citation24 and physical properties of a substrate or carrier.Citation24–Citation26
One of the major characteristics of MSCs is that they have migratory abilities,Citation27 that is, after administration, they specifically migrate to the sites of inflammation and tissue damage which is typically associated with cytokine outburstCitation28,Citation29 ().
Figure 1 Mechanisms of MSC homing toward damaged tissue.
Abbreviations: MSC, mesenchymal stem cell; VLA-4, very late antigen-4; VCAM-1, vascular cell adhesion molecule 1; FN, fibronectin; HA, hyaluronic acid; JAM, junction adhesion molecule; MMP-2, matrix metalloproteinase-2; bFGF, basic fibroblast growth factor; SDF-1, stromal cell-derived factor 1; ECM, extracellular matrix.
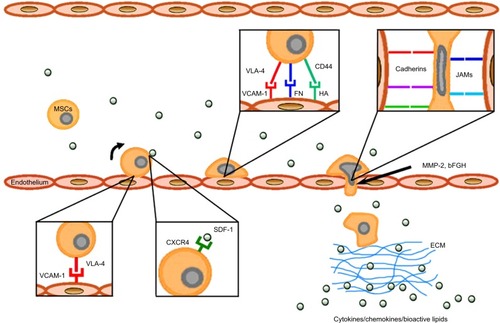
MSCs secrete a wide variety of different growth factors, cytokines, and adhesion molecules by which they affect the microenvironment of the inflamed and degenerating target tissue and thus maintain positive paracrine effect on the tissue repair.Citation30,Citation31 Since MSCs exhibit significant immunomodulatory properties, they are able to escape the immune system recognition mechanisms and modulate the defense mechanisms of the host. Considering their low immunogenicity, MSCs are poorly recognized by human leukocyte antigen incompatible hosts.Citation30,Citation32 Therefore, they may well perform specific tasks such as immunomodulators in the maintenance of peripheral or transplantation tolerance, in autoimmune response, and tolerance between mother and fetus.Citation8,Citation33,Citation34 The MSCs are able to influence many functions of activated T cells,Citation35 B cells,Citation36 and NK cellsCitation37 and can also affect the functions of dendritic cells (DCs),Citation38,Citation39 monocyte/macrophages,Citation40,Citation41 and neutrophils and mast cellsCitation42,Citation43 (). MSCs can mediate their immunomodulatory activity in a numerous models, such as graft versus host disease (GvHD),Citation34,Citation44 diabetes mellitus type 1,Citation45,Citation46 experimental autoimmune encephalomyelitis (EAE),Citation47 model of contusive spinal cord injury and its subsequent inflammation-related damage,Citation48,Citation49 and many others.Citation33 They may also act as primary matrices in the tissue repair processes caused by the inflammation and injury.Citation50,Citation51 Because of high affinity to the tumor tissue, MSCs can also serve as the targeted carriers of therapeutic agents, as part of the tumor stroma in anticancer therapy.Citation52,Citation53
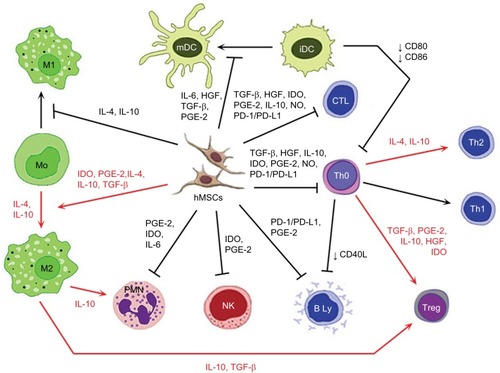
Figure 2 Immunomodulatory action of activated MSCs.
Notes: Red arrow: stimulation; black arrow: suppression; blunt-ended arrow: direct inhibition.
Abbreviations: iDC, immature dendritic cell; IL, interleukin; HGF, hepatocyte growth factor; TGF-β, transforming growth factor-β; PGE-2, prostaglandin E2; IDO, indoleamine 2,3-dioxygenase; NO, nitric oxide; PD-L1, programmed death ligand 1; hMSC, human mesenchymal stem cell; Treg, T regulatory; Th, T helper; CTL, cytotoxic T cell; mDC, mature dendritic cell; PD-1, programmed cell death protein 1; PMN, polymorphonuclear leukocyte; NK, NK cell.
Activities of MSCs in damaged tissue environment
A key factor of multicellular organism survival is the maintenance of homeostatic balance. Under normal circumstances, apoptotic cells are removed, without causing inflammation, by resident phagocytic cells. On the other hand, acute tissue damage is usually accompanied with inflammation and cell components released from necrotic cells, and microvascular damage leads to the increased vasopermeability and subsequent infiltration of macrophages and neutrophils.Citation54 The process of necrotic cell phagocytosis leads to the release of pro-inflammatory mediators, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), various chemokines, leukotrienes, and free radicals.Citation55 Besides the innate immune response, damage and repair of tissues is closely associated with the action of the adaptive immune response involving CD4+ and CD8+ T cells and B cells.Citation54
In recent years, MSCs have been recognized as one of the adult stem cell types, which actively participate in a tissue damage repair processes.Citation50,Citation51 In a case of tissue damage, the MSCs located in immediate vicinity or originated from bone marrow begin to migrate to the site of injury.Citation28,Citation29 Once the MSCs reach an injury site, they must cooperate closely with various types of stromal and inflammatory cells in order to participate in damage repair and regeneration processes.Citation51,Citation56 It has been shown that there are mutual interactions between the MSCs and inflammatory cells, which at the end determine the results of damaged tissue repair processes mediated by MSCs. These undergoing mechanisms are very complex where modulatory factors produced by the MSCs play the major role (). However, these mechanisms differ between various species.Citation31,Citation57 Many of these factors are released into exosomes, the small membranous vesicles participating in cell to cell communication. Regarding the repair and regeneration of damaged tissue, the exosomes from MSCs have similar functions as the MSCs, but little is known about the immunomodulatory effect of these vesicles.Citation58
Several studies have demonstrated that MSCs are capable of producing a wide range of growth factors such as transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), epidermal growth factor, fibroblast growth factor (FGF), vascular endothelial growth factor, platelet-derived growth factor (PDGF), insulin-like growth factor 1 (IGF-1), stromal cell-derived factor 1 (SDF-1), and angiopoietin-1.Citation30 Many of them are generated on the principle of nuclear factor-κB (NF-κB) activation, after prior exposure to the pro-inflammatory stimuli such as IFN-γ, TNF-α, IL-1β, lipopolysaccharide (LPS), or hypoxia.Citation59 These growth factors subsequently promote the development of tissue progenitor cells, fibroblasts, and endothelial cells which then support tissue regeneration and repair.Citation57
After an adequate pro-inflammatory stimulation, MSCs are also able to produce other important immunomodulatory factors such as prostaglandin E2 (PGE-2), indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), TNF-inducible gene 6 protein, TGF-β, and many others.Citation30,Citation31 There is also modification in the expressions of surface molecules, such as galectins, intracellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1).Citation30,Citation31
Within the course of action, MSCs are capable of in vitro and in vivo modulation of many types of immune cells. The ability to modulate the immune response is mediated by the action of complex mechanisms related mostly to the production of immunosuppressive trophic factors (). The MSCs are able to inhibit the proliferation of T helper (Th) and cytotoxic T cells through multiple pathways. Moreover, induction of Th 2 and regulatory T cells (Treg) differentiation result in the induction of an anti-inflammatory environment.Citation35 The maturation of immature dendritic cells (iDCs) is inhibited by IL-6, while reduction in the expression of co-stimulatory molecules CD40, CD80, and CD86 could cause an inhibition of T cell activation.Citation35,Citation39 Through the actions of MSCs, monocytes are directed to differentiate into alternative anti-inflammatory M2 phenotype. The most important mechanisms involved in this process are the effects induced by IDO, TGF-β, IL-10, and PGE-2.Citation41 IL-10 produced by M2 macrophages may subsequently increase the frequency of Treg cells associated with the decrease of tissue neutrophil migration.Citation41 The MSCs are also able to inhibit the proliferation of NK cells as well as limit their cytotoxic activity and cytokine secretion.Citation37 Correspondingly, the proliferation of B cells and antibody production are also negatively influenced.Citation36
MSCs also have the ability to induce complement activation. It has been observed that high levels of activated C3 correlate with increased immunomodulatory capacity of the MSCs.Citation60 In addition, MSCs express the complement regulating protein CD59 and complement factor H which protects cells from lysis.Citation60,Citation61
Therefore, MSCs have the potential to suppress uncontrolled immune responses, by in situ downregulation of the inflammatory response. Thus, it can be said that the immunomodulatory action of MSCs takes place in the microenvironment where inflammatory factors produced during the immune response directly affect the immunomodulatory capacity of MSCs.Citation30,Citation31,Citation57
Additionally, aside the immunomodulatory actions, MSCs also contribute to reparation and regeneration of damaged tissue with their differentiation potential. As seen in the study by Spakova et al, focused on immunomodulatory and regenerative properties of MSCs in osteoarthritis model in vitro, kartogenin (KGN) may be an effective accelerant promoting chondrogenic differentiation of MSCs but with no significant effect on hypertrophic differentiation. KGN caused an upregulation of Col II and aggrecan expression and downregulation of osteocalcin and MMP-13 and caused an increase in the expressions of chondrogenic markers (CD49e, CD26, and CD54) compared to control.Citation62
Activation of immunomodulatory capacity of MSCs
For the experimental and potential clinical applications, MSCs are typically used after various passages after in vitro expansion. Thus, their phenotype, differentiation, and immunomodulatory potential may significantly change during an in vitro expansion.Citation19,Citation20 Moreover, the therapeutic effects of the MSCs may differ due to the complexity and specific action of damaged tissue microenvironment.
Several results indicate that MSCs are not spontaneously immunosuppressive but that they require activation for the manifestation of their immunomodulatory properties. In particular, the most important priming factors of MSCs are IFN-γ, TNF-α, and IL-1β.Citation63–Citation65
One of the first modulatory factors that are produced after the T cell activation is IFN-γ. This cytokine would normally provide stimulatory signal for the activation and expansion of T cells, but in the presence of MSCs, it leads to the suppression of T cell proliferation and inhibition of biological functions.Citation35 The release and binding of IFN-γ on receptor expressed by the MSCs are key steps for the induction of the immunomodulatory properties not only for various T cell subtypes but also against B and NK cells, which are usually unresponsive to the IFN-γ action in the absence of MSCs.Citation35,Citation36,Citation39 Equally important factors that affect MSCs polarization are the concentration of IFN-γ and contemporary release of inflammatory cytokines. It has been shown that MSCs modified by IFN-γ can not only function as cells with immunomodulating properties but also act as effective antigen-presenting cells (APCs).Citation66,Citation67 However, the in vitro findings are difficult to associate with the in vivo microenvironment because of complexity and impossibility to measure IFN-γ at the local level.
The next step involved in the activation of immunomodulatory activities of MSCs is the release of inflammatory cytokines such as TNF-α and IL-1β which alone are not able to inhibit T cell proliferation mediated by the MSCs.Citation68 Synergic effect of IFN-γ with one of the pro-inflammatory cytokines is then sufficient to activate the MSCs. This is associated with significant augmentation of the immunosuppressive effect.Citation68 After either TNF-α or IL-1β action, there is a significant change in the MSCs phenotype. This includes an induction of MHC class I expression and increase of ICAM-1 and VCAM-1 expressions.Citation69 There is also a de novo expression of MHC class II molecules that could theoretically support the APC function of MSCs. Additionally, the expression of programmed death ligand 1 (PD-L1) is detected.Citation65,Citation68,Citation70 During the synergistic action of IFN-γ and TNF-α, stimulation of IL-8, IL-6,Citation71 HGF, and PGE-2 expression and cyclooxygenase-2 (COX-2) activity can be observed.Citation72 On the contrary, the use of IFN-γ alone may result in the induction of IDO and PD-L1 expressions.Citation65,Citation72
The activation of human MSCs by pro-inflammatory stimuli, through the induction of COX-2 has a reducing impact on the heme oxygenase-1 (HO-1) activity. This action negatively influences the suppressor effects of MSCs, induction of Treg, and subsequent IL-10 production.Citation64 Activation of MSCs through the interaction of IFN-γ and TNF-α leads to the activation of superoxide dismutase 3, which is an anti-inflammatory enzyme involved in the O2− catabolism.Citation73 Moreover, the coactivation of MSCs by IFN-γ and TNF-α induces the production of chemokines, such as CCR5, CCR10, CXCR3, CXCL9, and CXCL10, which are involved in chemotaxis and can inhibit proliferation of the immune system effector cells.Citation68,Citation71 These processes are essential for the inhibition of T cell function in vitro as well as for the prevention of delayed type hypersensitivity and GvHD in vivo.Citation34,Citation44 Through the complex interactions between the pro-inflammatory factors, the production and activity of various immunomodulatory molecules produced by MSCs are established.Citation30,Citation31
Besides these pro-inflammatory cytokines, in relation to the activation of MSCs, signaling through the Toll-like receptors (TLRs) was also previously described.Citation74 The MSCs priming through the TLR3 and TLR4 resulted in an intensification of their in vitro immunomodulation capacity. The MSCs were thus able to function through the expression of IDO induced through protein kinase R and IFN-β signaling.Citation75 However, the opposite effect was also observed. Binding of TLR3 and TLR4 ligands has reduced the expression of Jagged-1 which leads to the failure of MSCs’ ability to modulate T cell responses on cell contact-dependent manner.Citation76 Explanation of these different outcomes may be based on the findings that TLR3 and TLR4 activate MSCs in different ways. Activation through TLR4 with LPS induced the pro-inflammatory phenotype of MSCs where secretion of IL-6, IL-8, and TGF-β was intensified by co-stimulation with IFN-γ.Citation77–Citation79 On the other hand, activation through TLR3 with polyinosinic:polycytidylic acid (poly I:C) induced anti-inflammatory phenotype of MSCs where production of IDO, PGE-2, IL-4, and IL-1RA was detected.Citation77,Citation79 Similarly, in a recent work, there were observed important changes in the expression of immunomodulatory factors and adhesion molecules of chorion derived MSCs activated through TLR3–poly I:C interaction, after further cocultivation with activated lymphocytes. In particular, a great increase in IL-10 expression and positive changes in CD44 and ICAM-1 expression were also observed (Bačenková, unpublished data, 2016). These findings suggest that MSCs are susceptible to various environmental stimuli which significantly affect the activation of MSCs and their subsequent immunomodulatory functions.Citation63,Citation74
Migration of MSCs to the site of injury
Migration process is represented by several distinctive steps and starts with the resistance and adhesive interactions between cells flowing through the bloodstream and vascular endothelium. The first step is mediated by the homing receptors expressed on circulating cells which interact with the corresponding co-receptors presented on endothelium. Their interaction leads to the connection of circulating cells to the endothelium and induces rolling effect on the cell surface. This step is usually associated with chemokine-induced activation of integrins, tight adhesion of circulating cells to endothelium, and subsequent extravasation.Citation28,Citation29
For the successful targeted cell therapy, it is critically important to control cell adhesion to the target tissue extracellular matrix (ECM) through the expression of specific adhesion molecules.Citation28,Citation29,Citation69 The MSCs express a large number of adhesion molecules which include integrins and selectins. Heterodimeric protein α4/β1 integrin, also known as a very late antigen-4 (VLA-4), is a member of integrin family and a cell surface adhesion molecule that mediates the cell–matrix or cell–cell interactions. These interactions are provided through binding to the VCAM-1 and V-region of fibronectin.Citation80 The MSCs also express CD44 molecule which is a receptor for the hyaluronic acid and can interact with other ligands such as osteopontin, collagens, and matrix metalloproteinases (MMPs).Citation29,Citation81
It has been shown that α4/β1 is one of integrins that can mediate initial interaction, rolling, and firm adhesion during the process of MSCs homingCitation28,Citation29 (). The MSCs interact with endothelial cells in a coordinated manner through the α4/β1 integrin−VCAM-1 interaction, β1 integrin, P-selectin, MMP-2, and a cytokines secretion.Citation82 It has also been shown that VCAM-1 promotes adhesion of many other cells types and is the primary endothelial ligand for α4/β1, β1, and α4/β7 integrin.Citation83 Its over-expression occurs in endothelial cells as a response to the action of the TNF-α and IL-1β cytokines.Citation69,Citation84
Another factor involved in the homing process is fibronectin (FN), which binds to the ECM components such as collagen, fibrin, and heparan sulfate. These interactions play a fundamental role within the processes of cell adhesion, growth, migration, differentiation, and wound healing.Citation85 The FN fragmentation initiates additional exposure of the V-region containing a binding site for the α4/β1 integrin expressed on the MSCs. These FN fragments cause an increase in bonding strength of the α4/β1 integrin-expressing cells, thus allowing them to adhere to the surrounding matrix.Citation80,Citation85 Interaction between α4/β1 integrin and FN plays an important role in the process of transmigration of MSCs into the ECM. Final step in the process of transmigration of MSCs into ECM is also facilitated through the interactions of MSCs with integrins, junction adhesion molecules (JAMs), and cadherinsCitation86 ().
It was found that the migration ability of MSCs is under the strict control of a wide range of growth factors such as PDGF or IGF-1 operating under the receptor tyrosine kinase principle. However, the findings of the present study suggest that only a limited number of BMSCs used in systemic transplantations possess homing capacity toward injured tissue. A molecular signature that defines this population has been identified, and a surface marker PDGFRβ can be employed for potential isolation of the BMSCs with enhanced migratory capacity.Citation87 Recent data also demonstrate functional association between in vivo bone forming ability and homing capacity of BMSCs. Therefore, PDGFRβ can be used as a potential marker for future selection of BMSCs populations with high migration and bone formation capacity.Citation87
Various chemokines such as CCR2, CCR3, CCR4, and CCL5 are also involved in the homing process.Citation28,Citation29,Citation88 However, the resulting effect of most chemokines is more intensive after the stimulation with TNF-α and IL-1β.Citation89 Findings suggest that the mobilization and subsequent migration of MSCs to the site of injury depend on the state of local and systemic inflammatory microenvironmentCitation28,Citation29,Citation89 ().
Migration process is from a large part dependent on the chemokine attraction. A key molecule in this step is SDF-1, also known as CXC chemokine ligand 12 (CXCL12). It is a small chemotactic cytokine that is often induced by pro-inflammatory stimuli such as TNF-α and IL-1.Citation28,Citation29 The receptor for this chemokine is CXCR4, and the SDF-1–CXCR4 interaction is rather exclusive and plays an important role in the migration process of MSCsCitation29,Citation81 (). Since the SDF-1 is constitutively expressed on various cells and tissues, it was suggested that this molecule may play a preferable role in immune surveillance and basal extravasation.Citation90
It is also well known that culture conditions significantly influence the functions of MSCs. Since exogenously administrated MSCs always have to be expanded in vitro, it has been observed that MSCs can acquire or lose certain surface receptors during the cultivation which might subsequently affect their homing ability.Citation27,Citation91,Citation92 In particular, CXCR4 is expressed at high levels in the bone marrow and ischemic tissues but disappears from the MSCs surface after cultivation. However, hypoxic culture condition can enhance the expressions of CXCR4 and CX3CR1, and the addition of cytokines to the MSCs culture can also restore the CXCR4 levels.Citation93
Basic fibroblast growth factor (bFGF) and MMPs are other factors that can modulate MSCs homing (). bFGF can increase the migration activity of MSCs through the activation of Akt signaling pathway. However, depending on the bFGF concentration, its function can also have the opposite effect on MSCs homing. It has been shown that low concentrations of bFGF result in the attraction of cells, whereas higher concentration has the opposite effect. This ambivalent action of bFGF provides another opportunity for targeting the MSCs homing.Citation94 MMP-2 is another active molecule expressed by MSCs, involved in the degradation of collagen IV, a major component of basement membrane. It was shown that MMP-2 expressed by MSCs is functionally involved in the process of transmigration across the endothelium.Citation86,Citation95
Conclusion
In the last few years, there have been significant advances in the process of understanding the complex steps involved in the MSCs-mediated modulation of immune responses. The final fate of MSCs in local microenvironment and the precise mechanisms through which these cells act in a paracrine manner against neighboring somatic and progenitor cells are yet to be elucidated in detail. Some of the significant immunomodulatory effects are based on distinct properties of MSCs. However, to carry out the modulatory functions, MSCs have to be primed by pro-inflammatory cytokines. Initial data support the paradigm that MSCs are susceptible to the action of the microenvironment. This study proposes that both the concentration and duration of the stimulus have an important role in the process of polarization of MSCs. Therefore, the final modulatory activity of MSCs depends on the action of various factors associated with the contradictory effects. However, in general, these interactions ultimately result in functional polarization of the MSCs toward inhibitory and less to the pro-inflammatory phenotype. It also seems that even long-term implantation, or in some cases even the mere location of MSCs within lesion, is not necessary for MSCs to exert their immunomodulatory and pro-reparatory ability. The MSCs have the potential to modulate and reprogram the functions of the immune system cell and thus support the host immune defense or inhibit the inflammatory processes. Additionally, due to low immunogenicity and ability to migrate to the sites of tissue injury associated with cytokine outburst, MSCs may serve as carriers for the therapeutic agents. As a component of the stroma in some tumors, MSCs can be possibly used in anticancer therapy. However, it is necessary to investigate further not only the final mechanisms of action but also the series of events occurring at different steps of immunomodulation. It is also necessary to address the questions how to manipulate and influence MSCs to achieve the desired degree of differentiation and how to modulate their homing abilities.
Author contributions
All the authors participated in the design of the article, interpretation of the studies, and critical revision of the manuscript.
Acknowledgments
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-0684-12, by the grant of the Operational Program Research and Development, cofinanced by the European Regional Development Fund OPVaV-2012/2.2/08-RO – Medical University Science Park in Košice (MEDIPARK, Košice; ITMS: 26220220185), and by the grant VEGA No. 1/0217/16.
Disclosure
The authors report no conflicts of interest in this work.
References
- FriedensteinAJPetrakovaKVKurolesovaAIFrolovaGPHeterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissuesTransplantation196862302475654088
- CorselliMChenCWCrisanMLazzariLPéaultBPerivascular ancestors of adult multipotent stem cellsArterioscler Thromb Vasc Biol20103061104110920453168
- HoshinoAChibaHNagaiKIshiiGOchiaiAHuman vascular adventitial fibroblasts contain mesenchymal stem/progenitor cellsBiochem Biophys Res Commun200836830531018230345
- BačenkováDRosochaJTóthováTRosochaLŠarisskýMIsolation and basic characterization of human term amnion and chorion mesenchymal stromal cellsCytotherapy20111391047105621916779
- BunnellBAFlaatMGagliardiCPatelBRipollCAdipose-derived stem cells: Isolation, expansion and differentiationMethods20084511512018593609
- HarvanováDTóthováTSarišskýMAmrichováJRosochaJIsolation and characterization of synovial mesenchymal stem cellsFolia Biol (Praha)201157311912421888835
- HassRKasperCBöhmSJacobsRDifferent populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSCCell Commun Signal201191221569606
- HwangNSZhangCHwangYSVargheseSMesenchymal stem cell differentiation and roles in regenerative medicineWiley Interdiscip Rev Syst Biol Med2009119710620835984
- FriedensteinAJChailakhjanRKLalykinaKSThe development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cellsCell Tissue Kinet197033934035523063
- DonnenbergADMeyerEMRubinJPDonnenbergVSThe cell-surface proteome of cultured adipose stromal cellsCytometry A201587766567425929697
- DominiciMLe BlancKMuellerIMinimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statementCytotherapy2006831531716923606
- PhinneyDGSensebéLMesenchymal stromal cells: misconceptions and evolving conceptsCytotherapy20131514014523321325
- PaciniSDeterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs)Front Cell Dev Biol201425025364757
- SensebéLGadelorgeMFleury-CappellessoSProduction of mesenchymal stromal/stem cells according to good manufacturing practices: a reviewStem Cell Res Ther2013436623751270
- DischerDEMooneyDJZandstraPWGrowth factors, matrices, and forces combine and control stem cellsScience200932459351673167719556500
- WangYKChenCSCell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiationJ Cell Mol Med201317782383223672518
- ZippelNLimbachCARatajskiNPurinergic receptors influence the differentiation of human mesenchymal stem cellsStem Cells Dev201221688490021740266
- ChoudheryMSBadowskiMMuiseAPierceJHarrisDTDonor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiationJ Transl Med2014121824397850
- BinatoRde Souza FernandezTLazzarotto-SilvaCStability of human mesenchymal stem cells during in vitro culture: considerations for cell therapyCell Prolif2013461102223163975
- MadeiraAda SilvaCLdos SantosFCamafeitaECabralJMSá-CorreiaIHuman mesenchymal stem cell expression program upon extended ex-vivo cultivation, as revealed by 2-DE-based quantitative proteomicsPLoS One201278e4352322916271
- WagnerWHornPCastoldiMReplicative senescence of mesenchymal stem cells: a continuous and organized processPLoS One20083e221318493317
- JansenJHvan der JagtOPPuntBJVerhaarJAvan LeeuwenJPWeinansHJahrHStimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro studyBMC Musculoskelet Disord20101118819920731873
- HessRDouglasTMyersKAHydrostatic pressure stimulation of human mesenchymal stem cells seeded on collagen-based artificial extracellular matricesJ Biomech Eng2010132216
- TsimbouriPMAdult stem cell responses to nanostimuliJ Funct Biomater20156359862226193326
- EnglerAJSenSSweeneyHLDischerDEMatrix elasticity directs stem cell lineage specificationCell200612667768916923388
- GuilakFCohenDMEstesBTGimbleJMLiedtkeWChenCSControl of stem cell fate by physical interactions with the extracellular matrixCell Stem Cell200951172619570510
- NagyovaMSlovinskaLBlaskoJA comparative study of PKH67, DiI, and BrdU labeling techniques for tracing rat mesenchymal stem cellsIn Vitro Cell Dev Biol Anim201450765666324737277
- EggenhoferELukFDahlkeMHHoogduijnMJThe life and fate of mesenchymal stem cellsFront Immunol2014514824904568
- SohniAVerfaillieCMMesenchymal stem cells migration homing and trackingStem Cells Int2013201313076324194766
- EnglishKMechanisms of mesenchymal stromal cell immunomodulationImmunol Cell Biol201391192623090487
- ZacharLBačenkováDSoltysJRosochaJBioactive mediators associated with mesenchymal stem cells-mediated immunomodulationJ J Bone Stem Res201512006
- ShiYSuJRobertsAIShouPRabsonABRenGHow mesenchymal stem cells interact with tissue immune responsesTrends Immunol201233313614322227317
- FariniASitziaCErraticoSMeregalliMTorrenteYClinical applications of mesenchymal stem cells in chronic diseasesStem Cells Int2014201430657324876848
- YinFBattiwallaMItoSBone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responsesStem Cells20143251278128824452962
- DuffyMMRitterTCeredigRGriffinMDMesenchymal stem cell effects on T-cell effector pathwaysStem Cell Res Ther2011243421861858
- FranquesaMHoogduijnMJBestardOGrinyóJMImmunomodulatory effect of mesenchymal stem cells on B cellsFront Immunol2012321222833744
- CasadoJGTarazonaRSanchez-MargalloFMNK and MSCs crosstalk: the sense of immunomodulation and their sensitivityStem Cell Rev20139218418923397451
- SpaggiariGMAbdelrazikHBecchettiFMorettaLMSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2Blood2009113266576658319398717
- SpaggiariGMMorettaLInteractions between mesenchymal stem cells and dendritic cellsAdv Biochem Eng Biotechnol201313019920822869087
- CutlerAJLimbaniVGirdlestoneJNavarreteCVUmbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferationJ Immunol20101856617662320980628
- FrançoisMRomieu-MourezRLiMGalipeauJHuman MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiationMol Ther20122018719521934657
- BrownJMNémethKKushnir-SukhovNMMetcalfeDDMezeyEBone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanismClin Exp Allergy20114152653421255158
- LombardoEvan der PollTDelaRosaODalemansWMesenchymal stem cells as a therapeutic tool to treat sepsisWorld J Stem Cells20157236837925815121
- NewellLFDeansRJMaziarzRTAdult adherent stromal cells in the management of graft-versus-host diseaseExpert Opin Biol Ther201414223124624397853
- KatuchovaJTothovaTFarkasova IannacconeSImpact of different pancreatic microenvironments on improvement in hyperglycemia and insulin deficiency in diabetic rats after transplantation of allogeneic mesenchymal stromal cellsJ Surg Res2012178118819522480834
- KatuchovaJHarvanovaDSpakovaTMesenchymal stem cells in the treatment of type 1 diabetes mellitusEndocr Pathol20152629510325762503
- GlennJDSmithMDCalabresiPAWhartenbyKAMesenchymal stem cells differentially modulate effector CD8+ T cell subsets and exacerbate experimental autoimmune encephalomyelitisStem Cells201432102744275524911892
- CizkovaDNovotnaISlovinskaLVanickyIJergovaSRosochaJRadonakJRepetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injuryJ Neurotrauma20112891951196120822464
- CizkovaDDevauxSLe Marrec-CroqFModulation properties of factors released by bone marrow stromal cells on activated microglia: an in vitro studySci Rep20144751425524416
- HanZJingYZhangSLiuYShiYWeiLThe role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growthCell Biosci201221822390479
- KalininaNISysoevaVYRubinaKAParfenovaYVTkachukVAMesenchymal stem cells in tissue growth and repairActa Naturae201134303722649702
- Barcellos-de-SouzaPGoriVBambiFChiarugiPTumor microenvironment: Bone marrow-mesenchymal stem cells as key playersBiochim Biophys Acta20131836232133524183942
- GjorgievaDZaidmanNBosnakovskiDMesenchymal stem cells for anti-cancer drug deliveryRecent Pat Anticancer Drug Discov20138331031823688246
- MedzhitovRInflammation 2010: new adventures of an old flameCell201014077177620303867
- KryskoDVDeneckerGFestjensNGabrielsSParthoensED‘HerdeKVandenabeelePMacrophages use different internalization mechanisms to clear apoptotic and necrotic cellsCell Death Differ2006132011202216628234
- ChenYXiangLXShaoJZPanRLWangYXDongXJZhangGRRecruitment of endogenous bone marrow mesenchymal stem cells towards injured liverJ Cell Mol Med2010141494150819780871
- MaSXieNLiWYuanBShiYWangYImmunobiology of mesenchymal stem cellsCell Death Differ201421221622524185619
- BlazquezRSanchez-MargalloFMde la RosaODalemansWAlvarezVTarazonaRCasadoJGImmunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cellsFront Immunol2014555625414703
- CrisostomoPRWangYMarkelTAWangMLahmTMeldrumDRHuman mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanismAm J Physiol Cell Physiol2008294C675C68218234850
- MollGJitschinRvon BahrLMesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responsesPLoS One20116e2170321747949
- TuZLiQBuHLinFMesenchymal stem cells inhibit complement activation by secreting factor HStem Cells Dev201019111803180920163251
- SpakovaTPlsikovaJHarvanovaDLackoMRosochaJEffect of in vitro differentiation of human mesenchymal stem cells on cartilage repair in osteoarthritis of knee jointEur Cell Mater201632Suppl 361
- KramperaMMesenchymal stromal cell ‘licensing’: a multistep processLeukemia2011251408141421617697
- MougiakakosDJitschinRJohanssonCCOkitaRKiesslingRLe BlancKThe impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cellsBlood20111174826483521389316
- ShengHWangYJinYA critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1Cell Res20081884685718607390
- ChanJLTangKCPatelAPBonillaLMPierobonNPonzioNMRameshwarPAntigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gammaBlood20061074817482416493000
- Sánchez-AbarcaLIAlvarez-LaderasIDíez CampeloMUptake and delivery of antigens by mesenchymal stromal cellsCytotherapy201315667367823522868
- RenGZhangLZhaoXMesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxideCell Stem Cell2008214115018371435
- RenGZhaoXZhangLInflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppressionJ Immunol20101842321232820130212
- RameshwarPIFN-gamma and B7-H1 in the immunology of mesenchymal stem cellsCell Res20081884685718607390
- HemedaHJakobMLudwigAKGiebelBLangSBrandauSIFN-gamma and TNF-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cellsStem Cells Dev20101969370620067407
- EnglishKBarryFPField-CorbettCPMahonBPIFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cellsImmunol Lett20071109110017507101
- KempKGrayEMallamEScoldingNWilkinsAInflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cellsStem Cell Rev2010654855920683679
- DelaRosaODalemansWLombardoEToll-like receptors as modulators of mesenchymal stem cellsFront Immun20123182
- OpitzCALitzenburgerUMLutzCToll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase RStem Cells20092790991919353519
- LiottaFAngeliRCosmiLToll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signalingStem Cells20082627928917962701
- HwangSHChoHKParkSHToll like receptor 3 & 4 responses of human turbinate derived mesenchymal stem cells: stimulation by double stranded RNA and lipopolysaccharidePLoS One201497e10155825004159
- Romieu-MourèzRFrançoisMBoivinMNBouchentoufMSpanerDEGalipeauJCytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotypeJ Immunol20091827963797319494321
- WatermanRSTomchuckSLHenkleSLBetancourtAMA new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotypePLoS One20105e1008820436665
- GuanJLHynesROLymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1Cell199060153612295088
- Marquez-CurtisLAJanowska-WieczorekAEnhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axisBiomed Res Int2013201356109824381939
- RusterBGottigSLudwigRJBistrianRMullerSSeifriedEMesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cellsBlood2006108123938394416896152
- SpringerTAAdhesion receptors of the immune systemNature199034662834254341974032
- WuTCThe role of vascular cell adhesion molecule-1 in tumor immune evasionCancer Res200767136003600617616653
- ValenickLVHsiaHCSchwarzbauerJEFibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrixExp Cell Res20053091485515992798
- SteingenCBrenigFBaumgartnerLSchmidtJSchmidtABlochWCharacterization of key mechanisms in transmigration and invasion of mesenchymal stem cellsJ Mol Cell Cardiol20084461072108418462748
- AndersenRKZaherWLarsenKHAssociation between in vivo bone formation and ex vivo migratory capacity of human bone marrow stromal cellsStem Cell Res Ther2015619626450135
- HonczarenkoMLeYSwierkowskiMGhiranIGlodekAMSilbersteinLEHuman bone marrow stromal cells express a distinct set of biologically functional chemokine receptorsStem Cells20062441030104116253981
- PonteALMaraisEGallayNThe in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activitiesStem Cells20072571737174517395768
- BleulCCFuhlbriggeRCCasasnovasJMAiutiASpringerTAA highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1)J Exp Med19961843110111099064327
- KarpJMLeng TeoGSMesenchymal stem cell homing: the devil is in the detailsCell Stem Cell2009420621619265660
- RomboutsWJPloemacherREPrimary murine MSC show highly efficient homing to the bone marrow but lose homing ability following cultureLeukemia20031716017012529674
- HungSCPochampallyRRHsuSCSanchezCChenSCSpeesJProckopDJShort-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivoPLoS One20072e41617476338
- SchmidtALadageDSchinkotheTBasic fibroblast growth factor controls migration in human mesenchymal stem cellsStem Cells20062471750175816822883
- De BeckerAVan HummelenPBakkusMVan de BroekIDe WeverJDe WaeleMVan RietIMigration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3Haematologica200792444044917488654

