Abstract
Chronic nasal mucosal inflammatory disease is a common nasal disease, which is involved by inflammatory cells and a variety of cytokines. Its main pathological features are inflammatory reaction, increased secretion, mucosal swelling and thickening of nasal cavity or paranasal sinuses.It mainly includes chronic rhinitis (divided into allergic rhinitis, non-allergic rhinitis), chronic sinusitis (divided into with nasal polyps, without nasal polyps type), etc.The main symptoms of chronic rhinitis are nasal itching, sneezing, runny nose, and nasal congestion. The main symptoms of chronic sinusitis are nasal congestion, purulent or sticky nasal discharge, headache, and reduced sense of smell. They are a type of disease with a high incidence rate and seriously affect the quality of human life.Although the etiology and treatment of this type of disease have been extensively studied, there are still many aspects that are unclear.Currently, oxidative stress is believed to be an important link in the pathogenesis of chronic inflammatory diseases of the nasal mucosa. Therefore, anti-oxidative stress is a direction of research for the treatment of chronic nasal mucosal inflammatory diseases.Hydrogen, as a medically therapeutic gas, has been extensively studied for its antioxidant, anti-inflammatory, and anti-damage properties, and has been used in the treatment of various diseases.Although there are relatively few studies on the use of hydrogen for nasal inflammation, its positive effects have also been found. This article systematically summarizes the relevant research on the use of hydrogen to improve chronic nasal mucosal inflammation, with the aim of clarifying the ideas and indicating the direction for further research in the future.
Introduction
Chronic inflammatory diseases of the nasal mucosa are characterized by inflammation cells (such as eosinophils, neutrophils, and macrophages) and various cytokines jointly involved, with nasal mucosa inflammation reaction, high secretion of mucus, and tissue remodeling as the main pathological features, including Chronic rhinitis (CR), chronic sinusitis (CRS). Chronic rhinitis (CR) affects approximately 30% of the global populationCitation1,Citation2 and has become an important chronic inflammatory respiratory disease, causing serious impacts on patients’ quality of life and socio-economic status.CR is a chronic inflammatory disease of the nasal mucosa, characterized mainly by symptoms such as nasal itching, sneezing, anterior or posterior nasal discharge, and nasal congestion. It lasts for at least 1 hour every day and for more than 12 weeks each year.CR can be divided into allergic rhinitis (AR) and non-allergic rhinitis (NAR) based on the presence or absence of specific allergen sensitization.Citation2,Citation3 It is estimated that approximately 500 million people worldwide suffer from AR,Citation4 while NAR affects the lives of over 200 million people.Citation5 Chronic rhinosinusitis (CRS) is a chronic inflammation of the nasal sinus mucosa, with common symptoms including nasal congestion, runny nose accompanied by thick mucus or purulent secretions, decreased sense of smell, and facial pain lasting over 12 weeks.Citation6 The incidence of CRS is 8%~15%,Citation6 severely affecting people’s quality of life and causing a huge economic burden.Citation7,Citation8 However, the etiology and pathogenesis of these types of diseases are extremely complex and not yet fully understood. Currently, oxidative stress is believed to be an important link in the pathogenesis of chronic inflammatory diseases of the nasal mucosa,Citation9–14 and many scholars are exploring treatment methods targeting oxidative stress.Citation15–18
Since 2007, the research team led by Japanese scientist OhsawaI first discovered that hydrogen (H2) has good therapeutic value in treating cerebral ischemia-reperfusion injury.Citation19 Since then, H2 as a gas molecule with therapeutic effects has received attention from researchers. H2 has been proven to have significant therapeutic effects in various disease models, involving nearly 100 types of diseases,Citation20 and some studies have extended to clinical trials.Currently, it has been determined that hydrogen gas has selective antioxidant and anti-inflammatory properties. Scholars have conducted research in recent years on whether it can play a targeted therapeutic role in the oxidative stress pathway of chronic nasal mucosal inflammation. The following is a summary of basic research and clinical research related to the current field, in order to facilitate further in-depth research in the future.
Oxidative Stress Mechanism of Rhinitis and Therapeutic Mechanism of Hydrogen
Oxidative Stress Mechanism of Rhinitis
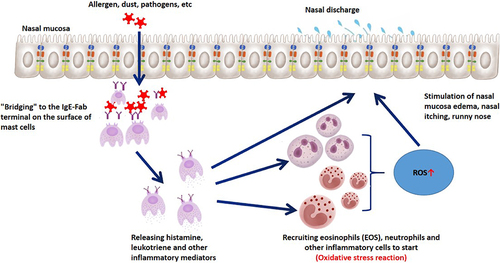
The nasal mucosal epithelium plays an important role in oxygen exchange in the respiratory tract. It continuously contacts pathogenic factors such as dust, allergens, and pathogens in the air, recruiting inflammatory cells (eosinophils, neutrophils, and lymphocytes) and releasing reactive oxygen species (ROS), directly or indirectly activating oxidative stress mechanisms (). Oxidative stress is an imbalance between oxidation and antioxidant defense, resulting in excessive ROS production. This can activate inflammasomes and various transcription factors, leading to the production of inflammatory cytokines, compromising the integrity of nasal mucosal cilia, altering the expression of adhesion molecules, resulting in changes in permeability and increased mucus production, causing symptoms of nasal inflammation such as congestion and runny nose (). At present, there has been a considerable amount of research on the oxidative stress mechanism of CR, especially AR. Inflammation promotes oxidative stress response, and oxidative stress in turn leads to aggravated allergic inflammation, both of which promote each other.Citation21,Citation22
There are also many studies on the oxidative stress mechanism of CRS. Literature has shown that the expression of cellular protective enzymes increases in CRS patients, indicating that oxidative stress plays a role in the pathophysiology of CRS.Citation14,Citation23 It has also been found that the oxygen free radical content in nasal polyp tissue is higher, indicating that oxygen free radical damage is related to the onset of nasal polyps.Citation24,Citation25 Studies have shown that the destruction of Nrf2 in nasal sinus epithelial cells enhances susceptibility to sinusitis in mouse models,Citation13 and Nrf2 is a transcription factor involved in regulating multiple antioxidant genes, thus confirming from a genetic level that oxidative stress mechanisms are involved in the pathogenesis of CRS.
Therapeutic Mechanism of Hydrogen
Research on hydrogen therapy for diseases has been extensive in recent years,Citation26–29 and the most prominent and clear mechanism of its therapeutic effect is the antioxidative effect. Hydrogen can selectively neutralize highly toxic free radicals,Citation30–32 such as hydroxyl radicals (-OH) and peroxynitrite anions (ONOO-), while having no neutralizing effect on free radicals with physiological activity. Hydrogen gas can also exert an indirect antioxidant effect by increasing the activity of antioxidant enzymes, including heme oxygenase 1 (HMOX1),Citation33 superoxide dismutase,Citation34 catalase,Citation35 and myeloperoxidase.Citation36 Research has found that hydrogen may regulate the activity and expression levels of antioxidant enzymes through the Nrf2 signaling pathway.Citation37
Inflammation is a common pathological process in various diseases. The mechanisms of inflammation that cause damage to the body include promoting excessive activation of the immune system and the release of inflammatory factors. Multiple studies in recent years have found that molecular hydrogen can reduce the production of inflammatory factorsCitation28,Citation38,Citation39 and increase the release of anti-inflammatory factors, thereby playing an anti-inflammatory role. Hydrogen gas also has anti-apoptotic effectsCitation32,Citation40,Citation41 and affects the hormone levels and activity.Citation42,Citation43
Based on the above research, we found that the formation of chronic nasal mucosal inflammation is closely related to oxidative stress mechanisms. Therefore, research on anti-oxidative stress targeting oxidative stress mechanisms can not only alleviate inflammatory reactions but also prevent further development of chronic nasal mucosal inflammation, which is expected to become an effective way to treat or improve chronic inflammation of the nasal mucosa.The main treatment mechanism of hydrogen is anti-oxidative stress, which theoretically can be used for improving chronic inflammation of the nasal mucosa.
Basic Study of Hydrogen in Improving Nasal Mucosal Inflammation
One of the important achievements in medical development in recent years is the discovery of signal gases as important regulatory factors in oxidative/antioxidative imbalance, including nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and H2. Among them, the physiological effects of H2 have received increasing attention since it was reported in the international renowned journal “Nature Medicine” in 2007 that H2 can effectively scavenge oxygen free radicals. In recent years, basic research on hydrogen in improving nasal mucosal inflammation has indicated that hydrogen can improve nasal mucosal inflammation.
Hydrogen Reduces Oxidative Stress in Inflamed Nasal Mucosa
Due to its small molecular size, H2 can easily penetrate biological membranes and reach target sites such as mitochondria and nuclei that most antioxidants cannot effectively reach. H2 efficiently scavenges the major substances that cause oxidative damage, such as OH and ONOO-, which has been demonstrated by Ohsawa et al in cellular level studies.Citation19
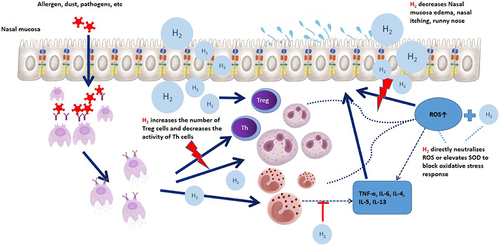
Yu et alCitation44 divided guinea pigs into 2 groups: the guinea pig AR model group and the normal guinea pig group. Each group was further divided into 2 subgroups, with one subgroup receiving injections of hydrogen-rich normal saline and the other receiving injections of normal saline. The levels of serum IgE, blood eosinophil count, and eosinophil cationic protein (ECP) were measured and observed. Oxidative stress was detected using serum malondialdehyde (MDA) and superoxide dismutase (SOD) analysis.MDA is a biomarker for lipid peroxidation, and SOD can eliminate ROS, reduce lipid peroxidation, and protect cells from damage caused by toxic oxygen free radicals. It is an important enzyme for defending against superoxides from the body or external environment.The results showed that hydrogen-rich normal saline could reduce the ROS and MDA levels, increase the SOD level, and reduce the frequency of sneezing and scratching in the AR-HRS group of guinea pigs. At the same time, it was found that the number of eosinophils in the blood and the level of ECP in the serum of the AR-HRS group both decreased. The study suggested that hydrogen gas can reduce oxidative stress and alleviate allergy symptoms by increasing SOD levels, neutralizing ROS (MDA decreased).
Therefore, H2 achieves its antioxidant effect by directly neutralizing free radicals and indirectly increasing SOD levels.
Hydrogen Reduces the Inflammatory Response of Nasal Mucosa
The long-term presence of inflammatory factors in the nasal mucosa, such as immune reactions, involving many inflammatory mediators, ultimately leads to chronic and persistent damage to the mucosa, resulting in chronic inflammation of the nasal mucosa. These inflammatory mediators include histamine, kinins, and many cytokines such as IL-1, IL-4, IL-6, IL-11, and IL-13. IL-4 and IL-13 are mainly secreted by activated Th2 cells and positively regulate immune function.Citation45 They are widely considered to be key mediators in the pathogenesis of AR, directly affecting the maturation and migration of Th2 cells. IL-4 and IL-13 share the same receptor subunit and play a critical role in IgE-dependent inflammatory reactions, acting on B cells to induce the production of IgE.
Zhao et alCitation39 conducted an animal experiment on AR. They divided 18 guinea pigs into three groups: a normal saline control group, an AR sensitization group, and a group treated with hydrogen-rich normal saline (HRS). They measured the levels of IgE and cytokines (IL-4 and IL-13) in their serum and used real-time reverse transcription-polymerase chain reaction and Western blot to detect the expression levels of IL-4 and IL-13 mRNA and protein in the nasal mucosa. The results showed that the AR guinea pigs treated with HRS had significantly lower levels of serum IgE, IL-4, and IL-13 (p < 0.05), and the expression levels of IL-4 and IL-13 mRNA and protein in their nasal mucosa were also lower than those in the AR group not treated with HRS. This suggests that HRS can alleviate inflammation and promote physiological function recovery of the nasal mucosa by inhibiting the MAPK signaling pathway in the AR guinea pig model, thereby reducing the transcription and expression of inflammatory factors such as IL-4, IL-13.
CD4+CD25+ regulatory T cells (Tregs) are a subset of CD4+ T cells derived from the thymus, which can secrete high levels of anti-inflammatory cytokines, including transforming growth factor (TGF)-β and interleukin (IL)-10. CD4+CD25+ Treg cells play important roles in balancing Th1/Th2 cell differentiation and participating in allergic inflammation. Defects in Treg function or decreased Treg cell numbers can trigger the development and progression of AR. A basic study by Xu et alCitation46 demonstrated that HRS can suppress allergic inflammatory responses by increasing the number of AR guinea pig CD4+CD25+Foxp3+Treg cells, promoting the expression of IL-10, TGF-β, and Foxp3.
Study also found that by inhaling H2, AR mouse models can reduce the infiltration of inflammatory cells into the mucosa and lower the levels of IL-5, IL-13, and monocyte chemotactic protein-1 (MCP-1) in the serum, suggesting that H2 may be valuable in the treatment of allergic diseases.Citation18
In summary, hydrogen gas can improve nasal mucosal inflammation by increasing the number and function of Treg cells, increasing the release of anti-inflammatory factors (TGF-β and IL-10), or by inhibiting the secretion of inflammatory cytokines (IL-4, IL-13, etc.) by Th2 cells ().
By Modulating Cellular Signaling Pathways to Improve Nasal Mucosal Inflammation
Hydrogen gas is a small molecular gas with strong diffusion ability that can directly enter organelles such as mitochondria that are related to cellular functions. In recent years, more and more studies have shown that hydrogen gas is involved in the regulation of cell signaling pathways and exerts antioxidative and anti-inflammatory effects by activating certain signaling pathways, such as lowering levels of TNF-α and IL-6,Citation47–49 and inhibiting phosphorylation signaling pathways related to hypersensitivity reactions. Hydrogen gas can also regulate the antioxidant activity of antioxidant enzymes by acting as a signaling factor through Nrf2.Citation35,Citation50 Nrf2 is an important factor in the body for removing oxidative free radicals, which not only inhibits the occurrence and development of oxidative stress reactions but also regulates the expression of antioxidant enzymes such as SOD, catalase, and glutathione peroxidase. Itoh et alCitation49 reported that drinking hydrogen water can regulate the phosphorylation and downstream signaling of the high-affinity IgE receptor FcεRI and relieve immediate-type hypersensitivity reactions, indicating that hydrogen gas may also exert its effects by regulating certain signaling pathways.
As mentioned earlier, the basic research on hydrogen gas for chronic inflammation of the nasal mucosa has been proven to improve symptoms of rhinitis in terms of oxidative stress, inflammatory mediators, and signaling pathways, as shown in . These studies are still in early stages and the observation periods are relatively short (7–14 days), thus lacking evidence of improvement on remodeling of nasal mucosal tissue.
Table 1 Basic Research on Hydrogen Improving Chronic Inflammation of Nasal Mucosa
Hydrogen in Clinical Research on Improving Nasal Mucosal Inflammation
Based on the basic research, some scholars have proposed the use of hydrogen-rich normal saline for the clinical treatment of chronic nasal mucosal inflammatory diseases. Due to its non-irritating, high safety, no obvious side effects, and the ability to directly act on the nasal mucosa through nasal administration, it can quickly exert antioxidant and anti-inflammatory effects. Therefore, it is worth studying its applicability in clinical practice.
CR includes two major types of rhinitis: AR and NAR. The treatment mainly involves nasal glucocorticoids, antihistamines, and nasal decongestants.Citation3,Citation51–54 However, due to the side effects of drugs, in recent years, many patients tend to prefer side-effect-free nasal saline irrigation.Citation55 But simple normal saline irrigation only has a physical flushing effect and does not have an anti-inflammatory treatment effect.
Jin et alCitation56 conducted a pilot study on a small sample of AR patients and found that hydrogen-rich normal saline nasal irrigation can significantly improve the nasal mucosal physiological function of AR patients. Based on this, the sample size was further expanded, and a total of 120 CR patients, including AR and NAR, were randomly divided into two groups, hydrogen-rich normal saline group and normal saline group, for a double-blind controlled study. After treatment, TNSS and nasal ECP levels decreased significantly in both groups (P<0.05). The TNSS level in the hydrogen-rich normal saline group was significantly lower than in the normal saline group (P<0.05). Combined with other evaluation indicators, it was found that nasal irrigation with hydrogen-rich normal saline can improve the clinical symptoms of CR. Subgroup analysis found that AR patients had more significant treatment effects, so it is believed that HRS can be effectively used in the clinical treatment of CR patients.Citation56
There are also clinical studies on other AR treatment methods, such as a 2021 meta-analysis that summarized 56 clinical studies on “phototherapy” for AR.Citation57 Although this therapy has a positive effect on AR, there are still shortcomings such as a small sample size, lack of summary on long-term side effects, and a high risk of research bias. There are also clinical studies on CR treatments such as cryotherapy, therapeutic ultrasound, and botulinum toxin.Citation58–60 These clinical studies also have limitations and require further improvement and exploration. Therefore, clinical studies on the use of hydrogen for chronic inflammation of the nasal mucosa are a new exploration and inspiration.
The main types of CRS are with or without nasal polyps. Clinical studies on treatment are also constantly being carried out, such as Lianhua Qingwen and specific immunotherapy.Citation61–63 Among these, some monoclonal biological products are more popular (Dupilumab, Omalizumab, Mepolizumab, Benralizumab),Citation64,Citation65 but they are still in the process of further validation and research, and they are expensive and have a longer treatment cycle.Therefore, nasal endoscopy surgery for CRS is still indispensable and serves as the beginning of systematic treatment. The postoperative treatment and care are also important guarantees for promoting inflammation improvement and preventing recurrence. Therefore, we propose to apply hydrogen saline to nasal lavage after CRS surgery, which has entered the clinical research stage. We hope to further confirm through clinical research the role of hydrogen saline in improving and restoring nasal mucosa inflammation and nasal physiological function after injury.
Conclusion
Oxidative stress is the primary cause of chronic inflammatory diseases of the nasal mucosa, where harmful substances such as free radicals and peroxides cause oxidative damage to cell membranes and cell death. However, hydrogen, due to its strong antioxidant properties and characteristic features of rapid diffusion, anti-inflammation, and anti-allergy, can prevent the production and further damage of these harmful substances, thereby alleviating the symptoms of chronic rhinitis. In terms of basic research, hydrogen has been shown to improve symptoms of chronic rhinitis through aspects such as oxidative stress, inflammatory mediators, and signaling pathways. Clinical studies have also found that irrigating the nasal cavity with hydrogen-rich normal saline can significantly improve the physiological function of CR nasal mucosa. However, both basic research and clinical studies are still at the observational level, with small sample sizes and short study duration, and there is a lack of evidence for improvement at the level of mucosal tissue remodeling.Therefore, in the future, it is necessary to further expand the sample size, increase the types of chronic inflammatory diseases of the nose, extend the observation time of treatment, enrich the evaluation criteria of the study (such as pathological sections, gene sequencing, etc.), and explore the transport, distribution, and pharmacokinetics of hydrogen in the body, to further investigate whether hydrogen can become a new method for treating chronic inflammatory diseases of the nose.
Data Sharing Statement
The literature used and cited in this study is available in peer-reviewed journals and is publicly accessible.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Ling Jin, Shiwang Tan and Kai Fan share first authorship.
Additional information
Funding
References
- Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. 2017;72(11):1657–1665. doi:10.1111/all.13200
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. 2008;63:8–160. doi:10.1111/j.1398-9995.2007.01620.x
- Agnihotri NT, McGrath KG. Allergic and nonallergic rhinitis. Allergy Asthma Proc. 2019;40(6):376–379. doi:10.2500/aap.2019.40.4251
- Ahmed MA. Comparative data review on prevalence, economic burden and epidemiology of allergic rhinitis between the USA and India. JEMDS. 2016;5(44):2840–2842. doi:10.14260/jemds/2016/663
- Bousquet J, Fokkens W, Burney P, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008;63(7):842–853. doi:10.1111/j.1398-9995.2008.01715.x
- Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:3–298.
- Papadopoulos NG, Bernstein JA, Demoly P, et al. Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy. 2015;70(5):474–494. doi:10.1111/all.12573
- Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275–1279. doi:10.1111/all.12470
- Naclerio R, Ansotegui IJ, Bousquet J, et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: impact of air pollution on patients with AR: current knowledge and future strategies. World Allergy Organ J. 2020;13(3):100106. doi:10.1016/j.waojou.2020.100106
- Chung DH, Lee KH, Kim SW, Shin SY, Cho JS. Comparison of pre- and post-operative stress levels in patients with allergic rhinitis and non-allergic rhinitis. Eur Arch Otorhinolaryngol. 2012;269(11):2355–2359. doi:10.1007/s00405-012-1946-2
- Asher BF, Guilford FT. Oxidative stress and low glutathione in common ear, nose, and throat conditions: a systematic review. Altern Ther Health Med. 2016;22(5):44–50.
- Hong Z, Guo Z, Zhang R, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016;239(2):117–125. doi:10.1620/tjem.239.117
- Ramanathan M Jr, Tharakan A, Sidhaye VK, Lane AP, Biswal S, London NR Jr. Disruption of Sinonasal Epithelial Nrf2 enhances susceptibility to rhinosinusitis in a mouse model. Laryngoscope. 2021;131(4):713–719. doi:10.1002/lary.28884
- Yu Z, Wang Y, Zhang J, et al. Expression of heme oxygenase-1 in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps: modulation by cytokines. Int Forum Allergy Rhinol. 2015;5(8):734–740. doi:10.1002/alr.21530
- Popov IB, Shcherbakov DA, Tyryk OB, Aleksanyan TA. Novyi vzlyad na lechenie polipoznogo rinosinusita [New approach to treatment of polypous rhinosinusitis]. Vestn Otorinolaringol. 2020;85(3):48–51. doi:10.17116/otorino20208503148
- Kim JS, Oh JM, Choi H, et al. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Complement Med Ther. 2020;20(1):101. doi:10.1186/s12906-020-02886-8
- Sadowska-Woda I, Bieszczad-Bedrejczuk E, Rachel M. Influence of desloratadine on selected oxidative stress markers in patients between 3 and 10 years of age with allergic perennial rhinitis. Eur J Pharmacol. 2010;640(1–3):197–201. doi:10.1016/j.ejphar.2010.04.060
- Fang S, Li X, Wei X, et al. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp Ther Med. 2018;16(6):5178–5184. doi:10.3892/etm.2018.6880
- Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi:10.1038/nm1577
- Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi:10.1186/s13618-015-0035-1
- Han M, Lee D, Lee SH, Kim TH. Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants. 2021;10(8):1266. doi:10.3390/antiox10081266
- Emin O, Hasan A, Aysegul D, Rusen D. Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. J Investig Allergol Clin Immunol. 2012;22(3):188–192.
- Mrowicka M, Zielinska-Blizniewska H, Milonski J, Olszewski J, Majsterek I. Evaluation of oxidative DNA damage and antioxidant defense in patients with nasal polyps. Redox Rep. 2015;20(4):177–183. doi:10.1179/1351000215Y.0000000001
- Uneri C, Oztürk O, Polat S, Yüksel M, Haklar G. Determination of reactive oxygen species in nasal polyps. Rhinology. 2005;43(3):185–189.
- Karlidağ T, Ilhan N, Kaygusuz I, Keles E, Yalçin S, Yildiz M. Roles of free radicals, nitric oxide, and scavenging enzymes in nasal polyp development. Ann Otol Rhinol Laryngol. 2005;114(2):122–126. PMID: 15757191. doi:10.1177/000348940511400207
- Huang L. Molecular hydrogen: a therapeutic antioxidant and beyond. Med Gas Res. 2016;6(4):219–222. doi:10.4103/2045-9912.196904
- Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144(1):1–11. doi:10.1016/j.pharmthera.2014.04.006
- Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44(9):971–982. doi:10.3109/10715762.2010.500328
- Zheng ZG, Sun WZ, Hu JY, et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir Res. 2021;22(1):149. doi:10.1186/s12931-021-01740-w
- Li Q, Yu P, Zeng Q, et al. Neuroprotective effect of hydrogen-rich saline in global cerebral ischemia/reperfusion rats: up-regulated tregs and down-regulated miR-21, miR-210 and NF-κB expression. Neurochem Res. 2016;41(10):2655–2665. doi:10.1007/s11064-016-1978-x
- Yokota T, Kamimura N, Igarashi T, Takahashi H, Ohta S, Oharazawa H. Protective effect of molecular hydrogen against oxidative stress caused by peroxynitrite derived from nitric oxide in rat retina. Clin Exp Ophthalmol. 2015;43(6):568–577. doi:10.1111/ceo.12525
- Liu CL, Zhang K, Chen G. Hydrogen therapy: from mechanism to cerebral diseases. Med Gas Res. 2016;6(1):48–54. doi:10.4103/2045-9912.179346
- Li Y, Li Q, Chen H, et al. Hydrogen gas alleviates the intestinal injury caused by severe sepsis in mice by increasing the expression of heme oxygenase-1. Shock. 2015;44(1):90–98. doi:10.1097/SHK.0000000000000382
- Zhai X, Chen X, Shi J, et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic Biol Med. 2013;65:731–741. doi:10.1016/j.freeradbiomed.2013.08.004
- Yu Y, Yang Y, Bian Y, et al. Hydrogen gas protects against intestinal injury in wild type but not NRF2 knockout mice with severe sepsis by regulating HO-1 and HMGB1 Release. Shock. 2017;48(3):364–370. doi:10.1097/SHK.0000000000000856
- Diao M, Zhang S, Wu L, et al. Hydrogen gas inhalation attenuates seawater instillation-induced acute lung injury via the Nrf2 pathway in rabbits. Inflammation. 2016;39(6):2029–2039. doi:10.1007/s10753-016-0440-1
- Iuchi K, Imoto A, Kamimura N, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971. doi:10.1038/srep18971
- Zhang CB, Tang YC, Xu XJ, Guo SX, Wang HZ. Hydrogen gas inhalation protects against liver ischemia/reperfusion injury by activating the NF-κB signaling pathway. Exp Ther Med. 2015;9(6):2114–2120. doi:10.3892/etm.2015.2385
- Zhao C, Yu S, Li J, Xu W, Ge R. Changes in IL-4 and IL-13 expression in allergic-rhinitis treated with hydrogen-rich saline in Guinea-pig model. Allergologia et Immunopathologia. 2017;45(4):350–355. doi:10.1016/j.aller.2016.10.007
- Zhang YG, Sheng QS, Wang ZJ, et al. Hydrogen-rich saline promotes motor functional recovery following peripheral nerve autografting in rats. Exp Ther Med. 2015;10(2):727–732. doi:10.3892/etm.2015.2518
- Wang JL, Zhang QS, Zhu KD, et al. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen Res. 2015;10(6):958–964. doi:10.4103/1673-5374.158361
- Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi:10.1016/bs.mie.2014.11.038
- Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. doi:10.1038/srep03273
- Yu S, Zhao C, Che N, Jing L, Ge R. Hydrogen-rich saline attenuates eosinophil activation in a Guinea pig model of allergic rhinitis via reducing oxidative stress. J Inflamm (Lond). 2017;14:1. doi:10.1186/s12950-016-0148-x
- Chen X, Li XM, Gu W, Wang D, Chen Y, Guo XJ. LAT alleviates Th2/Treg imbalance in an OVA-induced allergic asthma mouse model through LAT-PLC-γ1 interaction. Int Immunopharmacol. 2017;44:9–15. doi:10.1016/j.intimp.2016.12.029
- Xu F, Yu S, Qin M, et al. Hydrogen-rich saline ameliorates allergic rhinitis by reversing the imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and membrane-bound transforming growth factor-β in guinea pigs. Inflammation. 2018;41(1):81–92. doi:10.1007/s10753-017-0666-6
- Cardinal JS, Zhan J, Wang Y, et al. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77(2):101–109. doi:10.1038/ki.2009.421
- Buchholz BM, Kaczorowski DJ, Sugimoto R, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8(10):2015–2024. doi:10.1111/j.1600-6143.2008.02359.x
- Itoh T, Fujita Y, Ito M, et al. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389(4):651–656. doi:10.1016/j.bbrc.2009.09.047
- Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12(5):e0176992. doi:10.1371/journal.pone.0176992
- Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. 2013;68(9):1102–1116. doi:10.1111/all.12235
- Kompelli AR, Janz TA, Rowan NR, Nguyen SA, Soler ZM. Cryotherapy for the treatment of chronic rhinitis: a qualitative systematic review. Am J Rhinol Allergy. 2018;32(6):491–501. doi:10.1177/1945892418800879
- Sur DKC, Plesa ML. Chronic Nonallergic Rhinitis. Am Fam Physician. 2018;98(3):171–176.
- Sur DK, Plesa ML. Treatment of allergic rhinitis. Am Fam Physician. 2015;92(11):985–992.
- Inthavong K, Shang Y, Wong E, Singh N. Characterization of nasal irrigation flow from a squeeze bottle using computational fluid dynamics. Int Forum Allergy Rhinol. 2020;10(1):29–40. doi:10.1002/alr.22476
- Jin L, Fan K, Tan S, et al. The beneficial effects of hydrogen-rich saline irrigation on chronic rhinitis: a randomized, double-blind clinical trial. J Inflamm Res. 2022;15:3983–3995. doi:10.2147/JIR.S365611
- Costa TMR, Carneiro FM, Oliveira KAS, Souza MFB, Avelino MAG, Wastowski IJ. Rhinophototherapy, an alternative treatment of allergic rhinitis: systematic review and meta-analysis. Braz J Otorhinolaryngol. 2021;87(6):742–752. doi:10.1016/j.bjorl.2020.12.016
- Del Signore AG, Greene JB, Russell JL, Yen DM, O’Malley EM, Schlosser RJ. Cryotherapy for treatment of chronic rhinitis: 3-month outcomes of a randomized, sham-controlled trial. Int Forum Allergy Rhinol. 2022;12(1):51–61. doi:10.1002/alr.22868
- da Silva GS, Santos Isoppo K. Therapeutic ultrasound as a treatment for chronic rhinosinusitis: a systematic review. Clin Respir J. 2021;15(12):1275–1285. doi:10.1111/crj.13441
- Rinzin K, Hoang MP, Seresirikachorn K, Snidvongs K. Botulinum toxin for chronic rhinitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2021;11(11):1538–1548. doi:10.1002/alr.22813
- Lin L, Dai F, Ren G, Wei J, Chen Z, Tang X. Efficacy of lianhuaqingwen granules in the management of chronic rhinosinusitis without nasal polyps. Am J Otolaryngol. 2020;41(1):102311. doi:10.1016/j.amjoto.2019.102311
- Li J, Kang H, Hong S, Shen Y. Effect of postoperative specific immunotherapy combined with nasal irrigation on chronic rhinosinusitis with allergic rhinitis. Iran J Allergy Asthma Immunol. 2021;20(4):432–440.
- Patel GB, Kern RC, Bernstein JA, Hae-Sim P, Peters AT. Current and future treatments of rhinitis and sinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1522–1531. doi:10.1016/j.jaip.2020.01.031
- Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74(4):743–752. doi:10.1111/all.13685
- Brañes R, Rosenbaum A, Callejas C, Winter M. Omalizumab for chronic rhinosinusitis. [Omalizumab para la rinosinusitis crónica]. Medwave. 2018;18(7):e7347. doi:10.5867/medwave.2018.07.7346