Abstract
Background
Community-acquired pneumonia (CAP) is a global health concern due to its high rates of morbidity and mortality. Bacterial pathogens are common causes of CAP. It is one of the most common causes of acute respiratory distress syndrome (ARDS), a common severe respiratory system manifestation threatening human health. This study aimed to establish a predictive model for ARDS in patients with bacterial pneumonia, which was conducive to early identification of the occurrence and effective prevention of ARDS.
Methods
We collected the clinical data of hospitalized patients with bacterial pneumonia in Affiliated Huzhou Hospital of Zhejiang University School of Medicine from January 2022 to November 2022. The independent risk factors for ARDS in patients with bacterial pneumonia were determined by univariate and multivariate binary logistic regression analyses. The nomogram was constructed to display the predictive model, and the receiver-operating characteristic curve was plotted to evaluate the predictive value of ARDS.
Results
This study included 254 patients with bacterial pneumonia, of which 114 developed ARDS. The multivariate logistic regression analysis revealed age [odds ratio (OR) = 1.041, P = 0.003], heart rate (OR = 1.020, P = 0.028), lymphocyte count (OR = 0.555, P = 0.033), white blood cell count (OR = 1.062, P = 0.033), bilateral lung lesions (OR = 7.352, P = 0.011) and pleural effusion (OR = 2.512, P = 0.002) as the independent risk factors for ARDS. The predictive model was constructed based on the six independent factors, which was valuable in predicting ARDS with area under the curve of 0.794.
Conclusion
The predictive model was beneficial to evaluate the disease progression in patients with bacterial pneumonia and identify ARDS. Further, our nomogram might help doctors predict the incidence of ARDS and conduct treatment as early as possible.
Introduction
Community-acquired pneumonia (CAP) is associated with high rates of morbidity and mortality globally, imposing a huge burden on healthcare system and the economy.Citation1 Approximately 40% of patients with CAP require hospitalization, and 5% of them are admitted to the intensive care unit.Citation2 The mortality rate of severe CAP (sCAP) has significantly increased.Citation3 Hence, evaluating the severity and risk factors of CAP for physicians, and implementing suitable therapeutic strategies to improve prognosis are of great significance. Bacteria, including Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, and Haemophilus influenzae, are considered to be common causes of CAP.Citation1,Citation4,Citation5 Also, CAP is one of the most common causes of acute respiratory distress syndrome (ARDS).Citation6
ARDS is characterized by hypoxemia and bilateral radiographic opacities. It is a type of acute diffuse inflammatory lung injury associated with a predisposing risk factor, which leads to increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue.Citation7 At present, ARDS is diagnosed in accordance with the Berlin definition, which evaluates patients based on four aspects: respiratory symptoms, chest imaging, origin of edema and oxygenation.Citation8 ARDS progresses rapidly; however, no useful biomarker is available to enhance the diagnostic sensitivity and specificity, and effective treatments for ARDS are limited at present.Citation9,Citation10 The mortality of ARDS remains high, ranging from 34.9% to 46.1%, with different degrees of lung injury severity.Citation11 Therefore, early diagnosis and intervention are essential to improve survival rate. Pneumonia, mainly CAP, is the most common cause of acute ARDS.Citation11,Citation12 Previous studies have demonstrated that the dysregulation of lung microbiota and immune defenses may have an important influence in patients with ARDS.Citation13 The alterations may be associated with the respiratory complications of the patients,Citation14 playing an essential role in the risk of early-stage ARDS.Citation15 Pneumonia is commonly diagnosed by the comprehensive assessment of clinical manifestations, radiological findings, laboratory tests, and microbiological findings. The precise diagnosis and proper treatment of pulmonary infection in ARDS are challenging due to the imperfection of the existing techniques. The prognosis of ARDS with pulmonary superinfections is poor.Citation16 Therefore, the risk factors of ARDS in patients with bacterial pneumonia need to be urgently explored to provide doctors with instructive information for the timely selection of suitable therapeutic strategies. The purpose of this study was to analyze the clinical data of patients with bacterial pneumonia, screen the characteristic factors of these patients, construct and verify the nomogram model, and provide a reference for the clinical diagnosis and treatment of the disease.
Materials and Methods
Patients
Patients with bacterial pneumonia hospitalized in Affiliated Huzhou Hospital, Zhejiang University School of Medicine (Huzhou Central Hospital) between January 2022 and November 2022 were eligible for this retrospective study.
The inclusion criteria were as follows: (1) diagnosis consistent with guidelines for the diagnosis and treatment of adult CAP in China (2016 edition);Citation17 (2) clear bacterial pathogens;Citation17 (3) ARDS identified within the first 24 h of hospital admission based on the Berlin definition; and (4) patients with complete clinical data, including their medical history and treatment.
The exclusion criteria were as follows:(1) immune dysfunction [blood diseases, organ transplantation, human immunodeficiency virus, malignancies, and infectious diseases]; (2) age <18 years or incomplete data; and (3) clear diagnosis of viral infection, fungal infection, atypical pneumonia, lung cancer, and other diseases.
Data Collection
The following variables were included in this study: (1) demographic information and vital signs upon admission; (2) comorbidities such as hypertension, diabetes, coronary heart disease, cerebral infarction/cerebral hemorrhage, heart failure, hyperlipidemia, and anemia; (3) laboratory tests: lymphocytes, neutrophils, platelets, C-reactive protein (CRP), white blood cell (WBC), prothrombin time, D-dimer, albumin/globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST/ALT ratio, creatinine, lactate dehydrogenase (LDH), globulin, homocysteine, and albumin; and (4) computed tomography (CT) manifestations such as bilateral lung lesions, pleural effusion and pericardial effusion. The laboratory test results from the initial sample taken upon admission were recorded for statistical analysis. The retrospective study was approved by the medical ethics committee of Huzhou Central Hospital (Approval No: 202311018-01), and the requirement for patient consent was waived.
Statistical Analysis
The normality of the included variables was confirmed by the Kolmogorov–Smirnov test. Normally distributed variables were presented as mean ± standard deviation and analyzed using independent-sample Student t test. Non-normally distributed variables were presented as median (interquartile range) and compared using the Mann–Whitney U-test. In addition, the χ2 test or Fisher’s exact test was used to compare the differences in categorical variables. The univariate and multivariate logistic regression analyses were performed to find independent risk factors of ARDS in included patients with bacterial pneumonia. We constructed a predictive model for ARDS using logistic regression with significant factors identified in multivariate logistic regression. Finally, the receiver operating-characteristic (ROC) curve was drawn to validate the predictive value and constructed model, and area under the ROC curve (AUC) was calculated. A nomogram corresponding to the predictive model was drawn to illustrate the probability of developing ARDS. Statistical analyses were performed using SPSS Version 26.0 (SPSS, Inc., IL, USA) software, with statistical significance set at P < 0.05. R (version 3.6.1; R Foundation) was used for all statistical analyses and for drawing figures.
Results
Baseline Characteristics
A total of 254 patients with bacterial pneumonia were finally included in this study, of which 114 developed ARDS, with an incidence of 44.88% (). The patients in the ARDS group were of higher age (74.5 vs 71, P < 0.001) than those in the non-ARDS group. The ARDS group included more patients with diabetes (18.42% vs 6.43%, P = 0.003). The heart rate of patients was significantly lower in the non-ARDS group than in the ARDS group (99 vs 91.5, P = 0.002). Regarding the laboratory tests, neutrophil count (9.45 vs 5.9, P <0.001), CRP level (59.1 vs 29.35, P = 0.008) and WBC count (10.5 vs 7.65, P <0.001) were found to be higher in the ARDS group than in the non-ARDS group. The lymphocyte count was lower in the ARDS group (0.6 vs 1, P < 0.001). The prothrombin time (12.95 vs 12.6, P = 0.020) and D-dimer level (1.33 vs 0.69, P <0.001) were higher in the ARDS group. The creatinine (71.85 vs 65.75, P = 0.002), LDH (239.4 vs 197.5, P < 0.001), and homocysteine levels (13.15 vs 10.7, P = 0.008) were higher in patients in the ARDS group compared with those in the non-ARDS group. However, the albumin level (30.78 vs 33.51, P <0.001) was lower in the ARDS group than in the non-ARDS group. The CT imaging results showed that patients with ARDS had a higher proportion of bilateral lung lesions (98.25% vs 83.57%, P <0.001), pleural effusion (68.42% vs 37.14%, P <0.001) and pericardial effusion (21.93% vs 8.57%, P = 0.003).
Table 1 Baseline Characteristics of Included Patients
Risk Factors of ARDS in Patients with Bacterial Pneumonia
The univariate logistic regression showed that age (OR = 1.044, P <0.001), diabetes (OR = 3.287, P = 0.005), heart rate (OR = 1.021, P = 0.004), lymphocyte count (OR = 0.333, P < 0.001), neutrophil count (OR = 1.062, P =0.013), CRP level (OR = 1.004, P = 0.026), WBC count (OR = 1.080, P = 0.003), prothrombin time (OR = 1.222, P =0.005), ALT level (OR = 1.004, P = 0.046), creatinine level (OR = 1.007, P = 0.004), LDH level (OR = 1.004, P = 0.006), albumin level (OR = 0.874, P < 0.001), bilateral lung lesions (OR = 11.009, P = 0.001), pleural effusion (OR = 3.667, P < 0.001), and pericardial effusion (OR = 2.996, P =0.004) were associated with ARDS (). The multivariate logistic regression analysis revealed that the factors independently associated with the development of ARDS were age (OR = 1.041, P =0.003), heart rate (OR = 1.020, P = 0.028), lymphocyte count (OR = 0.555, P = 0.033), WBC count (OR = 1.062, P = 0.033), bilateral lung lesions (OR = 7.352, P = 0.011), and pleural effusion (OR = 2.512, P = 0.002).
Table 2 Univariate and Multivariate Logistic Regression Analyses of Risk Factors for ARDS in Patients with Bacterial Pneumonia
Predictive Value of the Novel Constructed Model
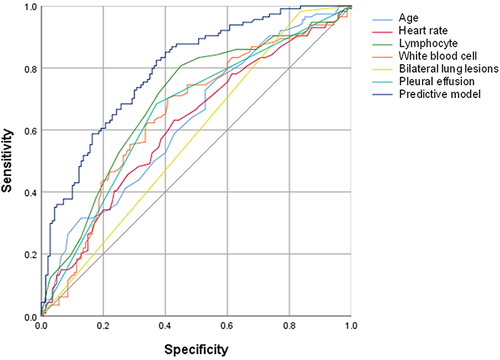
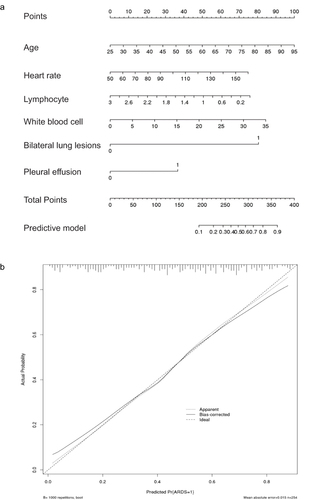
The novel model incorporating age, heart rate, lymphocyte count, WBC count, bilateral lung lesions and pleural effusion for predicting ARDS during hospitalization had an AUC value of 0.794 with a sensitivity and specificity of 0.825 and 0.629, respectively (). The model was presented in the form of nomogram for visualizing its clinical use (). The calibration plot indicated a good consistency between the predicted and observed values ().
Discussion
In ARDS, bacterial infection, endotoxins, and subsequent inflammation result in capillary endothelial barrier disruption and pulmonary venous congestion. This disrupts the balance of pulmonary capillary fluid and excessive alveolocapillary permeability, eventually leading to the acute onset of hypoxemia and bilateral pulmonary oedema.Citation18,Citation19 The pathogenesis of ARDS encompasses a confluence of intense inflammatory cascade, endothelial injury, epithelial injury, coagulation disorder, fibrosis, and apoptosis.Citation20 The occurrence of ARDS has an important impact on the morbidity and mortality of critically ill patients. Summarizing and analyzing the clinical characteristics and risk factors related to bacterial pneumonia with ARDS may be of immense value to the early identification of ARDS and an improvement in the therapeutic effect.
In our study, the incidence of ARDS in hospitalized patients with bacterial pneumonia was 44.88%. Additionally, this study identified six factors associated with ARDS in bacterial pneumonia: age, heart rate, lymphocyte count, WBC count, bilateral lung lesions and pleural effusion. Incorporating these factors, the predictive model achieved an AUC value of 0.794. This provided clinicians with a valuable tool to evaluate the probability of developing ARDS and taking preventive measures against acute lung injury. We can easily acquire these six factors through clinical investigations and employ our model to assess the scores of patients with bacterial pneumonia, thereby obtaining their probability of developing ARDS for treatment guidance.
The patients in the ARDS group were older compared with those in the non-ARDS group. The incidence and mortality rate of ARDS were much higher in elderly patients, which might be related to excessive inflammatory responses and larger changes in lung permeability.Citation21–23 Elderly patients exhibit a higher prevalence of underlying diseases, impaired visceral metabolism, and compromised reserve function. Consequently, the prognosis for them with ARDS is unfavorable, leading to increased mortality rates.
The results of our study showed that the heart rate was significantly higher in the ARDS group than in the non-ARDS group. The heart rate could reflect respiratory function, cardiac function and immune response.Citation24–28 Several studies showed that the heart rate could be exploited for the early identification of infections and respiratory diseases.Citation29,Citation30 The vagus nerve was an important neuroimmunomodulator in the inflammatory pathway, and heart rate could reflect the function of the vagus nerve.Citation25 Heart rate was a key element in the morbidity and mortality of patients with pulmonary disease.Citation31 Higher heart rate could be used as an independent predictor of adverse events of pneumonia, which was consistent with our results.Citation25,Citation32
Inflammation played a crucial role in both the prognosis and symptoms of ARDS, underscoring its significance in the development of the condition.Citation33 After pathogens entered the lung, numerous pattern recognition receptors recognized the microbial structures and endogenous molecules released after cell injury through pathogen-associated molecular patterns and danger-associated molecular patterns. Then, the innate immune response was activated. Subsequently, the production of inflammatory cytokines, interferons and chemokines led to the activation of local cells and the recruitment of macrophages and neutrophils. Eventually, pathogens and infected cells were eliminated.Citation34 Compared with viral-related ARDS, the level of IL-8, IL-17 and TNF-α was significantly higher in bacterial-related ARDS.Citation35 Interestingly, the research showed antibiotics could destroy bacterial cell walls, releasing a large number of products to promote inflammation and worsen lung injury.Citation33 In our study, lymphocyte count, neutrophil count, CRP level and WBC count were four meaningful inflammation indexes with statistically significant differences between the two groups. The neutrophil count, WBC count and CRP level were higher in the ARDS group than in the non-ARDS group. In contrast, the patients in the ARDS group had lower lymphocyte count. Lymphocyte and WBC counts were independent risk factors of ARDS in patients with bacterial pneumonia. The accumulation of WBCs, especially neutrophils, played an important role in the development of pulmonary edema associated with ARDS.Citation36 Studies showed that the changes in the neutrophil and lymphocyte counts were related to extensive activation of the immune system and immune dysfunction.Citation37,Citation38 The neutrophil count was positively correlated with the severity of ARDS.Citation39 Neutrophils were the first WBCs recruited to the sites of inflammation in ARDS.Citation40 They released reactive oxygen species, antimicrobial peptides, and multiple proteinases, which formed extracellular traps to execute the function of neutrophils.Citation41 However, the inappropriate or excessive activation of neutrophils could lead to lung tissue injury and an increased permeability of lung epithelium and endothelium by releasing toxic mediators. The pathology ultimately resulted in respiratory failure and ARDS.Citation42,Citation43 Lymphopenia was associated with a more severe clinical presentation and early mortality of CAP.Citation44,Citation45 Persistent lymphopenia was found to predict sepsis mortality.Citation46 Besides, reduced lymphocyte count was also an independent predictor of mortality risk in patients with severe pulmonary infection.Citation46–48 Lymphocytes were essential in the immune response, and lymphopenia might lead to a harmful inflammatory status.Citation49 The exhaustion and apoptosis of T cells seemed to be central to lymphocytic defects, especially in critically ill patients.Citation50
Patients with ARDS had a higher proportion of bilateral lung lesions and pleural effusion. Levitt et al established a definition of early ALI.Citation51 The score could be used to evaluate the likelihood of patients with bilateral infiltrates and hypoxemia progressing to ARDS excluding heart failure as a cause.Citation52 A study by Ko et al showed that 27% of patients with severe acute respiratory syndrome (SARS) (14/52) had initial bilateral lung involvement, of which 9 died. When the disease progressed to maximal severity (before the occurrence of ARDS), 63% of patients (33/52) displayed bilateral lung involvement. Besides, the patients had more lung lesion distributions, and the mortality was extremely high. The bilateral lung involvement by SARS indicated the wide distribution of the viral-load droplets, which had an unfavorable prognosis.Citation53
Pleural effusion was independently associated with the mortality of patients with CAP and sepsis.Citation44 The lung weight increased, and lung collapse occurred due to non-cardiogenic pulmonary edema in ARDS. The clinical factors of lung edema formation could influence the normal efficient absorptive mechanisms and lead to pleural effusion. An increasing accumulation of pleural effusion had significant effects on lung and chest wall distention. The influence of respiratory mechanics reduced lung gas volume and increased shunt fraction, leading to impaired gas exchange.Citation54 Pieces of evidence showed that the extent of these abnormalities depended on the perfusion of compressed airspaces, such as hypoxic vasoconstriction and vascular compression.Citation55
Despite a practical model established in this study, certain limitations need to be addressed in our future investigations. First, this was a single-center study and selection bias was inevitable. Hence, more clinical data in multiple centers should be analyzed to validate our findings. Second, we did not collect the risk scores for patients, such as acute physiology and chronic health evaluation II and sequential organ failure assessment. Therefore, our risk score should be compared with previously developed scores for improved scores in future studies. Finally, our study lacked external verification of the risk model using an independent cohort. The aforementioned factors need to be addressed in further studies to improve our evaluation accuracy.
Conclusion
This study found that old age, high heart rate, low lymphocyte count, high WBC count, bilateral lung lesions and pleural effusion were independent risk factors for ARDS in patients with bacterial pneumonia. The predictive model incorporating these six factors can facilitate clinicians to identify and diagnose ARDS as early as possible. Clinicians can adjust therapeutic strategies to prevent ARDS in patients with bacterial pneumonia by evaluating the risk of ARDS during hospitalization.
Ethical Standards Disclosure
This study was approved by the medical ethics committee of Huzhou Central Hospital (Approval No: 202311018-01). The requirement to obtain patient consent was waived due to the retrospective, non-interventional design of the study. All patient data were kept confidential, and our study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors drafted, revised or reviewed the article, and agreed on the journal in which the article will be submitted, gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Cilloniz C, Torres A, Niederman M, et al. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016;42(9):1374–1386.
- Ferrer M, Travierso C, Cilloniz C, et al. Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One. 2018;13(1).
- Martin-Loeches I, Torres A, Nagavci B, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023;49(6):615–632.
- Sheam MM, Syed SB, Nain Z, et al. Community-acquired pneumonia: aetiology, antibiotic resistance and prospects of phage therapy. J Chemother. 2020;32(8):395–410.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67.
- Cilloniz C, Ferrer M, Liapikou A, et al. Acute respiratory distress syndrome in mechanically ventilated patients with community-acquired pneumonia. Eur Respir J. 2018;51(3):67.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582.
- Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: advances in Diagnosis and Treatment. JAMA. 2018;319(7):698–710.
- Beitler JR, Goligher EC, Schmidt M, et al. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med. 2016;42(5):756–767.
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800.
- Laffey JG, Madotto F, Bellani G, et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5(8):627–638.
- Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113.
- Dickson RP, Erb-Downward JR, Falkowski NR, Hunter EM, Ashley SL, Huffnagle GB. The Lung Microbiota of Healthy Mice Are Highly Variable, Cluster by Environment, and Reflect Variation in Baseline Lung Innate Immunity. Am J Respir Crit Care Med. 2018;198(4):497–508.
- Dickson RP, Schultz MJ, van der Poll T, et al. Lung Microbiota Predict Clinical Outcomes in Critically Ill Patients. Am J Respir Crit Care Med. 2020;201(5):555–563.
- Luyt CE, Bouadma L, Morris AC, et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020;46(12):2168–2183.
- Qu JM, Cao B. Guidelines for the diagnosis and treatment of adult community acquired pneumonia in China (2016 Edition). Zhonghua Jie He He Hu Xi Za Zhi. 2016;39(4):241–242.
- Gonzales JN, Lucas R, Verin AD. The Acute Respiratory Distress Syndrome: mechanisms and Perspective Therapeutic Approaches. Austin J Vasc Med. 2015;2(1).
- Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637.
- Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18.
- Killien EY, Mills B, Vavilala MS, Watson RS, O’Keefe GE, Rivara FP. Association between age and acute respiratory distress syndrome development and mortality following trauma. J Trauma Acute Care Surg. 2019;86(5):844–852.
- Manzano F, Yuste E, Colmenero M, et al. Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care. 2005;20(3):274–280.
- Luo L, Shaver CM, Zhao Z, et al. Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest. 2017;151(4):755–763.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958.
- Mol MBA, Strous MTA, van Osch FHM, et al. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS One. 2021;16(10).
- Gonzales TI, Jeon JY, Lindsay T, et al. Resting heart rate is a population-level biomarker of cardiorespiratory fitness: the Fenland Study. PLoS One. 2023;18(5).
- Baumert M, Javorka M, Kabir MM. Joint symbolic analyses of heart rate, blood pressure, and respiratory dynamics. J Electrocardiol. 2013;46(6):569–573.
- Frasch MG. Heart Rate as a Non-Invasive Biomarker of Inflammation: implications for Digital Health. Front Immunol. 2022;13:930445.
- Mehrabadi MA, Aqajari SAH, Azimi I, Downs CA, Dutt N, Rahmani AM. Detection of COVID-19 Using Heart Rate and Blood Pressure: lessons Learned from Patients with ARDS. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:2140–2143.
- Shashikumar SP, Stanley MD, Sadiq I, et al. Early sepsis detection in critical care patients using multiscale blood pressure and heart rate dynamics. J Electrocardiol. 2017;50(6):739–743.
- van Gestel AJ, Kohler M, Steier J, et al. Cardiac autonomic function and cardiovascular response to exercise in patients with chronic obstructive pulmonary disease. COPD. 2012;9(2):160–165.
- Kolditz M, Ewig S, Klapdor B, et al. Community-acquired pneumonia as medical emergency: predictors of early deterioration. Thorax. 2015;70(6):551–558.
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med. 2017;195(3):331–338.
- Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181(12):1294–1309.
- Hagau N, Slavcovici A, Gonganau DN, et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14(6).
- Bdeir K, Higazi AA, Kulikovskaya I, et al. Neutrophil alpha-defensins cause lung injury by disrupting the capillary-epithelial barrier. Am J Respir Crit Care Med. 2010;181(9):935–946.
- Riche F, Gayat E, Barthelemy R, Le Dorze M, Mateo J, Payen D. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Crit Care. 2015;19:439.
- Hwang SY, Shin TG, Jo IJ, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med. 2017;35(2):234–239.
- Yang SC, Tsai YF, Pan YL, Hwang TL. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J. 2021;44(4):439–446.
- Wiedermann FJ, Mayr AJ, Kaneider NC, Fuchs D, Mutz NJ, Schobersberger W. Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest. 2004;125(1):212–219.
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489.
- Zemans RL, Matthay MA. What drives neutrophils to the alveoli in ARDS? Thorax. 2017;72(1):1–3.
- Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796.
- Cilloniz C, Peroni HJ, Gabarrus A, et al. Lymphopenia Is Associated With Poor Outcomes of Patients With Community-Acquired Pneumonia and Sepsis. Open Forum Infect Dis. 2021;8(6).
- Marrie TJ, Wu L. Factors influencing in-hospital mortality in community-acquired pneumonia: a prospective study of patients not initially admitted to the ICU. Chest. 2005;127(4):1260–1270.
- Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391.
- Cao Y, Han X, Gu J, et al. Prognostic value of baseline clinical and HRCT findings in 101 patients with severe COVID-19 in Wuhan, China. Sci Rep. 2020;10(1):17543.
- Hamilton F, Arnold D, Payne R. Association of prior lymphopenia with mortality in pneumonia: a cohort study in UK primary care. Br J Gen Pract. 2021;71(703):e148–e156.
- Heffernan DS, Monaghan SF, Thakkar RK, Machan JT, Cioffi WG, Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16(1).
- Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605.
- Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943.
- Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit Care Med. 2013;41(8):1929–1937.
- Ko SF, Lee TY, Huang CC, et al. Severe acute respiratory syndrome: prognostic implications of chest radiographic findings in 52 patients. Radiology. 2004;233(1):173–181.
- Mitrouska I, Klimathianaki M, Siafakas NM. Effects of pleural effusion on respiratory function. Can Respir J. 2004;11(7):499–503.
- Ahmed SH, Ouzounian SP, Dirusso S, Sullivan T, Savino J, Del Guercio L. Hemodynamic and pulmonary changes after drainage of significant pleural effusions in critically ill, mechanically ventilated surgical patients. J Trauma. 2004;57(6):1184–1188.