Abstract
Despite enormous progress in the field of pain management over the recent years, pain continues to be a highly prevalent medical condition worldwide. In the developing countries, pain is often an undertreated and neglected aspect of treatment. Awareness issues and several misconceptions associated with the use of analgesics, fear of adverse events – particularly with opioids and surgical methods of analgesia – are major factors contributing to suboptimal treatment of pain. Untreated pain, as a consequence, is associated with disability, loss of income, unemployment and considerable mortality; besides contributing majorly to the economic burden on the society and the health care system in general. Available guidelines suggest that a strategic treatment approach may be helpful for physicians in managing pain in real-world settings. The aim of this manuscript is to propose treatment recommendations for the management of different types of pain, based on the available evidence. Evidence search was performed by using MEDLINE (by PubMed) and Cochrane databases. The types of articles included in this review were based on randomized control studies, case–control or cohort studies, prospective and retrospective studies, systematic reviews, meta-analyses, clinical practice guidelines and evidence-based consensus recommendations. Articles were reviewed by a multidisciplinary expert panel and recommendations were developed. A stepwise treatment algorithm-based approach based on a careful diagnosis and evaluation of the underlying disease, associated comorbidities and type/duration of pain is proposed to assist general practitioners, physicians and pain specialists in clinical decision making.
Introduction
Pain, as defined by the International Association for the Study of Pain (IASP) is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”.Citation1 Pain can be experienced as an acute, chronic or intermittent sensation, or as a combination of the three, and is reported to be the common reason for medical visits. However, since pain has always been regarded as a symptom, not a disease state,Citation2 there has been an enormous gap between prevalence and treatment, and it remains largely undertreated.Citation3,Citation4 Acute pain, the most commonly experienced type of pain, may be a result of injuries, acute illnesses, surgeries or labor.Citation5 The overall prevalence of acute pain in hospital and ambulatory settings is reported to range between 30% and 80%.Citation6–Citation8 In addition to discomfort, uncontrolled acute pain translates to chronic pain, which may lead to delayed healing, prolonged hospitalization and increased morbidity.Citation9 Globally, approximately 20% of adults suffer from pain, of which, 10% report persistent pain.Citation10 Contrary to acute pain, chronic pain is seldom considered an individual entity, the focus majorly being on symptom relief. Chronic pain is generally associated with chronic illnesses such as neuropathy, cancer or human immunodeficiency virus infection–acquired immune deficiency syndrome (HIV-AIDS), and it is difficult to predict the nature and severity of pain.Citation11 Several studies report a significantly high prevalence of chronic pain with certain neuropathic characteristics (6%–10%), which is of higher severity and longer duration.Citation12–Citation14 Untreated chronic pain significantly affects the quality of life (QoL) of the patients,Citation15 is associated with considerable mortality,Citation16 and can be a major cause of absenteeism from work or reduced work performance as well as loss of productivity.Citation17
In developed countries too, pain is a commonly reported medical problem and comes with immense personal costs and a huge societal health care burden;Citation18,Citation19 the cost of chronic pain in the US has been estimated at $560–$635 billion.Citation19 It is, therefore, not surprising to note an uncharacteristically high prevalence of pain in a developing economy like India, which is already struggling with a growing disease burden and lack of quality health care resources.Citation20 According to the Global Burden of Diseases (GBD) survey, low-back pain (LBP) and migraine were amongst the top five medical conditions resulting in years lived with disability in the Indian population in 2013.Citation21 In India, around 100,000 patients with cancer or HIV-AIDS die every year due to inadequate pain treatment.Citation22 A survey of chronic pain conducted across eight major cities in India highlighted a high point prevalence (13%) of chronic pain, with 30% of the patients without treatment and 56% reporting unsatisfactory treatment.Citation23
Currently, there is a need to develop treatment recommendations based on available evidence to guide Indian physicians in the management of pain.
Unmet needs of India
According to a survey by the World Health Organization (WHO), the incidence of chronic pain was demonstrated to range between 5% and 33% in 15 centers across Asia, Africa, Europe and the USA.Citation10 India has a high burden of chronic diseases and injuries, which are the leading causes of disability and mortality and have pain as comorbidity.Citation24,Citation25 Globally, pain prevalence in the elderly population is highCitation26 and is further expected to rise,Citation27,Citation28 owing to the presence of multiple comorbiditiesCitation29 and barriers related to communication and cognition.Citation30 With a considerable increase in life expectancy in the past decade, India has witnessed an unprecedented rise in the ailing population, who are struggling to get adequate treatment for pain.Citation31,Citation32 Furthermore, there are some additional challenges, such as lack of adequate health care facilities, poverty, inadequate health care expenditure and lack of awareness among health care practitioners and patients.Citation33 Health care centers in India are often not well equipped to provide adequate treatment for pain, with physicians relying mostly on over-the-counter analgesics.Citation34,Citation35 This generalized approach seldom works in most complex pain disorders, which require a detailed diagnosis and thoughtful, planned management.Citation36 Another concern stemming from lack of awareness is the use of irrational and inappropriate multidrug prescriptions in India. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the second most common components of prescriptions in India.Citation37–Citation39 Rampant polypharmacy, coupled with lack of awareness, has had a huge impact on analgesic efficacy and presents tolerability issues.Citation40 Several misconceptions regarding use of analgesics and fear of adverse events associated with the use of opioids or surgical methods of analgesia result in suboptimal treatment of pain.Citation41,Citation42 These inadequacies might be overcome by the amalgamation of expert opinions and available evidence to form standardized treatment guidelines for pain therapy. Hence, an expert panel was convened to delve on these lacunae and frame an appropriate treatment paradigm for pain management. The objective of this publication is to summarize the evidence-based consensus recommendations of this expert panel for the pharmacological management of pain in India.
Methodology
Literature search of the current evidence was performed using the MEDLINE (by PubMed) and Cochrane databases. The articles included randomized control studies, case–control or cohort studies, prospective and retrospective studies, systematic reviews, meta-analyses, clinical practice guidelines and evidence-based consensus recommendations. Evidence grading was performed as per the following criteria used by the National Guidelines Clearinghouse:Citation43
Level 1: evidence from randomized control studies, systematic reviews and meta-analyses
Level 2: evidence from nonrandomized, prospective or retrospective studies
Level 3: evidence from comparative, case–control and descriptive studies
Level 4: expert committee reports or clinical opinions of respected authorities
A multidisciplinary expert panel, composed of pain specialists, orthopedic surgeons, gastroenterologists, cardiologists, neurologists and nephrologists, with clinical and research expertise in the diagnosis and treatment of pain was convened, and recommendations were formulated based on expert opinions and available evidence. Based on the level of evidence, recommendations were graded as follows:
Grade A: based on evidence Level 1
Grade B: based on evidence Level 2 or extrapolated from Level 1
Grade C: based on evidence Level 3 or extrapolated from Level 1 and 2
Grade D: based on evidence Level 4 or extrapolated from Levels 1–3
Recommendation statements were developed, circulated among panel members and modified based on their feedback until unified consensus was achieved.
Types of pain
Several methods of classification of pain have been developed to assist the physician in the appropriate diagnosis and treatment of pain in routine clinical practice. Pain can be classified into different types on the basis of duration or intensity, pathological mechanism and anatomic location ().
Table 1 Different types of pain
Based on underlying pathophysiology
Nociceptive pain
Nociceptive pain is elicited through A, δ and C nociceptive receptors present in peripheral tissues, which are activated by chemical, mechanical or thermal stimuli.Citation44 These receptors play an important role in the regulation of natural defense mechanisms and elicit pain as a response to potential tissue damage. A number of chemical substances aggravate the nociceptive response to pain; the most common ones are bradykinin, histamine, serotonin and prostaglandins (cyclooxygenase [COX] 1 and 2).
Neuropathic pain
Neuropathic pain primarily refers to pain initiated or caused by a primary lesion or damage to the central nervous system and includes disorders such as peripheral neuropathy, diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, causalgia and certain types of cancer pain.Citation45,Citation46 The pathways attributed to the generation of neuropathic pain involve abnormal excitability of afferent neurons, central sensitization following peripheral activation of neurons, loss of primary afferent response and activation of proinflammatory cytokines.Citation47,Citation48 Each of these factors may correspond to a particular mechanistic pathway, causing differences in the pattern and intensity of the somatosensory response.Citation49,Citation50
Mixed pain
In certain cases, pain may result from a combination of nociceptive and neuropathic processes.Citation51 This type of pain is considered as the mixed type and is most frequently experienced by patients with cancer and those with some complex neuropathies.Citation52–Citation54
Based on duration
Acute pain
Acute pain is caused due to an existing disease or injury, usually lasting for hours, days or months or until the disease or injury is healed.Citation55 This type of pain has a certain identifiable biological origin and is predictable in nature.Citation56 Acute pain is a part of the normal biological defense against a potential threat or injury and is accompanied by protective reflexes.Citation57 The sources of acute pain could be an acute illness (eg, appendicitis), perioperative (pain due to existing disease and surgical procedure), major trauma (vehicle accident), burns or due to childbirth.
Chronic pain
Chronic pain persists even after the healing of the disease or injury and may be physically and emotionally distressing. Often, there is no single biological point of origin for this pain, resulting in a complex pathophysiology that necessitates the use of multiple therapeutic interventional strategies for alleviation.Citation58 This type of pain may be a result of an existing chronic disorder (examples are cancer or noncancer pain such as HIV-AIDS, rheumatoid arthritis and LBP) or may have no known cause (idiopathic pain). Moreover, some secondary causes may include increased sensitivity to external stimuli (hyperalgesia) or pain due to innocuous stimuli (allodynia).Citation59
Breakthrough pain
Breakthrough pain is defined as transient flare-ups of severe and uncontrolled pain, in adequately controlled pain settings.Citation60 These episodic exacerbations of pain are characteristically observed in patients with cancer and last usually from a few seconds to hours. Breakthrough pain may be idiopathic (without known cause), incidental and associated with a precipitating factor (eg, movement), or may be experienced in the interim between two doses.Citation61
Based on etiology
Cancer pain
Pain is the most common symptom experienced by patients with cancer. The origin of cancer pain may be nociceptive (somatic or visceral) when there are changes in the underlying tissue pathology and neuropathic, when there is an involvement of neuronal damage. The severity of cancer pain increases as the disease progresses, with patients in the metastatic stage reporting the most severe pain.Citation62 Cancer pain may be felt at the site of tumor or may be referred to a secondary region of the body.Citation63 Breakthrough pain is another feature of cancer pain, which may be a consequence of repeated nociceptive sprouting or synaptic changes at the spinal dorsal horn (DH).Citation64 Optimization of opioid therapy may be useful in preventing episodic exacerbations at the end of dose.Citation65
Noncancer pain
Chronic pain due to nonmalignant causes is referred to as chronic noncancer pain (CNCP). Unlike cancer pain, CNCP may present no identifiable underlying pathology, may show increased heterogeneity and may be more challenging to treat.Citation66 CNCP may be classified further as neuropathic pain (peripheral and central), inflammatory pain (infectious, arthritic and postoperative pain), muscle pain (myofascial pain syndrome) and mechanical pain (LBP, neck-and-shoulder pain). Various practice guidelines advocate the use of opioids as first-, second- or third-line therapy in CNCP, following failure of nonopioid analgesics.Citation67,Citation68
Based on anatomic location
For this purpose, anatomic location refers to a region or site where the actual pain may be localized, rather than the location of the body system where the pain originated.Citation69 As per the IASP, pain is anatomically classified, regardless of origin, as localized to head, face and mouth pain; cervical pain; upper shoulder and upper limb pain; thoracic pain; abdominal pain; LBP, lumbar spine and coccyx pain; lower limb pain; pelvic pain; as well as anal, perineal and genital pain.Citation70 Although this categorization helps in differential diagnosis, it cannot be used as a basis for therapeutic management.Citation71
Somatic pain
Somatic pain is a sharp, well-localized pain emanating from the skin due to superficial damage and activation of peripheral nociceptors on the skin or mucous membranes.Citation72 Alternatively, the pain may be deep and central in origin, originating from muscles, tendons or joints. Examples are LBP, neck pain, musculoskeletal pain and pain due to burns, injuries or wounds.Citation73
Visceral pain
Visceral pain is deep, poorly localized pain resulting from pressure or damage to internal organs; it may be accompanied by low blood pressure, nausea or sweating as a response to stimulation of nerve endings that are at the site of pain and that relay signals to the CNS. Examples of visceral pain are gall/kidney stones, gastric ulcers or intestinal spasms.Citation74,Citation75
Referred pain
Pain that is felt at a site away from the site of injury is called referred pain. The origin of referred pain may be somatic (involving muscles or joints) or visceral (involving visceral organs).Citation76 Several mechanistic theories have been suggested to be involved in the cause of referred pain. Referred visceral pain may be attributed to the hyperexcitability of adjacent spinal neurons in response to nociceptive stimuli or a possible activation of the peripheral reflex arc via the efferent pathway.Citation77 Examples of referred pain are myocardial pain and anginal pain.
International Classification of Diseases, Eleventh Revision (ICD-11) criteria for classification of chronic pain
Although several methods of classification are available for the categorization of pain, there is a lack of accurate and consistent terminology for the coding of chronic pain in compliance with the ICD system used by the WHO. Responding to this, the IASP developed a system of classification of chronic pain based on the ICD criteria, which takes into consideration the etiology, location and pathophysiology of pain for better categorization of the disease. Categorization is based on multiple parenting, wherein each diagnosis falls under one primary category/parent but corresponds to more-than-one secondary categories.Citation78 According to this classification, chronic pain may be categorized as primary pain, cancer pain, posttraumatic or postsurgical pain, neuropathic pain, headache and orofacial pain, and musculoskeletal and visceral pain.
Pathophysiology of pain
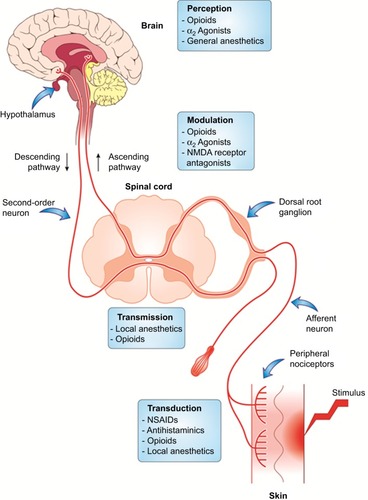
Regardless of its categorization, pain cannot be distinctly attributed to any isolated pathological event. Pain experience is a complex process that involves the activation of multiple neuronal signaling pathways within the peripheral nervous system (PNS) and central nervous system (CNS).Citation73,Citation79,Citation80 The subjective experience of pain may be summarized as a four-stage process - transduction, transmission, modulation and perception.Citation81 In stage 1 (transduction), nociceptive stimuli of tissue-damaging potential (including mechanical, chemical and thermal stimuli) are converted by the sensory cells into action potentials. Stage 2 (transmission) involves the conduction of these action potentials along afferent neurons to the DH of the spinal cord. In stage 3 (modulation), coding of nociceptive information occurs at the level of spinal DH. Modulation at the DH can be excitatory or inhibitory, thereby increasing or decreasing the resulting pain. Stage 4 involves generation of autonomic, affective, cognitive and behavioral responses to the painful stimulus, leading to pain perception.
Sensitization
Peripheral sensitization
Peripheral sensitization is characterized by abnormal sensitivity of afferent nociceptors to noxious stimuli. Nociceptors on the skin and deeper tissues sometimes become extremely sensitive to intense noxious stimuli in the presence of inflammation. This further lowers the threshold of nociceptor activation to normally innocuous or less-painful stimuli, accompanied by an increase in the degree or magnitude of response. Additionally, extreme sensitization may lead to the activation of sleeping or silent nociceptors, which upon excitation amplify the pain response manifold. Drugs acting at the peripheral nociceptor level are NSAIDs, opioids, cannabinoids and transient-receptor potential vanilloid (TRPV1) receptor antagonists.
Central sensitization
Afferent pain signals from the peripheral nociceptors to the spinal neurons may sometimes activate low-threshold mechanoceptors at the DH, thus amplifying the central neuronal response to noxious stimuli.Citation82 This phenomenon of altered sensitivity of neuronal cells at the level of the second-order neuron is known as central sensitization. Central sensitization is implicated in the transition of acute pain to chronic degenerative pain. As observed with peripheral sensitization, primary hyperalgesia is the first manifestation of altered threshold at the central neuronal level.Citation82 Under pathological conditions, receptors that are normally associated with sensory response to stimuli such as touch may acquire the ability to produce pain, resulting in secondary hyperalgesia, an important aspect of central sensitization.Citation64 Unlike peripheral nociception, a number of neurochemical drivers are involved in the modulation of pain perception at the central level, resulting in a complex interplay of events that underlie the pathology of many chronic and neuropathic pain conditions ().Citation80,Citation83
Figure 1 Mechanism of peripheral versus central sensitization.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ATP, adenosine triphosphate; ASIC, acid-sensing ion channels; BDNF, brain-derived neurotropic factor; 5-HT, 5-hydroxytryptamine; IL, interleukin; NMDA, N-methyl-d-aspartate receptor; NGF, nerve growth factor; PG, prostaglandin; NK1, neurokinin-1; TNF, tumor necrosis factor; TrkB, tyrosine receptor kinase B; TRPV, transient receptor potential vanilloid receptor.
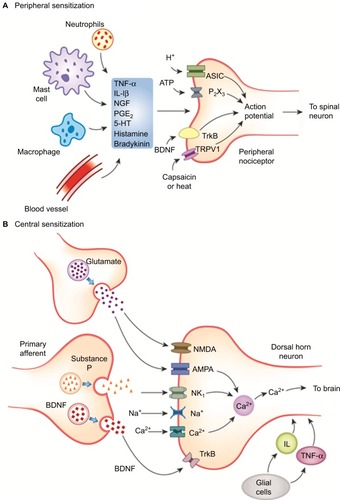
Drugs that modulate the pathways leading to central sensitization act by increasing the level of biogenic amines and reducing the spontaneous ectopic discharges. These include serotonin–norepinephrine reuptake inhibitors (SNRIs, such as duloxetine, paroxetine, venlafaxine), anticonvulsants (gabapentin and pregabalin) and tricyclic antidepressants (TCAs, such as amitriptyline, imipramine and desipramine).Citation84–Citation86
Wind-up phenomenon
Wind-up is the temporal summation of excitatory impulses following repeated firing of spinal dorsal neurons due to C-fiber discharges.Citation64 The process of wind-up begins with the release of excitatory neurotransmitter glutamate, which binds to the N-methyl-d-aspartate receptor (NMDA)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor.Citation87 Activation of these receptors, together with release of substance P from the C-fibers, initiates the depolarization of the cell membrane and triggers the intracellular release of Ca2+. Repetitive sensitization of NMDA receptors further contributes to hyperexcitability of the membrane, which aggravates the resulting response to pain, resembling an unending loop.Citation80,Citation88 Antagonism of NMDA receptor by drugs such as ketamine prevents the generation of excitatory response and provides therapeutic benefit in chronic neuropathic pain.Citation89
Neuroplasticity and pain
Apart from central sensitization, inflammation induces significant changes in the neuroplastic structure of the brain and spinal cord. Altered neuroplasticity is a characteristic feature of neuropathic pain and is often a consequence of tissue damage or injury.Citation90 The positive feedback-mediated structural repair following an injury or nerve damage may also contribute to selective degeneration and alteration of function, often progressing to the neuropathic state.Citation91 Modulation of peripheral nociceptor function may result in painful neuropathies, characterized by heightened sensitivity to noxious or normal stimuli and a pricking, tingling or burning pain. On the contrary, central nerve damage triggered by infection, metabolic disorders or mechanical insult may result in loss of pain sensation such as in diabetic neuropathy.Citation90
Modulation of nociceptive traffic
All events in the DH do not facilitate nociception. Spinal interneurons release amino acids (eg, γ-aminobutyric acid [GABA]) and neuropeptides (endogenous opioids) that bind to receptors on primary afferent and DH neurons and inhibit nociceptive transmission by presynaptic and postsynaptic mechanisms.
Descending transmission pathways are responsible for abolishing the ascending signals traveling up the central nervous system so that the pain perception is reduced,Citation64 via release of endorphins and monoamines.Citation92 Opioids like morphine act by modulation of both ascending and descending pain pathways ().Citation93
Progression from acute to chronic pain
Acute and chronic pain cannot be segregated into two distinct types; chronic pain is rather an extension of acute pain, resulting from poorly managed acute pain.Citation94 The metamorphosis of acute to chronic pain is believed to proceed through one of the following processes: central sensitization, modulation or modification of nociceptors, and altered neuroplasticity.Citation95,Citation96 Under normal circumstances, as a healing process begins, the noxious stimuli reduce and pain diminishes. However, in cases of persistent pain, peripheral and central sensitization most frequently transcends to hyperalgesia (increased response to less-painful stimuli) and allodynia (pain response to normally nonpainful stimuli). Altered spinal neuroplasticity as a result of glial cells modifying the neuronal cytoarchitecture is another important aspect of chronic pain and may be a consequence of repetitive nociceptive firing in the central neurons (reversible) or nerve damage due to neuropathy (irreversible).Citation97 Studies have reported substantial loss of gray matter in the brain and changes in the structure, sensitivity and activity of neurons as well as internal rewiring in certain chronic pain conditions.Citation91,Citation98,Citation99 These changes may be clinically manifested in the form of fibromyalgia or complex regional pain syndrome.Citation98 There is also a significant decline in neurotransmitter receptors and their function, which may explain the lack of efficacy with central opioid agonists in neuropathic pain.Citation91 While management of acute pain symptoms might seem to be the most rational approach from a clinician’s perspective, it is essential to evaluate the risk factors associated with pain chronification.Citation100 Increased pain intensity, loss of functionality and affected psychosocial ability are a few predictive factors for development of chronic pain.Citation101,Citation102 Studies have shown that assessment of functional and psychosocial outcomes is a highly favorable measure in predicting the risk of chronic pain.Citation103,Citation104
Undertreatment of pain
Pain is the most prevalent, yet frequently undertreated, medical condition globally.Citation105,Citation106 Untreated or inadequately treated pain is the major cause of depression and eventually leads to loss of productivity and functionality, as well as poor health-related QoL, of patients.Citation107,Citation108 There are several impediments that contribute to the undertreatment of pain in India, which include lack of awareness, regulatory restrictions on opioid use, physician attitudes, overuse of NSAIDs and insufficient resources for managing pain in primary care settings.Citation42,Citation109–Citation111 It is essential to minimize these barriers to ensure optimal treatment of pain.
Therapeutic management of pain
Evolution of analgesics
In the wake of increased understanding of the pathophysiology of pain, various receptors have been identified as potential targets for drug therapy over the years. In the early 1970s, Vane et alCitation112 proposed the involvement of prostaglandin inhibition as the mechanism of action of aspirin-like drugs. These findings paved the path for development of NSAIDs, targeting the COX-1 enzyme system involved in the synthesis of prostaglandins, thereby reducing both pain and associated inflammation. The earliest known marketed NSAIDs were ibuprofen and aspirin, which were followed up by similar compounds, with varying efficacies.Citation113 Acetaminophen, the safest analgesic till date was developed in 1946, after two synthetic antipyretics, acetanilide and phenacetin, were developed and had significant associated toxicities.Citation114 In the search for a more effective and safer analgesic, research was focused on the development of the selective COX-2 inhibitors celecoxib and rofecoxib.Citation115 However, there were severe concerns of cardiac toxicities observed with these agents, limiting their use.
Morphine and codeine were the earliest natural opioid analgesics to be developed and used in clinical practice. Further research led to the development of synthetic opioids such as fentanyl and pethidine, with increased efficacy and long-term activity. However, in the light of associated risk of overuse, addiction and misuse of these analgesics, it was recommended to follow a risk-management and monitoring program during opioid therapy. Tramadol, an opioid analgesic different from conventional opioids, was developed in 1977 and has since been routinely used in moderate-to-severe pain, owing to its high safety and efficacy.
Further scientific investigation into pain pathology has led to the development of anti-nerve growth factor (anti-NGF)- and anti-TRPV1-based therapies. However, these agents are still under clinical development, and there is a lack of conclusive evidence supporting their efficacy and safety in routine clinical practice.Citation116,Citation117 Deeper insights into the central pain pathways have uncovered some overlapping pathological factors implicated in pain, depression and other psychological disorders.Citation118 Antidepressant-induced increase in the neurotransmitters serotonin and norepinephrine has been thought to alleviate pain, the postulation being reinforced by the remarkable efficacy shown by antidepressants in chronic pain, particularly of neuropathic origin.Citation119 Similarly, the efficacy of the anticonvulsants pregabalin, gabapentin and carbamazepine was demonstrated in several clinical studies.Citation120 As a guidance to physicians treating chronic cancer pain, the WHO established an evidence-based three-step analgesic ladder that provides a stepwise approach for deciding treatment according to the intensity of pain.Citation121 This ladder has been widely adopted for use in noncancer pain settings.Citation122 However, the three-step ladder is not considered applicable anymore and has been modified to suit different clinical settings, due to the practical delays in administering pain relief for patients with severe pain and also due to concerns over the efficacy of low-dose opioids used in step two.
Evidence from several studies has highlighted the importance of nonpharmacological interventions for pain relief.Citation123 Exercises such as stretching and strengthening, relaxation, postural stabilization and yoga; therapeutic heat and cold packs; ergonomic adaptations; modification of daily activities; chiropractic treatment; massage or traction are some of the nonpharmacological approaches that are routinely used with pharmacological therapy.Citation124–Citation129
Therapeutic drug classes
A list of available therapies for pain management is represented in .
Table 2 Available therapies for treatment of pain
Need for individualized pain management
An individual’s perception of pain may be greatly influenced by the emotional and behavioral responses and is strongly correlated to his/her culture, personal history and genetic makeup.Citation130–Citation133 For instance, presence of different haplotypes of the catecholamine-O-methyltransferase gene has been linked to variability in pain perception in humans.Citation134 Thus, treatment for pain must be tailored for each individual and should focus on interruption of reinforcement of the pain behavior and modulation of the pain response. Important factors for consideration for treatment recommendation include individual’s response to prior appropriate treatment management, compliance and drug abuse/dependence behavior.Citation56 The biopsychosocial model of pain encompasses the biological, psychological and social aspects of pain and has been increasingly used in a variety of chronic pain settings.Citation135,Citation136 Since the experience of pain is heterogenic in nature; a “one size fits all” approach would not be the most ideal way to manage pain. In short, an individualized approach to treatment based on the genetic, cultural, social and behavioral aspects of the patient may help in the optimal management of pain across different clinical settings.Citation56,Citation137
Role of combination therapies
Combination therapies may consist of combination of two or more drugs administered separately or as fixed-dose combinations (FDCs) of two or more active ingredients in a single-dosage form.Citation138 Use of FDCs of analgesics represents a multimodal approach that is based on the integration of various mechanisms to result in pain relief.Citation139 FDCs have been hugely preferred to treat a variety of disorders owing to their safety and efficacy. Additionally, use of FDCs may improve patient adherence to therapy and reduce the cost associated with multiple medications.Citation140 However, not all FDCs are rational, and the majority of them may present no evidence of improved efficacy or tolerability.Citation141,Citation142 In India, FDCs have been used generously, notwithstanding the complete lack of efficacy with some combinations.Citation143 The Central Drugs Standard Control Organization (CDSCO) has taken cognizance of the increased use of irrational drug combinations by physicians in India and has recently banned more than 300 FDCs from the market.Citation144 From a physician’s perspective, the rationale for use of FDCs must identify clear association between FDC use and increased efficacy or reduced adverse events. Furthermore, from a regulatory perspective, the components of an FDC must possess complementary pharmacokinetic profiles and should not have supra-additive toxicity.
The US Food and Drug Administration (USFDA) draft guidance for industry mandates the fulfillment of some necessary conditions prior to the approval of analgesic FDCs. Thus, an FDC awaiting FDA approval must demonstrate strong evidence of efficacy over the available single-agent therapy in well-controlled single- or multiple-dose clinical studies. Moreover, each component of the FDC must contribute to the overall efficacy and safety of the product, in order to justify its use over single-agent therapies.Citation145 A similar guidance document has been developed by the CDSCO to guide the development of FDCs in India.Citation146 A list of approved analgesic FDCs in India is provided by the CDSCO.Citation147
Reasons for mismanagement of pain
Adequate treatment according to national and international treatment guidelines can result in sufficient pain relief in majority of the patients. However, barriers to the use of analgesics can result in undertreatment of pain. Other than institutional barriers such as government regulations regarding opioid prescription and irregularities in the treatment of patients with pain, physician and patient barriers hinder effective management of pain.
Physician barriers
Physician’s knowledge and understanding regarding patients’ pain, principles of treating pain, analgesics ladder and knowledge about the efficacy of different routes of opioid administration are very important for pain management.Citation148–Citation152 Another factor affecting inadequate assessment of pain by physicians included concerns regarding side effects or tolerance to opioids.Citation43,Citation153–Citation155 In cancer patients, inadequate pain assessment was considered a major barrier (20%–80%) in pain assessment.Citation42,Citation148,Citation149 The physicians either do not use an appropriate method to measure the pain intensity or fail to appropriately classify the pain. Misconceptions regarding the regulations governing opioid use also exist.Citation156
Concerns regarding addiction to or physical dependence on, as well as side effects of opioids prevent physicians from prescribing opioids according to standardized guidelines.Citation68,Citation127,Citation157–Citation159 According to the guidelines by the WHO, strong opioids should be the first choice of treatment for moderate-to-severe pain and the use of these opioids must be continuously personalized based on patients’ response in terms of pain relief. In a study by Tournebize et al,Citation160 more than 50% of surveyed physicians reported insufficient adherence to treatment guidelines for chronic pain. Studies have also shown that physicians hesitate to administer adequate doses of opioids because of the fear of the potential development of tolerance by the patient.Citation42,Citation161,Citation162 Most often, physicians do not consider assessment of pain as a priority and, as a result, do not give pain relief its due importance. Furthermore, a patient’s self-report of pain, considered the best indication of pain, is viewed with skepticism.
Patient barriers
Cognitive barrier results from patients’ lack of understanding about the different options of pain management, along with attitudes and beliefs that not only affect patients’ pain communication and adherence to analgesic regimen but also have a negative impact on the outcomes of pain management.Citation163
Many patients lack the knowledge of the principles involved in effective pain management and have concerns about taking pain medication (mostly concerns about addiction and side effects of pain medication). A few researchers believe that there is a relationship between better knowledge and less pain intensity or between more negative beliefs and more intensive pain.Citation164–Citation169 However, this association was either partially confirmed or not confirmed by other researchers.Citation170,Citation171
Patients beliefs and attitudes that hinder pain management include the following: fear of addiction, that originates from a misunderstanding of the relationship between psychological addiction, physical dependence and tolerance; concerns about analgesic use (side effects); concerns about pain communication due to age, language, cultural traditions or other illness (communication issues between patients, nurses and doctors [sensory barrier]; willingness to tolerate pain to be a good patient and to not trouble the family); not adapting adequately to the possibility of controlling pain in general (stigma of using pain medication, stoicism about dealing with pain, assuming that pain is a part of the disease and suffering, concerns regarding the capability of the caregiver to understand their needs, hesitation to question authority); and inadequate adherence to pain medication (sensory barrier).Citation42,Citation155,Citation166,Citation169,Citation172–Citation175 Financial barriers could also result in reduced adherence to treatment.
Management myths
Several myths about pain and its treatment exist in our society, which ultimately prevent the patient from receiving adequate care. According to the American Chronic Pain Association, some of the commonly accepted beliefs revolve around the perception (pain is an emotional and not a physical disorder) and treatment of pain (analgesic therapies may not be effective or may lead to addiction).Citation176 In addition, undertreatment of pain in the elderly and children may be primarily due to the assumption that pain cannot be felt by newborns and babies but is an acceptable and natural part of the aging process and therefore may not warrant medical attention.Citation177 It is essential to disprove these myths by promoting awareness among patients, physicians and the society in general.
Comorbidities
Presence of comorbid medical conditions may not only influence the response to analgesics but may present difficulties in diagnosis as well. The most commonly associated psychological comorbidities in pain are depression and anxiety.Citation178–Citation180 These may be preexisting or may result from the pain itself, often aggravated in untreated or undertreated chronic pain.Citation181 Presence of depression is known to have considerable impact on outcomes of pain treatment, signifying the two-way relationship between pain and depression. A systematic review of studies correlating the effect of depression on pain showed increased severity, intensity and duration of pain in depressed patients.Citation124 Functional assessment using valid tools may indicate the presence of underlying symptoms, necessitating the use of antidepressant therapy, which can in turn effectively help control pain.Citation126,Citation182 Until recently, the outcome of pain therapies was evaluated on the basis of reduction in pain intensity and improvement in sensory/motor ability, seldom delving into the psychological or cognitive aspects that frequently led to mismanaged pain. Clinical investigations over the years have resulted in the use of cognitive behavioral therapy in pain treatment programs, with remarkable efficacy in the acute and chronic pain settings.Citation183,Citation184
Management recommendations
General considerations (evaluating a patient with pain)
The recommendations are based on graded evidence as explained previously. The grades are mentioned in parenthesis next to each recommendation.
History
A thorough evaluation of the patient profile, patient history and presence of comorbid conditions is necessary as a first step toward pain diagnosis (C).
Diagnosis should involve measurement of pain intensity, physical examination, sensory examination, motor examination and evaluation of cognitive and functional well-being (B).
Assessment and monitoring
Identify and use appropriate pain assessment tools (A) For the purpose of designing treatment strategies for managing pain, it is essential to assess the severity of the pain. Use of pain assessment scales may help in the diagnosis of the disease, design of treatment strategies, monitoring and evaluation of outcome of therapy or for predicting the impact of pain on functional outcomes of a patient ().Citation185 Factors that should be taken into consideration while selecting the appropriate pain assessment method include sensitivity and specificity, usefulness or value of the assessment tool, objective of the assessment, age of the patient and extent of symptoms presented by the patient.Citation186
Table 3 Different pain assessment scales for measuring the intensity of pain
Sensory examination
Components of a sensory examination include light touch, pinprick, temperature (hot or cold) and vibration.Citation187 Quantitative sensory testing (QST) might help distinguish between various types of neuropathic pain.Citation188,Citation189 In patients with neuropathy, the threshold for pinprick and thermal sensations are increased, while increased sensitivity to light touch may indicate presence of allodynia or hyperalgesia, which may point toward painful inflammation or fibromyalgia with a central neuropathic origin.Citation190–Citation192 The QST has also been used in the diagnosis of osteoarthritis and other musculoskeletal conditions, although not as a sole measure.Citation193
Motor examination
Evaluation of motor function is an important method of diagnosing musculoskeletal pain, especially back pain and neck pain. Exercises such as neck rotation and neck flexion or extension tests may help determine the localization and intensity of pain (D).Citation194
Diagnostic tests
Examination by X-rays is the most common method used to confirm the localization of pain, particularly in LBP, neck pain, joint pain or pain associated with internal organs.Citation147 Use of computed tomography (CT) scan or magnetic resonance imaging (MRI) might be useful in cases in which diagnosis by physical examination or X-ray may be insufficient or if any underlying damage to the CNS is suspected to be involved.Citation195 Further details are presented in the Supplementary material section.
Comorbidities
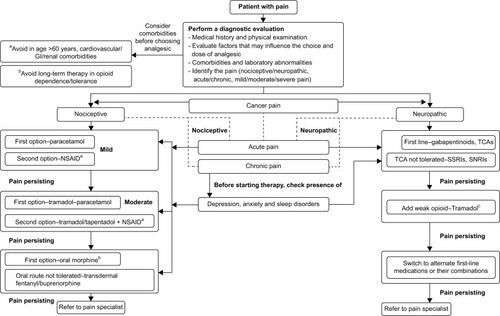
Since comorbidities may influence pain perception and affect the treatment outcomes, it is necessary to carry out screening of the patient for possible physical illnesses (liver impairments, gastrointestinal diseases, renal impairments, physical dependence to opioids and so on) and psychiatric comorbidities (depression, anxiety, psychological disorders) prior to initiating treatment ().
Figure 3 Pain algorithm.
Abbreviations: GI, gastrointestinal; NP, neuropathic pain; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Decide treatment goals
Prior to starting treatment, it is essential to decide the treatment goals so as to prevent undertreatment. It is important to consider the ethical implications of the associated treatment. The following questions may help: how severe is the pain? Is the pain associated with disability or loss of function? What are the existing comorbidities? Is psychosocial function impaired? Is opioid therapy required?
According to Cohen and Jangro,Citation196 treatment goals need to be broader, achieving a little more than pain reduction (must include QoL and the functional impact of pain treatment), and must be realistic, in order to ensure optimal therapy with minimal side effects.
Investigations for neuropathic pain
A detailed diagnosis of neuropathic pain involves, in addition to pain assessment tools (using neuropathic pain scales) and QST, evaluation of neurological function using functional neuroimaging, neurophysiology studies, skin biopsy techniques and other laboratory testing methods.Citation197 Additional laboratory investigations include microneurography studies, laser-evoked potentials, electrophysiology studies and nociceptive reflexes.Citation189
Pharmacological management
In order to understand the nature of pain and to determine the treatment course, it is essential to distinguish the physical causes of pain in addition to assessing the mental and environmental factors.
The choice of analgesic prescribed is based on the type of pain, duration of pain and comorbidities. A few years ago, NSAIDs, acetaminophen and opioids were prescribed in the treatment of all types of pain. This methodology is now considered ineffective, and with an increase in the awareness regarding different classifications of pain, mechanism-specific treatment is recommended.
Comorbidities influencing analgesic use
Studies have shown that a number of comorbidities influence the treatment outcomes of analgesic therapy. It is widely known that several chronic pain conditions are accompanied by psychosocial comorbidities such as anxiety and depression, which may amplify the pain perception, physical function, adaptation and treatment response.Citation198,Citation199 Systematic screening of patients for any associated psychiatric comorbidity may support the physician in deciding the treatment plan. In the case of suspected depression, antidepressants may be added to the therapeutic regimen to control the clinical symptoms.Citation200 While psychiatric comorbidities significantly affect the emotional response to pain, any coexisting physical comorbidities may affect the efficacy and tolerability of the analgesic. For example, decreased liver function may significantly affect the metabolism of NSAIDs and opioids, leading to increased levels in blood and associated toxicity. Preliminary screening by performing liver function tests and assessment of the Child–Pugh score may be important in determining liver failure.Citation201 Similar considerations are necessitated in suspected gastrointestinal or renal comorbidities.Citation202,Citation203
Duration of pain
Duration of pain is a decisive factor guiding the choice of an appropriate analgesic regimen.Citation204 However, duration of pain should always be correlated with the pain intensity.Citation158,Citation199
Acute pain
The primary pain management goal here is early intervention to reduce the pain to acceptable levels. Most of the acute pain is nociceptive and treatment for acute pain involves a combination of paracetamol, opioids and some topical agents. Mild somatic acute pain could be treated with paracetamol, NSAIDs, topical anesthetics and physical treatments such as ice, compression and so on. For acute visceral pain, NSAIDs, opioids, corticosteroids and agents modulating the central neuronal activity (TRPV1 antagonists) may be used, depending on the underlying comorbidity. For moderate-to-severe pain, a combination of opioids and nonopioids could offer optimal pain relief. In cases of pain from trauma or surgical procedures, systemic medication would have to be administered.
Chronic pain
Management goals for chronic pain include reducing the suffering due to pain and the associated emotional distress, in addition to increasing the coping ability and psychosocial well-being of the patient, by targeting the hypersensitive nervous system.Citation205 Multimodal therapy involving a combination of treatments is recommended for treatment of chronic cancer or noncancer pain. The treatment for CNCP could include nonopioids (paracetamol, NSAIDs and selective COX-2 inhibitors), opioids, analgesics, TCAs, corticosteroids, muscle relaxants, sedatives and anxiolytics.
Nociceptive pain
Nociceptive pain is generally time limited as the pain resolves when the tissue damage heals, but it may also be persistent when associated with inflammatory disorders such as rheumatoid arthritis. Treatment for localized pain should be started with the use of topical or oral NSAIDs.Citation56 Moderate-to-severe pain unresponsive to NSAIDs may be treated with opioids to manage symptoms and control inflammation.Citation206 Additionally, corticosteroids and immune-modulating agents (biologics) can also be used to manage inflammation in arthritic pain.Citation207
Neuropathic pain
Peripheral neuropathic pain manifests as stimulus-independent pain or stimulus-evoked pain (pain hypersensitivity triggered by a stimulus following damage to sensory neurons). Neuropathic pain may persist for months or years beyond the normal healing time of any damaged tissues. Evidence from randomized controlled studies (RCTs) and systematic reviews have confirmed the efficacy of TCAs (amitriptyline and nortriptyline), antiseizure medications (pregabalin, gabapentin and carbamazepine) and opioids in the treatment of various neuropathic conditions.Citation85,Citation86,Citation208–Citation212 Evidence supports the use of opioids as second- or third-line therapies for neuropathic pain, particularly of peripheral origin () (A).Citation208,Citation209 However, further confirmation from well-controlled studies is warranted to establish the efficacy of these agents in complex neuropathies.Citation189,Citation213
Mild, moderate and severe pain
Treatment recommendations are also dependent on the severity of pain. Acetaminophen and other mild NSAIDs such as aspirin are the preferred treatment for mild chronic pain and may be used to supplement other agents in treating mild-to-moderate pain.Citation56 However, acetaminophen lacks anti-inflammatory effects, limiting its use in pain associated with inflammatory diseases such as arthritis. NSAIDs are recommended for periodic flare-ups of mild-to-moderate inflammatory or nonneuropathic pain. For management of persistent moderate-to-severe pain, opioid therapy is the most sought-after treatment. Opioids are considered effective in the treatment of neuropathic pain that is not responsive to initial therapies, but not in the treatment of inflammatory, mechanical or compressive pain. Certain strong opioids, however, do not necessarily improve functional or psychological status and should be administered under adequate supervision.Citation214–Citation216
A person’s experience of pain generally manifests itself in emotional and behavioral responses and is strongly correlated to the culture, personal history and perceptions of the individual.Citation130 Thus, treatment for pain must be tailored for each individual patient and should focus on interruption of reinforcement of the pain behavior and modulation of the pain response. Important factors to be considered for treatment recommendation include individual’s response to prior appropriate treatment management, compliance and drug abuse/dependence behavior.Citation56 An understanding of cultural-based attitudes about pain is critical in the management of pain.
Treatment in special populations
Undertreatment of pain in special populations, such as the elderly, children or patients with renal/hepatic impairment, results from inadequate pain assessment and efficacy issues. Pain treatment in these populations must be performed following careful consideration of adverse events and the pharmacokinetic profile of the analgesic.
Elderly
Studies report that the prevalence of pain is higher in the elderly population, with high proportion of patients with disability and poor QoL.Citation62,Citation217 The most commonly occurring pain conditions in the elderly are rheumatoid or osteoarthritis, cancer and diabetic neuropathy.Citation218 In addition, elderly patients with chronic pain present several clinical comorbidities, which may influence the outcome of pain therapy. These may include gastrointestinal/renal/hepatic dysfunction, depression, anxiety and cognitive impairment, among others.Citation219,Citation220 Due to the limited evidence from clinical studies on the geriatric population with pain,Citation221 the health care practitioner needs to evaluate the possible risk factors that may impede effective analgesia. NSAIDs should be avoided in such patients due to the potential for gastrointestinal bleeding and renal toxicity. Opioids such as morphine, codeine, fentanyl or oxycodone may cause constipation or respiratory depression and should be used with caution, preferably in low doses and along with laxatives.Citation222 Paracetamol is the analgesic of choice for the geriatric population with pain.
Children
Pain in children may be mostly due to injuries, burns, inflammation and chronic illnesses such as cancer, HIV/AIDS or postoperative status. The WHO guidelines on management of chronic pain in children emphasize the importance of pain assessment in children.Citation223 Since self-reports of pain severity cannot be obtained in infants or small children, pain assessment entirely depends upon the clinician’s reports. Symptoms of crying, agitation, irritation or facial expression may be indicative of pain in infants. For children >4 years of age, Faces Pain Scale may be used for pain assessment.Citation224,Citation225 Pharmacological therapy of pain in children should take into account the age, pain intensity, underlying disease or comorbidities as well as the functional ability. The first-line therapy generally includes safer analgesics such as paracetamol, followed by ibuprofen.Citation223 The second-line therapy may include other NSAIDs. Strong opioids should only be used in moderate-to-severe pain.Citation226 The most preferred route of medication administration should be oral, either as tablets or as syrups.Citation134
Pregnant and lactating women
During pregnancy, women may experience LBP, joint pain, as well as abdominal and pelvic pain, among other conditions. Analgesic treatment during pregnancy and lactation may be difficult considering certain medications can potentially harm the fetus or may pass through breast milk and affect a nursing infant. Administration of NSAIDs in the first trimester has been linked to premature ductal closure and should be avoided.Citation227 Aspirin may be used in the first trimester but should be avoided near term, as it may delay labor and cause hemostatic abnormalities.Citation228 Paracetamol and opioids are relatively safer analgesics to use in pregnant women. In the case of lactating women, paracetamol and NSAIDs may be safe to use, while opioids should be avoided due to the risk of toxicity and dependence.Citation229
Renal impairment
A majority of analgesics are excreted by the renal route, and it is important to assess kidney function prior to administering analgesics for chronic pain. Since impaired renal function may lead to accumulation of drugs, there are chances of toxicity in such patients. Clinicians should conduct adequate assessment of kidney function by estimating serum creatinine values and the estimated glomerular filtration rate (eGFR).Citation203 An eGFR of <60 mL/min/1.73m2 may warrant dose adjustment.Citation230 NSAIDs, such as indomethacin, naproxen, diclofenac and ibuprofen, have been shown to decrease the GFR and increase the possibility of edema due to prostaglandin-mediated inhibition of renin.Citation231 Therefore, NSAIDs are best avoided in patients with renal impairment. Alternatives to NSAIDs are paracetamol and opioids such as codeine, tramadol or buprenorphine;Citation232 morphine and codeine are undialyzable and should be avoided in patients who are undergoing hemodialysis.Citation231 For moderate-to-severe pain, fentanyl can be safely administered to patients with renal dysfunction or those who require dialysis (although caution needs to be exercised in such patients as fentanyl is poorly dialyzable).Citation232,Citation233
Liver failure
Deciding analgesic therapy for patients with hepatic impairment can be a challenge, since most analgesics undergo hepatic metabolism. Cirrhosis is the most prevalent manifestation of liver disease, and it may be caused due to excessive alcohol intake, hepatitis infection or drug-induced toxicity. Since liver is the main site of metabolism for paracetamol, NSAIDs and opioids such as morphine, its dysfunction may lead to increased blood levels of these drugs, particularly those that are primarily metabolized by cytochrome P450 (CYP) enzymes.Citation234 On the contrary, changes in CYP enzymes may result in decreased efficacy of codeine and tramadol, which undergo biotransformation to active metabolites.Citation235 Thus, a thorough understanding of the pharmacokinetic profile of analgesics may help in deciding the appropriate pharmacotherapy for patients with liver dysfunction.Citation236 For instance, although paracetamol undergoes metabolic conversion to a hepatotoxic metabolite N-acetyl-p-benzoquinone imine,Citation237 clinically significant toxicity is observed only at large doses, and small doses of this drug may appear to be safe in such patients.Citation238 Dose adjustment should be considered while administering NSAIDs and opioids to patients with hepatic impairment.
Disease-specific treatment recommendations are presented in the Supplementary materials section.
Author contributions
All authors contributed towards drafting and critically revising the review and agree to be accountable for all aspects of the work. All authors met International Committee of Medical Journal Editors criteria and all those who fulfilled those criteria are listed as authors. All authors provided direction and comments on the manuscript, made the final decision about where to publish as well as approved the submission of the manuscript to the journal.
Acknowledgments
We wish to acknowledge the efforts of other members of the Pain Working Group who contributed significantly to the development of this manuscript, namely, SS Sukumar, Hammad Usmani, Rajeev Rao, Ananth Hazare, PR Krishnan, Poorna Chandra and Umesh Gupta. Padmini Deshpande (Siro Clinpharm Pvt Ltd) provided writing assistance, and Dr Sangita Patil (SIRO Clinpharm Pvt Ltd) provided additional editorial support for the development of this manuscript. This study was funded by Johnson & Johnson Pvt Ltd, Mumbai, Maharashtra, India.
Supplementary materials
Pain Assessment Scales
Brief Pain Inventory (BPI)
Originally developed for assessment of pain in cancer patients, the BPI has been successfully used in a variety of clinical settings.Citation1 In the BPI, severity of pain is measured across four grades, and the degree of interference of pain is evaluated across seven parameters: general activity, walking, work, mood, enjoyment of life, relationship with others and sleep.Citation2 Several modified versions of the BPI have been developed to adapt to the requirements of the target population across different geographical settings. The reliability of the Hindi version of the BPI was demonstrated in a clinical trial in North India by Saxena et al.Citation3
McGill Pain Questionnaire (MPQ)
Developed by Melzack and Torgerson in 1971,Citation4 the MPQ is a multidimensional pain assessment tool consisting of three major classes of word descriptors (total of 78 words) reflecting sensory, affective and evaluative components. It also contains a pain intensity scale ranging from one to five to rate the severity of the pain.Citation5 MPQ and the short-form MPQ (SF-MPQ) are valid and reliable tools to assess chronic pain, particularly cancer pain, in adults.Citation6–Citation9 The MPQ can also be used in the assessment of neuropathic and arthritic pain.Citation10–Citation12
Visual Analog Scale (VAS)
The VAS is a simple, easily administered, single-item tool to assess pain severity, especially in acute pain.Citation13 VAS consists of a horizontal or vertical rating scale (1–10 cm) with verbal descriptors of “no pain” and “pain as bad as it could be.” The patient is asked to mark the level of pain on the numerical scale of 1–10 cm (or 1–100 mm). Several studies have validated the use of VAS in acute and chronic pain.Citation13–Citation15
Numerical Rating Scale (NRS)
The NRS consists of a numerical scale similar to the VAS, however, with numbers marked from one to ten, ranging from “no pain” to “worst pain imaginable”. The NRS is easy to administer via paper-and-pencil, telephone, fax or any computerized system and has demonstrated superiority over the VAS across different studies,Citation16,Citation17 especially in illiterate or elderly populations.Citation18–Citation20
Verbal Rating Scale (VRS)
The VRS scale is composed of a series of adjectives along with numbers to best describe the intensity of the pain. VRS has been reported to be well correlated with the VAS, in terms of simplicity and reliability.Citation21,Citation22 However, the VRS scale has been found to be less sensitive to changes in pain intensity and is considered highly subjective.Citation22–Citation24
Faces Pain Scale (FPS)
For the self-reporting of pain severity in children, elderly and other special populations, FPS may be used. In this scale, pain is graded by means of facial expressions. FPS and the revised FPS have demonstrated reliability in predicting the severity of pain in children aged 4–8 years and in patients with cognitive impairment.Citation25–Citation28
Disease-specific assessment scales
Due to the methodological problems faced during the assessment of pain using standard tools,Citation29 disease-specific pain measurement questionnaires have been developed with an aim to correctly capture the nature and severity of pain in different pathophysiological conditions.Citation30 Use of Neuropathic Pain Scale (NPS), Neuropathic Pain Questionnaire (NPQ) and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale is recommended to differentiate between nociceptive and neuropathic pain (A).
Cognitive and behavioral assessment scales
Pain perception is greatly influenced by the psychological and emotional status of the patient. Chronic pain may induce notable changes in the behavior, cognition and social abilities of a person. Pain is also accompanied by a state of fear, anxiety, depression and negative beliefs. These psychological traits may serve as important predictors of chronic pain and disability and may determine treatment outcomes. Therefore, a comprehensive pain management program should also involve psychological, cognitive and behavioral assessment of the patient. For this purpose, tools such as Pain Beliefs and Perceptions Inventory (PBPI), Pain Anxiety Symptoms Scale (PASS), Cognitive Evaluation Questionnaire and the Patient Attitude Questionnaire (PAQ) may be used.
Assessment in elderly or cognitively impaired patients
Patients’ self-reports of pain have been proven to be more reliable tools for accurate diagnosis of pain and treatment of pain. However, obtaining detailed self-reports in both patients with cognitive impairment and the elderly can prove to be a major challenge and, therefore, requires the use of pain assessment tools that are relatively easy and adequately describe the pain experience.Citation31 For cognitively sound older adults, the VRS, NRS or FPS may be easy and reliable to use (A), whereas in elderly with symptoms of dementia or cognitive impairment,Citation32 the Abbey’s Pain Scale, Pain Assessment in Advanced Dementia Scale (PAINAD) and Pain Assessment for the Dementing Elderly (PADE) have demonstrated reliability (A).Citation33–Citation35
Disease-specific treatment recommendations
Low-back pain (LBP)
Classification
LBP is localized to the lumbar vertebrae (L1–l5) just above the gluteus muscle.
LBP can be classified by duration as acute (<6 weeks), subacute (6–12 weeks) and chronic (>12 weeks).
LBP may be classified by cause as mechanical (strains/sprains or fractures), nonmechanical (tumors) and referred pain (kidney or gall stones or pain in nearby tissue).Citation36
LBP is classified by mechanism or “diagnostic triage” into serious spinal pathology (accompanied by “red flags”), nerve root pain/radicular pain and nonspecific LBP.
Diagnosis
Use diagnostic methods such as physical examination, X-rays, ultrasonography, computed tomography (CT) scans, magnetic resonance imaging (MRI), diskography, bone scans and blood tests (B).Citation37–Citation39
Identify “red flags”, which are signs/symptoms that are unlikely to be observed in normal LBP and generally indicate involvement of serious pathology. Identifying the red flags may help diagnose underlying chronic conditions, such as cancer (C).
Identify “yellow flags’, which represent the psychosocial barriers that may influence the subjective response to pain and which may delay recovery or indicate the risk of chronification or disability (C).
Treatment
Initiate treatment with acetaminophen and mild NSAIDs (ibuprofen, diclofenac) as first-line therapy (A).Citation40,Citation41
Second-line therapy should typically involve a combination of NSAIDs and mild opioids (codeine, tramadol) (A),Citation41,Citation42 followed by moderate-to-strong opioids (morphine, fentanyl) in severe pain.Citation42,Citation43
Third-line therapy should be initiated in case a central origin is suspected or if pain significantly affects the physical, mental and social behavior of patients; it may include anticonvulsants (gabapentin, pregabalin),Citation44 sedatives (benzodiazepines) and antidepressants (amitriptyline, nortriptyline) (A).Citation45–Citation47
Neck pain
Classification
Neck pain can be classified into four classes according to the impact of pain on body function and movement, as follows:
Pain with mobility deficits
Pain with headache
Pain with radiating pain
Pain with impaired movement.
Based on the causative mechanism, neck pain may be classified as follows:
Axial neck pain caused due to incorrect posture, physical stress or fatigue
Whiplash-associated disorder (WAD) due to pain in the muscles, joints, tendons and ligaments due to injuries or impact
Cervical radiculopathy and myelopathy due to changes to the sensory/motor function of cervical nerves or compression of spinal cord.
Diagnosis
Diagnosis should be preceded by careful evaluation of patient, the nature and extent of pain and any additional symptoms such as headaches, nausea, vomiting, vertigo or other distress (C).Citation48
Symptom assessment may help in differentiating different types of neck pains. Sharp, shooting or tingling pain may indicate a radicular origin, whereas dull, aching pain accompanied with soreness or tenderness may point toward axial pain (C).
Physical examination and self-report questionnaires are more valid assessment tools and should be preferred over diagnostic tests such as imaging, electrophysiology or blood tests, which have failed to demonstrate reliability (C).
Identify the “red flags”, which may include trauma, infections, history of pain, malignancy or major trauma, in addition to weight loss, chest pain, nausea, vomiting or fever (C).Citation49
Treatment
Exercise and physiotherapy are the most effective interventional methods with proven efficacy in neck pain (A).Citation50–Citation53
Analgesics such as acetaminophen and NSAIDs may be used for acute pain, and weak opioids may be used for chronic episodes, as first-line therapies (A).Citation49
Antidepressants, muscle relaxants and strong opioids may be reserved for refractory cases or after failure of other interventional methods (C).Citation48
Headache
Classification
According to the International Headache Society, primary headaches can be classified into migraine headaches, cluster headaches and tension-type headaches. Migraine headaches result from migraine disorders, which are further categorized as migraine with aura, migraine without aura, childhood migraine or retinal migraine depending upon the presentation.Citation54–Citation56
Secondary headaches may be caused due to an underlying disorder such as tumors.
Diagnosis
Preliminary diagnosis can be performed through symptom assessment and detailed evaluation of patient profile and history or presence of underlying disorders.
A typical migraine attack is triggered by physical activity and may be suspected if symptoms involve severe pulsating or throbbing headache on one or both sides of the head, sometimes accompanied by visual aura, sensitivity to light, nausea or vomiting. Frequently occurring migraine attacks (≥15 days per month for >3 months) despite continuing medication may be regarded as chronic migraine; the duration of headache may be 4–72 hours.
Tension-type headaches, also known as ordinary headaches, may be triggered by stress. The pain is usually bilateral, with pressing/tightening quality and is not usually accompanied by nausea, vomiting or light sensitivity. Persistent tension-type headache may indicate the presence of serious underlying disorders. These headaches may last from a few minutes to days (up to 7 days).
Cluster headaches usually involve severe unilateral pain around the eyes (orbital pain), forehead or sides of the head (temporal pain). These headaches may last for 10 minutes to 4 hours.
Diagnosis by neuroimaging techniques should be reserved for patients who have persistent pain lasting 6-month duration, often in the absence of migraine and accompanied by neurologic symptoms such as confusion, visual problems and hallucinations; also to be used in the case of patients with history of such disorders in the past (B).Citation57
Treatment
Migraine headache: begin initial treatment with oral triptans (sumatriptan or zolmitriptan), followed by the antiemetics prochlorperazine or chlorpromazine in case of nausea and vomiting (A); opioids and antidepressants may be considered as second-line therapy. In children, initial treatment may be initiated with acetaminophen and ibuprofen, followed by sumatriptan nasal spray or oral triptans (A).Citation58–Citation68
Cluster and tension-type headaches: treatment for cluster and tension-type headaches should consist of acetaminophen, low-dose NSAIDs (ibuprofen, naproxen and diclofenac), followed by an NSAID–caffeine or acetaminophen–caffeine combination as second-line therapy (A).Citation69–Citation72
Arthritic pain
Classification
Although arthritic pain involves both rheumatoid arthritis and osteoarthritis, primarily it can be classified as inflammatory arthritis, noninflammatory arthritis or arthralgia. Inflammatory pain involves the inflammation of the joints, synovial cavity or synovium. Noninflammatory arthritic pain may result from altered joint mechanics, while arthralgia represents joint tenderness in absence of inflammation, usually resulting from an underlying condition such as fibromyalgia.Citation73
Diagnosis
Carefully observe symptoms of swelling, tenderness, redness and stiffness, as well as checking joint movement, wrist movement, rotation, movement or function (B).Citation73
The diagnosis may be confirmed using bone aspiration tests and imaging tests (B).
Treatment
First-line therapy should consist of acetaminophen and oral or topical NSAIDs (ibuprofen, diclofenac or naproxen) (A).Citation73–Citation75
Second-line therapy with opioids (tramadol, morphine and oxycodone) should be considered only if the patient is unable to tolerate NSAIDs or if pain persists despite NSAID medication (A).
Table S1 Treatment recommendations
References
- CleelandCRyanKPain assessment: global use of the Brief Pain InventoryAnn Acad Med Singapore19942321291388080219
- CleelandCSRyanKThe Brief Pain InventoryPain Research Group1991 Available from: http://sosmanuals.com/manuals/cae48184b-7bae3b1d85105ea85375b60.pdfAccessed June 2016
- SaxenaAMendozaTCleelandCSThe assessment of cancer pain in north India: the validation of the Hindi Brief Pain Inventory – BPI-HJ Pain Symptom Manag19991712741
- MelzackRTogersonWSOn the language of painAnesthesiology197134150594924784
- MelzackRThe McGill Pain Questionnaire: major properties and scoring methodsPain1975132772991235985
- NgamkhamSVincentCFinneganLHoldenJEWangZJWilkieDJThe McGill pain questionnaire as a multidimensional measure in people with cancer: an integrative reviewPain Manag Nurs2012131275122341138
- DudgeonDRaubertasRFRosenthalSNThe short-form McGill pain questionnaire in chronic cancer painJ Pain Symptom Manag199384191195
- GrahamCBondSSGerkovichMMCookMRUse of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistencyPain1980833773877402695
- KremerEFAtkinsonJHIgnelziRJPain measurement: the affective dimensional measure of the McGill Pain questionnaire with a cancer pain populationPain19821221531637070825
- LovejoyTITurkDCMorascoBJEvaluation of the psychometric properties of the revised Short-Form McGill Pain Questionnaire (SF-MPQ-2)J Pain201213121250125723182230
- BurckhardtCSJonesKDAdult measures of pain: The McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short-Form McGill Pain Questionnaire (SF-MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven-Yale Multidisciplinary Pain Inventory (WHYMPI)Arthritis Care Res200349S5S96S104
- KachooeiAREbrahimzadehMHErfani-SayyarRSalehiMSalimiERaziSShort Form-McGill Pain Questionnaire-2 (SF-MPQ-2): a cross-cultural adaptation and validation study of the Persian version in patients with knee osteoarthritisArch Bone Jt Surg201531455025692169
- BijurPESilverWGallagherEJReliability of the visual analog scale for measurement of acute painAcad Emerg Med20018121153115711733293
- SeymourRThe use of pain scales in assessing the efficacy of analgesics in post-operative dental painEur J Clin Pharmacol19822354414447151849
- PriceDDMcGrathPARafiiABuckinghamBThe validation of visual analogue scales as ratio scale measures for chronic and experimental painPain198317145566226917
- PaiceJACohenFLValidity of a verbally administered numeric rating scale to measure cancer pain intensityCancer Nurs199720288939145556
- JensenMPKarolyPBraverSThe measurement of clinical pain intensity: a comparison of six methodsPain19862711171263785962
- FerrazMBQuaresmaMAquinoLAtraETugwellPGoldsmithCReliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritisJ Rheumatol1990178102210242213777
- HjermstadMJFayersPMHaugenDFStudies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature reviewJ Pain Symptom Manag201141610731093
- BriggsMClossJSA descriptive study of the use of visual analogue scales and verbal rating scales for the assessment of postoperative pain in orthopedic patientsJ Pain Symptom Manag1999186438446
- LittmanGSWalkerBRSchneiderBEReassessment of verbal and visual analog ratings in analgesic studiesClin Pharmacol Ther198538116233891192
- HoldgateAAshaSCraigJThompsonJComparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute painEmerg Med (Fremantle)2003155–644144614992058
- AicherBPeilHPeilBDienerHPain measurement: Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headacheCephalalgia201232318519722332207
- LangleyGSheppeardHProblems associated with pain measurement in arthritis: comparison of the visual analogue and verbal rating scalesClin Exp Rheumatol198323231234
- BieriDReeveRAChampionGDAddicoatLZieglerJBThe Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale propertiesPain19904121391502367140
- GoodenoughBAddicoatLChampionGDPain in 4-to 6-year-old children receiving intramuscular injections: a comparison of the Faces Pain Scale with other self-report and behavioral measuresClin J Pain199713160739084953
- WareLJEppsCDHerrKPackardAEvaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale, and Iowa pain thermometer in older minority adultsPain Manag Nurs20067311712516931417
- HicksCLvon BaeyerCLSpaffordPAvan KorlaarIGoodenoughBThe Faces Pain Scale–Revised: toward a common metric in pediatric pain measurementPain200193217318311427329
- OhnhausEEAdlerRMethodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scalePain197514379384800639
- SalaffiFCiapettiACarottiMPain assessment strategies in patients with musculoskeletal conditionsReumatismo201264421622923024966
- HerrKCoynePJMcCafferyMManworrenRMerkelSPain assessment in the patient unable to self-report: position statement with clinical practice recommendationsPain Manag Nurs201112423025022117755
- HerrKBjoroKDeckerSTools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science reviewJ Pain Symptom Manag2006312170192
- AbbeyJPillerNDe BellisAThe Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementiaInt J Palliat Nurs200410161314966439
- WardenVHurleyACVolicerLDevelopment and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) ScaleJ Am Med Dir Assoc20034191512807591
- VillanuevaMRSmithTLEricksonJSLeeACSingerCMPain assessment for the dementing elderly (PADE): reliability and validity of a new measureJ Am Med Dir Assoc2003411812807590
- BogdukNEvidence-based clinical guidelines for the management of acute low back painAustralian faculty of musculoskeleltal medicine1999
- LateefHPatelDWhat is the role of imaging in acute low back pain?Curr Rev Musculoskelet Med200922697319468875
- JarvikJGDeyoRADiagnostic evaluation of low back pain with emphasis on imagingAnn Intern Med2002137758659712353946
- O’SullivanPDiagnosis, classification management of chronic low back pain Available from: http://www.smly.fi/@Bin/172109/lumbo-pelvic_workshoplevi07handouts.pdfAccessed June 2016
- Van TulderMWScholtenRJKoesBWDeyoRANonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review GroupSpine200025192501251311013503
- RuoffGERosenthalNJordanDKarimRKaminMProtocol CAPSS-112 Study GroupTramadol/Acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient studyClin Ther20032541123114112809961
- SchnitzerTJGrayWLPasterRZKaminMEfficacy of tramadol in treatment of chronic low back painJ Rheumatol200027377277810743823
- AllanLRicharzUSimpsonKSlappendelRTransdermal fentanyl versus sustained release oral morphine in strong-opioid naïve patients with chronic low back painSpine200530222484249016284584
- MooreRAStraubeSWiffenPJDerrySMcQuayHJCochrane Database Syst Rev20093CD00707619588419
- StraubeAAicherBFiebichBLHaagGCombined analgesics in (headache) pain therapy: shotgun approach or precise multi-target therapeutics?BMC Neurol2011114311521208452
- SteinDPeriTEdelsteinEElizurAFlomanYThe efficacy of amitriptyline and acetaminophen in the management of acute low back painPsychosomatics199637163708600497
- PilowskyIHallettECBassettDLThomasPGPenhallRKA controlled study of amitriptyline in the treatment of chronic painPain19821421691796757842
- ChildsJDClelandJAElliottJMNeck pain: clinical practice guidelines linked to the International classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy AssociationJ Orthop Sports Phys Ther2008389A1A34
- DouglassABBopeETEvaluation and treatment of posterior neck pain in family practiceJ Am Board Fam Pract200417SupplS13S2215575026
- FortierMAAndersonCTKainZNEthnicity matters in the assessment and treatment of children’s painPediatrics2009124137838019564322
- CampbellCMEdwardsRREthnic differences in pain and pain managementPain Manag20122321923023687518
- CallisterLCCultural influences on pain perceptions and behaviorsHome Health Care Manag Pract2003153207211
- ShiptonEThe pain experience and sociocultural factorsNew Zealand Med J2013126137079
- BarnesTRA rating scale for drug-induced akathisiaBr J Psychiatry198915456726762574607
- SimpsonGMAngusJWA rating scale for extrapyramidal side effectsActa Psychiatr Scand197045S2121119
- KaySRFiszbeinLAOplerLThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- EvansRWRozenTDAdelmanJUNeuroimaging and other diagnostic testing in headacheWolff’s Headache and Other Head Pain7th edSilbersteinSDLiptonRBDalessioDJNew York, NYOxford University Press20012749
- AhonenKHämäläinenMLRantalaHHoppuKNasal sumatriptan is effective in treatment of migraine attacks in children: a randomized trialNeurology200462688388715037686
- BrandesJLKudrowDStarkSRSumatriptan-naproxen for acute treatment of migraine: a randomized trialJAMA2007297131443145417405970
- BrennerMLewisDThe treatment of migraine headaches in children and adolescentsJ Pediatr Pharmacol Ther2008131172423055860
- ColmanIBrownMDInnesGDGrafsteinERobertsTERoweBHParenteral dihydroergotamine for acute migraine headache: a systematic review of the literatureAnn Emerg Med200545439340115795718
- EngindenizZDemircanCKarliNIntramuscular tramadol vs. diclofenac sodium for the treatment of acute migraine attacks in emergency department: a prospective, randomised, double–blind studyJ Headache Pain20056314314816355295
- GoldsteinJSilbersteinSDSaperJRRyanRELiptonRBAcetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled studyHeadache200646344445316618262
- HamalainenMLHoppuKValkeilaESantavuoriPIbuprofen or acetaminophen for the acute treatment of migraine in children a double-blind, randomized, placebo-controlled, crossover studyNeurology19974811031079008503
- LewisDAshwalSHersheyAOHirtzDYonkerMSilbersteinSPractice parameter: pharmacological treatment of migraine headache in children and adolescents Report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology SocietyNeurology200463122215222415623677
- PriorMJCodispotiJRFuMA randomized, placebo-controlled trial of acetaminophen for treatment of migraine headacheHeadache201050581983320236342
- SilbersteinSDConsortiumUHPractice parameter: evidence-based guidelines for migraine headache (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology200055675476210993991
- WinnerPRothnerADSaperJA randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescentsPediatrics2000106598999711061765
- HolmesAChristelisNArnoldCDepression and chronic painMed J Aust2013199Suppl 6S17S20
- CastroMQuarantiniLCDaltroCComorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of lifeArch Clin Psychiatry (São Paulo)201138126129
- CovicTCummingSRPallantJFDepression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS)BMC Psychiatry20121211022230388
- GallowaySKBakerMGiglioPDepression and anxiety symptoms relate to distinct components of pain experience among patients with breast cancerPain Res Treat2012201285127623227331
- JordanKArdenNDohertyMEULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)Ann Rheum Dis200362121145115514644851
- EmeryPZeidlerHKvienTKCelecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparisonLancet199935491962106211110609815
- LinJZhangWJonesADohertyMEfficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trialsBMJ2004329746132415286056
Disclosure
Prashant Narang and Jaishid Ahdal are employees and/or shareholders of Johnson & Johnson Private Limited, Mumbai, Maharashtra, India. Gur Prasad Dureja, Rajagopalan N Iyer, and Gautam Das report no conflicts of interest in this work.
References
- International Association for the Study of Pain [webpage on the Internet] (IASP)Taxonomy2012 Available from: http://www.iasp-pain.org/TaxonomyAccessed April 2016
- GoldbergDSMcGeeSJPain as a global public health priorityBMC Public Health20111177021978149
- McPhillips-TangumCACherkinDCRhodesLAMarkhamCReasons for repeated medical visits among patients with chronic back painJ Gen Intern Med19981352892959613883
- MäntyselkäPKumpusaloEAhonenRPain as a reason to visit the doctor: a study in Finnish primary health carePain2001892–317518011166473
- MooreRJBiobehavioral Approaches to PainBerlinSpringer2009
- GregoryJMcGowanLAn examination of the prevalence of acute pain for hospitalised adult patients: a systematic reviewJ Clin Nurs2016255–658359826778249
- MurphyAMcCoySO’ReillyKA prevalence and management study of acute pain in children attending emergency departments by ambulancePrehosp Emerg Care2016201525826024309
- GalinskiMRuscevMGonzalezGPrevalence and management of acute pain in prehospital emergency medicinePrehosp Emerg Care201014333433920507221
- GatchelRJTurkDCPsychosocial Factors in Pain: Critical PerspectivesNew York, NYGuilford Press1999
- JacksonTPStabileVSMcQueenKKThe global burden of chronic painASA Newsl2014782427
- ButchartAKerrEAHeislerMPietteJDKreinSLExperience and management of chronic pain among patients with other complex chronic conditionsClin J Pain200925429329819590477
- BouhassiraDLantéri-MinetMAttalNLaurentBTouboulCPrevalence of chronic pain with neuropathic characteristics in the general populationPain2008136338038717888574
- TorranceNSmithBHBennettMILeeAJThe epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population surveyJ Pain20067428128916618472
- Van HeckeOAustinSKKhanRASmithBTorranceNNeuropathic pain in the general population: a systematic review of epidemiological studiesPain2014155465466224291734
- MäntyselkäPTTurunenJHAhonenRSKumpusaloEAChronic pain and poor self-rated healthJAMA2003290182435244214612480
- TorranceNElliottAMLeeAJSmithBHSevere chronic pain is associated with increased 10 year mortality. A cohort record linkage studyEur J Pain201014438038619726210
- Facts and Figures on PainAmerican Academy of Pain Medicine Available from: http://www.painmed.org/files/facts-and-figures-on-pain.pdfAccessed April 2016
- BreivikHEisenbergEO’BrienTOPENMindsThe individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate careBMC Public Health2013131122924365383
- GaskinDJRichardPThe economic costs of pain in the United StatesJ Pain201213871572422607834
- BondMPain education issues in developing countries and responses to them by the International Association for the Study of PainPain Res Manag201116640440622184547
- Institute for Health Metrics and EvaluationGlobal burden of diseases, injuries, and risk factors study 2010: IndiaInstitute for Health Metrics and Evaluation2013 Available from: http://www.healthdata.org/sites/default/files/files/country_profiles/GBD/ihme_gbd_country_report_india.pdfAccessed April 2016
- hrw.orgGlobal state of pain treatment – access to medicines and palliative careThe Human Rights Watch2011 Available from: https://www.hrw.org/sites/default/files/reports/hhr0511W.pdfAccessed April 2016
- DurejaGPJainPNShettyNPrevalence of chronic pain, impact on daily life, and treatment practices in IndiaPain Pract2014142E51E6224304963
- Srinath ReddyKShahBVargheseCRamadossAResponding to the threat of chronic diseases in IndiaLancet200536694981744174916291069
- PatelVChatterjiSChisholmDChronic diseases and injuries in IndiaLancet2011377976341342821227486
- AbdullaAAdamsNBoneMGuidance on the management of pain in older peopleAge Ageing201342suppl 1i1i5723420266
- FerrellBAPain management in elderly peopleJ Am Geriatr Soc199139164731670940
- TsangAVon KorffMLeeSCommon chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disordersJ Pain200891088389118602869
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older PersonsPharmacological management of persistent pain in older persons. American geriatrics society panel on the pharmacological management of persistent pain in older personsPain Med20091061062108319744205
- HerrKAGarandLAssessment and measurement of pain in older adultsClin Geriatr Med200117345747811459715
- VisserAWBoyesenPHaugenIKInstruments measuring pain, physical function, or patient’s global assessment in hand osteoarthritis: a systematic literature searchJ Rheumatol201542112118213426472412
- SmithJPMajmundarMAging in Asia: Findings from New and Emerging Data InitiativesWashington, DCNational Academies Press2012
- GolechhaMHealthcare agenda for the Indian governmentIndian J Med Res2015141215115325900948
- MaheshwariRWChincholkarAGuptaMPrescription pattern monitoring of non-steroidal anti-inflammatory drugs in urban health center in Talegaon: a retrospective studyIJPBS20156596602
- GuptaMMalhotraSJainSAggarwalAPandhiPPattern of prescription of non-steroidal antiinflammatory drugs in orthopaedic outpatient clinic of a north Indian tertiary care hospitalIndian J Pharmacol2005376404
- JainNJainSSymposium: pain management: current trendsJIMSA2013264243244
- KhadeABashirMSMSheethalAPrescription pattern in the department of surgery in a tribal district hospital of Andhra Pradesh, IndiaAnn Med Health Sci Res20133343844124116329
- PatelVVaidyaRNaikDBorkerPIrrational drug use in India: a prescription survey from GoaJ Postgrad Med2005511915793331
- ShaliniMJoshiMStudy of polypharmacy and associated problems among elderly patientsInternet J Med Update2012713539
- BhatnagarSFreedom from pain (Sixteenth International Conference of Indian Association of Palliative Care)Caregiver Burden of Chronic Pain in IndiaNew DelhiI. K. International Pvt Ltd2009
- ZallmanLRubensSLSaitzRSametJHLloyd-TravagliniCLiebschutzJAttitudinal barriers to analgesic use among patients with substance use disordersPain Res Treat2012201216706222685649
- Von RoennJHCleelandCSGoninRHatfieldAKPandyaKJPhysician attitudes and practice in cancer pain management: a survey from the Eastern Cooperative Oncology GroupAnn Intern Med199311921211268099769
- University of Minnesota [webpage on the Internet]Levels of evidence and grades of recommendationsUniversity of Minnesota: National Guidelines Clearinghouse Available from: https://hsl.lib.umn.edu/biomed/help/levels-evidence-and-grades-recommendationsAccessed May 2016
- BessonJThe complexity of physiopharmacologic aspects of painDrugs199653219
- JensenTSBaronRTranslation of symptoms and signs into mechanisms in neuropathic painPain20031021–21812620591
- BaronRMechanisms of disease: neuropathic pain – a clinical perspectiveNat Clin Pract Neurol2006229510616932531
- BridgesDThompsonSWNRiceASCMechanisms of neuropathic painBr J Anaesth2001871122611460801
- NickelFTSeifertFLanzSMaihöfnerCMechanisms of neuropathic painEur Neuropsychopharmacol2012222819121672666
- HanssonPTDickensonAHPharmacological treatment of peripheral neuropathic pain conditions based on shared commonalities despite multiple etiologiesPain2005113325125415661430
- TreedeR-DJensenTSCampbellJNeuropathic pain redefinition and a grading system for clinical and research purposesNeurology200870181630163518003941
- SussmanCBates-JensenBMWound Care: A Collaborative Practice Manual3rd edPhiladelphia, PALippincott Williams & Wilkins2007
- FallonMTNeuropathic pain in cancerBr J Anaesth2013111110511123794652
- BaronRBinderAHow neuropathic is sciatica? The mixed pain conceptOrthopade200433556857515067505
- CampbellJNMeyerRAMechanisms of neuropathic painNeuron2006521779217015228
- BonicaJJNeurophysiologic and pathologic aspects of acute and chronic painArch Surg1977112675076116580
- HootenWTimmingRBelgradeMAssessment and Management of Chronic PainBloomington, MNInstitute for Clinical Systems Improvement2013106
- BerryPChapmanCCovingtonEPain: Current Understanding of Assessment, Management, and TreatmentsReston, VANational Pharmaceutical Council and the Joint Commission for the Accreditation of Healthcare Organizations2001b44
- AshburnMAStaatsPSManagement of chronic painLancet199935391671865186910359427
- HenryJLPathophysiology of Chronic Pain2008 Available from: http://fhs.mcmaster.ca/paininstitute/documents/pathophysiology_of_chronic_pain.pdfAccessed May 2016
- PortenoyRKHagenNABreakthrough pain: definition, prevalence and characteristicsPain19904132732811697056
- MishraSBhatnagarSChaudharyPRanaSPSBreakthrough cancer pain: review of prevalence, characteristics and managementIndian J Palliat Care2009151141820606850
- van den Beuken-van EverdingenMde RijkeJKesselsASchoutenHvan KleefMPatijnJPrevalence of pain in patients with cancer: a systematic review of the past 40 yearsAnn Oncol20071891437144917355955
- FoleyKPain syndromes in patients with cancerSwerdlowMVentafriddaVCancer PainNetherlandsSpringer19874554
- WallPDMelzackRBonicaJJTextbook of PainEdinburghChurchill Livingstone1994
- International Association for the Study of Pain (IASP)Breakthrough pain in cancer patients2006 Available from: http://iasp.files.cms-plus.com/Content/ContentFolders/Publications2/PainClinicalUpdates/Archives/PCU06-1_1390263807201_21.pdfAccessed May 2016
- JuskaMBalonRChronic non-cancer pain and substance use disorders: challenges and strategiesCurr Psychiatry20131273541
- HoKYChuaNGeorgeJMEvidence-based guidelines on the use of opioids in chronic non-cancer pain – a consensus statement by the Pain Association of Singapore Task ForceAnn Acad Med Singa-pore2013423138152
- KahanMMailis-GagnonAWilsonLSrivastavaANational Opioid Use Guideline GroupCanadian guideline for safe and effective use of opioids for chronic noncancer pain clinical summary for family physicians. Part 1: general populationCan Fam Physician201157111257126622084455
- PainCPain management: classifying, understanding, and treating painHosp Physician20023862330
- Iasp-pain.orgInternational Association for the Study of Pain (IASP): Classification of chronic pain2002 Available from: http://www.iasp-pain.org/files/Content/ContentFolders/Publications2/FreeBooks/Classification-of-Chronic-Pain.pdfAccessed May 2016
- World Health OrganizationWHO guidelines on pharmacological treatment of persisting pain in children with medical illnesses2012 Available from: http://apps.who.int/iris/bitstream/10665/44540/1/9789241548120_Guidelines.pdfAccessed May 2016
- MurphyMPSomatic painSchmidtFRWillisDWEncyclopedia of PainBerlin, HeidelbergSpringer Berlin Heidelberg200721902192
- WoolfCSomatic pain-pathogenesis and preventionBr J Anaesth19957521691767577250
- GiamberardinoMAVisceral Pain: Clinical, Pathophysiological and Therapeutic AspectsOxfordOxford University Press2009
- DavisMPDrug management of visceral pain: concepts from basic researchPain Res Treat2012201218
- VecchietLVecchietJGiamberardinoMAReferred muscle pain: clinical and pathophysiologic aspectsCurr Rev Pain19993648949810998708
- GiamberardinoMAReferred muscle pain/hyperalgesia and central sensitisationJ Rehabil Med Suppl20031Suppl 418588
- TreedeRDRiefWBarkeAA classification of chronic pain for ICD-11Pain201515661003100725844555
- WoolfCRecent advances in the pathophysiology of acute painBr J Anaesth19896321391462669905
- WoolfCJCentral sensitization: implications for the diagnosis and treatment of painPain20111523 supplS2S1520961685
- KiddBUrbanLMechanisms of inflammatory painBr J Anaesth200187131111460811
- RochaAPCKraycheteDCLemonicaLPain: current aspects on peripheral and central sensitizationRev Bras Anestesiol20075719410519468623
- LatremoliereAWoolfCJCentral sensitization: a generator of pain hypersensitivity by central neural plasticityJ Pain200910989592619712899
- MarksDMShahMJPatkarAAMasandPSParkG-YPaeC-USerotonin-norepinephrine reuptake inhibitors for pain control: premise and promiseCurr Neuropharmacol20097433133620514212
- SindrupSHOttoMFinnerupNBJensenTSAntidepressants in the treatment of neuropathic painBasic Clin Pharmacol Toxicol200596639940915910402
- JensenTSAnticonvulsants in neuropathic pain: rationale and clinical evidenceEur J Pain20026suppl A616811888243
- SchaibleHGPeripheral and central mechanisms of pain generationSteinCAnalgesiaBerlinSpringer Berlin Heidelberg2006328
- CrossSAPathophysiology of painMayo Clinic Proc1994694375383
- PetrenkoABYamakuraTBabaHShimojiKThe role of N-methyl-d-aspartate (NMDA) receptors in pain: a reviewAnesth Analg20039741108111614500166
- FeinANociceptors and the Perception of Pain. Vol. 4Farmington, CTUniversity of Connecticut Health Center201246167
- PuretićMBDemarinVNeuroplasticity mechanisms in the patho-physiology of chronic painActa Clin Croat20125121
- BasbaumAIFieldsHLEndogenous pain control mechanisms: review and hypothesisAnn Neurol197845451462216303
- OssipovMHDussorGOPorrecaFCentral modulation of painJ Clin Invest2010120113779378721041960
- SinatraRCauses and consequences of inadequate management of acute painPain Med201011121859187121040438
- PergolizziJVRaffaRBTaylorRTreating acute pain in light of the chronification of painPain Manag Nurs201415138039024602441
- VoscopoulosCLemaMWhen does acute pain become chronic?Br J Anaesth2010105suppl 1i69i8521148657
- SiddallPJNeuroplasticity and pain: what does it all mean?Med J Aust2013198417717823451946
- RayALNeuroplasticity, sensitization, and painTreatment of Chronic Pain by Integrative Approaches: the AMERICAN ACADEMY of PAIN MEDICINE Textbook on Patient ManagementDeerTRLeong MichaelSRayALSpringer2013759768
- MayAChronic pain may change the structure of the brainPain2008137171518410991
- DworkinRHToward a clearer specification of acute pain risk factors and chronic pain outcomesPain Forum199762148150
- Young CaseyCGreenbergMANicassioPMHarpinREHubbardDTransition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factorsPain20081341–2697917504729
- DworkinRHWhich individuals with acute pain are most likely to develop a chronic pain syndrome?Pain Forum199762127136
- van der WindtDAKuijpersTJellemaPvan der HeijdenGJBouterLMDo psychological factors predict outcome in both low-back pain and shoulder pain?Ann Rheum Dis200766331331916916857
- HunterJPhysical symptoms and signs and chronic painClin J Pain2001174 supplS26S3211783828
- BreivikHCollettBVentafriddaVCohenRGallacherDSurvey of chronic pain in Europe: prevalence, impact on daily life, and treatmentEur J Pain200610428733316095934
- World Health OrganizationAchieving balance in national opioids control policy: guidelines for assessment2000 Available from: http://apps.who.int/medicinedocs/pdf/whozip39e/whozip39e.pdfAccessed May 2016
- AnderssonHIIncreased mortality among individuals with chronic widespread pain relates to lifestyle factors: a prospective population-based studyDisabil Rehabil200931241980198719874076
- MacfarlaneGJCrombieIKMcBethJSilmanAJWidespread body pain and mortality: prospective population based study. Commentary: an interesting finding, but what does it mean?BMJ2001323731466211566829
- ClearyJSimhaNPanieriAFormulary availability and regulatory barriers to accessibility of opioids for cancer pain in India: a report from the Global Opioid Policy Initiative (GOPI)Ann Oncol201324suppl 11xi33xi4024285227
- BerterameSErthalJThomasJUse of and barriers to access to opioid analgesics: a worldwide, regional, and national studyLancet2016387100281644165626852264
- LeBaronVBeckSLMaurerMBlackFPalatGAn ethnographic study of barriers to cancer pain management and opioid availability in IndiaOncologist201419551552224755460
- VaneJBakhleYBottingRCyclooxygenases 1 and 2Annu Rev Pharmacol Toxicol1998381971209597150
- RaoPKnausEEEvolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyondJ Pharm Pharm Sci200811281s110s19203472
- healthbrk.com/[webpage on the Internet]AcetaminophenThe new world encylopedia2016 Available from: http://healthbrk.com/health-problems/drug/10503-acetaminophen-new-world-encyclopediaAccessed May 2016
- HawkeyCJCOX-2 chronologyGut200554111509151416227351
- BannwarthBKostineMTargeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists?Drugs201474661962624691709
- WongGYGavvaNRTherapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacksBrain Res Rev200960126727719150372
- BairMJRobinsonRLKatonWKroenkeKDepression and pain comorbidity: a literature reviewArch Intern Med2003163202433244514609780
- SansoneRASansoneLAPain, pain, go away: antidepressants and pain managementPsychiatry (Edgmont)20085121619
- RyderS-AStannardCFTreatment of chronic pain: antidepressant, antiepileptic and antiarrhythmic drugsContin Educ Anaesth Crit Care Pain2005511821
- WHOCancer Pain Relief: with a Guide to Opioid AvailabilityGeneva, SwitzerlandWHO1996
- Vargas-SchafferGIs the WHO analgesic ladder still valid? Twenty-four years of experienceCan Fam Physician201056651451720547511
- ChouRHuffmanLHAmerican Pain Society, American College of PhysiciansNonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American pain society/American college of physicians clinical practice guidelineAnn Intern Med2007147749250417909210
- WrightASlukaKANonpharmacological treatments for musculo-skeletal painClin J Pain2001171334611289087
- BronfortGEvansRNelsonBAkerPDGoldsmithCHVernonHA randomized clinical trial of exercise and spinal manipulation for patients with chronic neck painSpine200126778879711295901
- ChiuTTHui-ChanCWCheingGA randomized clinical trial of TENS and exercise for patients with chronic neck painClin Rehabil200519885086016323384
- ShermanKJCherkinDCErroJMigliorettiDLDeyoRAComparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trialAnn Intern Med20051431284985616365466
- WalkerMJBoylesREYoungBAThe effectiveness of manual physical therapy and exercise for mechanical neck pain: a randomized clinical trialSpine200833222371237818923311
- YlinenJTakalaENykänenMActive neck muscle training in the treatment of chronic neck pain in women: a randomized controlled trialJAMA2003289192509251612759322
- FortierMAAndersonCTKainZNEthnicity matters in the assessment and treatment of children’s painPediatrics2009124137838019564322
- CampbellCMEdwardsRREthnic differences in pain and pain managementPain Manag20122321923023687518
- CallisterLCCultural influences on pain perceptions and behaviorsHome Health Care Manag Pract2003153207211
- ShiptonEThe pain experience and sociocultural factorsNew Zealand Med J2013126137079
- DiatchenkoLSladeGDNackleyAGGenetic basis for individual variations in pain perception and the development of a chronic pain conditionHum Mol Gen200514113514315537663
- NielsonWRWeirRBiopsychosocial approaches to the treatment of chronic painClin J Pain2001174 supplS114S12711783824
- FerrariRSchraderHThe late whiplash syndrome: a biopsychosocial approachJ Neurol Neurosurg Psychiatry200170672272611385003
- KimHDionneRAIndividualized pain medicineDrug Discov Today Ther Strateg200963838721399745
- Nature Publishing Group [webpage on the Internet]Combination Drug Therapy2016 Available from: http://www.nature.com/subjects/combination-drug-therapy?WT.ac=search_subjects_combination_drug_therapyAccessed May 2016
- StraubeAAicherBFiebichBLHaagGCombined analgesics in (headache) pain therapy: shotgun approach or precise multi-target therapeutics?BMC Neurol201111111521208452
- World Health OrganizationGuidelines for registration of fixed-dose combination medicinal productsTechnical Report Series2005 Available from: http://apps.who.int/medicinedocs/documents/s19979en/s19979en.pdfAccessed May 2016
- GoswamiNGandhiAPatelPDikshitRAn evaluation of knowledge, attitude and practices about prescribing fixed dose combinations among resident doctorsPerspect Clin Res20134213023833738
- SibalVRajuSMondalMAhmadMNCombination drugs: are they rational?Curr Sci2006914406
- GautamCSSahaLFixed dose drug combinations (FDCs): rational or irrational: a view pointBr J Clin Pharmacol200865579579618294326
- The Indian Express [webpage on the Internet]Complete list: Do you take one of these 300 banned drug combinations?2016 Available from: http://indianexpress.com/article/india/india-news-india/do-you-take-one-of-these-300-banned-drugs/Accessed June 2016
- US Food and Drug AdministrationGuidance for industry Analgesic Indications: developing drug and biological products2014 Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregu-latoryinformation/guidances/ucm384691.pdfAccessed June 2016
- Central Drugs Standard Control OrganizationGuidance for industry on fixed dose combinations (FDCs)2010 Available from: http://www.cdsco.nic.in/writereaddata/FDC%20Guidelines%20_%20Revised1.pdfAccessed June 2016
- Central Drugs Standard Control Organization (CDSCO)List of approved Fixed Drug Combinations (FDCs) in India Available from: http://cdsco.nic.in/writereaddata/SND-Approval-Drugs-may-List1-2016.pdfAccessed June 2016
- MacDonaldNFindlayHPBrueraEDudgeonDKramerJA Canadian survey of issues in cancer pain managementJ Pain Symptom Manag1997146332342
- SapirRCataneRStrauss-LiviatanNChernyNICancer pain: knowledge and attitudes of physicians in IsraelJ Pain Symptom Manag1999174266276
- YuSWangXSChengYYangJCleelandCSSpecial aspects of cancer pain management in a Chinese general hospitalEur J Pain20015suppl A152011798212
- VainioATreatment of terminal cancer pain in France: a questionnaire studyPain19956221551628545140
- EnsinkFBBautzMTVossMCGorlitzAHanekopGGIndicators of structural quality in palliative care for cancer pain patients in Lower-SaxonySchmerz200216425526212192434
- ElliottTEElliottBAPhysician attitudes and beliefs about use of morphine for cancer painJ Pain Symptom Manag199273141148
- GreenwaldBDNarcessianEJPomeranzBAAssessment of physiatrists’ knowledge and perspectives on the use of opioids: review of basic concepts for managing chronic painAm J Phys Med Rehabil199978540841510493451
- AndersonKORichmanSPHurleyJCancer pain management among underserved minority outpatients: perceived needs and barriers to optimal controlCancer20029482295230412001130
- WolfertMZGilsonAMDahlJLClearyJFOpioid analgesics for pain control: wisconsin physicians’ knowledge, beliefs, attitudes, and prescribing practicesPain Med201011342543420002590
- RipamontiCBandieriERoilaFESMO Guidelines Working GroupManagement of cancer pain: ESMO clinical practice guidelinesAnn Oncol201122Suppl 6vi69vi7721908508
- ChouRFanciulloGJFinePGClinical guidelines for the use of chronic opioid therapy in chronic noncancer painJ Pain200910211313019187889
- TrescotAMBoswellMVAtluriSLOpioid guidelines in the management of chronic non-cancer painPain Physician20069113916700278
- TournebizeJGibajaVMuszczakAKahnJPAre physicians safely prescribing opioids for chronic noncancer pain? A systematic review of current evidencePain Pract201616337038325865462
- BhambBBrownDHariharanJAndersonJBalousekSFlemingMFSurvey of select practice behaviors by primary care physicians on the use of opioids for chronic painCurr Med Res Opin20062291859186516968589
- CleelandCSJanjanNAScottCBSeiferheldWFCurranWJCancer pain management by radiotherapists: a survey of radiation therapy oncology group physiciansInt J Radiat Oncol Biol Phys200047120320810758325
- JacobsenRMoldrupCChristrupLSjogrenPPatient-related barriers to cancer pain management: a systematic exploratory reviewScand J Caring Sci200923119020818785917
- ElliottBAElliottTEMurrayDMBraunBLJohnsonKMPatients and family members: the role of knowledge and attitudes in cancer painJ Pain Symptom Manag1996124209220
- PaiceJAToyCShottSBarriers to cancer pain relief: fear of tolerance and addictionJ Pain Symptom Manag199816119
- PotterVTWisemanCEDunnSMBoyleFMPatient barriers to optimal cancer pain controlPsychooncology200312215316012619147
- RiddellAFitchMIPatients’ knowledge of and attitudes toward the management of cancer painOncol Nurs Forum19972410177517849399275
- WardSECarlson-DakesKHughesSHKwekkeboomKLDonovanHSThe impact on quality of life of patient-related barriers to pain managementRes Nurs Health19982154054139761138
- WardSEGoldbergNMiller-McCauleyVPatient-related barriers to management of cancer painPain19935233193247681557
- KroenkeKTheobaldDWuJLozaJKCarpenterJSTuWThe association of depression and pain with health-related quality of life, disability, and health care use in cancer patientsJ Pain Symptom Manag2010403327341
- VallerandAHHasenauSTemplinTCollins-BohlerDDisparities between black and white patients with cancer pain: the effect of perception of control over painPain Med20056324225015972088
- Randall-DavidEWrightJPorterfieldDSLesserGBarriers to cancer pain management: home-health and hospice nurses and patientsSupport Care Cancer2003111066066512898368
- YatesPMEdwardsHENashREBarriers to effective cancer pain management: a survey of hospitalized cancer patients in AustraliaJ Pain Symptom Manag2002235393405
- BreitbartWPassikSMcDonaldMVPatient-related barriers to pain management in ambulatory AIDS patientsPain1998761–29169696454
- FitzcharlesMADaCostaDWareMAShirYPatient barriers to pain management may contribute to poor pain control in rheumatoid arthritisJ Pain200910330030519070549
- The American Chronic Pain AssociationThe Myths of Pain Control2006 Available from: https://theacpa.org/uploads/documents/chronicle_fall06_82806.pdfAccessed June 2016
- BistreSStraussYErrors in pain management: a Mexican perspectiveJ Pain Palliat Care Pharmacother201226326627022973916
- HolmesAChristelisNArnoldCDepression and chronic painMed J Aust2013199Suppl 6S17S20
- CastroMQuarantiniLCDaltroCComorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of lifeArch Clin Psychiatry (São Paulo)2011384126129
- CovicTCummingSRPallantJFDepression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS)BMC Psychiatry201212611022230388
- GallowaySKBakerMGiglioPDepression and anxiety symptoms relate to distinct components of pain experience among patients with breast cancerPain Res Treat2012201285127623227331
- StaigerTOGasterBSullivanMDDeyoRASystematic review of antidepressants in the treatment of chronic low back painSpine200328222540254514624092
- RoditiDRobinsonMEThe role of psychological interventions in the management of patients with chronic painPsychol Res Behav Manag20114414922114534
- TurnerJAChapmanCRPsychological interventions for chronic pain: a critical review. I. Relaxation training and biofeedbackPain19821211217036049
- TurkDCMelzackRHandbook of Pain AssessmentNew York, NYGuilford Press2011
- Morón MerchanteIPergolizziJVvan de LaarMTramadol/paracetamol fixed-dose combination for chronic pain management in family practice: a clinical reviewISRN Family Med2013201363846924959571
- CruccuGTruiniATools for assessing neuropathic painPLoS Med200964e100004519360134
- BackonjaMMAttalNBaronRValue of quantitative sensory testing in neurological and pain disordersPain201315491807181923742795
- CruccuGAnandPAttalNEFNS guidelines on neuropathic pain assessmentEur J Neurol200411315316215009162
- RolkeRBaronRMaierCQuantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference valuesPain2006123323124316697110
- Arendt-NielsenLYarnitskyDExperimental and clinical applications of quantitative sensory testing applied to skin, muscles and visceraJ Pain200910655657219380256
- LautenbacherSRollmanGBMcCainGMulti-method assessment of experimental and clinical pain in patients with fibromyalgiaPain199459145537854801
- WyldeVPalmerSLearmonthIDDieppePTest–retest reliability of quantitative sensory testing in knee osteoarthritis and healthy participantsOsteoarthritis Cartilage201119665565821329759
- ChildsJDClelandJAElliottJMNeck pain: clinical practice guidelines linked to the International classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy AssociationJ Orthop Sports Phys Ther2008389A1A34
- LateefHPatelDWhat is the role of imaging in acute low back pain?Curr Rev Musculoskelet Med200922697319468875
- CohenMJangroWA clinical ethics approach to opioid treatment of chronic noncancer painAMA J Ethics201517652152926075979
- NurcanÃÃSommerCNeuropathic pain assessment – an overview of existing guidelines and discussion points for the futureEur Neurol Rev201162128131
- TurkDCAudetteJLevyRMMackeySCStanosSAssessment and treatment of psychosocial comorbidities in patients with neuropathic painMayo Clin Proc2010853 supplS42S5020194148
- HeckmanBDHolroydKAHimawanLDo psychiatric comorbidities influence headache treatment outcomes? Results of a naturalistic longitudinal treatment studyPain20091461–2566419660866
- LeoRJChronic pain and comorbid depressionCurr Treat Options Neurol20057540341216079044
- AmarapurkarDNPrescribing medications in patients with decompensated liver cirrhosisInt J Hepatol2011201151952621994861
- RifkinBSPerazellaMAAnalgesic therapy in patients with chronic kidney disease: a case-based approachHosp Physician2005431322
- RadnerHRamiroSvan der HeijdeDMLandeweRBuchbinderRAletahaDHow do gastrointestinal or liver comorbidities influence the choice of pain treatment in inflammatory arthritis? A Cochrane systematic reviewJ Rheumatol2012907480
- BillaGGabhaneMBiswasSPractice of pain management by Indian healthcare practitioners: results of a paper based questionnaire surveyPain Res Treat201520158
- GatchelRJPengYBPetersMLFuchsPNTurkDCThe biopsychosocial approach to chronic pain: scientific advances and future directionsPsychol Bull2007133458162417592957
- VasudevanSVPottsEEMehrotraCPain management in arthritis: evidence-based guidelinesWis Med J200310271418
- SinghJASaagKGBridgesSL2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritisArthritis Rheum2016681126
- DworkinRHO’ConnorABBackonjaMPharmacologic management of neuropathic pain: evidence-based recommendationsPain2007132323725117920770
- DworkinRHBackonjaMRowbothamMCAdvances in neuropathic pain: diagnosis, mechanisms, and treatment recommendationsArch Neurol200360111524153414623723
- Van SeventerRBachFWTothCCPregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trialEur J Neurol20101781082108920236172
- WatsonCPNMoulinDWatt-WatsonJGordonAEisenhofferJControlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathyPain20031051–2717814499422
- EisenbergEMcNicolEDCarrDBEfficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trialsJAMA2005293243043305215972567
- SmithHSOpioids and neuropathic painPain Physician2012153 SupplES93ES11022786465
- HowardFACOG Practice Bulletin No. 51. Chronic pelvic painObstet Gynecol2004103358960514990428
- FallMBaranowskiAPElneilSEAU guidelines on chronic pelvic painEur Urol201057354819733958
- JarrellJFVilosGAAllaireCConsensus guidelines for the management of chronic pelvic painJ Obstet Gynaecol Can200527986988719830953
- BernabeiRGambassiGLapaneKManagement of pain in elderly patients with cancerJAMA199827923187718829634258
- CavalieriTAManaging pain in geriatric patientsJ Am Osteopath Assoc2007107Suppl 41016
- McLachlanAJBathSNaganathanVClinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairmentBr J Clin Pharmacol201171335136421284694
- FalzoneEHoffmannCKeitaHPostoperative analgesia in elderly patientsDrugs Aging2013302819023288604
- BresslerHBKeyesWJRochonPABadleyEThe prevalence of low back pain in the elderly: a systematic review of the literatureSpine19992417181310488512
- FormanWBOpioid analgesic drugs in the elderlyClin Geriatr Med19961234895008853941
- WHOWHO guidelines for the management of persistent pain in children with medical illnesses2012 Available from: http://apps.who.int/iris/bitstream/10665/44540/1/9789241548120_Guidelines.pdfAccessed June 2016
- BieriDReeveRAChampionGDAddicoatLZieglerJBThe Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale propertiesPain19904121391502367140
- GoodenoughBAddicoatLChampionGDPain in 4-to 6-year-old children receiving intramuscular injections: a comparison of the Faces Pain Scale with other self-report and behavioral measuresClin J Pain199713160739084953
- VergheseSTHannallahRSAcute pain management in childrenJ Pain Res2010310512321197314
- KorenGFlorescuACosteiAMBoskovicRMorettiMENon-steroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysisAnn Pharmacother200640582482916638921
- SchiffEMashiachSThe use of low dose aspirin in pregnancyAm J Reprod Immunol1992283–41531561285867
- MalhotraSKhannaSSafety of analgesics in pregnancyInt J Obstet Gynaecol Res201631208212
- Doncaster and Bassetlaw Hospitals, NHS Foundation TrustAnalgesia in Patients with Impaired Renal Function – Formulary Guidance2014 Available from: https://usa1990fa.files.wordpress.com/2014/10/analgesia-in-patients-with-impaired-renal-function.pdfAccessed June 2016
- SakataRKNunesMHGAnalgesics use for kidney failureRevista Dor2014153224229
- PhamP-CTToscanoEPhamP-MTPhamP-ATPhamSVPhamP-TTPain management in patients with chronic kidney diseaseNDT Plus20092211111825949305
- JohnsonSJOpioid safety in patients with renal or hepatic dysfunctionPain Treat Topics200761
- BosilkovskaMWalderBBessonMDaaliYDesmeulesJAnalgesics in patients with hepatic impairmentDrugs201272121645166922867045
- ImaniFMotavafMSafariSAlavianSMThe therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendationsHepat Mon20141410e2353925477978
- HamiltonJGoldbergEChopraSChiefJTFRunyonBAManagement of Pain in Patients with Advanced Chronic Liver Disease or Cirrhosis WolterKluwerWaltham, MA2012
- BensonGDKoffRSTolmanKGThe therapeutic use of acetaminophen in patients with liver diseaseAm J Ther200512213314115767831
- ChandokNWattKDSPain management in the cirrhotic patient: the clinical challengeMayo Clin Proc201085545145820357277
- McPhersonMLCanadayBRHeitHARospondRMA Pharmacist’s Guide to the Clinical Assessment and Management of PainAmerican Pharmacists Association2004 Available from: http://www.ulm.edu/~biglane/4058%20Pain%20-pharmacists-guide.pdfAccessed June 2016
- BellGMSchnitzerTJCox-2 inhibitors and other nonsteroidal anti-inflammatory drugs in the treatment of pain in the elderlyClin Geriatr Med200117348950211459717
- AntmanEMBennettJSDaughertyAFurbergCRobertsHTaubertKAUse of nonsteroidal antiinflammatory drugs. An update for clinicians: a scientific statement from the American Heart AssociationCirculation20071151634164217325246
- DworkinRHO’ConnorABAudetteJRecommendations for the pharmacological management of neuropathic pain: an overview and literature updateMayo Clin Proc2010853 supplS3S14
- CleelandCRyanKPain assessment: global use of the Brief Pain InventoryAnn Acad Med Singapore19942321291388080219
- CleelandCSRyanKThe Brief Pain InventoryPain Research Group1991 Available from: http://sosmanuals.com/manuals/cae48184b-7bae3b1d85105ea85375b60.pdfAccessed June 2016