Abstract
The objective of this article is to review published clinical data on diclofenac epolamine topical patch 1.3% (DETP) in the treatment of acute soft tissue injuries, such as strains, sprains, and contusions. Review of published literature on topical nonsteroidal anti-inflammatory drugs (NSAIDs), diclofenac, and DETP in patients with acute soft tissue injuries was included. Relevant literature was identified on MEDLINE using the search terms topical NSAIDs, diclofenac, diclofenac epolamine, acute pain, sports injury, soft tissue injury, strain, sprain, and contusion, and from citations in retrieved articles covering the years 1978–2008. Review of published, randomized clinical trials and meta-analyses shows that topical NSAIDs are significantly more effective than placebo in relieving acute pain; the pooled average relative benefit was 1.7 (95% confidence interval, 1.5–1.9). In a limited number of comparisons, topical and oral NSAIDs provided comparable pain relief, but the use of topical agents produced lower plasma drug concentrations and fewer systemic adverse events (AEs). The physical–chemical properties of diclofenac epolamine make it well suited for topical use. In patients with acute soft tissue injuries treated with DETP, clinical data report an analgesic benefit within hours of the first application, and significant pain relief relative to placebo within 3 days. Moreover, DETP displayed tolerability comparable with placebo; the most common AEs were pruritus and other application site reactions. Review of published literature suggests that DETP is generally safe and well tolerated, clinically efficacious, and a rational treatment option for patients experiencing acute pain associated with strains, sprains, and contusions, and other localized painful conditions.
Introduction
Muscle and musculoskeletal injuries, including sprains, strains, and contusions, comprise the majority of sports-related injuries.Citation1–Citation3 Sprains resulting from joint injuries are graded from I to III (mild to severe or by number and extent of ligaments involved) and most frequently affect the ankle.Citation2 Sprains involve some degree of tearing and stretching of a supporting ligament,Citation2 whereas strains refer to partial or complete tearing of the muscle–tendon unit.Citation1 Muscle contusions result from direct blunt trauma to muscle fibers, causing damage to the structure and function of the muscle.Citation3 Each of these injuries produces local tissue inflammation, which causes pain, swelling, tenderness, limited mobility, and if sufficiently severe, disability.Citation2,Citation3 Moreover, initial treatment is similar depending on the severity of the injury. In general, initial treatment consists of rest, ice, compression, and elevation (RICE) and use of nonsteroidal anti-inflammatory drugs (NSAIDs) to limit the extent of inflammation and pain and promote their early resolution.Citation3–Citation5 Depending on the injury, exercise and rehabilitation are then used to restore strength and range of motion, and to promote proper healing.
Soft tissue injury
Acute ankle injuries account for an estimated 20% of all sports-related injuries and, of these, 85% are sprains typically involving inversion injuries to the lateral stabilizing ligaments (ie, anterior talofibular, calcaneofibular, and posterior talofibular).Citation2,Citation6–Citation8 About 5% result from eversion injuries of the deltoid or medial ligaments.Citation8 Conservative treatment with early weight bearing, bracing, and functional rehabilitation is generally the accepted approach for managing ankle sprains.Citation2 A recent, systematic review of 24 high-quality clinical studies assessing the clinical course of ankle sprains revealed that there is a rapid decrease in patient reports of pain over the first 2 weeks following the injury.Citation9 The initial pain and swelling following an ankle sprain may limit the patient’s ability to perform the rehabilitation necessary for proper healing.Citation10 Moreover, fluid accumulation around the site of injury may exacerbate tissue damage, delay healing, and eventually lead to some degree of chronic disability.Citation11 Accordingly, the initial emphasis of treatment after injury is to rapidly reduce pain and inflammation with RICE and use of NSAIDs during post-injury and the initial rehabilitation program.Citation4 NSAIDs have been shown to effectively reduce pain and inflammation, improve function, and allow more rapid return to normal activity after ankle sprains.Citation6 Although most ankle sprains do not lead to significant disability, residual pain is prevalent in 5%–33% of patients for up to 1 year after injury; in some patients, ankle symptoms, such as lateral instability, also persist.Citation9
Acute muscle strains are partial tears of the tendinous insertion into the muscle at the distal myotendinous junction, which are caused by excessive stretching while the muscle is being activated or during excessive use.Citation5,Citation12 In general, muscles that cross two or more joints and those with a higher percentage of type II muscle fibers are particularly susceptible to strains (eg, hamstring, quadriceps, gastrocnemius).Citation1,Citation5 The degree of damage depends on how much force was placed on the muscle in relation to the muscle resistance and, in turn, is associated with the magnitude of pain and inflammation.Citation5 Hemorrhage and hematoma formation are commonly seen in acute strains. Treatment is based on the severity of the injury and includes RICE and NSAIDs in the acute setting.Citation3,Citation5 During the healing phase, stretching and strengthening exercises are often added.Citation3
Contusions resulting from direct blunt trauma to muscle fibers may cause capillary rupture, bleeding, hematoma formation, and eventually an intense inflammatory response.Citation5 Mild contusions cause localized tenderness with little, if any, loss in range of motion, whereas severe contusions present with marked tenderness and swelling and less than 50% range of motion.Citation5 RICE is used initially to limit the range of motion of the affected extremity and minimize the risk of hemorrhage, and NSAIDs are introduced in the acute setting to provide early resolution of pain and inflammation.Citation3 This is followed by restoration of active and active-assisted range-of-motion exercises and, subsequently, by functional rehabilitation and resistance exercises.Citation5
Use of oral NSAIDs in the management of soft tissue injuries
NSAIDs provide the cornerstone for the treatment of acute pain and inflammation.Citation11,Citation13 Clinical studies in patients with soft tissue injuries demonstrate that NSAIDs provide symptomatic relief of pain and allow faster recovery and return to normal activities when compared with placebo.Citation11,Citation13 On the basis of these benefits, NSAIDs have a role in the short-term management of soft tissue injuries. They should be used judiciously for as short a period as possible and at the lowest possible effective dose, based on the type of injury and level of dysfunction and pain.Citation13 In general, a short course of NSAID therapy lasting 3–7 days after ligament sprains and muscle injury is likely to be beneficial, but the effects of their long-term use remain controversial.Citation13 Use of NSAIDs in acute tendonitis and tenosynovitis is also reasonable in the days immediately after injury, when some amount of inflammation is expected.Citation13
The analgesic and anti-inflammatory effects of NSAIDs are mediated via their inhibition of prostaglandin biosynthesis. Traditional NSAIDs, such as diclofenac, inhibit both cyclooxygenase-1 (COX-1) and COX-2 isoenzymes, which are rate-limiting enzymes in the prostaglandin biosynthetic pathway.Citation14,Citation15 At pharmacologically effective doses, systemic NSAID therapy may produce a variety of adverse events (AEs) related to the inhibition of prostaglandin synthesis involved in maintaining normal physiological processes. For example, inhibition of the COX-1 isoenzyme in gastrointestinal mucosa is known to be responsible for the increased risk of gastrointestinal AEs associated with traditional NSAID use. This led to the design and development of the COX-2–selective inhibitors, such as celecoxib and etoricoxib, which display reduced risk of gastrointestinal AEs compared with traditional NSAIDs.Citation16
Gastrointestinal toxicity, including serious gastrointestinal perforations, ulcers, or bleeding, can often limit the use of traditional NSAIDs.Citation17 Poor tolerability due to upper gastrointestinal symptoms, often categorized as dyspepsia, is a significant cause of treatment discontinuation.Citation18 The prevalence of dyspepsia in patients treated with traditional NSAIDs can be high, ranging from 5%–50%.Citation19 Due to the role of the COX-2 isoenzyme in renal prostaglandin biosynthesis, both traditional and selective NSAIDs are associated with renovascular AEs; risk may be increased in agents with a longer half-life.Citation20,Citation21
The AEs associated with oral NSAID therapy also have a significant economic consequence. A retrospective database analysis found that direct medical costs were increased by nearly $1 per day due to gastrointestinal events related to NSAID use in an elderly population.Citation22 Other studies have shown that reducing gastrointestinal events by switching from oral NSAIDs to topical agents would reduce medical costs.Citation23
Topical NSAIDs
General
Topical NSAIDs are designed to be applied over the site of injury and exert their analgesic and anti-inflammatory effects in the underlying superficial, musculoskeletal soft tissue. Topical NSAIDs work locally and are not dependent on systemic absorption and subsequent redistribution into peripheral tissues.Citation24 Accordingly, topical application of NSAIDs differs from transdermal drug delivery, which is designed to provide active drug to the systemic circulation and exert effects at sites distant from the application site.Citation25
Benefits
Topical application of NSAIDs offers a number of potential benefits compared with oral systemic delivery. NSAIDs, when administered topically, are generally well tolerated with AEs mainly limited to mild skin irritation – erythema, dermatitis, and pruritus – that resolves upon discontinuation.Citation26 Topical administration results in significantly lower systemic drug exposure, reducing the risks of dose-dependent NSAID-associated AEs.Citation27 In the event of an untoward effect, the topical dose can be easily terminated.Citation25 Topical application can also avoid extensive first-pass metabolism, as is seen with oral diclofenac formulations. Other advantages of topical drugs include direct access, delivery, localization at site of action, and the option of prolonged use;Citation28–Citation30 ease of use may lead to improved patient compliance and adherence to the prescribed regimen.Citation31–Citation33 Moreover, topical drugs may be a viable option for patients who cannot use oral medications.Citation25
Pharmacokinetics
Pharmacokinetic studies, which included diclofenac, ketorolac, and ketoprofen in various formulations, including gels, ointments, and patches, found that peak plasma NSAID concentrations following topical application are 0.2%–8% of those seen after oral dosing.Citation15 Additionally, the time to achieve peak levels following topical application was approximately 10-fold longer than the corresponding time following an equivalent oral dose and ranged from 2.2 to 23 hours.Citation15 With multiple topical doses, steady state is achieved within 2–5 days, and Cmax is about 2.5 times higher than Cmax following a single topical dose; however, serum concentrations remain >90% lower than those achieved after oral NSAIDs.Citation34,Citation35 Application of a diclofenac epolamine topical patch (DETP) in 10 healthy male volunteers (twice daily for 7 days) produced peak plasma drug levels nearly 100 times lower than those achieved after a single 50-mg, oral dose of diclofenac with area under curve values that were 10–20 times lower over 48 hours.Citation36,Citation37
Efficacy
The efficacy of topical NSAIDs was assessed in a meta-analysis of 61 randomized controlled trials that involved a total of 7,727 patients with acute pain.Citation27 Trials were included if a topical NSAID was compared with placebo, another topical NSAID, or an oral NSAID in recent soft tissue injury, sprain, strain, or trauma. Of 37 placebo-controlled trials, 27 showed that the topical NSAID was significantly superior to placebo, and 9 additional studies showed a trend favoring the topical drug ().Citation27 Overall, the pooled relative benefit was 1.7 (95% confidence interval [CI]: 1.5–1.9), and the number needed to treat (NNT) to achieve a successful outcome with topical NSAIDs was 3.9 (95% CI: 3.4–4.4). The 24 studies that compared topical NSAIDs with one another or with an oral NSAID found no difference in efficacy between treatments.Citation27 Overall, the incidence of local AEs (2.6% vs 3.0%), systemic AEs (0.8% vs 0.7%), and AEs leading to withdrawal of treatment (0.6% vs 0.4%) did not differ significantly between the topical NSAIDs and placebo.Citation27 This meta-analysis also included 25 additional trials involving 2,433 patients with chronic pain conditions, including osteoarthritis and other rheumatologic disorders.Citation27 In this analysis, topical NSAIDs were more effective than placebo, with a pooled relative benefit of 2.0 (95% CI: 1.5–2.7) and an NNT of 3.1 (95% CI: 2.7–3.8).Citation27
Figure 1 Success rates with topical nonsteroidal anti-inflammatory drugs (NSAIDs) vs topical placebo in the treatment of acute (open circles) and chronic (filled squares) painful conditions. Outcomes were assessed after 7 days in acute conditions and 14 days in chronic conditions. Copyright © 1998, BMJ Publishing Group Ltd. Reproduced with permission from Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs, BMJ. 1998;316:333–338.
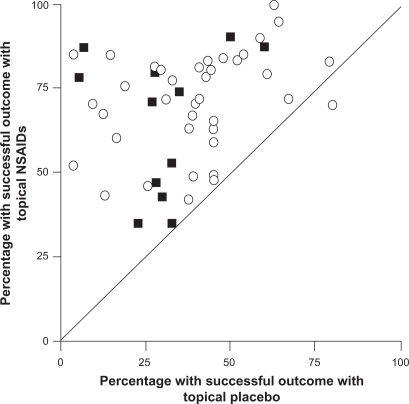
A subsequent meta-analysis evaluated 26 randomized controlled trials involving 2,853 adults with acute pain resulting from strains, sprains, or sports injuries.Citation38 Topical NSAIDs demonstrated significantly better analgesic efficacy than placebo in 19 of the 26 trials and overall produced a relative benefit of 1.6 (95% CI: 1.4–1.7) and an NNT of 3.8 (95% CI: 3.4–4.4).Citation38 When only high-quality and/or high-validity studies were retained in the meta-analysis, the superiority of topical NSAIDs over placebo was still evident with NNT values of 3.7–4.0. Five topical NSAIDs were evaluated in at least 3 clinical trials (ketoprofen, ibuprofen, felbinac, piroxicam, and indomethacin) and all were found to be superior to placebo.Citation38 Consistent with the first meta-analysis, incidence of local AEs (4.4% vs 4.7%), systemic AEs (2.8% vs 2.4%), and AE–related withdrawals (0.8% vs 0.7%) was similar between topical NSAIDs and placebo.Citation38 Together, these analyses demonstrate that topical NSAIDs are effective and well tolerated in the short-term treatment of acute soft tissue injuries.
Limitations
Several limitations are inherent to the use of topical NSAIDs. Most notably, since systemic exposure is minimized, these formulations are only effective for the treatment of localized pain at the site of application. Due to the continued contact of the formulation with the skin, localized skin reactions, such as irritant dermatitis and erythema, are observed.Citation27,Citation38 However, as noted previously, meta-analyses have shown that these local AEs occur only in a small minority of patients treated for acute soft tissue injuries with topical NSAIDs.Citation27,Citation38
Diclofenac
Diclofenac is a traditional NSAID that possesses anti-inflammatory, analgesic, and antipyretic properties through inhibition of COX isoenzymes and blockade of prostaglandin synthesis.Citation39 It is among the most widely prescribed NSAIDs worldwide,Citation21 and unlike other traditional NSAIDs, such as ibuprofen and naproxen, does not interfere with the anti-platelet effects of low-dose aspirin.Citation21,Citation40,Citation41 Diclofenac also has the potential to affect polymorphonuclear leukocyte function in vitro, which may contribute to its anti-inflammatory effects by reducing the ability of these cells to infiltrate into an inflammatory site and produce damaging oxygen radicals and proteases.Citation39 Noncomparative clinical studies showed that oral diclofenac sodium has a rapid onset of action in treating soft tissue injuries, including acute strains, sprains, and contusions, with doses of 75–150 mg/d producing clinical improvement in as little as 2–3 days.Citation42 Comparative clinical trials demonstrated that diclofenac sodium administered as a conventional enteric-coated tablet formulation was at least as effective and well tolerated as other NSAIDs in patients with acute soft tissue injuries.Citation43–Citation45 In a randomized controlled trial of 139 patients with acute sports injuries, diclofenac sodium 150 mg/d allowed an earlier return to sport-playing fitness than aspirin 3.6 g/d,Citation44 and in another randomized controlled trial of 151 patients with acute strains, diclofenac sodium 100 mg/d was as effective as indomethacin 100 mg/d but produced fewer AEs and fewer discontinuations due to AEs.Citation43
Diclofenac dispersible, a drinkable formulation of diclofenac, also has been shown to provide a rapid onset of pain relief in patients with acute soft tissue injuries.Citation46 In a randomized, blinded study of 48 adults with acute minor sports injuries and moderate to severe pain on movement, a single 50-mg dose of diclofenac dispersible was more effective than 500 mg of naproxen granular in reducing pain intensity on movement and on pressure during the 4-hour period after dosing (P ≤ 0.04), and in producing rapid pain relief in the first hour (P = 0.034 at 15 minutes).Citation47 Both agents were equally well tolerated.
Regardless of the formulation, the most common AEs associated with oral diclofenac have been gastrointestinal effects, including abdominal pain or cramping, constipation, indigestion, and nausea and, less frequently, abdominal distention, flatulence, vomiting, and upper and lower gastrointestinal complicated events including bleeding.Citation16,Citation18,Citation39,Citation48 The transient effect of oral antiarthritic doses on inhibition of platelet aggregationCitation49 most likely accounts for the lower rates of ulcer complications associated with diclofenac use compared with other traditional NSAIDs.Citation50–Citation52 An analysis of data from clinical trials involving more than 85,000 patients, as well as postmarketing surveillance, found that oral diclofenac produces AEs in about 12% of patients and gastrointestinal AEs in 8%–10% of patients; approximately 1.5%–2% of patients discontinue treatment due to AEs.Citation39 However, trials evaluating NSAID and COX-2 inhibitor used in patients with rheumatoid arthritis and osteoarthritis have reported discontinuation rates due to gastrointestinal AEs of 6–19 events per 100 patient-years.Citation16,Citation18,Citation48 In a randomized controlled trial, the cumulative discontinuation rate due to gastrointestinal AEs in 3,518 patients receiving diclofenac sodium 50 mg three times daily for osteoarthritis was 19.2 events per 100 patient-years.Citation18
Considerations for topical administration of diclofenac
The stratum corneum – a heterogeneous structure containing protein (keratin), water, and lipids (cholesterol, phospholipids) – plays a crucial role in controlling the percutaneous absorption of drug molecules.Citation53,Citation54 Other key factors affecting absorption include drug-related factors (eg, lipophilicity, water solubility, molecular size, and vehicle), application conditions (open vs closed), and skin integrity, which is affected by disease, body site, and age.Citation53
Hydration of the stratum corneum is an important determinant of drug absorption. Free diclofenac displays very low water solubility due to its high hydrophobicity and lipophilicity and, therefore, is paired with a salt to facilitate topical absorption.Citation54,Citation55 Several different salts of diclofenac are used in commercial products, including sodium, potassium, diethylamine, and N-(2-hydroxyethyl) pyrrolidine (also called epolamine) salts, and they have been considered for both oral and topical administration.Citation56
Several formulations of diclofenac have also been developed, including gel, patch, and lotion formulations, which differ in their pharmacokinetics and systemic exposure. For example, a diclofenac aqueous gel formulation has been shown to produce Cmax levels more than double those seen with an emulsion gel.Citation57,Citation58 The topical solution, a 1.5% weight/weight diclofenac sodium topical solution, utilizes the permeation enhancer dimethyl sulfoxide (DMSO). Continuous application of the solution (80 drops 4 times daily for 7 days) is associated with a mean Tmax of 4.0 hours and a mean Cmax of 19.4 ng/mL, while continuous application of the diclofenac sodium 1% gel (4 g 4 times daily for 7 days) is associated with a median Tmax of 14 hours and a mean Cmax of 15 ng/mL.Citation59,Citation60 Additionally, continuous use of the topical solution resulted in approximately one-third of the systemic exposure seen after 1 month of applying a 3% diclofenac sodium gel twice daily.Citation59
Of the available diclofenac formulations, the epolamine salt of diclofenac offers specific advantages for topical administration. The surfactant property of diclofenac epolamine improves hydration of the stratum corneum, thereby lowering surface tension at the interface between the skin and the topical pharmaceutical preparation, which favors drug absorption through the skin.Citation54 The use of epolamine as the ion-pairing agent enhances lipophilicity and water solubility compared with simple sodium or potassium salts of diclofenac and the permeation of diclofenac through the stratum corneum and epidermis.Citation54–Citation56,Citation61,Citation62 This was demonstrated by a direct comparison that showed the mean absorption rate (ka) of diclofenac epolamine into plasma was 1.4-fold greater than that of diclofenac sodium (P < 0.001).Citation63 Interaction of the ion pair with cell membrane lecithins has also been demonstrated to enhance membrane permeability, further facilitating absorption of diclofenac through the external layer of the skin.Citation54,Citation64 This property omits the need for permeation enhancers, such as DMSO, which is used in other topical diclofenac formulations.Citation65,Citation66 When applied topically in a patch, diclofenac epolamine produces consistent and sustained drug release for 12 hours, with systemic drug exposure being 10–20 times lower over 48 hours than that following a single oral diclofenac dose.Citation36 A recent report has indicated that, over 12 hours, plasma concentrations of diclofenac after twice-daily dosing for 4 consecutive days with the patch formulation of diclofenac epolamine were <1% of those after a single 50-mg dose of oral diclofenac sodium enteric-coated tablets.Citation37,Citation67 The patch formulation of diclofenac epolamine has been shown to have a bioavailability of approximately 30% relative to a 1% gel formulation following continuous use over 7 days. The administration of diclofenac epolamine 1% gel led to a mean Tmax of 3.1 hours and mean Cmax of 28.1 ng/mL, while the patch formulation led to a mean Tmax and Cmax of 5.4 hours and 17.4 ng/mL, respectively.Citation36
Pharmacokinetic studies have shown that topically applied diclofenac not only penetrates the stratum corneum and deeper skin layers, but also reaches joints, muscles, and synovial fluid in sufficiently high concentrations to exert, in principle, local therapeutic activity.Citation68,Citation69 In one study, the plasma bioavailability of a topical 4% spray gel of diclofenac sodium applied to a defined area on the thigh was 50 times lower than oral administration of diclofenac sodium tablets. However, bioavailability in subcutaneous adipose tissue and skeletal muscle tissue was 2–3 times higher with topical dosing than with oral dosing.Citation70 In healthy volunteers, topical diclofenac was shown to improve the pain threshold of the quadriceps muscle, providing further evidence of deep penetration after topical application.Citation71
Efficacy and tolerability of topical diclofenac
The efficacy and tolerability of various topical diclofenac formulations, including 1.5% diclofenac sodium solution, 1.16% diclofenac diethylamine gel, 140 mg diclofenac hydroxyethylpyrrolidine patch, and 2% diclofenac lecithin organogel, in 19 clinical trials in more than 3,000 patients have been recently reviewed.Citation72 Topical diclofenac agents have been shown to provide effective analgesia for both acute and chronic pain, including indications such as blunt impact injuries, soft tissue injuries, sprains, osteoarthritis, and epicondylitis.Citation72 Efficacy of topical diclofenac was comparable with other topical NSAIDs (such as indomethacin gel, ketoprofen gel, piroxicam gel, and indomethacin patch) and some oral NSAIDs (diclofenac, naproxen, ibuprofen) in relieving acute and chronic pain.Citation72
Topical diclofenac formulations are well tolerated; local skin irritation was the most frequent AE and was easily resolved. Incidence of systemic AEs has been shown to be similar to placebo,Citation65 and the incidence of gastrointestinal AEs has been shown to be lower than oral diclofenac (35% vs 48%, P = 0.0006).Citation73 The use of DMSO to enhance percutaneous absorption of diclofenac sodium may contribute to the localized skin irritation, and in some patients, cause halitosis and body odor.Citation65
Unlike conventional topical formulations (eg, gels), patches permit constant delivery of the active agent into the affected area by means of an occlusive bandage that promotes controlled and slow release of the drug.Citation36,Citation74 Occlusion has a favorable impact on drug absorption, sometimes improving penetration by 200%–300%, which likely reflects changes in skin hydration and temperature.Citation30
Diclofenac epolamine topical patch
The DETP 1.3% (FLECTOR® Patch; King Pharmaceuticals® Inc, Bristol, TN), the first topical NSAID patch approved in the United States for the treatment of acute pain of minor strains, sprains, and contusions, is a topical delivery system composed of an adhesive material containing 1.3% diclofenac epolamine, which is applied to a nonwoven polyester felt backing.Citation75 The 10 cm × 14 cm patch is covered by a polypropylene film release liner that is removed before the patch is applied to the skin. Each adhesive patch contains 180 mg of diclofenac epolamine and inactive excipients, which enhance skin hydration and facilitate plaster adherence. Following application of DETP on the upper arm, peak plasma concentrations of 0.7–6.0 ng/mL were found at 10–20 hours with plasma concentrations increasing to 1.3–8.8 ng/mL after twice-daily application of DETP for 5 days.Citation75 Once absorbed, diclofenac is highly bound to serum albumin (>99%), with a half-life of approximately 12 hours.Citation75 Diclofenac undergoes metabolism and subsequent urinary and biliary excretion of the sulfate and glucuronide conjugates.Citation75
DETP is indicated for the topical treatment of acute pain resulting from minor strains, sprains, and contusions.Citation75 One patch is applied to the painful area and changed every 12 hours.Citation75
Efficacy and tolerability of DETP in treating strains, sprains, and contusions
The efficacy and tolerability of DETP in relieving acute pain due to minor sports injury were evaluated in a multicenter, randomized, placebo-controlled, parallel-group study of 222 adults who were injured within the 72 hours prior to study enrollment.Citation76 The patch was applied directly over the injured site and changed twice daily for 2 weeks. Measures of pain intensity were evaluated on days 3, 7, and 14. DETP was significantly more effective than placebo in relieving pain during daily activities as measured by a 100-mm visual analog scale (VAS) and analyzed by the summed pain intensity difference during clinic visits on days 3 (P = 0.036) and 14 (P = 0.048).Citation76 In addition, DETP was more effective than placebo based on daily diary recordings on days 3, 7, and 14 (P ≤ 0.044). DETP was well tolerated; the incidence and intensity of AEs were comparable with those seen with the placebo patch. Rash was reported by 5% of patients in each treatment group, whereas pruritus was less common with DETP than placebo (5% vs 17%).Citation76
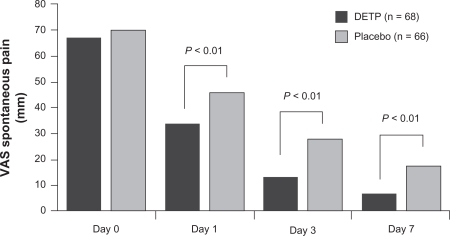
Comparable results were reported in a randomized, placebo-controlled, multicenter study conducted in the United States, which included 365 patients with minor sports injuries occurring within the previous 72 hours and pain ≥ 5 on a numeric pain scale from 0 to 10.Citation77 DETP significantly reduced the median time to pain resolution (primary end point) compared with placebo (8.8 vs 12.4 days, P = 0.009). In addition, DETP significantly reduced spontaneous pain compared with placebo, starting on day 6 and continuing until day 13 (all P < 0.05). The tolerability of DETP was comparable with placebo, with no difference between treatments in the incidence or distribution of AEs.Citation77
DETP was also assessed in a single-center, open-label study involving 101 patients with minor sports and overload injuries.Citation78 DETP was applied topically twice daily for 14 days. Spontaneous pain – measured using a 4-point verbal scale ranging from 0 (no pain) to 3 (severe pain) – was reduced by 28% on day 7 and 60% on day 14.Citation78 Comparable results were found for spontaneous pain, measured using a 10-cm VAS, and pain on pressure, evaluated using the 4-point verbal scale. DETP was well tolerated, with all patients rating the tolerability of DETP to be good to excellent.Citation78
Two 7-day, multicenter, randomized controlled studies evaluated DETP in the treatment of acute pain due to ankle sprains. In the first study, which enrolled 140 patients with benign ankle sprains, DETP significantly reduced spontaneous pain starting as early as 3 hours (P = 0.005) and continuing through day 3 (P = 0.004) and day 7 (P = 0.0008).Citation79 DETP also reduced all secondary efficacy parameters (pain at rest and upon palpation, provoked passive pressure, and monopedal support) compared with placebo starting on day 3 and persisting through day 7. Similarly, DETP produced greater anti-inflammatory activity than placebo assessed by the decrease in edema as measured from the perimalleolar perimeter. Tolerability was judged to be good or very good by physicians in 69 of 70 patients in each group.Citation79
In the second study, 134 patients with ankle sprains occurring within the previous 48 hours and having pain ≥ 50 mm on a 100-mm VAS were randomly allocated to DETP or placebo group.Citation80 DETP was superior to placebo in reducing pain on movement starting 4 hours after the first application (P < 0.02) and continuing up to the end of the 7-day study period (P < 0.01; ). DETP was also superior to placebo in improving the secondary study end points, including pain during passive stretch, on pressure, and when standing on one foot, by day 3 (P = 0.003, P = 0.007, P = 0.002, respectively).Citation80 No AEs were reported in this study.
Figure 2 Comparison of diclofenac epolamine topical patch (DETP) and placebo in reducing pain on movement in patient with acute ankle sprains. Pain was measured on a 100-mm visual analog scale (VAS) at baseline (day 0) and on days 1, 3, and 7 of twice-daily treatment. Copyright © 2003, Elsevier Masson SAS Editeur, all rights reserved. Adapted with permission from Joussellin E. Flector Tissugel® in the treatment of painful ankle sprain. J Traumatol Sport. 2003;20:1S5–1S9.
Efficacy and tolerability of DETP in other painful conditions
DETP administered for 14 days to patients with epicondylitis significantly reduced the percentage of patients reporting moderate to intense pain compared with baseline and placebo (P < 0.05).Citation81 At study completion, nearly a third fewer patients treated with DETP continued to experience intense pain compared with placebo. Pain on pressure was also significantly improved by DETP compared with placebo starting on day 7.Citation81 DETP was well tolerated, with mild, temporary skin reactions seen in 1 patient in each study group.
DETP or placebo was applied topically every 12 hours for 2 weeks to 60 patients with periarticular tendinous disorders (eg, tendonitis, epicondylitis), rheumatologic disorders (eg, osteoarthritis, rheumatoid arthritis), or extra-articular pathologies (eg, fibrositis).Citation74 In the subgroup with periarticular disorders, spontaneous pain, pain on pressure, and periarticular swelling improved progressively with DETP, with significant differences compared with placebo first seen on day 5 (P ≤ 0.05) and persisting to the end of the 14-day treatment period (P ≤ 0.01).Citation74 On day 7, the efficacy of DETP was judged to be good or excellent by 70% of patients compared with only 7% in the placebo group. The efficacy of DETP was also superior to placebo in the subgroup with fibrositis starting on day 7.Citation74 DETP applied twice daily was compared with 2 g of diclofenac diethylammonium emulgel 1.16% applied 4 times per day for 14 days to patients with localized periarticular or tendinous disorders.Citation82 Although both topical agents significantly improved spontaneous pain and pain on pressure, the effects of DETP were significantly greater than those of diclofenac diethylammonium (P < 0.001).Citation82 Furthermore, the patient and physician assessments of treatment efficacy favored DETP (both P < 0.0001). Both treatments were similarly well tolerated irrespective of the site or nature of the localized inflammation.
Safety profile
The most common AEs with DETP in placebo-controlled clinical trials were application site reactions (11%), most commonly pruritus (5%), occurring at rates comparable with placebo (12% and 8%, respectively).Citation75 DETP should not be applied to nonintact or damaged skin including areas with, for example, dermatitis, eczema, lesions, burns, or wounds.Citation75
Gastrointestinal AEs were reported in 9% of patients treated with DETP compared with 6% of those who received placebo,Citation75 representing a three fold to five fold improvement over the rates for traditional oral NSAIDs.Citation50 Dyspepsia was reported in only 1% of patients treated with DETP. Regardless, DETP should be used with caution in patients with a history of ulcers or gastrointestinal bleeding.Citation75
As with other NSAIDs, DETP has boxed warnings for cardiovascular and gastrointestinal risk and should be used with caution in patients with hypertension, congestive heart failure, and fluid retention; it is contraindicated in the treatment of perioperative pain following coronary artery bypass graft surgery.Citation75 Treatment with DETP is not recommended in patients with advanced renal disease but, if initiated, close monitoring of renal function is advised. Patients who develop abnormal liver function tests, anemia, or abnormal platelet function during use should also be monitored and further evaluated.
The general precautions recommended for all NSAIDs also apply to DETP. DETP should not be taken by patients who have shown hypersensitivity to diclofenac or other NSAIDs, and since there are no well-controlled studies in pregnant women, the benefit and risk should be carefully weighed.Citation75
The potential for drug–drug interactions should be considered when DETP is used by patients who are simultaneously using other drugs.Citation75 This may be especially relevant in older patients who may have age-related physiological changes and comorbid conditions that require concomitant medications.Citation83 In general, NSAIDs may reduce the antihypertensive effects of angiotensin-converting enzyme inhibitors and the natriuretic effects of furosemide and thiazide diuretics. NSAIDs can increase exposure to lithium and methotrexate, raising the possibility for greater toxicity by these agents, and augment the anticoagulant effects of warfarin, thereby raising risk of gastrointestinal bleeding. In addition, concomitant use of aspirin reduces binding of diclofenac to serum proteins, thereby increasing the possibility of AEs.Citation75
A large proportion of the adult population in the United States is currently treated with low-dose aspirin for cardiovascular prophylaxis. In a recent Internet survey of 1,000 general practitioners in the United States, 85% of providers indicated that more than 25% of their patients were taking aspirin for preventive reasons.Citation84 The concomitant use of low-dose aspirin and oral NSAIDs has been associated with an increased risk of serious gastrointestinal AEs.Citation19 Although the use of gastroprotective agents, such as proton pump inhibitors, appears to lower the risk of serious gastrointestinal AEs, not all patients on NSAID therapy receive these agents.Citation84 Therefore, patients currently being treated with low-dose aspirin and requiring concomitant NSAID therapy should be prescribed the lowest effective dose for the shortest time period. In this regard, topical NSAID formulations allow for the lowest systemic exposure, providing effective analgesic and anti-inflammatory clinical efficacy for the treatment of acute strains, sprains, and contusions. However, it is unlikely that controlled clinical studies will be performed to specifically examine the risk of NSAID-associated AEs in patients being treated for acute pain with topical NSAIDs and low-dose aspirin, as studies involving the treatment of acute pain usually include small sample sizes compared with those needed to adequately address this question.
Conclusion
Topical NSAIDs are effective in the treatment of acute pain due to soft tissue injuries. These agents are designed to work locally in the soft tissue and on peripheral nerves underlying the site of application and are not designed to be absorbed systemically in an appreciable amount. Controlled clinical trials have demonstrated that DETP is clinically effective in treating acute pain due to soft tissue injuries, producing clinical benefit within several hours of the first application and significant pain relief relative to placebo that is measurable within 3 days and maintained through the end of a 7-day to 14-day treatment period. Notably, DETP is well tolerated, with the incidence and intensity of AEs being comparable with placebo. On the basis of available evidence, DETP represents a generally safe and well-tolerated, clinically effective option for treating acute pain associated with strains, sprains, and contusions, along with other localized painful inflammatory conditions.
Acknowledgements
Writing and editorial assistance for this manuscript was provided by Jennifer Schwinn, RPh, of Quintiles Medical Communications, Parsippany, New Jersey, USA. Funding for writing and editorial support was provided by King Pharmaceuticals®, Inc., Bridgewater, New Jersey, USA. Funders were involved in the review of the manuscript for accuracy of content.
Disclosure
Dr Lionberger is a consultant for Pfizer Inc, Aesculp-B Braun, Smith & Nephew Pharmaceutical Ltd., and King Pharmaceuticals®, Inc.
Dr Brennan has served as a consultant and on speaker’s bureaus for Alpharma Pharmaceuticals, a wholly owned subsidiary of King Pharmaceuticals®, Inc., Cephalon, Inc., Purdue Pharma, Forest Laboratories, Inc., PriCara, Eli Lilly, and Endo Pharmaceuticals.
References
- GarrettWEJrMuscle strain injuries: clinical and basic aspectsMed Sci Sports Exerc19902244364432205779
- LiuSHNguyenTMAnkle sprains and other soft tissue injuriesCurr Opin Rheumatol199911213213710319217
- AlmekindersLCAnti-inflammatory treatment of muscular injuries in sport. An update of recent studiesSports Med199928638338810623981
- PetrellaREkmanEFSchullerRFortJGEfficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trialClin J Sport Med200414422523115273528
- ArringtonEDMillerMDSkeletal muscle injuriesOrthop Clin North Am19952634114227609956
- IvinsDAcute ankle sprain: an updateAm Fam Physician200674101714172017137000
- GerberJPWilliamsGNScovilleCRArcieroRATaylorDCPersistent disability associated with ankle sprains: a prospective examination of an athletic populationFoot Ankle Int199819106536609801078
- RubinASallisREvaluation and diagnosis of ankle injuriesAm Fam Physician1996545160916188857783
- van RijnRMvan OsAGBernsenRMLuijsterburgPAKoesBWBierma-ZeinstraSMWhat is the clinical course of ankle sprains? A systematic literature reviewAm J Med2008121432433118374692
- WolfeMWUhlTLMattacolaCGMcCluskeyLCManagement of ankle sprainsAm Fam Physician20016319310411195774
- Ogilvie-HarrisDJGilbartMTreatment modalities for soft tissue injuries of the ankle: a critical reviewClin J Sport Med1995531751867670974
- BoutinRDFritzRCSteinbachLSImaging of sports-related muscle injuriesRadiol Clin North Am2002402333362vii12118828
- MehalloCJDreznerJABytomskiJRPractical management: non-steroidal antiinflammatory drug (NSAID) use in athletic injuriesClin J Sport Med200616217017416603889
- PaulusHEBulpittKJNonsteroidal antiinflammatory agents and corticosteroidsSchumacherHRKlippelJHKoopmanWJPrimer on the Rheumatic Diseases10th edAtlanta, GAArthritis Foundation1993298303
- HeynemanCALawless-LidayCWallGCOral versus topical NSAIDs in rheumatic diseasesDrugs200060355557411030467
- LaineLCurtisSPKaurACannonCPfor the MEDAL Steering CommitteeAssessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomized comparisonLancet2007369956046547317292766
- LanasASerranoPBajadorEFuentesJSainzRRisk of upper gastrointestinal bleeding associated with non-aspirin cardiovascular drugs, analgesics and nonsteroidal anti-inflammatory drugsEur J Gastroenterol Hepatol200315217317812560762
- BarafSBFuentealbaCGreenwaldMfor the EDGE Study GroupGastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the Etoricoxib versus Diclofenac Sodium Gastrointestinal Tolerability and Effectiveness (EDGE) trialJ Rheumatol200734240842017304660
- SopenaFLanasAHow to advise aspirin use in patients who need NSAIDsCurr Pharm Des200713222248226017691998
- BraterDCEffects of nonsteroidal anti-inflammatory drugs on renal function: focus on cyclooxygenase-2-selective inhibitionAm J Med19991076AS65S70 discussion S70–S71.
- CannonCPCurtisSPFitzGeraldGAfor the MEDAL Steering CommitteeCardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparisonLancet200636895491771178117113426
- RahmeEJosephLKongSXWatsonDJLeLorierJCost of prescribed NSAID-related gastrointestinal adverse events in elderly patientsBr J Clin Pharmacol200152218519211488776
- HosieGBirdHThe topical NSAID felbinac versus oral NSAIDs: a critical reviewEur J Rheumatol Inflamm199414421287601178
- BalleriniRCasiniAChinolMMannucciCGiaccaiLSalviMStudy on the absorption of ketoprofen topically administered in man: comparison between tissue and plasma levelsInt J Clin Pharmacol Res19866169723957503
- StanosSPTopical agents for the management of musculoskeletal painJ Pain Symptom Manage200733334235517349504
- DreiserRLTopical antirheumatic drug therapy: current practice and future trendsEur J Rheumatol Inflamm1994144387601180
- MooreRATramerMRCarrollDWiffenPJMcQuayHJQuantitative systematic review of topically applied non-steroidal anti-inflammatory drugsBMJ199831671283333389487165
- BrownMBMartinGPJonesSAAkomeahFKDermal and transdermal drug delivery systems: current and future prospectsDrug Deliv200613317518716556569
- YangSIParkHYLeeSHTransdermal eperisone elicits more potent and longer-lasting muscle relaxation than oral eperisonePharmacology200471315015615161997
- VarvelJRShaferSLHwangSSAbsorption characteristics of transdermally administered fentanylAnesthesiology19897069289342729633
- ClearyGWTransdermal delivery systems: a medical rationaleShahVPMailbachHITopical Drug Bioavailability, Bioequivalence, and PenetrationNew York, NYPlenum Press19931768
- PayneRMathiasSDPastaDJWankeLAWilliamsRMahmoudRQuality of life and cancer pain: satisfaction and side effects with transdermal fentanyl versus oral morphineJ Clin Oncol1998164158815939552070
- HenzlMRLoombaPKTransdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practiceJ Reprod Med200348752554012953327
- SioufiAPommierFBoschetFGodbillonJLavoignatDSalliereDPercutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac emulgelBiopharm Drug Dispos19941564414497993982
- TaburetAMSinglasEGlassRCThomasFLeuteneggerEPharmacokinetic comparison of oral and local action transcutaneous flurbiprofen in healthy volunteersJ Clin Pharm Ther19952021011077650070
- AssandriACanaliSGiachettiCLocal tolerability and pharmacokinetic profile of a new transdermal delivery system, diclofenac hydroxyethylpyrrolidine plasterDrugs Exp Clin Res199319389958112203
- WillisJVKendallMJPharmacokinetic studies on diclofenac sodium in young and old volunteersScand J Rheumatol Suppl1978223641278178
- MasonLMooreRAEdwardsJEDerrySMcQuayHJTopical NSAIDs for acute pain: a meta-analysisBMC Fam Pract20045101915147585
- SmallREDiclofenac sodiumClin Pharm1989885455582670397
- Catella-LawsonFReillyMPKapoorSCCyclooxygenase inhibitors and the antiplatelet effects of aspirinN Engl J Med2001345251809181711752357
- GladdingPAWebsterMWFarrellHBZengISLParkRRuijneNThe antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteersAm J Cardiol200810171060106318359332
- MachteyIDiclofenac in the treatment of painful joints and traumatic tendinitis (including strains and sprains): a brief reviewSemin Arthritis Rheum1985152 Suppl 187924081796
- FurbergBLernerANyströmBRosénMWilligPAntiphlogistics in acute inflammatory conditions in the soft tissues of the musculo-skeletal system – a double blind comparison of diclofenac and indomethecinCurr Ther Res198538523527
- DuncanJJFarrJEComparison of diclofenac sodium and aspirin in the treatment of acute sports injuriesAm J Sports Med19881666566593149152
- MeadersMAWisemanPLA randomized multicenter comparison of piroxicam and diclofenac in the treatment of acute musculoskeletal injuriesCurr Ther Res199047971981
- BakshiRRotmanHShawMSussmanHDouble-blind, multicenter evaluation of the efficacy and tolerability of diclofenac dispersible in the treatment of acute soft-tissue injuriesClin Ther199517130377758059
- ColomboGGiombiniAPamichTDiclofenac dispersible provides superior analgesia with faster onset of action compared to naproxen granular in patients with acute, painful, minor sports injuriesJ Sports Med Phys Fitness19973732282339407756
- KruegerKLinoLDoreRGastrointestinal tolerability of etoricoxib in rheumatoid arthritis patients: results of the etoricoxib vs diclofenac sodium gastrointestinal tolerability and effectiveness trial (EDGE-II)Ann Rheum Dis200867331532217965424
- SchwartzJIDallobALLarsonPJComparative inhibitory activity of etoricoxib, celecoxib, and diclofenac on COX-2 versus COX-1 in healthy subjectsJ Clin Pharmacol200848674575418434566
- GoldsteinJLLowrySCLanzaFLSchwartzHIDogeWEThe impact of low-dose aspirin on endoscopic gastric and duodenal ulcer rates in users of a non-selective non-steroidal anti-inflammatory drug or a cyclo-oxygenase-2-selective inhibitorAliment Pharmacol Ther200623101489149816669964
- GoldsteinJLEisenGMLewisBGralnekIMZlotnickSFortJGVideo capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placeboClin Gastroenterol Hepatol20053213314115704047
- WilcoxCMAllisonJBenzulyKConsensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirinClin Gastroenterol Hepatol2006491082108916877048
- MorganCJRenwickAGFriedmannPSThe role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysisBr J Dermatol2003148343444312653734
- FiniAFazioGRapaportIDiclofenac/N-(2-hydroxyethyl)pyrrolidine: a new salt for an old drugDrugs Exp Clin Res199319381888112202
- FiniAFazioGGonzalez-RodriguezMCavallariCPasseriniNRodriguezLFormation of ion-pairs in aqueous solutions of diclofenac saltsInt J Pharm1999187216317310502622
- MinghettiPCilurzoFCasiraghiAEx vivo study of transdermal permeation of four diclofenac salts from different vehiclesJ Pharm Sci200796481482317286288
- HaroutiunianSDrennanDALipmanAGTopical NSAID therapy for musculoskeletal painPain Med201011453554920210866
- SethBLComparative pharmacokinetics and bioavailability study of percutaneous absorption of diclofenac from two topical formulations containing drug as a solution gel or as an emulsion gelArzneimittelforschung19924221201221610419
- Pennsaid [package insert]Varennes, QuebecNuvo Manufacturing2010
- Voltaren Gel [package insert]Parsippany, NJNovartis Consumer Health Inc2009
- LedwidgeMTCorriganOIEffects of surface active characteristics and solid state forms on pH solubility profiles of drug-salt systemsInt J Pharm1998174187200
- O’ConnorKMCorriganOIComparison of the physicochemical properties of the N-(2-hydroxyethyl) pyrrolidine, diethylamine and sodium salt forms of diclofenacInt J Pharm2001222228129311427358
- MaggiCALualdiPMautoneGComparative bioavailability of diclofenac hydroxyethylpyrrolidine vs diclofenac sodium in manEur J Clin Pharmacol19903822072082338120
- ConteARoncaGPetriniMMautoneGEffect of lecithin on epicutaneous absorption of diclofenac epolamineDrugs Exp Clin Res200228624925512776579
- BookmanAAWilliamsKSShainhouseJZEffect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trialCMAJ20041711433333815313991
- RovatiSGaravaniAResearch and development of a pharmaceutical technique allowing for improvement in clinical efficacy and simplicity of use of known drugsTribuna Medi Ticinese199661204207
- RuscaAMautoneGSunSMagelliMJohnsonFComparison of plasma pharmacokinetics of FLECTOR® Patch (diclofenac epolamine topical patch) and oral Voltaren® (diclofenac sodium enteric coated tablets) in healthy volunteersJ Pain200894 Suppl 14517950038
- DaviesNMAndersonKEClinical pharmacokinetics of diclofenac. Therapeutic insights and pitfallsClin Pharmacokinet19973331842139314611
- RadermacherJJentschDSchollMALustinetzFrölichJCDiclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint diseaseBr J Clin Pharmacol19913155375411888621
- BrunnerMDehghanyarPSeigfriedBMartinWMenkeGMüllerMFavourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulationBr J Clin Pharmacol20056057357716236050
- AffaitatiGVecchietFLerzaREffects of topical diclofenac (DHEP plaster) on skin, subcutis and muscle pain thresholds in subjects without spontaneous painDrugs Exp Clin Res2001272697611392056
- ZacherJAltmanRABellamyNTopical diclofenac and its role in pain and inflammation: an evidence-based reviewCurr Med Res Opin20082492595018279583
- TugwellPSWellsGAShainhouseJZEquivalence study of a topical diclofenac solution (Pennsaid®) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trialJ Rheumatol2004312002201215468367
- GaleazziMMarcolongoRA placebo-controlled study of efficacy and tolerability of a nonsteroidal anti-inflammatory drug, DHEP plaster, in inflammatory peri-and extra-articular rheumatological diseasesDrugs Exp Clin Res19931931071158112199
- Flector Patch [package insert]Bristol, TNKing Pharmaceuticals®, Inc.2009
- GalerBSRowbothamMPeranderJDeversAFriedmanETopical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trialJ Pain Symptom Manage200019428729410799795
- RowbothamMCGalerBSBlockJABackonjaMMFlector Tissugel®: efficacy and safety in the treatment of minor sports injuries. Data from a controlled trial in the United StatesJ Traumatol Sport2003201S151S20
- JenourePSegesserBLuhtiUGremionGA trial with diclofenac HEP plaster as topical treatment in minor sport injuriesDrugs Exp Clin Res19931931251318112201
- SaillantGAugustePHBoyerCStudy comparing the efficacy and tolerability of Flector Tissugel® to that of a placebo in the treatment of benign ankle sprainSports Med19987215
- JoussellinEFlector Tissugel® in the treatment of painful ankle sprainJ Traumatol Sport2003201S51S9
- JenourePJRostanAGremionGMulticenter, double-blind, controlled clinical study of diclofenac Tissugel plaster in patients with epicondylitisMedicina Dello Sport199750285292
- RosenthalMBahousIA controlled clinical study on the new topical dosage form of DHEP plasters in patients suffering from localized inflammatory diseasesDrugs Exp Clin Res1993193991058112205
- PodichettyVKMazanacDJBiscupRSChronic non-malignant musculoskeletal pain in older adults: clinical issues and opioid interventionPostgrad Med J20037993762763314654573
- ElnachefNScheimanJMFendrickAMChanging perceptions and practices regarding aspirin, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs among US primary care providersAliment Pharmacol Ther200828101249125818729848
