Abstract
Background
The buprenorphine transdermal system (BTDS) is approved in the US for the management of chronic pain. Due to its high affinity for μ-opioid receptors with a slow dissociation profile, buprenorphine may potentially displace or prevent the binding of competing μ-opioid-receptor agonists, including immediate-release (IR) opioids, in a dose-dependent manner. Health care professionals may assume that the use of IR opioids for supplemental analgesia during BTDS therapy is not acceptable.
Materials and methods
This post hoc analysis evaluated the use of IR opioids as supplemental analgesia during the management of moderate–severe chronic pain with BTDS at 52 US sites (BUP3015S, NCT01125917). Patients were categorized into IR-opioid and no-IR-opioid groups. At each visit of the extension phase, adverse events, concomitant medications, and information from the Brief Pain Inventory (BPI) were recorded.
Results
The most common supplemental IR opioids prescribed during BTDS treatment (n=354) were hydrocodone–acetaminophen and oxycodone–acetaminophen. The mean daily dose of IR opioids (morphine equivalents) for supplemental analgesia was 22 mg. At baseline, BPI – pain intensity and BPI – interference scores were higher for patients in the IR-opioid group. In both treatment groups, scores improved by week 4, and then were maintained throughout 6 months of the open-label extension trial. The incidence of treatment-emergent adverse events was similar in both groups.
Conclusion
Patients who were prescribed IR opioids reported lower scores for BPI pain intensity and pain interference to levels similar to patients receiving BTDS without IR opioids, without increasing the rate or severity of treatment-emergent adverse events. Patients prescribed concomitant use of IR opioids with BTDS had greater treatment persistence. The results of this post hoc analysis provide support for the concomitant use of IR opioids for supplemental analgesia during the management of moderate–severe chronic pain with BTDS.
Introduction
Chronic pain conditions affect approximately 100 million adults in the US.Citation1 The US Food and Drug Administration guidance has noted that prescription opioids are an important component of modern pain management.Citation2 Opioid medications can be classified as immediate-release (IR) or extended-release (ER)/long-acting (LA) opioid formulations on the basis of their duration of action. Usually, IR opioids are short-acting, intended for use every 3–6 hours, and are more appropriate for transient pain types, such as acute, breakthrough, or intermittent pain.Citation3,Citation4 Common IR opioids (eg, morphine, hydromorphone, oxymorphone, codeine, fentanyl, tramadol, tapentadol, oxycodone, and hydrocodone) may be available as single entity or in combination with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). Due to associated risks of hepatic and gastrointestinal toxicity with acetaminophen or NSAIDs, the maximum daily amount of these combination therapies may be limited.Citation4 On the other hand, ER/LA opioids have a prolonged half-life and deliver a dose over a longer period of time (greater than 8 hours), which makes them appropriate for patients with persistent chronic pain that requires stable, around-the-clock dosing.Citation3,Citation4 Generally, ER/LA opioids are intended to result in less frequent administration than IR-opioid formulations.Citation5
Buprenorphine hydrochloride was introduced in the US for pain management in a parenteral form in 1981.Citation6 Transdermal formulations for pain management were launched in Europe in 2001, which led to renewed interest in the use of buprenorphine to treat cancer pain and chronic nonmalignant pain.Citation7,Citation8 In 2010, the buprenorphine transdermal system (BTDS; Butrans®, Purdue Pharma LP, Stamford, CT, US) was approved in the US for the management of chronic pain.Citation9–Citation11 The BTDS is a transdermal patch that delivers an average of 5, 7.5, 10, 15, or 20 μg/h of buprenorphine over 7 days. It is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.Citation9 Several studies have demonstrated that the BTDS is effective, safe, and generally well tolerated among adults with moderate–severe chronic pain.Citation10–Citation19
Buprenorphine is a lipophilic, semisynthetic opioid derived from thebaine that is classified as a schedule III controlled substance.Citation9,Citation20 Buprenorphine demonstrates high binding affinity for μ-, κ-, and δ-receptors and low affinity for ORL1 (nociceptin).Citation21 Buprenorphine demonstrates different intrinsic activities as a partial agonist at μ-opioid receptors and at ORL1 receptors, an antagonist at κ-receptors, and an agonist at δ-opioid receptors in vitro.Citation9,Citation22,Citation23 The analgesic effects of buprenorphine appear to derive largely (if not solely) from its actions at the μ-opioid receptor,Citation24 while the contributions of actions at the other opioid receptors are unclear.Citation9 The slow dissociation of buprenorphine from μ-opioid receptors may contribute to its long duration of activity.Citation6,Citation25
Due to its high affinity and slow dissociation from opioid receptors, buprenorphine given at higher doses may effectively inhibit the binding of concomitantly administered opioids. In the higher-dose sublingual buprenorphine formulations, this results in a dose-related protective effect that is useful when treating opioid dependence in patients with higher opioid requirements.Citation26 Because buprenorphine at higher doses may inhibit concomitantly administered opioids, it has been assumed that IR opioids are generally less effective when used for supplemental analgesia during therapy with lower-dose buprenorphine formulations, such as the BTDS.
The risks of treating chronic pain with opioids should always be carefully assessed in each patient, and there are many patient- and drug-specific factors to consider. The BTDS shares the common side effects of opioid therapy, such as nausea, headache, dizziness, constipation, and somnolence, and also may cause application-site reactions, such as pruritus, erythema, and rash. Additionally, the risk of respiratory depression is greatest when initiating therapy or when increasing the dose. Although respiratory depression can occur at recommended doses, the risk of death due to overdose is linked to higher opioid doses.Citation27,Citation28 Specific patient populations may be at greater risk of respiratory depression, including elderly, cachectic, or debilitated patients and those with preexisting pulmonary diseases, taking concomitant central nervous system depressants, or impaired renal or hepatic function. Because buprenorphine is highly metabolized by CYP3A4, there is a risk of drug interactions with CYP3A4 inhibitors, leading to increased buprenorphine plasma concentrations and increased or prolonged opioid effects. Misuse, abuse, and addiction to opioids can also occur, even at recommended doses.Citation9
Breakthrough pain, usually presenting as incident pain or episodic pain, describes exacerbations of pain occurring in the background of adequately managed chronic pain.Citation29 Incorporating principles of the multimodal approach to pain management, it is common to manage breakthrough pain utilizing nonopioid or combination IR opioid–nonopioid products for supplemental analgesia.Citation30 As a balanced approach to pain management for some patients with chronic pain, IR opioids may be used to supplement ER/LA-opioid therapy. However, there is limited guidance and discussion on optimal treatment strategies for add-on therapy of IR opioids to ER/LA-opioid therapy to treat breakthrough pain.Citation30 The objective of this post hoc analysis is to describe the efficacy, safety, and tolerability of the BTDS when used concomitantly with IR opioids as supplemental analgesia during the management of moderate–severe chronic pain.
Materials and methods
Study population and design of open-label extension
Data in the current analysis were collected from the open-label extension phase (BUP3015S, NCT01125917) of a 12-week, randomized double-blind clinical trial of the BTDS in patients with moderate–severe chronic low-back pain (BUP3015, NCT00313014).Citation10 The open-label, long-term study was conducted from June 2004 to September 2005. Ethical approvals were obtained, and consent processes for the clinical trials used in this research were followed. The data from the clinical trials is freely available (ClinicalTrials.gov).
All patients, regardless of treatment, who had entered the double-blind period and who completed or discontinued due to lack of efficacy were eligible to enroll in the open-label extension phase of this study. Opioid-experienced patients (receiving 30–80 mg/day of oral morphine or equivalent) with moderate–severe chronic low-back pain who had received either the BTDS or IR oxycodone for up to 12 weeks in the double-blind study were eligible to be enrolled in the open-label, long-term study. IR opioids for supplemental analgesia were not permitted after the first week of the double-blind study.Citation10
Regardless of their dose level at the completion of the double-blind trial, all patients began the extension phase with BTDS 5 μg/h, and the dose was titrated to BTDS 10 μg/h or further to BTDS 20 μg/h if necessary. The maximum BTDS dose allowed was 20 μg/h, and patients with adequate analgesia were allowed to withdraw from the study. BTDS 7.5 and 15 μg/h were not available at the time this study was conducted. Titration to the next dose level occurred at the minimum titration interval of 3 days, when plasma concentrations were at a steady state. Downward titration was also permitted. Patients were able to titrate to their optimal dose, and at any given time could wear only one 5, 10, or 20 μg/h patch.
Enrolled patients were allowed non-sponsor-supplied supplemental analgesic medications, such as IR opioids and NSAIDs. Extended-release opioids, including transdermal fentanyl, were not allowed during the entire study course (ie, neither the core nor the extension).
At each visit of the extension phase, information recorded included adverse events (AEs), concomitant medication, and study-medication changes. Patients also completed an eleven-item Brief Pain Inventory – short form (BPI-SF), a self-administered questionnaire developed to assess the severity of pain and the interference of pain on daily functioning. The BPI pain-intensity scale measures, 1) pain at its worst in the last 24 hours, 2) pain at its least in the last 24 hours, 3) pain on average, and 4) pain right now. The BPI pain-interference scale measures how pain interferes with the patient’s general activity, mood, walking ability, work, relations with others, sleep, and enjoyment of life during the last 24 hours. Pain intensity was scored on an 11-point scale where 0 = “no pain” and 10 = “pain as bad as you can imagine”, while pain interference was scored as 0 = “does not interfere” and 10 = “completely interferes”.
Analysis
In this retrospective post hoc analysis, patients were categorized into two groups: 1) the IR-opioid group (those who were prescribed IR opioids as supplemental analgesia at least once during the extension trial), and 2) the no-IR-opioid group (those who were not prescribed IR opioids as supplemental analgesia during the extension trial). For each patient in the IR-opioid group, the average daily IR-opioid dose (in morphine equivalents) was calculated. The equianalgesic ratios used to convert from other opioids to morphine were those published in recent guidelines, where 30 mg of oral morphine per day equates to 30 mg per day of hydrocodone or 20 mg per day of oxycodone.Citation31
Pain scores from the BPI-SF (pain intensity and pain interference) were tabulated and plotted by group at baseline and at weeks 4, 12, 16, 20, and 24 of the extension phase. Baseline scores were the patient’s BPI scores at the end of the double-blind period, and were defined as the measurement at week 0 of the extension phase. P-values for BPI pain intensity and pain interference were generated from an analysis of covariance with treatment included (IR-opioid group/no-IR-opioid group) and adjusted by baseline. Because this study was terminated early for administrative reasons unrelated to safety or efficacy, BPI pain intensity and BPI interference were analyzed only for the 6-month period.
Treatment-emergent AEs (TEAEs) were tabulated for the entire extension phase for both the IR-opioid group and the no-IR-opioid group. The incidence of TEAEs was further calculated in a manner where patients in the IR-opioid group were separated by whether they were on IR opioids or not. TEAEs in patients who were on IR opioids were those that occurred within a 7-day period after reporting the use of a concurrent IR opioid. TEAEs in patients who were not on IR opioids were those that occurred outside the 7-day period of concurrent IR-opioid use. Total TEAEs in the IR-opioid group were also summarized, and included all TEAEs that occurred during the 24-week treatment period, regardless of concurrence of IR-opioid usage. The calculation for total TEAEs in the IR-opioid group may be numerically less than the sum of TEAEs during periods on IR opioids or not on IR opioids, since it removed any TEAEs that may have been reported twice (eg, when a patient was on IR opioids, then again when the patient was not). Since this was a post hoc analysis of an open-label, observational study, only minimal inferential analyses were performed, and the results presented are mainly descriptive.
Results
Patient characteristics
A total of 354 patients were enrolled in the extension trial, with 181 patients in the IR-opioid group and 173 patients in the no-IR-opioid group (). Patient characteristics were similar between the IR-opioid group and the no-IR-opioid group: mean age 50.9 years vs 51.5 years, sex 56% male vs 55% male, race 98% white vs 90% white, respectively. Of the 354 patients, 213 were exposed to the BTDS for at least 6 months, including 54 patients exposed for at least 1 year. Patients in the IR-opioid group remained in the extension phase a mean of 164 days compared with 122 days for patients in the no-IR-opioid group.
Table 1 Comparative characteristics, enrolled extension-trial population
IR-opioid use as supplemental analgesia
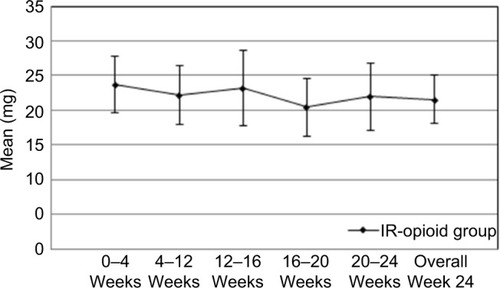
The most common IR opioids prescribed for supplemental analgesia during BTDS treatment were hydrocodone–acetaminophen and oxycodone–acetaminophen. The mean daily dose of IR opioids (reported in morphine equivalents, using a morphine:hydrocodone potency ratio of 1:1 and a morphine:oxycodone potency ratio of 3:2) prescribed for supplemental analgesia was 22 mg. Mean supplemental IR-opioid use was relatively consistent throughout the 24-week study period (). The median daily IR-opioid dose was 12 mg.
Pain scores
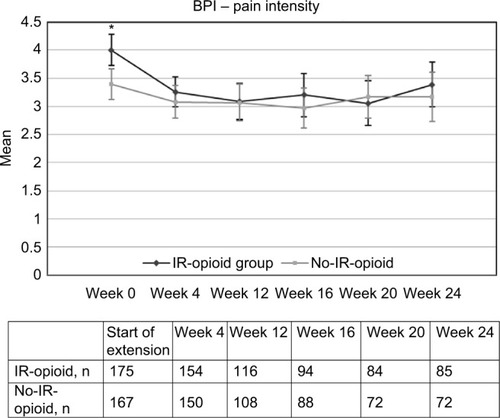
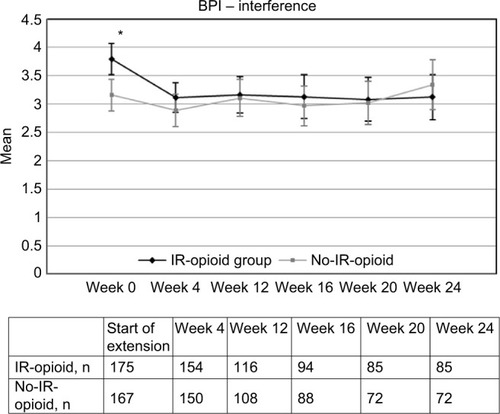
At baseline (end of core study), scores were higher (P<0.05) for both BPI pain intensity (rating of 4) and BPI interference (rating of 3.8) in patients in the IR-opioid group versus patients in the no-IR-opioid group (BPI scores of 3.4 and 3.16, respectively; and ). By the first scheduled visit after baseline at week 4, BPI pain intensity and BPI interference had improved for patients in the IR-opioid group, and in the 5 months thereafter remained similar to the no-IR-opioid group. The number of patients with a completed BPI assessment at baseline and at weeks 4, 12, 16, 20, and 24 was 175, 154, 116, 94, (84 for BPI pain intensity, 85 for BPI interference), and 85 for the IR-opioid group and 167, 150, 108, 88, 72, and 72 for the no-IR-opioid group, respectively. Reasons for discontinuations in each group were not evaluated for this post hoc analysis.
Treatment-emergent adverse events
In general, the incidence of TEAEs was similar in patients on IR opioids compared with patients in the no-IR-opioid group during the study (). The BTDS was well tolerated in both groups, with no remarkable increase in TEAEs in the IR-opioid group. In the IR-opioid group, 72.4% had a TEAE compared to 69.4% in the no-IR-opioid group. The most common TEAE was headache: 14% in the IR-opioid group compared with 16.8% in the no-IR-opioid group.
Table 2 Incidence of TEAEs (≥5%) during BTDS treatment for moderate–severe chronic pain: IR-opioid group vs no-IR-opioid group
The incidence of serious AEs (SAEs) reported during this extension study was 5% (19 of 354). Of the 19 patients who experienced SAEs, eleven were in the IR-opioid group and eight the no-IR-opioid group. One patient in the IR-opioid group experienced a fatal SAE (sudden cardiac death of a 74-year-old male). The majority of SAEs, including the one death, were considered by the investigator to be unrelated to BTDS use. One additional SAE was reported after study discontinuation.
Discussion
While more rigorous studies are needed, the results of this post hoc analysis provide support for the acceptable use of IR opioids for supplemental analgesia during BTDS therapy. Compared with the no-IR-opioid group, the IR-opioid group reported higher BPI pain and interference scores at the start of the open-label period, suggesting patients with a higher pain score required additional analgesia. While the use of IR opioids remained consistent throughout the 24-week period, BPI scores stabilized after the first visit/week 4 and were similar to those reported in the no-IR-opioid group for the remainder of the 24-week analysis period. These findings suggest that the use of IR opioids with the BTDS lowered BPI scores to levels comparable with BTDS use alone. The reporting of TEAEs in patients on IR opioids was similar to patients in the no-IR-opioid group.
Data for this post hoc analysis were obtained from an open-label clinical study, which introduces some inherent limitations, such as patient self-reporting of medication use, pain scores, and AEs. The division of patients into the IR-opioid group and no-IR-opioid group was based on investigator documentation of prescribing concomitant medications. The actual dose taken by the patient during the study was not verified. While the mean daily dose of IR opioids prescribed for supplemental analgesia was 22 mg of morphine equivalents, due to the trial design and methodology, it is not possible to confirm that all prescribed doses of supplemental opioid analgesic were actually taken by the patients in the IR-opioid group. This introduces a potential inconsistency between the prescribed dose and the dose of IR opioids actually taken by the patient for supplemental analgesia. This analysis should thus be considered exploratory research.
Consistent with most open-label trial designs, additional medical therapies deemed appropriate for the subject’s medical condition were permitted during the extension phase at the discretion of the investigator, with the exception of long-acting opioid analgesics. The addition of other medical therapies may impact the results of this analysis. In an open-label, usual-care study design, such as this one, investigators are generally allowed to individualize treatment for chronic pain, using their own clinical judgment regarding a target pain score balanced with safety considerations. Although AEs were evaluated, this post hoc analysis did not evaluate reasons for discontinuation by patients. Patients with inadequate analgesia could have discontinued from the extension study.
Despite the stated limitations, previous studies on both buprenorphine pharmacology and clinical experience with the BTDS further corroborate that the supplemental use of IR opioids during BTDS therapy is appropriate for some patients ().Citation6–Citation8,Citation17,Citation23,Citation25,Citation32–Citation48 Although buprenorphine is considered a partial agonist at the μ-opioid receptor in vitro and an antagonist at the κ-opioid receptor, studies have shown that buprenorphine can be expected to produce pharmacological effects similar to those of full μ-agonists, especially at the comparatively lower buprenorphine doses delivered by the BTDS.Citation9,Citation7,Citation22,Citation25 It has been reported that buprenorphine behaves like a full μ-opioid agonist at analgesic doses, and the partial agonistic property, high affinity binding, or slow dissociation of buprenorphine does not have a negative effect on the availability of μ-opioid receptors or on its interaction with full μ-opioid agonists.Citation23,Citation25 In animals pretreated with an analgesic dose of buprenorphine, the addition of morphine, oxycodone, or hydromorphone results in an additive or synergistic effect.Citation25
Table 3 Published studies where IR opioids were used as supplemental analgesia during transdermal buprenorphine therapy for chronic pain
Buprenorphine exhibits dose-dependent receptor occupancy. Due to its high affinity for μ-opioid receptors, buprenorphine may inhibit or displace other μ-opioid receptor agonists at higher concentrations. Receptor inhibition is likely dependent on the dose of buprenorphine or concentration of buprenorphine at the receptor.Citation49–Citation54 However, the buprenorphine doses provided by the BTDS in the US are relatively low. For example, the systemic buprenorphine-delivery rate from the BTDS averages 5, 7.5, 10, 15, and 20 μg/hour over 7 days. Since transdermal administration of buprenorphine bypasses the gastrointestinal tract and first-pass metabolism, bioavailability is 100% of the dose delivered. The BTDS doses in the US correspond to average daily buprenorphine doses of approximately 0.12 mg, 0.18 mg, 0.24 mg, 0.36 mg, and 0.48 mg.Citation9,Citation13 In the US, the sublingual and buccal buprenorphine products indicated for the treatment of opioid dependence are available in higher-dosage strengths than the BTDS, ranging 1.4–11.4 mg with naloxone and 2–8 mg without naloxone,Citation55–Citation58 and may have less than 100% bioavailability, since some of the dose is swallowed.Citation59,Citation60 At higher sublingual buprenorphine doses (2–32 mg), opioid receptors approach saturation, as evidenced in a study evaluating positron-emission tomography brain scans.Citation61 Buprenorphine produces analgesia in humans at less than full receptor occupancy.Citation62 Therefore, at lower doses of buprenorphine, such as those elicited by the BTDS, opioid receptors are unsaturated, which allows for the concomitant binding of other opioids to unoccupied receptors for effective analgesia for breakthrough pain.
To support the pharmacological perspective, we sought to understand the clinical perspective further, and conducted literature searches in the Medline, Embase, and Derwent Drug File databases, using search terms for use of IR opioids for breakthrough pain or supplemental analgesia during transdermal buprenorphine therapy (). We found numerous studies that reported the use of supplemental opioid analgesics, including codeine, morphine, tramadol, fentanyl, and buprenorphine, during transdermal buprenorphine therapy for chronic pain. Many of the studies identified included a higher-dose transdermal formulation of buprenorphine available in Europe that provides 35, 52.5, or 70 μg/hour of buprenorphine,Citation63 corresponding to average daily buprenorphine doses of 0.84 mg, 1.26 mg, and 1.68 mg.Citation64 Few studies were designed to evaluate the safety and efficacy of supplemental IR-opioid use along with transdermal buprenorphine.Citation34,Citation37,Citation41 The studies that did, reported the efficacious use of an IR opioid along with transdermal buprenorphine with an AE profile that was generally acceptable, and similar to that produced by opioids and transdermal systems. The results of this literature search indicate vast clinical experience with the concomitant use of IR opioids and buprenorphine, even at higher transdermal buprenorphine doses than those approved in the US ().Citation8,Citation17,Citation32–Citation48
Conclusion
The results of this post hoc analysis provide support for the use of IR opioids as an acceptable choice for supplemental analgesia during the management of moderate–severe chronic pain with the BTDS. In this study, patients who were concomitantly prescribed IR opioids with the BTDS reported lowered scores for BPI pain intensity and pain interference to levels similar to patients receiving the BTDS only. Patients concomitantly prescribed BTDS treatment with supplemental IR opioids did not report substantial increases in the rate or severity of TEAEs compared with BTDS use alone. Patients prescribed concomitant IR opioids with the BTDS remained in the study for a longer period of time than patients receiving the BTDS alone. Pharmacologic evidence and extensive published medical literature corroborate the clinical findings of this post hoc analysis, and together provide evidence and rationale to health care professionals regarding the acceptability of prescribing IR opioids for breakthrough pain during BTDS therapy.
Author contributions
All authors were involved in study design and data collection, and participated in data analysis, data interpretation, and writing the article. The authors had full access to all data, and had final responsibility for the decision to submit for publication.
Acknowledgments
This research was supported by funding from Purdue Pharma LP. The authors thank colleagues Ellie He and Zhai Qiang from Purdue Pharma who provided biostatistical expertise that assisted the research.
Disclosure
MJC, MK, and SRR are full-time employees of Purdue Pharma LP. SS is a speaker/consultant for Purdue Pharma and other companies. RBR is a speaker, consultant, and basic-science investigator for several pharmaceutical companies involved in analgesics research, but receives no royalty from the sale of any product. The abstract of this paper was presented at the American Pharmacists Association Annual Meeting and Exposition, March 9–12, 2012, New Orleans, LA as an abstract with interim findings, and then at the following conferences as posters: Pain Week, September 6–10, 2012, Las Vegas, NV; American Academy of Pain Management 23rd Annual Clinical Meeting, September 20–23, 2012, Phoenix, AZ.
References
- US Institute of MedicineRelieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and ResearchWashingtonNational Academies Press2011
- US Food Drug AdministrationAbuse-deterrent opioids — evaluation and labeling: guidance for industry2015 Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM334743.pdfAccessed April 26, 2017
- BrennanMJUpdate on prescription extended-release opioids and appropriate patient selectionJ Multidiscip Healthc2013626528023900563
- ArgoffCESilversheinDIA comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needsMayo Clin Proc200984760261219567714
- RauckRLWhat is the case for prescribing long-acting opioids over short-acting opioids for patients with chronic pain? A critical reviewPain Pract20099646847919874536
- JohnsonREFudalaPJPayneRBuprenorphine: considerations for pain managementJ Pain Symptom Manage200529329732615781180
- PergolizziJAloisiAMDahanACurrent knowledge of buprenorphine and its unique pharmacological profilePain Pract201010542845020492579
- LikarRKayserHSittlRLong-term management of chronic pain with transdermal buprenorphine: a multicenter, open-label, follow up study in patients from three short-term clinical trialsClin Ther200628694395216860176
- Butrans [prescribing information]Stamford (CT)Purdue Pharma LP2014
- SteinerDMuneraCHaleMRipaSLandauCEfficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind studyJ Pain201112111163117321807566
- SteinerDJSitarSWenWEfficacy and safety of the seven-day buprenorphine transdermal system in opioid-naïve patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled studyJ Pain Symptom Manage201142690391721945130
- LandauCJCarrWDRazzettiAJSesslerNEMuneraCRipaSRBuprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy: a multicenter, 5-week run-in and randomized, double-blind maintenance-of-analgesia studyClin Ther200729102179219318042474
- MuneraCDrehoblMSesslerNELandauCA randomized, placebo-controlled, double-blinded, parallel-group, 5-week study of buprenorphine transdermal system in adults with osteoarthritisJ Opioid Manage201063193202
- BreivikJLjosaaTMStengaard-PedersenKA 6-months, randomized, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naïve to potent opioidsScand J Pain20101122141
- ConaghanPGO’BrienCMWilsonMSchofieldJPTransdermal buprenorphine plus oral paracetamol vs an oral codeine-paracetamol combination for osteoarthritis of hip and/or knee: a randomised trialOsteoarthritis Cartilage201119893093821477658
- GordonACallaghanDSpinkDBuprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phaseClin Ther201032584486020685494
- GordonARashiqSMoulinDEBuprenorphine transdermal system for opioid therapy in patients with chronic low back painPain Res Manag201015316917820577660
- JamesIGO’BrienCMMcDonaldCJA randomized, double-blind, double-dummy comparison of the efficacy and tolerability of low-dose transdermal buprenorphine (BuTrans seven-day patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis painJ Pain Symptom Manage201040226627820541900
- KarlssonMBerggrenACEfficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 μg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority studyClin Ther200931350351319393841
- GutsteinHBAkilHOpioid analgesicsBruntonLLGoodman and Gilman’s Pharmacological Basis of Therapeutics11th edNew YorkMcGraw-Hill2006547590
- HuangPKehnerGBCowanALiu-ChenLYComparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonistJ Pharmacol Exp Ther2001297268869511303059
- RaffaRBDingZExamination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonistAcute Pain200793145152
- KögelBChristophTStrassburgerWFriderichsEInteraction of μ-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in miceEur J Pain20059559961116139189
- WalshSLEissenbergTThe clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinicDrug Alcohol Depend2003702 SupplS13S27
- KressHGClinical update on the pharmacology, efficacy and safety of transdermal buprenorphineEur J Pain200913321923018567516
- GreenwaldMKComerSDFiellinDABuprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policyDrug Alcohol Depend201414411125179217
- HuberERobinsonRCNoeCEVan NessOWho benefits from chronic opioid therapy? Rethinking the question of opioid misuse riskHealthcare (Basel)201642E2927417617
- BohnertASBValensteinMBairMJAssociation between opioid prescribing patterns and opioid overdose-related deathsJAMA2011305131315132121467284
- PortenoyRKBennettDSRauckRPrevalence and characteristics of breakthrough pain in opioid-treated patients with chronic noncancer painJ Pain20067858359116885015
- ChouRFancuilloFinePGClinical guidelines for the use of chronic opioid therapy in chronic noncancer painJ Pain200910211313019187889
- DowellDHaegerichTMChouRCDC guideline for prescribing opioids for chronic pain: United States, 2016MMWR Recomm Rep2016651149
- AurilioCPaceMCPotaVOpioids switching with transdermal systems in chronic cancer painJ Exp Clin Cancer Res2009286119422676
- BöhmeKLikarREfficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain: a randomised, double-blind, placebo-controlled studyPain Clin2003152193202
- de BarutellCGonzalez-EscaladaJEfficacy and safety of buprenorphine TDS in conjunction with oral tramadol or morphine as rescue medication in the treatment of 390 patients with chronic pain: a summary of two retrospective Spanish multicenter studiesJ Appl Ther Res2007621424
- FreyeEAnderson-HillemacherARitzdorfILevyJVOpioid rotation from high-dose morphine to transdermal buprenorphine (Transtec) in chronic pain patientsPain Pract20077212312917559481
- MentenJCarpentierIDeschutterHNuytsSVan BeekKThe use of transdermal buprenorphine to relieve radiotherapy related pain in head and neck cancer patientsCancer Invest201331641242023758187
- MercadanteSVillariPFerreraPSafety and effectiveness of intravenous morphine for episodic breakthrough pain in patients receiving transdermal buprenorphineJ Pain Symptom Manage200632217517916877185
- MercadanteSPorzioGFulfaroFSwitching from transdermal drugs: an observational “n of 1” study of fentanyl and buprenorphineJ Pain Symptom Manage200734553253817629666
- MercadanteSCasuccioATirelliWGiarratanoAEquipotent doses to switch from high doses of opioids to transdermal buprenorphineSupport Care Cancer200917671571819104845
- MordarskiSEfficacy and safety of buprenorphine in patients receiving haemodialysisJ Appl Ther Res2009724651
- NadezhdaAOsipovaNAbuzarovaGTransdermal buprenorphine for the treatment of chronic pain syndrome in oncology patientsJ Appl Ther Res2009726572
- PaceMCPassavantiMBGrellaEBuprenorphine in long-term control of chronic pain in cancer patientsFront Biosci2007121291129917127381
- PoulainPDenierWDoumaJEfficacy and safety of transdermal buprenorphine: a randomized, placebo-controlled trial in 289 patients with severe cancer painJ Pain Symptom Manage200836211712518411010
- RuggieroACocciaPArenaREfficacy and safety of transdermal buprenorphine in the management of children with cancer-related painPediatr Blood Cancer201360343343723034996
- SettiTSanfilippoFLeykinYTransdermal buprenorphine for postoperative pain control in gynecological surgery: a prospective randomized studyCurr Med Res Opin201228101597160822876835
- SittlRGriessingerNLikarRAnalgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trialClin Ther200325115016812637117
- SorgeJSittlRTransdermal buprenorphine in the treatment of chronic pain: results of a phase III, multicenter, randomized, double-blind, placebo-controlled studyClin Ther200426111808182015639693
- ZarthRComparison of buprenorphine, morphine sulfate, and fentanyl citrate as rescue medication for breakthrough pain in cancer patientsJ Appl Ther Res2008641519
- JohnsonREStrainECAmassLBuprenorphine: how to use it rightDrug Alcohol Depend2003702 SupplS59S7712738351
- StrainECPrestonKLLiebsonIAAcute effects of buprenorphine, hydromorphone and naloxone in methadone maintained volunteersJ Pharmacol Exp Ther199226139859931376362
- StrainECPrestonKLLiebsonIABuprenorphine effects in methadone-maintained volunteers: effects at two hours after methadoneJ Pharmacol Exp Ther199527226286387853176
- WalshSLJuneHLSchuhKJEffects of buprenorphine and methadone in methadone-maintained subjectsPsychopharmacology (Berl)199511932682767675960
- SchuhKJWalshSLBigelowGEBuprenorphine, morphine and naloxone effects during ascending morphine maintenance in humansJ Pharmacol Exp Ther199627828368468768738
- Center for Substance Abuse TreatmentClinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: A Treatment Improvement Protocol (TIP) 40Rockville (MD)US Department of Health and Social Services2004
- Suboxone [package insert]Slough (UK)Reckitt Benckiser Pharmaceuticals2014
- Subutex [package insert]Slough (UK)Reckitt Benckiser Pharmaceuticals2014
- Bunavail [package insert]RaleighBioDelivery Sciences International2014
- Zubsolv [package insert]Morristown (NJ)Orexo US2014
- NathRPUptonRAEverhartETBuprenorphine pharmacokinetics: relative bioavailability of sublingual tablet and liquid formulationsJ Clin Pharmacol199939561962310354966
- MendelsonJUptonRAEverhartETJacobPJonesRTBioavailability of sublingual buprenorphineJ Clin Pharmacol199737131379048270
- GreenwaldMKJohansonCEMoodyDEEffects of buprenorphine maintenance dose on μ-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteersNeuropsychopharmacology200328112000200912902992
- CowanAFriderichsStrassburgerWRaffaRBBasic pharmacology of buprenorphineBuddKRaffaBRBuprenorphine: The Unique Opioid AnalgesicStuttgartThieme2005321
- Electronic Medicines CompendiumTranstec 35, 52.5, and 70 micrograms transdermal patch2015 Available from: http://www.medicines.org.uk/emc/medicine/8864Accessed April 26, 2017
- LikarRTransdermal buprenorphine in the management of persistent pain: safety aspectsTher Clin Risk Manag20062111512518360586