Abstract
Background
Neuropathic pain is a debilitating condition that severely affects the quality of life for those with this pain condition, and treatment for pain relief is greatly sought-after. Zerumbone (Zer), a sesquiterpene compound isolated from the rhizomes of a Southeast Asian ginger plant, Zingiber zerumbet (L.) Roscoe ex Smith. (Zingiberaceae), showed antinociceptive and antiinflammatory properties when previously tested on models of nociception and inflammation.
Objective
This study investigated the effects of prophylactic administration of zerumbone on allodynia and hyperalgesia in a mouse model of chronic constriction injury (CCI)-induced neuropathic pain.
Methods
Intraperitoneal administration of Zer (5–50 mg/kg) from day 1 post-surgery was carried out to identify the onset and progression of the pain condition. Responses toward mechanical and cold allodynia, and mechanical and thermal hyperalgesia were assessed on days 3, 5, 7, 9, 11, and 14 post-surgery. Blood plasma and spinal cord levels of interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and IL-10 were screened using enzyme-linked immunosorbent assay on day 15.
Results
Zer (10 and 50 mg/kg) attenuated pain symptoms on all days of behavioral testing without any signs of sedation in the rotarod test. ED50 values for mechanical allodynia, cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia were 9.25, 9.507, 8.289, and 9.801 mg/kg, respectively. Blood plasma and spinal levels of IL-1β, IL-6, and tumor necrosis factor-α but not IL-10 were significantly (p<0.05) suppressed by zer treatment.
Discussion and conclusion
Zer exhibits its antiallodynic and antihyperalgesic properties via reduced sensitization at nociceptor neurons possibly through the suppression of inflammatory mediators. Zer may prove to be a novel and beneficial alternative for the management of neuropathic pain.
Introduction
Neuropathic pain is a highly prevalent global problem that affects almost 6.9%–10% of the population worldwide.Citation1 Approximately 40% of patients attending pain clinics for treatment of various forms of pain conditions exhibit neuropathic symptoms.Citation2,Citation3 Neuropathic pain arises due to factors that cause direct trauma to the nerve, tumour growth, cancer compression, and cancer therapy.Citation4 Apart from autoimmune diseases and metabolic diseases such as diabetes, lifestyle of individuals such as smoking and alcoholism could also lead to this condition.Citation5,Citation6
Neuropathic pain is defined as “pain that arises as a direct consequence of a lesion or diseases affecting the somatosensory system” and is often accompanied by symptoms of allodynia and hyperalgesia.Citation7 This debilitating pain condition severely affects the physical activities of patients and further leads to emotional distress.Citation8 As currently available conventional drugs are only partially effective, accompanied with undesired side effects, alternative pain management options are greatly sought-after.Citation9,Citation10
The pathophysiology of neuropathic pain shows that inflammation is a vital component of the progression and maintenance of this condition.Citation11 When a nerve is injured, Wallerian degeneration takes place whereby axons and myelin sheaths undergo degradation. The vascular permeability is compromised, permitting infiltration of macrophages and various other immune cells such as mast cells to the injury site. Inflammatory cytokines, principally interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, are secreted by resident and non-resident macrophages.Citation12,Citation13 Inflammatory mediators are signaling proteins that take up a role as intercellular messengers as an immune response.Citation14 They in turn recruit and activate macrophages, neutrophils, and surrounding glial cells at the site of injury.Citation14 Primary afferent neurons are directly sensitized by TNF-α, IL-1β, and IL-6 via their receptors.Citation15–Citation17
Inflammatory cytokines have a primary role that triggers the downstream cascade of the inflammatory process, followed by the antiinflammatory process during the recovery and repair process. An “inflammatory soup” is formed comprising adenosine triphosphate, bradykinin, histamine, serotonin, prostaglandin E2, leukotriene B4, nerve growth factor, substance P, and calcitonin gene-related peptide contributing to neurogenic inflammation.Citation18 Peripheral sensitization occurs, a result of which normally inactive and unresponsive “silent nociceptors” become sensitive to the development of spontaneous discharges, giving rise to allodynia.Citation19,Citation20 In addition, nociceptors, which usually transmit painful stimulus, are now hypersensitive and thus exhibit an exaggerated pain response, also known as hyperalgesia.Citation19
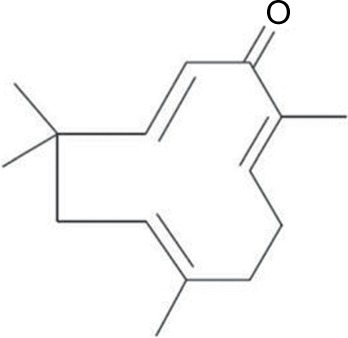
Considering that neuropathic pain is largely modulated and maintained by the neuroimmune and neuroinflammatory activities, treatments or drugs often target to either reverse or reduce the inflammatory process.Citation21 We have recently reported that zerumbone (Zer) (), a monocyclic, sesquiterpenoid phytochemical substance isolated from the rhizome oil of Zingiber zerumbet (L.) Roscoe ex Smith (Zingiberaceae), was able to therapeutically attenuate symptoms of neuropathic pain.Citation22 More recently, we have elucidated the involvement of the serotonergic and L-Arginine-Nitric Oxide-cGMP-K+ATP channel pathways in the analgesic action of Zer in neuropathic pain.Citation23,Citation24 This reinforces that Zer is a potential bioactive compound for the management of neuropathic pain.
Apart from its antinociceptive properties, Zer has also been widely reported for its antiinflammatory properties.Citation25–Citation27 Zerumbone successfully reversed the increase in IL-1, IL-6, and TNF-α production in the inflammatory response of diabetic rats.Citation28 Furthermore, zerumbone suppresses the synthesis of these inflammatory mediators in dextran sodium sulfate-induced colitis of mice.Citation29 Direct influence of zerumbone, specifically, on these aforementioned mediators has been well documented, not just in various other in vivo models but also in vitro.Citation30 However, suppression of the inflammatory process by zerumbone in a neuropathic pain model, specifically in the chronic constriction injury (CCI) model, is generally lacking and remains to be explored.
With this in mind, our study explored, for the first time, the prophylactic effects of Zer treatment administered from the initial stage throughout the progression stage of this neuropathic pain condition. We also provide evidence of the inflammatory components in the circulation as well as in spinal cord tissues following treatment with this compound. This approach with a prophylactic paradigm is expected to reverse the neuroimmune and neuroinflammatory processes. The significance of such treatments in clinical settings is that it serves as a preventive measure that could be started in advance for patients with higher risk of developing neuropathic pain, with the hope to delay or lessen the advancement of the condition.
Materials and methods
Zerumbone extraction and isolation
Zerumbone was prepared from rhizomes of Zingiber zerumbet using the same protocol as previously described by Perimal et al.Citation26 The plant was identified and confirmed by a botanist and a specimen was inserted at Institute of Bioscience, Universiti Putra Malaysia (Reference number: SK622/07). Briefly, rhizomes of Zingiber zerumbet were purchased from Chow Kit wet market, washed and sliced into small pieces of ~0.5–1 mm in size. The hydrodistillation process was carried out by placing the rhizome slices in the round bottom flask with water added to it. Soluble oils were collected in a vial 4 h after water and hexane were added to it. After the separation of the hexane and water, the water phase was removed. The crude essential oil was obtained from the hexane phase using a rotary evaporator before being placed at 4°C for 48 h. Only the pure crystallized product of the compound was subjected to column chromatography. The yield was 0.07%. The purity was determined by subjecting the eluate to thin-layer chromatography. This was followed by recrystallization before the compound was analyzed with high-performance liquid chromatography (HPLC) where the purity was determined to be more than 98%. Zerumbone was dissolved in Tween-20, dimethyl sulfoxide (DMSO), and physiological saline 0.9% at a ratio of 5:5:90 and was prepared freshly just before the experiments.
Animals
Male ICR mice (25–35 g at the beginning of the experiments) were housed eight mice per cage under a 12 h dark/light cycle at a room temperature of 24°C±2°C and a humidity level of 70%, with free access to water and standard laboratory chow. Mice were acclimatized for 1 week prior to any experimental procedures. All animal experiments were performed in accordance to the ethical guidelines for investigation of experimental pain in conscious animals. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Universiti Putra Malaysia (Reference number: UPM/IACUC/AUP-R060/2013).
Chemicals
Tween-20, DMSO, amitriptyline, and morphine sulfate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Morphine was dissolved in 0.9% normal saline. All treatments were administered intraperitoneally (ip) at a volume of 10 mL/kg.
Preselection of mice and body weights
Mice were preselected with rotarod (UgoBasile, Varese, Italy) spinning at 20 rpm and only mice that were able to remain on the wheels for 120 s without falling or rolling over were used in the experiment to eliminate possible occurrence of motor dysfunction. Mice with approximately similar body weights were preselected to be used in the experiment and the weights were measured daily throughout the experimental period to monitor the effect of treatments on their feeding habits and general well-being.
Experimental design
A total of 56 mice were allocated into 7 groups (n=8 mice per group) as follows:
Sham
CCI + vehicle
CCI + Zer (5 mg/kg ip)
CCI + Zer (10 mg/kg ip)
CCI + Zer (50 mg/kg ip)
CCI + morphine (1 mg/kg ip)
CCI + amitriptyline (20 mg/kg ip)
CCI surgery
The procedure of Bennett and XieCitation31 was adapted with minor modifications to induce neuropathy.Citation32,Citation33 Briefly, mice were anesthetized with tribromoethanol (250 mg/kg ip) before an incision was made to the skin to expose the left common sciatic nerve. Three loose ligations with 1 mm spacing between ligatures were made using 4/0 silk sutures. A brief twitch in the left hind limb marks the tightness of the ligatures. The skin incision was closed using a non-absorbable suture, before iodine was applied to the area. The entire surgery procedure was carried out on mice in the sham group but with no ligations made to the sciatic nerve to confirm the consequence of nerve injury.
Nociceptive assay
Responses toward mechanical allodynia, cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia were evaluated using von Frey, cold plate, Hargreaves, and Randall–Selitto tests, respectively. Baseline measurements of the nociceptive assays were obtained 1 day before CCI surgery (pre-CCI). Nociceptive assays were carried out 30 min following drug administration on days 3, 5, 7, 9, 11, and 14. The assays were carried out in a sequence of testing for mechanical allodynia, cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia whereby this sequence was consistent throughout the experiment.
von Frey filament test
Response toward mechanical allodynia was evaluated by von Frey filament test as described by Chaplan et alCitation34 and Campana and Rimondini.Citation35 Mice were placed in a Plexiglass chamber on an elevated wire mesh grid and were allowed to acclimate prior to testing. When all explorative and grooming behavior was reduced and the animal is on its four limbs, von Frey filament (IITC Life Science Inc., Woodland Hills, CA, USA) was applied to the middle of the dorsum of the ipsilateral paw followed by the contralateral paw. The mean of three readings was recorded as the withdrawal threshold.
Cold plate test
Response toward cold allodynia was evaluated by cold plate test as described by Nadal et al.Citation36 Mice were placed onto a cold plate (UgoBasile, Varese, Italy) at a temperature of 5°C±0.2°C. The number of times the mouse lifted, scratched, or flinched its paws were counted as the number of paw lifts. Each mouse was observed for a total of 5 min and measurements were obtained from both the ipsilateral and contralateral paws. The total number of lifts of the ipsilateral paw was then subtracted from the total number of lifts of the contralateral paws.
Hargreaves test
Response toward thermal hyperalgesia was assessed using Hargreaves test as described by Hargreaves et alCitation37 using Hargreaves apparatus (Model 37370, Gemonio, Varese, Italy). Mice were placed in a Plexiglass chamber which was placed on an elevated clear platform. Following a brief acclimatization period, a radiant heat source with an infrared radiation intensity of 80 W/sr was directed to the mid-plantar surface of its ipsilateral paw followed by the contralateral paw. The time taken for the mouse to either remove or flinch its paw was recorded as the withdrawal latency. The radiant heat source was removed after 20 s to prevent injury to the paws.
Randall–Selitto
Mice response toward mechanical hyperalgesia was carried out as previously described by Randall and Selitto.Citation38 Briefly, mice were restrained as such where they were able to flex their legs. A constantly increasing pressure was applied to the plantar surface of both the ipsilateral and contralateral hind paws through the tip of the paw pressure meter (IITC Life Science Inc., Woodland Hills, CA, USA). Readings were obtained when the mice attempted to withdraw the paw. The cut-off force was set at 200 g to prevent further injury to the paws.
Determination of ED50 values
The effective dose (ED50) was determined for each noci-ceptive assay using GraphPad Prism V.5. The doses were log[dose] transformed and plotted against % of inhibition. A nonlinear regression (curve fit) was generated to determine the ED50 values.
Motor coordination
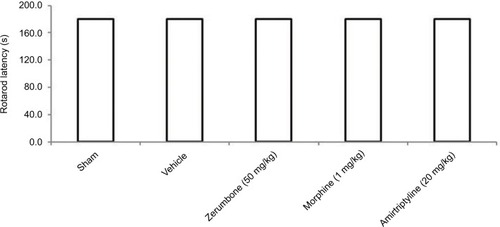
Possible sedative effects of the drug treatments were evaluated by the rotarod test (UgoBasile, Varese, Italy) as previously described by Zulazmi et al.Citation24 On day 14 post-CCI, mice were administered with their respective treatments. Exactly 30 min later, the animals were placed on the rotarod bar rotating at 20 rpm. The time spent on the bar for a period of up to 180 s was recorded. This method was carried out only in sham-operated and CCI mice treated with vehicle, Zer (50 mg/kg), morphine (1 mg/kg), and amitriptyline (20 mg/kg).
Euthanasia and organ collection
On day 15 post-CCI, treatments were given accordingly and mice were sacrificed 30 min posttreatment. Blood was withdrawn via cardiac puncture, centrifuged at 3000 g at 4°C and frozen at −20°C until assayed. The spinal cord at the lumbar 4–6 region was dissected out and homogenized using a Polytron device (Mississauga, Ont, Canada). Approximately, 750 μL PBS per 25 mg tissue was used to homogenize the tissues before samples were centrifuged for 10 min at 4°C at 12,000 g. The aliquot of the supernatant fraction was stored at −20°C until further use.
Enzyme-linked immunosorbent assay
Blood plasma and spinal cord cytokines (TNF-α, IL-1β, IL-10, and IL-6) levels were measured using mouse-specific, enzyme-linked immunosorbent assay (ELISA) kits purchased from eBioscience, Vienna, Austria. Assays were performed according to the manufacturer’s instructions. The samples were assayed in duplicates and the cytokines levels were expressed in pg/mg whereby 50 μL of blood plasma or tissue protein was used for each assay. Standards were run in every trial, and standard curves were generated for every assay. Sample concentrations were calculated using Bio-Plex Manager software.
Statistical analysis
All data obtained were checked for normality using Shapiro–Wilk Test for Normality before statistical testing was carried out. Data for behavioral testing were expressed as mean ± SEM and were analyzed using two-way ANOVA followed by Bonferroni’s post hoc analysis. Values for ELISA were expressed relative to the total protein. A confidence interval of 5% or less was considered to be statistically significant. All behavioral tests and ELISA assays were carried out where the investigator was blinded from the treatment group of the animals.
Results
von Frey filament test
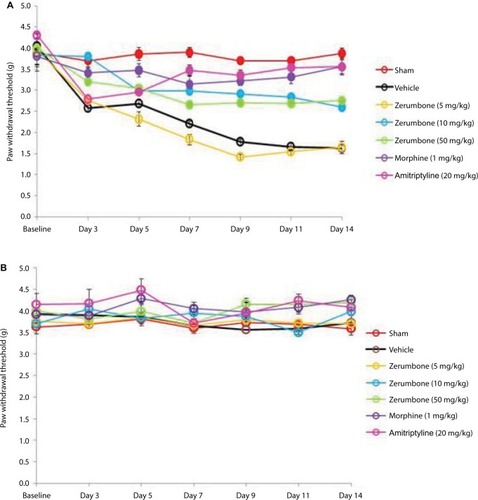
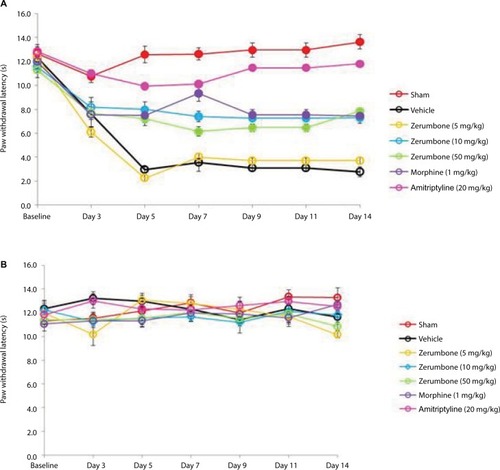
represents the mean body weights of mice throughout the experiment. Mice with similar (F (6, 49)=3.966, p=0.003) at the beginning of the experiment showed no significant (p>0.05) changes between experimental groups on all days. represents the pain response toward mechanical allodynia when tested with von Frey filaments. Mice that underwent CCI surgery show significantly increased sensitivity as early as day 3 (F (6, 49)=14.121, p=0.000) and were significantly different (p≤0.05) from sham group throughout the experimental period. Treatment with Zer at a dose of 5 mg/kg did not show significant (p>0.05) difference from the vehicle group, indicating the lack of effect of this treatment dose on mechanical allodynia. However, Zer administered at higher doses of 10 and 50 mg/kg ip significantly attenuated mechanical allodynia as the values were significantly different from the vehicle group on all days of testing. Both morphine, 1 mg/kg ip, and amitriptyline, 20 mg/kg ip, that serve as positive controls, completely alleviated mechanical allodynia and showed mean values similar (p>0.05) to that of the sham group. The withdrawal threshold mean values of the contralateral paws were not affected by either the surgery or the treatments as the values were not significantly different from the sham group (F (6, 49)=1.479, p=0.209).
Figure 2 Changes in the body weights of mice in different treatment groups throughout the experimental period of 14 days.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury.
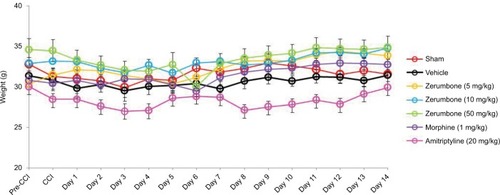
Figure 3 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal thresholds toward mechanical allodynia as tested with von Frey filament test.
Abbreviation: ANOVA, analysis of variance.
Cold plate test
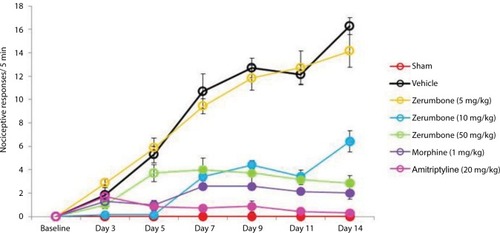
shows the response cold allodynia which was tested using the cold plate test. All mice did not show any paw lifting in the baseline data (0.00±0.00) (F (6, 49)=0.000; p=0.00). Following CCI and treatment with vehicle, the mean number of paw lifting was recorded to be 16.29±0.71 on day 14. Again, a significant (p<0.05) effect was observed only in Zer 10 and 50 mg/kg ip with mean values 6.43±0.90 and 2.86±0.53, respectively. Even though both positive control drugs alleviated (p<0.05) cold allodynia, amitriptyline, 20 mg/kg ip was more effective with a mean value of 0.29±0.18 on day 14. Cold allodynia was not observed in the contralateral paws.
Figure 4 Effect of treatments on cold allodynia withdrawal response as tested with cold plate.
Abbreviation: ANOVA, analysis of variance.
Hargreaves test
The CCI model produced thermal hyperalgesia as significant increased pain response was observed in CCI groups compared to the sham group (F (6, 49)=6.128, p=0.000) as shown in . Zerumbone at doses of 10 and 50 mg/kg ip alleviated thermal hyperalgesia on all days of behavioral testing. Both these doses showed similar (p>0.05) effect to morphine on days 9, 11, and 14. Amitriptyline completely reversed thermal hyperalgesia as the values were not significantly different (p>0.05) from the sham group. Thermal hyperalgesia was absent in the contralateral paws.
Figure 5 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal latency toward thermal hyperalgesia as tested with Hargreaves.
Abbreviation: ANOVA, analysis of variance.
Randall–Selitto test
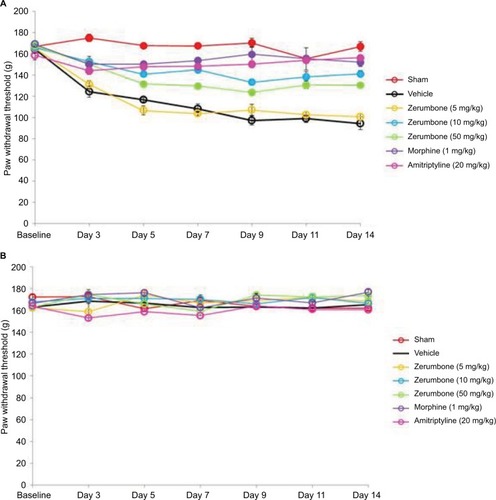
CCI surgery successfully developed mechanical hyperalgesia () as all groups subjected to CCI showed reduced threshold compared to sham-operated mice. Response toward mechanical hyperalgesia was significantly lowered (F (6, 49)=55.731; p=0.000) in higher doses of Zer (10 and 50 mg/kg), morphine (1 mg/kg ip), and amitriptyline (20 mg/kg ip) treated groups indicating the antihyperalgesic property of Zer and the positive control drugs. Lower dose of Zer (5 mg/kg), however, failed to exhibit antihyperalgesic effect as the threshold values were not significantly different (p>0.05) from the vehicle group. On the contralateral paw, mechanical hyperalgesia was absent and was not affected by the treatments.
Figure 6 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal threshold toward mechanical hyperalgesia as tested with Randall–Selitto in CCI and sham mice.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury.
Motor coordination test
Rotarod test was used to evaluate the presence of sedative effects of the treatments (). Neither vehicle nor the highest dose of Zer (50 mg/kg ip), morphine (1 mg/kg ip), or amitriptyline (20 mg/kg ip) showed reduced effect, as all mice were able to complete the entire rotarod session, which lasted for 3 min.
Enzyme-linked immunosorbent assay
Blood plasma
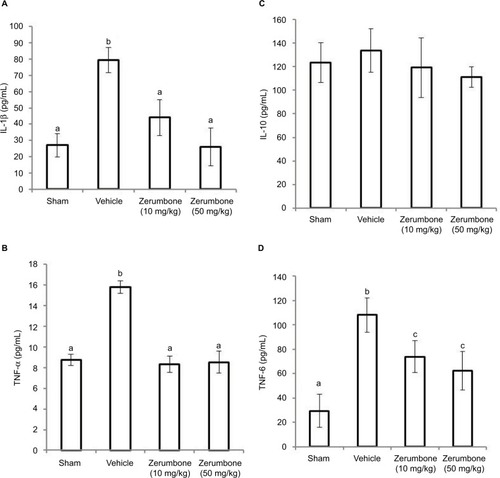
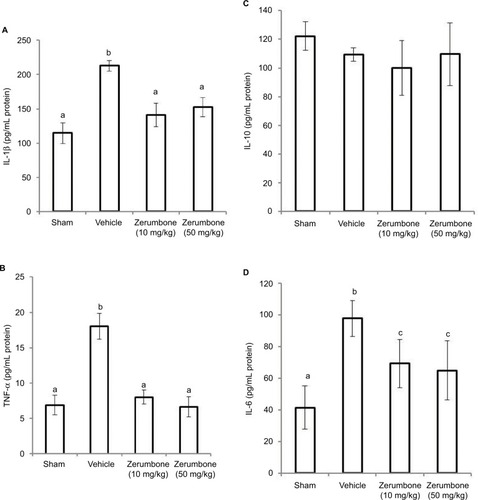
shows the circulating levels of inflammatory mediators in the CCI model and after the treatment of Zer (10 and 50 mg/kg). CCI caused an increase in pro-inflammatory cytokines, precisely IL-1β, TNF-α, and IL-6. Zerumbone treatment successfully reduced IL-1β, TNF-α, and IL-6 compared to the vehicle group. The levels IL-β and TNF-α were reduced to similar levels (p>0.05) of the sham group but IL-6 level was significantly higher (p≤0.05) than the sham group. The differences in the blood level of inflammatory mediators were not significantly different in both doses of Zer (10 and 50 mg/kg). However, blood plasma IL-10 showed no significant difference (p>0.05) in all the groups tested, indicating that neither the CCI surgery nor Zer treatments altered the levels.
Figure 8 Effect of zerumbone (10 and 50 mg/kg) treatments on the blood plasma levels of (A) IL-1β, (B) TNF-α, (C) IL-10, and (D) IL-6 in CCI and sham mice.
Abbreviations: CCI, chronic constriction injury; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.
Lumbar region of the spinal cord
represents the levels of inflammatory mediators in the lumbar 4–6 of spinal cord following CCI and treatment with Zer (10 and 50 mg/kg). The levels showed similar trend with the blood levels of inflammatory mediators where IL-1β, TNF-α, and IL-6 were reduced (p≤0.05) with Zer treatment but no changes (p>0.05) in IL-10 level was detected. Interleukin-6 level was significantly higher (p≤0.05) than its sham counterpart. Higher dose of zerumbone (50 mg/kg) was not able to exhibit a significant difference (p>0.05) from the lower dose of Zer (10 mg/kg) used in the assays.
Figure 9 Effect of zerumbone (10 and 50 mg/kg) treatment on the L4–L6 spinal cord levels of (A) IL-1β, (B) TNF-α, (C) IL-10, and (D) IL-6 in CCI and sham mice.
Abbreviations: CCI, chronic constriction injury; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.
Discussion
This study aimed to highlight the effects of prophylactic treatment of Zer at three different doses on attenuation of allodynia and hyperalgesia as well as the changes in production of inflammatory mediators. The outcome showed that all the drugs used as treatment did not cause any form of discomfort, change in appetite, or inability to access food throughout the period of 14 days as the body weights did not undergo any drastic changes. Hyperalgesia and allodynia were observed as early as our first day of behavioral testing on day 3 possibly due to the axon degeneration process that occurs from 24 to 48 h in mice.Citation39 Zerumbone successfully exerted analgesia at doses of 10 and 50 mg/kg but not 5 mg/kg throughout the experimental period. This outcome is consistent with our earlier study by Zulazmi et al.Citation22 This indicates that Zer treatment, both therapeutically and prophylactically, resulted in similar outcomes.
Morphine, the “gold standard” drug for pain treatment, and amitriptyline, a first-line drug for neuropathic pain treatment, were used as positive controls in this study. Even though these drugs were very effective in the attenuation of allodynia and hyperalgesia, reversing the threshold/latency similar to that of the sham group, Zer (10 and 50 mg/kg) was comparable with these drugs at some points. Most importantly, the effects of all the drugs were validated to be behavior specific since mice receiving the highest dose of drugs were able to maintain their balance on the rotarod throughout the 180 s time period. This ruled out the possibilities that the reduced pain response is due to suppression of general behavior as a result of the sedative effects of the drugs.Citation40 The ED50 values of Zer were calculated to be 9.245 mg/kg (R2=0.9894), 9.507 mg/kg (R2=0.9794), 8.289 mg/kg (R2=0.9105), and 9.801 mg/kg (R2=0.9157) for mechanical allodynia, cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia, respectively. Therefore, the best dose for prophylactic Zer treatment is 10 mg/kg.
We carried out this study on the CCI model of neuropathic pain as it involves both components of nerve injury and inflammation.Citation41 The results showed that the CCI model induced upregulation of plasma and spinal cord IL-1β, TNF-α, and IL-6 observed in the vehicle group on day 14, in line with previous studies.Citation41–Citation43 IL-1, TNF-α, and IL-6 are expressed as early as the second or third day post injury, during the first phase of Wallerian degeneration process. IL-1β reached maximum levels after 24 h of nerve injury and again showed peak production on day 14.Citation44 Within hours of nerve injury, mRNA coding of TNF-α and TNF receptors were rapidly increased in the sciatic nerve and subsequently within 1–3 days in the dorsal root ganglion (DRG), with the elevated levels displayed for at least 2 weeks post-injury.Citation45,Citation46
The action of Zer in pain modulation could take place at different levels of the pain pathway which include nerve terminals, DRG, spinal cord, and the ascending or descending tracts to the supraspinal regions. Furthermore, Zer could also act on various ion channels that are present along the peripheral nerves. In this study, Zer treatment suppresses inflammatory mediators, lowering pain transmission from primary afferent neurons to the ascending tract of the pain pathway. Pain impulses reaching the supraspinal regions will be modulated, and thus lower pain levels are perceived. This is then translated as lowered pain response that we have observed in our behavioral study.
The role of IL-6 in the pathogenesis of neuropathic pain has also been reported. In a sciatic cryoneurolysis (SCN) rat model where nerve injury is induced by freezing the sciatic nerve, elevated IL-6-like immunoreactivity and IL-6 mRNA were observed in the spinal cord dorsal and ventral horns of the rats.Citation47,Citation48 Furthermore, administration of human IL-6 intrathecally could also mimic and cause pain behavior after SCN.Citation47 The levels of IL-1β and TNF-α are also elevated in the DRG and spinal cord following peripheral nerve injury. Glial cells, principally astrocytes and microglia that are present within the dorsal horn, are activated to play essential roles in pain transmission and maintenance of this chronic disease.Citation12,Citation49 These glial cells secrete IL-1β, IL-6, and TNF-α which are characterized as messengers of the immune system to act on various cells, including neurons.Citation49
Since pro-inflammatory cytokines are critical in the initiation and maintenance of neuropathic pain, antagonism of these cytokines’ signaling inhibits neuronal hypersensitivity and inflammation due to nerve injury. TNF-α inhibitors have been reported to reduce peripheral nerve injury-associated thermal and mechanical pain.Citation50 Receptor fusion protein, etanercept, and humanized monoclonal antibody, infliximab, are examples of TNF-α inhibitors. Etanercept effectively reduced pain in clinical trials when tested in patients suffering from sciatica and spinal stenosis.Citation51,Citation52 Infliximab, on the other hand, benefited patients with L3–L4 and L4–L5 disc herniation.Citation53 Furthermore, TNF-α inhibitors are currently used to treat a number of immune disorders including psoriasis, Chrohn’s disease, and rheumatoid arthritis.Citation54
IL-1β alleviates thermal and mechanical pain hypersensitivity following spinal nerve injury and CCI in mice.Citation55,Citation56 Therapeutic targets that are currently approved and used in clinical settings include anakinra and rilonacept, which target IL-1 receptors. Canakinumab is an anti-IL-1β neutralizing antibody used in a few inflammatory diseases such as gout, rheumatoid arthritis, and Still’s disease.Citation54
IL-6 inhibitors have also been established as therapeutic targets. Tocilizumab, a humanized IL-6 receptor neutralizing antibody, could be used in the treatment of juvenile idiopathic arthritis, rheumatoid arthritis, and sciatica.Citation57 Intrathecal injection of anti-IL-6 antibody reduced mechanical allodynia induced by L5 spinal nerve transection.Citation48 Furthermore, attenuation and delay of mechanical allodynia were observed in IL-6 knockout mice following sciatic nerve ligation and CCI.Citation58,Citation59
Zerumbone’s antiinflammatory properties by suppression of these biomarkers have been extensively reflected across various models. Zerumbone administered intravenously successfully downregulates IL-1β expression and attenuates the severity of acute necrotizing pancreatitis induced by sodium taurocholate as well as pancreatitis-induced hepatic injury.Citation60 On the other hand, expression of TNF-α was inhibited by intraperitoneal injection of Zer in acute and chronic models of inflammation in mice.Citation27 The secretion of serum IL-6 and membrane-bound IL-6 receptors was inhibited by Zer in human cervical cancer and human ovarian cancer cell lines.Citation61
Furthermore, oral doses of Zer were able to inhibit TNF-α expression and cytosolic IL-6 in the ultraviolet B photokeratitis and cataractogenesis models.Citation62,Citation63 In addition, Zer significantly lowered IL-1β and TNF-α in the colonic mucosa of a dextran sodium sulfate-induced acute ulcerative colitis model.Citation29 The p38 MAPK activation of IL-1β, IL-6, and TNF-α was decreased by treatment with Zer in a streptozotocin-induced diabetic nephropathy model in rats.Citation64 These studies indicate that Zer is involved in the regulation of all these three inflammatory cytokines.
In this study, treatment of Zer from the first day of injury suppressed production of pro-inflammatory mediators as the levels of both the circularity and spinal cord IL-1β, TNF-α, and IL-6 were lowered. Upregulated levels of inflammatory cytokines (TNF-α and IL-1β) trigger the activation and expression of phospholipase-A2 (PLA2), an enzyme essential for the initial degradation of myelin sheath after nerve injury. Since the release of these cytokines was suppressed in Zer-treated mice, it was expected to stop or at least reduce myelin break down in the sciatic nerve.
The antiinflammatory cytokine, IL-10, however, assumes a role in attenuating the inflammatory process by counter-regulating the pro-inflammatory mediator productions and functions while upregulating endogenous anti-cytokines.Citation65 IL-10 is usually expressed after the recruitment of macrophages.Citation13 Apart from that, IL-10 also plays a protecting role against tissue damage and excessive immune responses. The immune homeostasis is usually maintained by the release of endogenous antiinflammatory cytokines, specifically IL-10, which follows a negative feedback mechanism by suppressing the genes that code pro-inflammatory cytokines and their respective receptors. In addition, IL-10 also has analgesic actions. By evaluating the changes in the production of this cytokine in this study, it would allow clearer understanding of the association of Zer with the inflammatory process that takes place within.Citation66
IL-10 was indifferent in this CCI model and Zer-treated groups on day 14, contrary to earlier reports of peak production of this mediator in the first 2 weeks after injury.Citation65 However, there are also reports stating that the expression of this mediator is more gradual and progressive whereby a significant mRNA expression is reached only at day 45 post-injury.Citation67
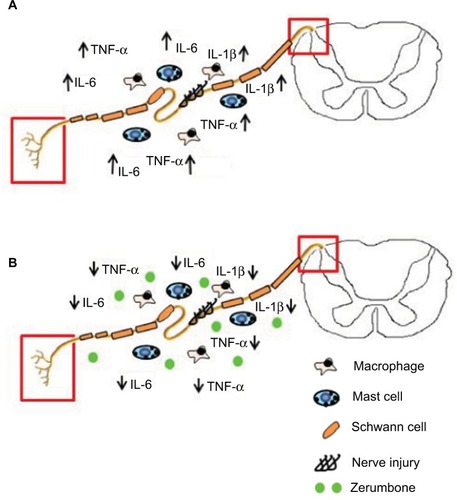
Besides these principal cytokines evaluated in this study, other key components of inflammation such as prostaglandin E2 (PGE2), nuclear factor (NF)-κB, nitric oxide (NO), and inducible nitric oxide synthase (iNOS) are also involved in peripheral nerve injury. Zerumbone’s antiinflammatory effect in many other components of inflammation has been reported in various other models in both in vivo and in vitro.Citation27–Citation29,Citation60,Citation62,Citation68–Citation75 Nevertheless, this study is the first report on the antiinflammatory role of Zer in the CCI mouse model of neuropathic pain. represents an overview of Zer’s antiinflammatory properties in attenuating allodynia and hyperalgesia.
Figure 10 Overview of the antiinflammatory actions of zerumbone on neuropathic pain symptoms allodynia and hyperalgesia.
Abbreviations: IL, interleukin; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.
Conclusion
In conclusion, Zer was consistent in exhibiting its analgesic properties toward mechanical allodynia, cold allodynia, mechanical hyperalgesia, and thermal hyperalgesia when Zer treatment was provided throughout the development and progression of neuropathic pain. This outcome is due to the antiinflammatory property exerted by Zer. We demonstrated that Zer successfully reversed the production of IL-1β, IL-6, and TNF-α but not IL-10 in blood plasma as well as in spinal cord tissues. Therefore, Zer is a highly potential drug for the management of pain in neuropathic conditions when administered prophylactically through suppressing the inflammatory event that occurs due to nerve injury.
Authors’ contribution
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This research was supported by Universiti Putra Malaysia under the Ministry of Science, Technology & Innovation, Science Fund Scheme, Grant No: 5450778, which is greatly appreciated.
Disclosure
The authors report no conflicts of interest in this work.
References
- van HeckeOAustinSKKhanRASmithBHTorranceNNeuropathic pain in the general population: a systematic review of epidemiological studiesPain2014155465466224291734
- ChongMSBajwaZHDiagnosis and treatment of neuropathic painJ Pain Symptom Manage2003255 Suppl41112565181
- ChaudakshetrinPA survey of patients with neuropathic pain at Siriraj pain clinicJ Med Assoc Thai200689335436116696420
- ShiptonEPost-surgical neuropathic painANZ J Surg200878754855518593408
- ColleoniMSacedotePMurine models of human neuropathic painBiochim Biophys Acta201018021092493319879943
- BoultonAJVinikAIArezzoJCDiabetic neuropathies: a statement by the American Diabetes AssociationDiabetes Care200528495696215793206
- TreedeRDJensenTSCampbellJNNeuropathic pain: redefinition and a grading system for clinical and research purposesNeurology200870181630163518003941
- SelvarajahDCashTSankarAThe contributors of emotional distress in painful diabetic neuropathyDiabetes Vasc Dis Res2014114218225
- McDiarmidTMacklerLSchneiderDMClinical inquiries. What is the addiction risk associated with tramadol?J Fam Pract2005541727315623411
- DaniellHWOpioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant painJ Pain200891283617936076
- ZhangJ-MAnJCytokines, inflammation and painInt Anesthesiol Clin2007452273717426506
- AustinPJMoalem-TaylorGThe neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokinesJ Neuroimmunol20102291–2265020870295
- RotshenkerSWallerian degeneration: the innate-immune response to traumatic nerve injuryJ Neuroinflammation2011810910921878125
- FregnanFMuratoriLSimõesARGiacobini-RobecchiMGRaimondoSRole of inflammatory cytokines in peripheral nerve injuryNeural Regen Res20127292259226625538747
- SchafersMSorkinLEffect of cytokines on neuronal excitabilityNeurosci Lett2008437318819318420346
- BinshtokAMWangHZimmermannKNociceptors are interleukin-1β sensorsJ Neurosci20082852140621407319109489
- ObrejaORatheePKLipsKSDistlerCKressMIL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase CFaseb J200216121497150312374772
- PezetSMcMahonSBNeurotrophins: mediators and modulators of painAnnu Rev Neurosci20062950753816776595
- SommerCKressMRecent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesiaNeurosci Lett20043611–318418715135924
- WoolfCJMannionRJNeuropathic pain: aetiology, symptoms, mechanisms, and managementLancet (London, England)1999353916819591964
- MoalemGTraceyDJImmune and inflammatory mechanisms in neuropathic painBrain Res Rev200651224026416388853
- ZulazmiNAGopalsamyBFaroukAAOSulaimanMRBharathamBHPerimalEKAntiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic painFitoterapia201510521522126205045
- ChiaJSMOmar FaroukAAMohamadASSulaimanMRPerimalEKZerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptorsBiomed Pharmacother2016831303131027570173
- ZulazmiNAGopalsamyBMinJCZerumbone alleviates neuropathic pain through the involvement of l-arginine-nitric oxide-cGMP-K(+) ATP channel pathways in chronic constriction injury in mice modelMolecules (Basel, Switzerland)2017224E555
- SulaimanMRPerimalEKZakariaZAMokhtarFAkhtarMNLajisNHPreliminary analysis of the antinociceptive activity of zerumboneFitoterapia200980423023219535012
- PerimalEKAkhtarMNMohamadASZerumbone-induced antinociception: involvement of the L-arginine-nitric oxide-cGMP-PKC-K+ATP channel pathwaysBasic Clin Pharmacol Toxicol2011108315516220955360
- SulaimanMRPerimalEKAkhtarMNAnti-inflammatory effect of zerumbone on acute and chronic inflammation models in miceFitoterapia201081785585820546845
- Thing-FongTShorong-ShiiLChia JuCI-MinLZerumbone, a tropical ginger sesquiterpene, ameliorates streptozotocin-induced diabetic nephropathy in rats by reducing the hyperglycemia-induced inflammatory responseNutrit Metabol201310164
- MurakamiAHayashiRTakanaTKwonKHOhigashiHSafitriRSuppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combinationBiochem Pharmacol20036671253126114505804
- RahmanHSRasedeeAYeapSKBiomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trialsBioMed Res Int2014201492074225025076
- BennettGJXieYKA peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in manPain1988331871072837713
- Ming-TattLKhalivullaSIAkhtarMNAnti-hyperalgesic effect of a benzylidene-cyclohexanone analogue on a mouse model of chronic constriction injury-induced neuropathic pain: participation of the κ-opioid receptor and K ATPPharmacol Biochem Behavior20131145863
- Ming-TattLKhalivullaSIAkhtarMNAntinociceptive activity of a synthetic curcuminoid analogue, 2, 6-bis-(4-hydroxy-3-methoxybenzylidene) cyclohexanone, on nociception-induced models in miceBasic Clin Pharmacol Toxicol2012110327528221967232
- ChaplanSRBachFWPogrelJWChungJMYakshTLQuantitative assessment of tactile allodynia in the rat pawJ Neurosci Methods199453155637990513
- CampanaGRimondiniRMechanical nociception measurement in mice and rats with automated von Frey equipmentSpampinatoSMOpioid Receptors: Methods and ProtocolsNew York, NYSpringer2015229231
- NadalXBanosJEKiefferBLMaldonadoRNeuropathic pain is enhanced in delta-opioid receptor knockout miceEur J Neurosci200623383083416487163
- HargreavesKDubnerRBrownFFloresCJorisJA new and sensitive method for measuring thermal nociception in cutaneous hyperalgesiaPain198832177883340425
- RandallLOSelittoJJA method for measurement of analgesic activity on inflamed tissueArch Int Pharmacodyn1957111440941913471093
- BeirowskiBAdalbertRWagnerDThe progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nervesBMC Neurosci20056615686598
- ChenLChenWQianXFangYZhuNLiquiritigenin alleviates mechanical and cold hyperalgesia in a rat neuropathic pain modelSci Rep20144567614
- CostaBComelliFBettoniIColleoniMGiagnoniGThe endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and antihyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factorsPain2008139354155018602217
- GuneliEOnalAAtesMEffects of repeated administered ghrelin on chronic constriction injury of the sciatic nerve in ratsNeurosci Lett201047922623020561937
- WellsMRRacisSPJrVaidyaUChanges in plasma cytokines associated with peripheral nerve injuryJ Neuroimmunol19923932612681644899
- PerrinFELacroixSAvilés-TriguerosMDavidSInvolvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β in Wallerian degenerationBrain2005128485486615689362
- SacerdotePFranchiSTrovatoAEValsecchiAEPaneraiAEColleoniMTransient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathyNeurosci Lett2008436221021318394803
- DubovyPJancalekRKlusakovaISvizenskaIPejchalovaKIntra- and extraneuronal changes of immunofluorescence staining for TNF-alpha and TNFR1 in the dorsal root ganglia of rat peripheral neuropathic pain modelsCell Mol Neurobiol2006267–81205121716705482
- DeLeoJAColburnRWNicholsMMalhotraAInterleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy modelJ Interferon Cytokine Res19961696957008887053
- ArrudaJLColburnRWRickmanAJRutkowskiMDDeLeoJAIncrease of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic painBrain Res Mol Brain Res19986222282359813345
- WhiteheadKJSmithCGDelaneySADynamic regulation of spinal pro-inflammatory cytokine release in the rat in vivo following peripheral nerve injuryBrain Behav Immun201024456957620035858
- IwatsukiKAraiTOtaHTargeting anti-inflammatory treatment can ameliorate injury-induced neuropathic painPloS One201382e5772123469058
- GenevaySStingelinSGabayCEfficacy of etanercept in the treatment of acute, severe sciatica: a pilot studyAnn Rheum Dis20046391120112315115710
- OhtoriSMiyagiMEguchiYEpidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized studySpine201237643944422020607
- KorhonenTKarppinenJPaimelaLThe treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trialSpine200631242759276617108825
- LeesJGDuffySSMoalem-TaylorGImmunotherapy targeting cytokines in neuropathic painFront Pharmacol2013414224319429
- WolfGGabayETalMYirmiyaRShavitYGenetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in micePain2006120331532416426759
- SommerCPetrauschSLindenlaubTToykaKVNeutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathyNeurosci Lett19992701252810454137
- OhtoriSMiyagiMEguchiYEfficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciaticaEur Spine J201221102079208422350007
- RamerMSMurphyPGRichardsonPMBisbyMASpinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout micePain19987821151219839821
- MurphyPGRamerMSBorthwickLGauldieJRichardsonPMBisbyMAEndogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in miceEur J Neurosci19991172243225310383613
- WenhongDJiaYWeixingWZerumbone attenuates the severity of acute necrotizing pancreatitis and pancreatitis-induced hepatic injuryMediators Inflammation2012201212
- AbdelwahabSIAbdulABZainZNMHadiAHAZerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cellsInt Immunopharmacol201212459460222330084
- ChenBYLinDPSuKCDietary zerumbone prevents against ultraviolet B-induced cataractogenesis in the mouseMol Vis20111772373021423870
- ChenBYLinDPWuCYDietary zerumbone prevents mouse cornea from UVB-induced photokeratitis through inhibition of NF-kappaB, iNOS, and TNF-alpha expression and reduction of MDA accumulationMol Vis20111785486321527993
- TzengTFLiouSSChangCJLiuIMThe ethanol extract of Zingiber zerumbet attenuates streptozotocin-induced diabetic nephropathy in ratsEvidence Based Complementary Altern Med20132013340645
- Siqueira MiettoBKronerAGirolamiEISantos-NogueiraEZhangJDavidSRole of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injuryJ Neurosci20153550164311644226674868
- Oliveira JúniorJOdPortella JuniorCSACohenCPInflammatory mediators of neuropathic painRevista Dor201617Suppl 13542
- OkamotoKMartinDPSchmelzerJDMitsuiYLowPAPro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic painExp Neurol2001169238639111358451
- MurakamiATakahashiDKinoshitaTKoshimizuKKimHWYoshihiroAZerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the a,b-unsaturated carbonyl group is a prerequisiteCarcinogenesis200223579580212016152
- SzabolcsATiszlaviczLJeaKaszakiZerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histologyPancreas200735324925517895846
- Al-SaffarFJGanabadiSFakuraziSYaakubHLipMChondroprotective effect of zerumbone on monosodium iodoacetate induced osteoarthritis in ratsJ Appl Sci2010104248260
- Al-SaffarFJGanabadiSFakuraziSYaakubHZerumbone significantly improved immunoreactivity in the synovium compared to Channa striatus extract in monosodium iodoacetate (MIA)-induced knee osteoarthritis in ratJ Med Plants Res20115917011710
- SomchitMNMakJHBustamamAAZerumbone isolated from Zingiber zerumbet inhibits inflammation and pain in ratsJ Med Plants Res201262177180
- GanabadiSKadirSFAZerumbone’s effect on major histocompatibility complex type II cells in synovial membrane of osteoarthritic jointRes J Vet Sci2009211420
- ZakariaZAMohamadASChearCTWongYYIsrafDASulaimanMRAntiinflammatory and antinociceptive activities of Zingiber zerumbet methanol extract in experimental model systemsMed Princ Pract201019428729420516705
- ChienTYHuangSKHLeeCJTsaiPWWangCCAntinociceptive and anti-inflammatory effects of zerumbone against mono-iodoacetate-induced arthritisInt J Mol Sci201617224926901193