Abstract
Objectives
To compare health care utilization of duloxetine initiators and pregabalin initiators among fibromyalgia patients in a real-world setting.
Methods
A retrospective cohort study was conducted based on a US national commercial health claims database (2006–2009). Fibromyalgia patients who initiated duloxetine or pregabalin in 2008, aged 18–64 years, and who maintained continuous health insurance coverage 1 year before and 1 year after initiation were assigned to duloxetine or pregabalin cohorts on the basis of their initiated agent. Patients who had pill coverage of the agents over the course of 90 days preceding the initiation were excluded. The two comparative cohorts were constructed using propensity score greedy match methods. Descriptive analysis and paired t-test were performed to compare health care utilization rates in the postinitiation year and the changes of these rates from the preinitiation year to the postinitiation year.
Results
Both matched cohorts (n=1,265 pairs) had a similar mean initiation age (49–50 years), percentage of women (87%–88%), and prevalence of baseline comorbid conditions (neuropathic pain other than diabetic peripheral neuropathic pain, low back pain, cardiovascular disease, hypertension, headache or migraine, and osteoarthritis). In the preinitiation year, both cohorts had similar inpatient, outpatient, and medication utilization rates (inpatient, 15.7%–16.1%; outpatient, 100.0%; medication, 97.9%–98.7%). The utilization rates diverged in the postinitiation year, with the pregabalin cohort using more fibromyalgia-related inpatient care (3.2% versus 2.2%; P<0.05), any inpatient care (19.3% versus 16.8%; P<0.05), and fibromyalgia-related outpatient care (62.1% versus 51.8%; P<0.05). From the preinitiation period to the postinitiation period, the duloxetine cohort experienced decreases in certain utilization rates, whereas the pregabalin cohort had increases (percentage of patients with a fibromyalgia-related admission, −1.2% versus 0.4% [P<0.01]; number of fibromyalgia-related outpatient claims, −1.7 versus 4.7 [P<0.01]).
Conclusion
Fibromyalgia patients initiating pregabalin tended to consume more fibromyalgia-related inpatient and outpatient care in the first postinitiation year, whereas fibromyalgia patients initiating duloxetine tended to have lower utilization rates of fibromyalgia-related inpatient care in the postinitiation year than in the preinitiation year.
Introduction
Fibromyalgia is a chronic pain disorder characterized by widespread musculoskeletal pain, tenderness, general fatigue, and sleep disturbances.Citation1–Citation10 It often presents with other clinical conditions, such as mood disorder, headache, irritable bowel syndrome, and interstitial cystitis.Citation5–Citation10 The disorder not only inflicts severe loss of functionality, productivity, and quality of lifeCitation11–Citation19 on 4–10 million Americans and about 3%–6% of the world’s population (especially women and people with family history)Citation20–Citation30 but also imposes a significant socioeconomic burden on patients, payers, and health care systems.Citation31–Citation39
Treatment for fibromyalgia includes pharmacological treatment, behavioral intervention, physical therapy, exercises, and alternative medicine.Citation40–Citation48 Although fibromyalgia patients often use analgesics, antidepressants, anticonvulsants, opioids, dopamine agonists, and other medications to alleviate their symptoms, the only pharmacologic treatments approved by the US Food and Drug Administration (FDA) for fibromyalgia are pregabalin (approved in 2007),Citation49–Citation53 duloxetine (approved in 2008),Citation53–Citation56 and milnacipran (approved in 2009).Citation57–Citation59
During the last 3 years, several real-world studies have been published to compare medication adherence, dosing patterns, direct medical costs, and health care utilization rates between fibromyalgia patients who initiated pregabalin and fibromyalgia patients who initiated duloxetine with different perspectives, methods, outcomes, and results.Citation1–Citation4,Citation11,Citation60–Citation72 Most of these published studiesCitation2–Citation4,Citation11,Citation60–Citation64,Citation66–Citation72 have a drawback in common: that they did not systematically examine the effect of medication choice between duloxetine and pregabalin on health care utilization rates within a period in which both medications were approved by FDA for fibromyalgia, and therefore, their results may not represent fibromyalgia patients who took these medications after FDA approval.
To address this common drawback, we conducted a real-world retrospective cohort study that compared health care utilization between fibromyalgia patients who initiated pregabalin and duloxetine in a post-FDA-approval year (2008) with a propensity score greedy match method. Our objectives were to corroborate postinitiation differences in health care utilization between two cohorts of fibromyalgia patients who initiated duloxetine or pregabalin in a post-FDA-approval year, to ascertain whether the changes of health care utilization from preinitiation year to postinitiation year differed across the two cohorts and to explore whether the specialty care utilization rates differed across the two cohorts in a postinitiation year.
Methods
Data sources
We used US national health care claims databases, collected by Thomson Reuters from large US employers, as our data sources. The databases contained electronically encrypted files of inpatient, outpatient, and medication claims, as well as enrollment records of 29 million unique patients for a period from 2006 through 2009. The inpatient, outpatient, and medication claims from the databases contained diagnosis codes from the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), Clinical Procedure Terminology codes, the National Drug Code, payment amount, and other pieces of information. The enrollment records provided us with detailed demographic information and benefit coverage of patients in the databases. Together, these databases allowed us to ascertain clinical and economic outcomes for a treatment either at a patient or cohort level.
Study design
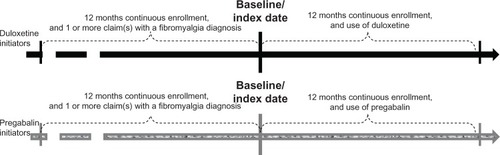
A retrospective cohort study design was used with a cohort of fibromyalgia patients who initiated duloxetine in 2008 and a cohort of fibromyalgia patients who initiated pregabalin in 2008. The first prescription date of an initiated agent (either duloxetine or pregabalin) was used as baseline or index date. Patients in both cohorts were observed for the 12 months immediately preceding and immediately after the index date. The scheme of our study design and duration can be expressed as seen in .
Sample selection
Our study selected commercially insured fibromyalgia patients who initiated duloxetine or pregabalin in 2008 and who were 18–64 years old on the initiation day. All selected patients had at least one claim with a fibromyalgia diagnosis code (ICD-9-CM, 729.1) in the 12-month preinitiation period and had 12-month continuous commercial health plan enrollment preceding and after the index date. Patients were excluded from this study if they had pill coverage of an initiated agent within the last 3 preindex months or if they received initial prescriptions for both agents (duloxetine and pregabalin) on the same day in the study period. The sample selection criteria are illustrated in .
Measurements
Patient characteristics
Demographic characteristics included sex, age, residential region (Northeast, North central, South, and West), and type of health plan on the index date, which included comprehensive, health maintenance organization, preferred provider organization, point-of-service, and others.
Clinical characteristics were measured for the preindex period. They included the most common fibromyalgia-related comorbid conditions (based on ICD-9-CM codes on inpatient or outpatient claims) and the history of fibromyalgia-related medications (based on National Drug Code codes on medication claims) in the 12-month preindex period.
The most common fibromyalgia-related comorbid conditionsCitation4,Citation73–Citation82 included neuropathic pain other than diabetic peripheral neuropathic pain, low back pain, cardiovascular diseases, headache and migraine, osteoarthritis, chronic pulmonary diseases, dyslipidemia, sleep disorder, and hypothyroidism. The fibromyalgia-related medicationsCitation4,Citation42–Citation44 included antidepressants (selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), serotonin/norepinephrine reuptake inhibitors (SNRIs), and other antidepressants), anticonvulsants, opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), sleep and antianxiety medications, skeletal muscle relaxants, dopamine agonists, topicals, and 5-HT3 antagonists.
Utilization outcomes
All patients’ inpatient, outpatient, and medication claims were differentiated into fibromyalgia-related and nonfibromyalgia-related categories on the basis of whether their claims contained a fibromyalgia diagnosis code (ICD-9-CM, 729.1) or a fibromyalgia-related National Drug Code. Then, percentages of patients who consumed these categories of care and the number of claims in these categories in the pre- and postindex periods were measured, respectively, as health care utilization outcomes.
To understand the effect of provider specialties on the utilization outcomes, we divided outpatient care into care provided by primary care providers and care provided by specialists, based on provider specialty information on the claims.
Statistical analysis
To adjust for preindex cross-cohort heterogeneity in observed patients’ characteristics and health care utilization rates, we used a propensity score greedy matching (ie, propensity score nearest-neighbor paired matching without replacement) methodCitation83–Citation86 involving two steps.
In the first step, a logistic regression model was developed to predict patients’ propensity scores of being duloxetine initiators. This logistic regression model had a binary response variable indicating whether a patient initiated duloxetine or pregabalin in 2008, as well as the following predictor variables: sex, age at initiation date, comorbid conditions with statistical significant cross-cohort differences in the preindex period, and histories of fibromyalgia-related medications with statistically significant cross-cohort differences in the preindex period, as well as utilization and direct health care costs (inpatient, outpatient, and medication) in the preindex period.
In the second step, each duloxetine initiator was pair-matched to a pregabalin initiator on the basis of a propensity score in the nearest neighbor and without replacement, so that the preindex cross-cohort heterogeneity in observed patients’ characteristics and health care utilization rates was minimized to a statistically nonsignificant level (P>0.05).
The differences in the postindex utilization of health care resources between the paired cohorts were examined either through cross-cohort comparison of postindex health care utilization rates or through cross-cohort comparison of the changes in health care utilization rates between the pre- and postindex periods.
All analyses were conducted using SAS (SAS Institute, Inc, Cary, NC, USA) programming language. Findings with P-values less than 0.05 were considered statistically significant.
Results
Study sample
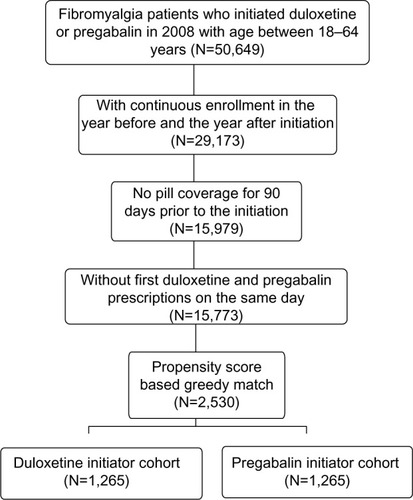
From our claims databases, we identified 50,649 fibromyalgia patients who initiated duloxetine or pregabalin in 2008 and who had an index date age between 18 and 64 years. Of these patients, 29,173 had continuous commercial health insurance for the last pre- and first postindex years. After excluding patients who had pill coverage of initiated agents in the last 3 preindex months or initiated both agents on the same day, we had 15,773 qualified patients. Using the propensity score greedy matching method, we selected 1,265 pairs of fibromyalgia patients who initiated duloxetine or pregabalin in 2008 to form our study cohorts (duloxetine, N=1,265; pregabalin, N=1,265). reveals the sample selection process.
Patient characteristics
After propensity score greedy matching, duloxetine and pregabalin cohorts had similar baseline demographic and clinical characteristics. Specifically, both duloxetine and pregabalin cohorts had a mean age around 49 years (49.3 versus 49.6 years; P>0.05), and 87%–88% were women (87.9% versus 87.4%; P>0.05). Most patients in both cohorts were from South or North central regions (77.8% versus 77.2%; P>0.05) and had health insurance provided by preferred provider organizations (63.3% versus 62.6%; P>0.05).
Both cohorts had the most common preindex comorbid conditions of neuropathic pain other than diabetic peripheral neuropathic pain (51.1% versus 50.5%; P>0.05), low back pain (45.6% versus 45.7%; P>0.05), cardiovascular diseases (39.3% versus 39.4%; P>0.05), hypertension (32.1% versus 31.5%; P>0.05), headache or migraine (24.1% versus 23.4%; P>0.05), osteoarthritis (21.8% versus 22.3%; P>0.05), and depression (18.4% versus 17.9%; P>0.05). The details of other preindex demographic and clinical characteristics are presented in .
Table 1 Patient demographic and clinical characteristics
Health care utilization rates in preindex year
After propensity score greedy matching, both cohorts had similar health care utilization in the preindex year, either in terms of percentages of patients consuming individual types of health care or in terms of average numbers of claims per patient per year.
About 16% of patients consumed inpatient care (15.7% versus 16.1%; P>0.05), with 0.2 admissions per patient per year, and about 3% received fibromyalgia-related inpatient care (3.4% versus 2.8%; P>0.05). All patients consumed outpatient care, and 98% of them consumed fibromyalgia-related outpatient care (98.1% versus 98.8%; P>0.05), with 6.1 claims per patient per year. About 63% were receiving antidepressants (63.0% versus 62.6%; P>0.05), with 4.4 versus 4.3 prescriptions per patient per year; 44% were receiving anticonvulsants (44.4% versus 43.6%; P>0.05), with 2.4 versus 2.2 prescriptions per patient per year; 77% were receiving opioids (76.9% versus 77.2%; P>0.05), with 6.8 versus 6.6 prescriptions per patient per year; 44% were receiving nonsteroidal anti-inflammatory drugs (44.0% versus 43.2%; P>0.05), with 1.4 versus 1.3 prescriptions per patient per year; 34% were receiving sleep and antianxiety medications (34.4% versus 34.0%; P>0.05), with 1.8 prescriptions per patient per year; 47% were receiving skeletal muscle relaxants (47.5% versus 46.7%; P>0.05), with 2.1 versus 1.9 prescriptions per patient per year; and 10% were receiving topical treatments (10.3% versus 9.8%; P>0.05), with 0.2 prescriptions per patient per year. Other utilization rates of each cohort can be found in .
Table 2 Health care utilization rates in pre- and postinitiation years
Healthcare utilization rates in postindex year
Statistically significant cross-cohort differences in certain health care utilization rates were observed in the postindex year, although such differences were not present in the preindex year because of the use of the propensity score greedy match.
Compared with those in the pregabalin cohort, patients in the duloxetine cohort were less likely to use inpatient care (fibromyalgia-related, 2.2% versus 3.2%; nonfibromyalgia-related, 14.5% versus 16%; both, 16.8% versus 19.3%; all P<0.01), fibromyalgia-related outpatient care (51.8% versus 62.1%; P<0.01), selective serotonin reuptake inhibitors (20.1% versus 34.0%; P<0.01), and nonduloxetine serotonin/norepinephrine reuptake inhibitors (4.7% versus 11.9%; P<0.01) but were more likely to use nonpregabalin anticonvulsants (43.6% versus 39.3%; P<0.01), as well as 5-HT3 antagonists (5.0% versus 3.4%; P<0.05). In addition, the duloxetine cohort was associated with fewer inpatient admissions (0.2 versus 0.5; P<0.01), fewer outpatient claims (fibromyalgia-related, 4.4 versus 10.8; nonfibromyalgia-related, 77.1 versus 79.1; specialty care, 28.4 versus 38.1; all outpatient, 81.5 versus 90.0; all P<0.01), and fewer prescriptions of nonduloxetine antidepressants (3.0 versus 4.3; P<0.01), but more prescriptions of nonpregabalin anticonvulsants (2.9 versus 2.1; P<0.01). Other postindex utilization rates for each cohort are presented in .
Changes in health care utilization rates
compares the changes of individual health care utilization rates from the preindex year with those of the postindex year between the two cohorts. It suggests that the cross-period changes of certain health care utilization rates differed between the two cohorts with statistical significance.
Table 3 Comparison of utilization changes
For example, from the preindex period to the postindex period, the percentages of patients with fibromyalgia-related inpatient admissions decreased 1.2% in the duloxetine cohort but increased 0.4% in the pregabalin cohort (P<0.01), whereas the percentages of patients with either type of inpatient admissions increased 1.1% in the duloxetine cohort and 3.2% in the pregabalin cohort. Both cohorts experienced significant pre–post reduction in the percentages of patients receiving fibromyalgia-related outpatient care (−46.3% versus −36.7%; P<0.01), but the numbers of claims per patient per year for fibromyalgia-related outpatient care, specialty care, and overall outpatient care increased in the pregabalin cohort and decreased in the duloxetine cohort (fibromyalgia-related outpatient care, −1.7 versus 4.7 [P<0.01]; specialty outpatient care, −0.1 versus 10.2 [P<0.01]; all outpatient care, −2.3 versus 6.3 [P<0.01]).
Discussion
This study examined and compared real-world health care utilization between two cohorts of fibromyalgia patients who initiated duloxetine or pregabalin in a period after FDA approval. Through propensity score greedy match, we were able to pair the two cohorts with the same demographic and clinical characteristics, as well as the same health care utilization rates, in the preinitiation year. However, these paired health care utilization rates diverged in the postinitiation year, with different trajectories.
Selecting an FDA-approved pharmaceutical treatment with a better effect on health care utilization is a potential approach that decision makers can use to control use of health care resources with optimal effectiveness and efficiency. To do so, payers, physicians and patients need information from comparative studies that depict real-world use of health care resources under different treatments.
To the best of our knowledge, published real-world studies for fibromyalgia patients who initiated duloxetine or pregabalinCitation1,Citation2,Citation4,Citation62–Citation64,Citation87 have not yet longitudinally and cross-sectionally examined and compared the health care utilization rates between duloxetine and pregabalin initiators after these medications received approval from the FDA for the treatment of fibromyalgia. We believe that this study is the first that examined and compared these utilization rates longitudinally and cross-sectionally through the use of propensity score greedy matching methods with a postinitiation year extending beyond the FDA approval date. The study results showed that compared with fibromyalgia patients initiating pregabalin in 2008, fibromyalgia patients initiating duloxetine in 2008 not only had fewer inpatient admissions per patient per year (0.2 versus 0.5; P<0.01) and fewer outpatient claims (fibromyalgia-related, 4.4 versus 10.8; nonfibromyalgia-related, 77.1 versus 79.1; specialty care, 28.4 versus 38.1; total, 81.5 versus 90.0, all P<0.01) in the first postinitiation year but also were associated with reduced percentages of patients using fibromyalgia-related inpatient care (cross-period changes, −1.2% versus 0.4%; P<0.01) and outpatient care (cross-period changes, −46.3% versus −36.7%; P<0.01) in a real-world setting.
Among all published real-world studies for fibromyalgia patients initiating duloxetine and pregabalin,Citation1,Citation2,Citation4,Citation62–Citation64,Citation87 only the current study and the study published by Sun et alCitation1 examined and compared the real-world use of health care resources between fibromyalgia patients who used duloxetine and pregabalin after FDA approval for treating fibromyalgia, although these two studies used different analytical methods (the former used propensity score greedy matching methods, the latter used propensity score stratification methods). Because the focus of that published studyCitation1 was on medication compliance and direct medical costs, it did not examine or compare the longitudinal changes of health care utilization rates from the preinitiation year to the postinitiation year between the two cohorts. Therefore, our study may represent a more holistic real-world evaluation of the effect of choice between duloxetine and pregabalin on the health care utilization rates among fibromyalgia patients. That said, the results from both studies are still consistent in terms of cross-sectional comparison of the health care utilization rates.
Similar to all real-world studies using health care claims databases, our study has its own limitations. First, our sample selection requirement for 12-month continuous health insurance coverage preceding and after the initiation might exclude patients with discontinued health insurance coverage. Second, our claims databases only represent a geographically diverse population with large employer-sponsored commercial health insurance, which might differ from other populations. Third, all clinical conditions were identified on the basis of ICD-9-CM diagnosis codes on health care claims and had not yet been validated with medical chart review; therefore, clinical conditions not recorded on health care claims or without a proper diagnosis code were not included in this study. Fourth, our study did not collect nonpharmacologic treatments and, therefore, did not assess the effect of nonpharmacologic treatments on health care utilization rates. Fifth, some of our patients initiated duloxetine in 2008, but before the June 2008 approval of duloxetine for the treatment of fibromyalgia. Finally, our study could not adjust for unobservable confounding factors, which might bias our study results.
Conclusion
Fibromyalgia patients who initiated duloxetine in 2008 used less fibromyalgia-related inpatient and outpatient care in the postinitiation year than fibromyalgia patients who initiated pregabalin in 2008. Further, these duloxetine initiators used less fibromyalgia-related inpatient care in the postinitiation year than in the preinitiation year, whereas the pregabalin initiators used more of the same care in the postinitiation year than in the preinitiation year. Further research is needed to identify the factors contributing to these cross-cohort and cross-period differences, so that payers, physicians, and patients can use the information to reduce fibromyalgia patients’ use of health care resources while achieving optimal clinical outcomes.
Disclosure
X Peng, D Novick and J Andrews are employees of Eli Lilly and Company, which produce duloxetine. P Sun and S Sun are employees of Kailo Research Group, which received research grants from pharmaceutical companies such as Eli Lilly and Company. The remaining authors report no conflicts of interest in this work.
References
- SunPPengXSunSDirect medical costs and medication compliance among fibromyalgia patients: duloxetine initiators vs pregabalin initiatorsPain Pract Epub3142013
- SunPZhaoYZhaoZWatsonPMedication dosing patterns associated with duloxetine and pregabalin among patients with fibromyalgiaCurr Med Res Opin20112791793180121810060
- ZhaoYSunPBernauerMComparing common reasons for inpatient and outpatient visits between commercially-insured duloxetine or pregabalin initiators with fibromyalgiaJ Pain Res2015443451
- ZhaoYSunPWatsonPMitchellBSwindleRComparison of medication adherence and healthcare costs between duloxetine and pregabalin initiators among patients with fibromyalgiaPain Pract201111320421620807351
- MarcusDARichardsKLChambersJFBhowmickAFibromyalgia family and relationship impact exploratory surveyMusculoskeletal Care201311312513423172797
- ForsEALandmarkTBakkeØContextual and time dependent pain in fibromyalgia: an explorative studyBMC Res Notes2012564423163972
- Castro-SánchezAMMatarán-PeñarrochaGALópez-RodríguezMMLara-PalomoICArendt-NielsenLFernández-de-las-PeñasCGender differences in pain severity, disability, depression, and widespread pressure pain sensitivity in patients with fibromyalgia syndrome without comorbid conditionsPain Med201213121639164723171037
- AuquierLBontouxDLöoHLa fibromyalgie. [Fibromyalgia]Rev Med Interne2008292161168 French17976867
- WolfeFHawleyDJGoldenbergDLRussellIJBuskilaDNeumannLThe assessment of functional impairment in fibromyalgia (FM): Rasch analyses of 5 functional scales and the development of the FM Health Assessment QuestionnaireJ Rheumatol20002781989199910955343
- WolfeFSmytheHAYunusMBThe American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria CommitteeArthritis Rheum19903321601722306288
- Al-AllafAWWork disability and health system utilization in patients with fibromyalgia syndromeJ Clin Rheumatol200713419920117762453
- RobinsonRLJonesMLIn search of pharmacoeconomic evaluations for fibromyalgia treatments: a reviewExpert Opin Pharmacother2006781027103916722813
- BennettRMJonesJTurkDCRussellIJMatallanaLAn internet survey of 2,596 people with fibromyalgiaBMC Musculoskelet Disord200782717349056
- ShaverJLWilburJRobinsonFPWangEBuntinMSWomen’s health issues with fibromyalgia syndromeJ Womens Health (Larchmt)20061591035104517125422
- RutledgeDNJonesKJonesCJPredicting high physical function in people with fibromyalgiaJ Nurs Scholarsh200739431932418021131
- BergerADukesEMartinSEdelsbergJOsterGCharacteristics and healthcare costs of patients with fibromyalgia syndromeInt J Clin Pract20076191498150817655684
- PantonLBKingsleyJDTooleTA comparison of physical functional performance and strength in women with fibromyalgia, age- and weight-matched controls, and older women who are healthyPhys Ther200686111479148817079747
- VerbuntJAPernotDHSmeetsRJDisability and quality of life in patients with fibromyalgiaHealth Qual Life Outcomes20086818211701
- KurtzeNGundersenKTSvebakSQuality of life, functional disability and lifestyle among subgroups of fibromyalgia patients: the significance of anxiety and depressionBr J Med Psychol199972Pt 447148410616131
- WolfeFPetriMAlarcónGSFibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activityJ Rheumatol2009361828819004039
- Better MedicinePrevalence and incidence of fibromyalgia Available from: http://www.localhealth.com/article/fibromyalgiaAccessed May 5, 2009
- National Fibromyalgia AssociationPrevalence of fibromyalgia Available from: http://fmaware.org/PageServera6cc.html?pagename=fibromyalgia_affectedAccessed May 5, 2009
- BrancoJCBannwarthBFaildeIPrevalence of fibromyalgia: a survey in five European countriesSemin Arthritis Rheum201039644845319250656
- BannwarthBBlotmanFRoué-Le LayKCaubèreJPAndréETaïebCFibromyalgia syndrome in the general population of France: a prevalence studyJoint Bone Spine200976218418718819831
- MasAJCarmonaLValverdeMRibasBEPISER Study GroupPrevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in SpainClin Exp Rheumatol200826451952618799079
- TodaKThe prevalence of fibromyalgia in Japanese workersScand J Rheumatol200736214014417476621
- WeirPTHarlanGANkoyFLThe incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codesJ Clin Rheumatol200612312412816755239
- TopbasMCakirbayHGulecHAkgolEAkICanGThe prevalence of fibromyalgia in women aged 20–64 in TurkeyScand J Rheumatol200534214014416095011
- SardiniSGhirardiniMBetelemmeLArpinoCFattiFZaniniFStudio epidemiologico sulla fibromialgia primaria in eta pediatrica. [Epidemiological study of a primary fibromyalgia in pediatric age.]Minerva Pediatr19964812543550 Italian9091773
- WolfeFRossKAndersonJRussellIJHebertLThe prevalence and characteristics of fibromyalgia in the general populationArthritis Rheum199538119287818567
- KimSKKimSHLeeCKEffect of fibromyalgia syndrome on the health-related quality of life and economic burden in KoreaRheumatology (Oxford)201352231132023024016
- RobinsonRLKroenkeKMeasePBurden of illness and treatment patterns for patients with fibromyalgiaPain Med201213101366137622958298
- PerrotSSchaeferCKnightTHufstaderMChandranABZlatevaGSocietal and individual burden of illness among fibromyalgia patients in France: association between disease severity and OMERACT core domainsBMC Musculoskelet Disord2012132222340435
- ChandranASchaeferCRyanKBaikRMcNettMZlatevaGThe comparative economic burden of mild, moderate, and severe fibromyalgia: results from a retrospective chart review and cross-sectional survey of working-age US adultsJ Manag Care Pharm201218641542622839682
- SchaeferCChandranAHufstaderMThe comparative burden of mild, moderate and severe fibromyalgia: results from a cross-sectional survey in the United StatesHealth Qual Life Outcomes201197121859448
- SpaethMEpidemiology, costs, and the economic burden of fibromyalgiaArthritis Res Ther200911311719591654
- KleinmanNHarnettJMelkonianABurden of fibromyalgia and comparisons with osteoarthritis in the workforceJ Occup Environ Med200951121384139319952796
- AnnemansLLe LayKTaïebCSocietal and patient burden of fibromyalgia syndromePharmacoeconomics200927754755919663526
- DoronYPelegRPelegANeumannLBuskilaDThe clinical and economic burden of fibromyalgia compared with diabetes mellitus and hypertension among Bedouin women in the NegevFam Pract200421441541915249530
- CarvilleSFArendt-NielsenSBliddalHEULAREULAR evidence-based recommendations for the management of fibromyalgia syndromeAnn Rheum Dis200867453654117644548
- GuymerEKLittlejohnGOFibromyalgia. Diagnosis and managementAustralas Chiropr Osteopathy2002102818417987178
- SumptonJEMoulinDEFibromyalgia: presentation and management with a focus on pharmacological treatmentPain Res Manag200813647748319225604
- BuckhardtCSGoldenbergDCroffordLGuideline for the Management of Fibromyalgia Syndrome. Pain in Adults and Children APS Clinical Practice Guideline Series No. 4Glenview, ILAmerican Pain Society2005
- HäuserWThiemeKTurkDCGuidelines on the management of fibromyalgia syndrome – a systematic reviewEur J Pain201014151019264521
- ForsethK KØGranJTManagement of fibromyalgia: what are the best treatment choices?Drugs200262457759211893227
- BrosseauLWellsGATugwellPOttawa Panel MembersOttawa Panel evidence-based clinical practice guidelines for aerobic fitness exercises in the management of fibromyalgia: part 1Phys Ther200888785787118497301
- BrosseauLWellsGATugwellPOttawa Panel MembersOttawa Panel evidence-based clinical practice guidelines for strengthening exercises in the management of fibromyalgia: part 2Phys Ther200888787388618497302
- CroffordLJPain management in fibromyalgiaCurr Opin Rheumatol200820324625018388513
- CroffordLJRowbothamMCMeasePJPregabalin 1008–1105 Study GroupPregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trialArthritis Rheum20055241264127315818684
- CalandreEPMorillas-ArquesPRodriguez-LopezCMRico-VillademorosFHidalgoJPregabalin augmentation of quetiapine therapy in the treatment of fibromyalgia: an open-label, prospective trialPharmacopsychiatry2007402687117447176
- CroffordLJMeasePJSimpsonSLFibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalinPain2008136341943118400400
- MeasePJRussellIJArnoldLMA randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgiaJ Rheumatol200835350251418278830
- García-CampayoJSerrano-BlancoARoderoBEffectiveness of the psychological and pharmacological treatment of catastrophization in patients with fibromyalgia: a randomized controlled trialTrials2009102419389246
- RussellIJMeasePJSmithTREfficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trialPain2008136343244418395345
- ArnoldLMRosenAPritchettYLA randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorderPain20051191–351516298061
- ArnoldLMLuYCroffordLJA double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorderArthritis Rheum20045092974298415457467
- ClauwDJMeasePPalmerRHGendreauRMWangYMilnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trialClin Ther200830111988200419108787
- GendreauRMThornMDGendreauJFEfficacy of milnacipran in patients with fibromyalgiaJ Rheumatol200532101975198516206355
- VittonOGendreauMGendreauJKranzlerJRaoSGA double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgiaHum Psychopharmacol200419Suppl 1S27S3515378666
- ReedCBirnbaumHGIvanovaJIReal-world role of tricyclic antidepressants in the treatment of fibromyalgiaPain Pract201212753354022226400
- GoreMTaiKSChandranAZlatevaGLeslieDClinical characteristics, pharmacotherapy, and healthcare resource use among patients with fibromyalgia newly prescribed pregabalin or tricyclic antidepressantsJ Med Econ2012151324421970698
- GoreMTaiKSChandranAZlatevaGLeslieDClinical comorbidities, treatment patterns, and healthcare costs among patients with fibromyalgia newly prescribed pregabalin or duloxetine in usual careJ Med Econ2012151193121970699
- BurkeJPSanchezRJJoshiAVCappelleriJCKulakodluMHalpernRHealth care costs in patients with fibromyalgia on pregabalin vs duloxetinePain Pract2012121142221615857
- HarnettJMargolisJCaoZReal-world evaluation of health-care resource utilization and costs in employees with fibromyalgia treated with pregabalin or duloxetinePain Pract201111321722921199319
- Rodriguez-RevengaLMadrigalIBlanch-RubióJScreening for the presence of FMR1 premutation alleles in women with fibromyalgiaGene2013512230530823111161
- BergerASadoskyADukesEMEdelsbergJZlatevaGOsterGPatterns of healthcare utilization and cost in patients with newly diagnosed fibromyalgiaAm J Manag Care201016Suppl 5S126S13720586521
- BergerASadoskyADukesEMartinSEdelsbergJOsterGCharacteristics and patterns of healthcare utilization of patients with fibromyalgia in general practitioner settings in GermanyCurr Med Res Opin20082492489249918664319
- HorndaschSHohe Inanspruchnahme des Gesundheitswesens durch Fibromyalgiepatienten. [High utilization of health care services by fibromyalgia patients.]Schmerz20092317071 German19153777
- PalacioAUribeCLLiHFinancial and clinical characteristics of fibromyalgia: a case-control comparisonAm J Manag Care201016Suppl 5S118S12520586520
- SanchezRJUribeCLiHLongitudinal evaluation of health care utilization and costs during the first three years after a new diagnosis of fibromyalgiaCurr Med Res Opin201127366367121241205
- WhiteKPNelsonWRHarthMOstbyeTSpeechleyMDoes the label “fibromyalgia” alter health status, function, and health service utilization? A prospective, within-group comparison in a community cohort of adults with chronic widespread painArthritis Rheum200247326026512115155
- WolfeFAndersonJHarknessDA prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgiaArthritis Rheum1997409156015709324009
- HudsonJIGoldenbergDLPopeHGJrKeckPEJrSchlesingerLComorbidity of fibromyalgia with medical and psychiatric disordersAm J Med19929243633671558082
- RaphaelKGJanalMNNayakSComorbidity of fibromyalgia and posttraumatic stress disorder symptoms in a community sample of womenPain Med200451334114996235
- BradleyLAPsychiatric comorbidity in fibromyalgiaCurr Pain Headache Rep200592798615745615
- Chamizo-CarmonaE¿Existe asociación entre la fibromialgia, el aumento de la comorbilidad por enfermedad neoplásica, cardiovascular e infecciones, y el de la mortalidad? [Is there an association between fibromyalgia and an increase in comorbidity: neoplastic and cardiovascular diseases, infections and mortality?]Reumatol Clin200514200210 Spanish21794265
- BuskilaDCohenHComorbidity of fibromyalgia and psychiatric disordersCurr Pain Headache Rep200711533333817894922
- Gil YuberoJLlensa CubarsíIMas MarquèsMBuñuel AlvarezJCComorbilidad registrada en los pacientes diagnosticados de fibromialgia en un centro de atención primaria. [Comorbidity recorded in patients diagnosed with fibromyalgia at a primary care centre.]Aten Primaria2007394217 Spanish17428429
- WhiteLABirnbaumHGKaltenboeckATangJMallettDRobinsonRLEmployees with fibromyalgia: medical comorbidity, healthcare costs, and work lossJ Occup Environ Med2008501132418188077
- de TommasoMSardaroMSerpinoCFibromyalgia comorbidity in primary headachesCephalalgia200929445346419170692
- GonzálezEElorzaJFaildeIFibromyalgia and psychiatric comorbidity: their effect on the quality of life patientsActas Esp Psiquiatr2010385295300 Spanish.21117004
- ShillamCRDupree JonesKMillerLFibromyalgia symptoms, physical function, and comorbidity in middle-aged and older adultsNurs Res201160530931721873914
- RosenbaumPRRubinDBThe central role of the propensity score in observational studies for causal effectsBiometrika19837014155
- GuoSYFraserMWPropensity Score Matching and Related Models. Propensity Score Analysis: Statistical Methods and ApplicationsLos AngelesSEGA2010145149
- D’AgostinoRBJrPropensity score methods for bias reduction in the comparison of a treatment to a non-randomized control groupStat Med19981719226522819802183
- DehejiaRHWahbaSPropensity score-matching methods for nonexperimental causal studiesRev Econ Stat2002841151161
- KleinmanNLSanchezRJLynchWDCappelleriJCBerenIAJoshiAVHealth outcomes and costs among employees with fibromyalgia treated with pregabalin vs standard of carePain Pract201111654055121392253