Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs), which act via inhibition of the cyclooxygenase (COX) isozymes, were discovered more than 100 years ago. They remain a key component of the pharmacological management of acute and chronic pain. The COX-1 and COX-2 isozymes have different biological functions; analgesic activity is primarily (although not exclusively) associated with inhibition of COX-2, while different side effects result from the inhibition of COX-1 and COX-2. All available NSAIDs, including acetaminophen and aspirin, are associated with potential side effects, particularly gastrointestinal and cardiovascular effects, related to their relative selectivity for COX-1 and COX-2. Since all NSAIDs exert their therapeutic activity through inhibition of the COX isozymes, strategies are needed to reduce the risks associated with NSAIDs while achieving sufficient pain relief. A better understanding of the inhibitory activity and COX-1/COX-2 selectivity of an NSAID at therapeutic doses, based on pharmacokinetic and pharmacodynamic properties (eg, inhibitory dose, absorption, plasma versus tissue distribution, and elimination), and the impact on drug tolerability and safety can guide the selection of appropriate NSAIDs for pain management. For example, many NSAIDs with moderate to high selectivity for COX-2 versus COX-1 can be administered at doses that maximize efficacy (~80% inhibition of COX-2) while minimizing COX-1 inhibition and associated side effects, such as gastrointestinal toxicity. Acidic NSAIDs with favorable tissue distribution and short plasma half-lives can additionally be dosed to provide near-constant analgesia while minimizing plasma concentrations to permit recovery of COX-mediated prostaglandin production in the vascular wall and other organs. Each patient’s clinical background, including gastrointestinal and cardiovascular risk factors, should be taken into account when selecting appropriate NSAIDs. New methods are emerging to assist clinicians in the selection of appropriate NSAIDs and their doses/schedules, such as biomarkers that may predict the response to NSAID treatment in individual patients.
Introduction
Pain represents a major public health problem worldwide,Citation1–Citation3 with chronic pain affecting approximately 27% of the adult population in EuropeCitation4 and more than 100 million adults in the United States.Citation1 Undertreated acute pain can be associated with an increased risk of deleterious health consequences (eg, delayed wound healing, immune dysfunction, cardiovascular problems related to the stress response, and respiratory problems, such as pneumonia) and the development of chronic pain.Citation5 In addition, unrelieved chronic severe pain can negatively impact an individual’s quality of life, day-to-day functioning, sleep quality, interpersonal relationships, and work productivity, and is associated with a substantial economic burden.Citation1,Citation6
There are two major options for the pharmacological management of pain: non-steroidal anti-inflammatory drugs (NSAIDs), which act via inhibition of cyclooxygenase (COX) isozymes, and opiate/opioid analgesics.Citation7,Citation8 NSAIDs, which are among the most widely used medications worldwide,Citation9,Citation10 are often preferred because of their low abuse potential, robust efficacy, and long history of clinical use.Citation11 Guidelines for pain management tend to be specific to medical conditions or settings. Current guidelines for chronic low back pain and osteoarthritis generally recommend the use of oral acetaminophen (or topical NSAIDs for osteoarthritis) for first-line pain management, with other oral NSAIDs recommended in the first-line or second-line setting depending on the specific guideline; some guidelines also recommend opioids, although typically not as first-line therapy.Citation8,Citation12–Citation15 It has, however, recently been shown that acetaminophen is not measurably effective in acute low back pain (ie, not more effective than placebo).Citation16 Other work in patients with osteoarthritis demonstrated that ibuprofen was associated with greater improvements compared with acetaminophen in measures of osteoarthritis pain, function, and quality of life.Citation17 Acetaminophen and other oral NSAIDs are also recommended for acute pain, such as postoperative pain management.Citation7
The first non-opioid analgesics (phenazone, paracetamol, and aspirin) were found by serendipity more than 100 years ago.Citation18 Later, systematic research identified their mode of action as inhibition of prostanoid production.Citation18 The development of animal models of pain and inflammation led to the discovery of a host of new NSAIDs (eg, diclofenac, ibuprofen, ketoprofen, naproxen), many of which were acidic, highly protein-bound compounds that generally showed similar therapeutic effects and side effects related to their inhibitory activity on prostanoid synthesis.Citation18
NSAIDs inhibit prostanoid biosynthesis through their activity on the COX enzymes COX-1 and COX-2.Citation19 The two COX isoforms (COX-1 and COX-2) have different functions, and the inhibition of these isoforms results in different therapeutic effects and side effects.Citation11 Efforts to avoid the gastrointestinal side effects associated with COX-1 inhibitionCitation11 led to the development of selective COX-2 inhibitors called “coxibs”, which were designed to inhibit COX-2 while sparing COX-1 at therapeutic doses.Citation18 Evidence of increased risk of myocardial infarction and other thrombotic events led to concerns over the cardiovascular safety of coxibs, resulting in the withdrawal of rofecoxib from the market in 2004.Citation10 However, traditional NSAIDs, which inhibit COX-2 without sustained inhibition of platelet COX-1, may also be associated with an increased risk of cardiovascular events, particularly at high doses.Citation11,Citation20–Citation23
Although novel compounds are being developed (eg, COX-inhibiting nitrous oxide donors) to improve safety and tolerability, it is unlikely that an ideal oral NSAID that avoids all potential side effects will be available in the near future.Citation11,Citation24 Thus, strategies are needed to reduce the risks associated with currently available NSAIDs. Drug choice should be driven by the clinical and demographic features (eg, age) of the patient, and the lowest effective dose of drug should be used for the shortest period of timeCitation25 to reduce the risk of cardiovascular and gastrointestinal side effects related, at least in part, to drug exposure.Citation11
The development of whole-blood assays of COX isozyme inhibition in vitro has proved useful for differentiating NSAIDs based on their pharmacodynamic features, such as COX-2 versus COX-1 selectivity (ie, experimental COX isoenzyme selectivity),Citation26–Citation28 while ex vivo assessment after NSAID dosing allows evaluation of COX isozyme selectivity achieved at circulating drug levels. Additionally, the use of biochemical markers of COX isozyme inhibition in vivo, such as the assessment of major enzymatic urinary metabolites of the parent prostanoid, permits assessment of the actual inhibition of prostanoid biosynthesis following NSAID dosing. The use of these biomarkers of COX inhibition have provided a mechanistic interpretation of the efficacy, adverse events, and interindividual variability associated with NSAID treatment.Citation21,Citation22,Citation29–Citation31
This review provides an overview of these and other key aspects that differentiate orally administered NSAIDs, with a focus on the role of these factors in establishing the safety and tolerability of individual NSAIDs for analgesia. Characterization of the pharmacokinetic and pharmacodynamic differences between these compounds and their relationship with the administered oral dose may allow for better tailoring of NSAID therapy to individual patient needs.
Mechanism of action of NSAIDs: COX-1 and COX-2 inhibition
The anti-inflammatory, analgesic, and antipyretic activities of NSAIDs are mediated by their inhibition of prostanoid biosynthesis.Citation11,Citation32,Citation33 Prostanoids are synthesized from arachidonic acid, a fatty acid present in cell membranes as a phospholipid ester.Citation11,Citation19,Citation32 COX isozymes convert arachidonic acid first to prostaglandin (PG)G2 and then to PGH2, which undergoes a series of subsequent conversion reactions, ultimately producing five bioactive prostanoids, ie, PGD2, PGE2, PGF2α, PGI2 (prostacyclin), and thromboxane A2 (TxA2)Citation19. These bioactive prostanoids exhibit various cell-specific and tissue-specific activities through their interaction with different receptors, mediating a range of diverse, and often opposing, physiological and pathological processes, including induction and resolution of the inflammatory response, protection of and damage to the gastrointestinal mucosa, promotion and inhibition of blood clotting and atherosclerosis, and renal control of blood pressure and renal disease.Citation23,Citation34
As described previously, there are two distinct isoforms of COX: the constitutively expressed COX-1 isoform and the inducible COX-2 isoform.Citation11,Citation32,Citation35 COX-1 is present in the majority of cells and tissues, including the endothelium, monocytes, gastrointestinal epithelial cells, and platelets.Citation32 In contrast, COX-2 is constitutively expressed in only a few tissues.Citation32 However, expression of COX-2 is upregulated in a variety of cells and tissues, such as vascular endothelium, rheumatoid synovial endothelial cells, monocytes, and macrophages, during inflammation through the actions of various inflammatory mediators (eg, bacterial endotoxins, tumor necrosis factor α, interleukins); the increase in COX-2 protein levels is the primary driving force for enhanced production of prostanoids at inflammatory sites.Citation32,Citation35 Although COX-2 is the primary pathway, evidence suggests that COX-1 may also contribute to the initial phase of prostanoid-dependent pain and inflammation; in vivo, COX-1 is expressed, along with COX-2, in circulating inflammatory cells and in inflamed tissue.Citation19,Citation34 The roles of COX-1 and COX-2 in different systems are summarized in .
Figure 1 Mechanism of action of NSAIDs.
Abbreviations: COX, cyclooxygenase; cPGES, cytosolic PGE2 synthase; CRTH2, chemoattractant receptor–homologous molecule expressed on T helper 2 cells; DP, PGD2 receptor; EP, PGE receptor; FP, PGF receptor; GI, gastrointestinal; H-PGDS, hematopoietic PGD synthase; IP, PGI2 receptor; L-PGDS, lipocalin-type PGD synthase; mPGES, membrane-associated PGE2 synthase; PG, prostaglandin; PGFS, PGF synthase; PGIS, PGI2 synthase; tNSAIDs, traditional non-steroidal anti-inflammatory drugs; TP, TX receptor; TxA2, thromboxane A2; TXS, thromboxane synthase.
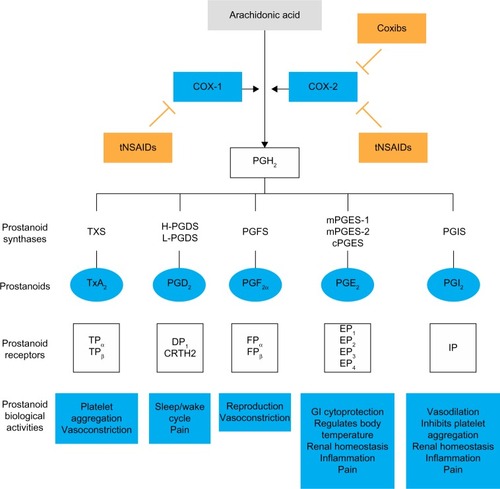
During the inflammatory response, COX-dependent prostanoids play a role in the development of hyperalgesia. PGE2 and PGI2 increase the sensitivity of pain receptors (or nociceptors) in the periphery and enhance the activity of various pain mediators.Citation34,Citation36 Peripheral inflammation is also associated with upregulation of COX-2 and an increase in PGE2 in the central nervous system, while contributing to the development of central hyperalgesia.Citation34 The role of COX-2 in the development of central hyperalgesia has been demonstrated in an engineered mouse model, in which conditional deletion of the gene for COX-2 in neurons and glial cells of the central nervous system resulted in the reduction of peripheral inflammation–induced COX-2 expression in the spinal cord and of mechanical hypersensitivity related to joint movement or tenderness to touch.Citation37
In general, NSAIDs inhibit prostanoid synthesis by competitive and transient inhibition of arachidonic acid binding to the COX active site.Citation19 The therapeutic effects of NSAIDs largely result from COX-2 inhibition at sites of inflammation, while many of the side effects associated with NSAIDs, particularly gastrointestinal side effects, are attributed to inhibition of the protective effects of prostanoids produced by COX-1.Citation11,Citation23,Citation33 Although all NSAIDs generally act by inhibition of arachidonic acid binding at COX, the mechanism of action of aspirin (acetylsalicylic acid) differs fundamentally from that of non-aspirin NSAIDs in that it causes an irreversible inactivation of COX-1 and COX-2 in most patients, preventing the oxidative conversion of arachidonic acid to PGG2 and PGH2 Citation38. In non-nucleated platelets, this effect confers persistent inhibition of COX-1–mediated TxA2 production and platelet function throughout the dosing interval.Citation38,Citation39 Acetaminophen also differs from other NSAIDs in that it has no measurable anti-inflammatory effects at therapeutic doses,Citation40 but primarily inhibits prostanoid-mediated hyperalgesia through suppression of PGE2 biosynthesis.Citation40–Citation42
Inhibition of prostanoid biosynthesis by NSAIDs is responsible for the therapeutic activities of these drugs;Citation11,Citation32 however, this inhibitory activity also suppresses other effects of these prostanoids, resulting in the side effects associated with NSAID treatment.Citation22,Citation23,Citation33 For example, inhibition of PGE2 production may reduce hyperalgesia but may also reduce the tissue-protective effects of this prostanoid in various organs, including the gastrointestinal tract, vascular wall, kidney, and lung.Citation11
Pharmacokinetics and pharmacodynamics of NSAIDs
COX isozyme selectivity
Different NSAIDs display different levels of selectivity for the COX-1 and COX-2 isoforms in vitro as a consequence of the chemical features of the drug.Citation11,Citation21,Citation22,Citation33 Selectivity for the COX-1 and COX-2 isoforms, as well as the extent of inhibition reached after dosing with NSAIDs, can be determined using whole-blood assays.Citation27,Citation28,Citation43 Maximal platelet COX-1 activity is assessed via measurement of TxB2 (the inactive metabolite of TxA2, generated non-enzymatically) formed in whole blood allowed to clot for 1 hour at 37°C. This assay uses endogenously generated thrombin to release arachidonic acid from platelets; arachidonic acid is converted to PGH2 by COX-1 and then transformed to TxA2 by the activity of thromboxane synthase.Citation27,Citation28 To evaluate maximal monocyte COX-2 activity, PGE2 levels are measured in whole blood following 24 hours of stimulation with exogenously added lipopolysaccharide to induce COX-2 expression in circulating monocytes.Citation27
Several studies have been performed to define the relationships between the extent of inhibition of COX-1 and COX-2 in whole blood after dosing with NSAIDs (COX isozyme inhibition ex vivo) and the degree of inhibition of prostanoid biosynthesis in vivo by assessing urinary levels of the major enzymatic metabolite of TxB2, 11-dehydro-TxB2, and the major metabolite of prostacyclin, 2,3-dino-6-keto-PGF1α.Citation44,Citation45 The use of low-dose aspirin, which is a selective inhibitor of platelet COX-1, and coxibs has shown that 11-dehydro-TxB2 is primarily of platelet origin (~70%), while 2,3-dino-6-keto-PGF1α is primarily derived by vascular COX-2 (~60%). Importantly, inhibition of 11-dehydro-TxB2 after dosing with aspirin is initially detected when the maximal capacity of platelet COX-1 is reduced by >97%.Citation45 Thus, the relationship between inhibition of platelet TxB2 biosynthesis ex vivo and in vivo is non-linear ().Citation44 In contrast, the relationship between inhibition of COX-2 activity measured ex vivo and the reduction in PGI2 biosynthesis measured in vivo is apparently linear ().Citation19,Citation45
Figure 2 Selectivity of NSAIDs for COX-1 and COX-2.
Abbreviations: ASA, acetylsalicylic acid; COX, cyclooxygenase; IC50, half-maximal inhibitory concentration; NSAID, non-steroidal anti-inflammatory drug; PGI-M, 2,3-dino-6-keto-prostaglandin F1α; TxA2, thromboxane A2.
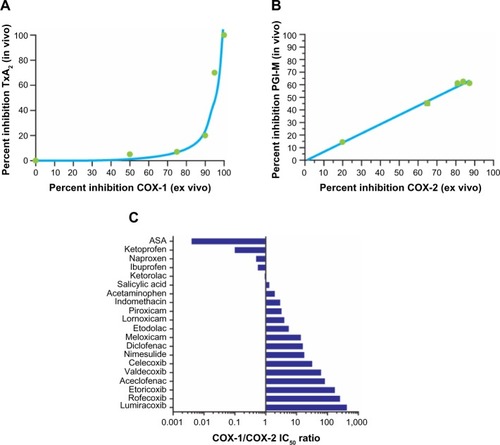
This knowledge allows prediction of the clinical consequences of COX isozyme inhibition by NSAIDs. NSAIDs may affect platelet function in vivo when the inhibition of platelet COX-1 ex vivo is >97%.Citation45 This effect occurs primarily with low-dose aspirin and persists throughout the dose interval. Naproxen, which is a reversible inhibitor of COX isozymes and has a higher potency for COX-1 than COX-2 and a long half-life (~14 hours), may have an aspirin-like effect on platelet function in some individuals when administered at high (500 mg twice daily) doses.Citation46 The use of whole-blood assays allows for characterization of COX isozyme selectivity of NSAIDs in vitro. Concentration-dependent relationships for inhibition of whole-blood COX-1 and COX-2 activities as a function of the addition of an NSAID in vitro allow for assessment of half-maximal inhibitory concentration (IC50) values for COX-1 and COX-2, as well as calculation of IC50 ratios for COX-1/COX-2. COX-2 selectivity assessed in human whole blood in vitro is a continuous variable, and it is not possible to separate traditional NSAIDs from coxibs ().Citation21,Citation27,Citation42,Citation47–Citation52
All NSAIDs are essentially COX-2 inhibitors with differing degrees of COX-1 inhibition as a “side effect”. From a clinical perspective, COX-2 selectivity can be considered as a variable describing the probability of sparing COX-1 activity and avoiding associated side effects (eg, in the gastrointestinal mucosa and platelets) at therapeutic concentrations of the NSAID.Citation53 Drugs that inhibit COX-1 and COX-2 with comparable potency (known as nonselective NSAIDs; eg, ibuprofen and ketoprofen) will not spare COX-1 activity after dosing, while drugs with intermediate COX-2 selectivity (eg, nimesulide, meloxicam, diclofenac, celecoxib) or highly selective COX-2 inhibitors (eg, rofecoxib, etoricoxib, lumiracoxib [not available in the United States]) have greater potential for sparing COX-1 activity.Citation23
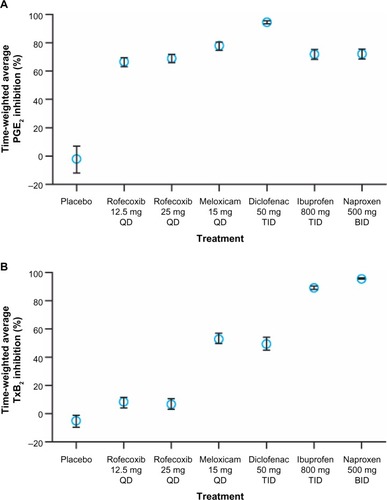
Although in vitro COX-2 selectivity is a chemical feature of the NSAID, COX-2 selectivity measured in whole blood following dosing depends on the dose administered.Citation23,Citation54 NSAIDs are administered at doses that are not bioequivalent with respect to the extent of inhibition of monocyte COX-2 (which represents a target for drug efficacy) obtained at maximal concentration of drug in the systemic circulation after dosing (Cmax). In particular, NSAIDs with short half-lives (eg, diclofenac) are often given at higher doses to extend their duration of clinical efficacy (analgesic or anti-inflammatory effect). As shown in , diclofenac 50 mg three times daily is associated with a higher inhibitory effect (>90% inhibition) over 8 hours following dosing compared with other NSAIDs.Citation54 Different NSAIDs are associated with a differential impact of platelet COX-1 (which represents an index of drug-associated side effects) depending upon their COX isozyme selectivity. Thus, administration of high-dose diclofenac and meloxicam, which have comparable and intermediate COX-2 selectivity in vitro, results in partial inhibition of COX-1. Further, the anti-inflammatory and full analgesic effects of NSAIDs are associated with plasma concentrations that inhibit whole-blood COX-2 activity by 80% (IC80).Citation55 Therefore, for drugs such as diclofenac, lowering the dose will lead to circulating concentrations near the COX-2 IC80 values, which correlate with adequate analgesia while reducing COX-1–mediated side effects.Citation23,Citation53
Figure 3 Average inhibition of whole-blood COX-2 activity (A) and COX-1 activity (B) by NSAIDs. Inhibition was observed over 8 hours following dosing with different NSAIDs.
Abbreviations: BID, twice-daily dosing; COX, cyclooxygenase; NSAID, non-steroidal anti-inflammatory drug; PGE2, prostaglandin E2; QD, once-daily dosing; TID, dosing three times per day; TxB2, thromboxane B2.
Absorption, distribution, and elimination
In addition to dose, the therapeutic activity and side effects of NSAIDs depend on their absorption, distribution, and elimination; these pharmacokinetic parameters can differ substantially between different NSAIDs.Citation56 As with any analgesic class, the rate of absorption is a key factor in the selection of an NSAID; those with rapid absorption are preferable for most patients, particularly those with severe or acute pain.Citation56 For example, celecoxib, which has a relatively slow rate of absorption, can be administered at standard doses to effectively treat osteoarthritis pain, but is not ideal for treatment of acute pain as it takes considerable time for absorption and often requires a loading dose to achieve clinically meaningful analgesia.Citation56
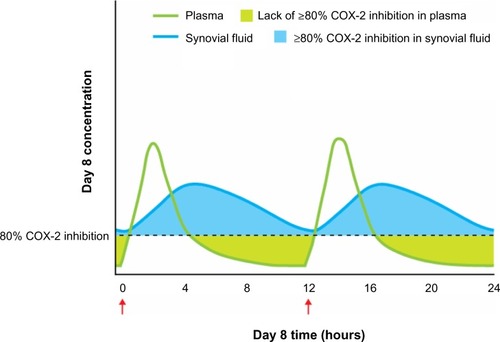
The distribution of NSAIDs in injured tissues, blood, and other areas of the body is a particularly crucial consideration for ensuring activity at the site of inflammation, as well as reducing the risk of side effects unrelated to therapeutic activity on COX enzymes throughout the body.Citation56,Citation57 NSAIDs can be classified as acidic or non-acidic based on their chemical structure, and the acidity of the drug can have an effect on its distribution. NSAIDs with acidic functional groups (eg, diclofenac, ibuprofen, ketoprofen) and with a high degree of protein binding have been shown to selectively accumulate and persist at sites of inflammation,Citation56–Citation59 while non-acidic NSAIDs (eg, acetaminophen, celecoxib, rofecoxib) tend to be distributed homogenously throughout the body.Citation56 Acidic NSAIDs with a high degree of protein binding may remain in inflamed tissues or synovial fluid for a longer time than in the plasma (). For example, diclofenac 75 mg (enteric-coated) reaches peak concentrations in the synovial fluid that exceed plasma concentrations at 4 hours after oral administration in patients with arthritis;Citation60 these high and persistent synovial levels are maintained above or near the expected IC80 for COX-2 for up to 12 hours after dosing, with rapid disappearance of diclofenac from the plasma, even after repeated dosing.Citation60 The persistence of certain NSAIDs (eg, diclofenac, ibuprofen) in the synovial fluid is associated with a sustained therapeutic effect despite relatively rapid clearance from the plasma, vascular wall, and kidneys, indicating that accumulation at sites of inflammation may allow for continued anti-inflammatory and analgesic activity.Citation57 The localization of COX activity to inflamed tissue and the resulting ability to use lower doses than might otherwise be required also minimizes COX inhibition at sites associated with potential side effects (eg, hepatic, renal, cardiovascular, and gastrointestinal adverse effects).Citation57,Citation58
Figure 4 NSAID concentration in inflamed tissue/synovial fluid and plasma.
Abbreviations: COX, cyclooxygenase; NSAID, non-steroidal anti-inflammatory drug.
The accumulation of NSAIDs at sites of inflammation and accompanying therapeutic effects are also affected by their pharmacokinetic properties (including extended-release or immediate-release formulations). Acidic NSAIDs with short plasma half-lives (eg, diclofenac, ibuprofen, ketoprofen) may be associated with tolerability benefits compared with drugs with a long plasma half-life due to rapid clearance from the plasma and non-targeted tissues, allowing for recovery of COX activity in other tissues (eg, production of vasoprotective prostanoids by endothelial COX-2) even while the drug continues to actively inhibit COX-2 at sites of inflammation. The use of excessively high doses or slow-release formulations of these analgesics could negate these benefits.Citation21,Citation29,Citation56,Citation58 Further studies using in vivo markers of COX-2 inhibition (such as urinary metabolites of PGE2 and PGI2) should ideally be conducted to verify this hypothesis. Persistence in the plasma is also a key factor with respect to the gastrointestinal safety and tolerability of NSAIDs.Citation61,Citation62
Interactions of NSAIDs with other drugs
NSAIDs are also differentiated by their potential for pharmacodynamic interactions with other drugs, such as aspirin, corticosteroids, and selective serotonin reuptake inhibitors, which are often administered concomitantly for different reasons.Citation56,Citation63,Citation64 Aspirin irreversibly acetylates the COX active site, preventing generation of TxA2, a prothrombotic prostanoid involved in platelet activation and aggregation.Citation38,Citation39 NSAIDs characterized by a high affinity for platelet COX-1 (eg, ibuprofen, naproxen) may interfere with the antiplatelet effects of aspirin; in contrast, this effect has not been detected with NSAIDs having intermediate or high selectivity for COX-2.Citation65–Citation67 Moreover, no interaction has been detected with low-dose paracetamol.Citation66 Although further randomized controlled trials are needed to confirm the clinical relevance of these pharmacodynamic interactions, an analysis of the medical records of 7,107 patients with cardiovascular disease who received aspirin alone or aspirin plus an NSAID indicated that the risks of all-cause mortality and cardiovascular-related mortality were significantly higher for patients who received aspirin plus ibuprofen compared with those who received aspirin alone (P<0.05), but not for patients who received aspirin plus diclofenac or aspirin plus any other NSAID (drugs not specified).Citation68
NSAID therapy in patients taking anticoagulant therapy with coumarins (vitamin K antagonists) should also be approached with caution. Coadministration of NSAIDs with coumarins may be associated with an increased risk of bleeding complications, particularly for naproxen.Citation69 The increased risk of bleeding associated with concomitant use of NSAIDs and coumarins (eg, warfarin) results from displacement of the anticoagulant from plasma proteins by the NSAID, thereby increasing the plasma concentration of free coumarin and associated anticoagulant activity and bleeding risk.Citation70
NSAID safety and tolerability
Gastrointestinal safety and tolerability
Gastrointestinal complications are among the most common and well-known side effects of NSAID treatment and generally result from inhibition of COX-1.Citation11,Citation32 COX-1 is expressed throughout the gastrointestinal tract and mediates the production of mucosal PGs that confer a number of protective effects, including increasing blood flow to the mucosa and promoting mucus and bicarbonate secretion;Citation11,Citation32 inhibition of COX-1 may therefore result in an increased susceptibility for mucosal damage (eg, bleeding).Citation11,Citation23,Citation32 Additionally, COX-2 may promote healing of gastric lesions; thus, inhibition of COX-2 may also play a role in the formation of ulcers.Citation23
A recent meta-analysis of data for more than 220,000 patients from 280 placebo-controlled trials of NSAIDs showed that all evaluated NSAIDs (coxibs, diclofenac, ibuprofen, and naproxen) increased the early risk of upper gastrointestinal complications (eg, ulcer perforations, bleeding, obstructions) to some extent.Citation20 There is, however, considerable variability in the risk of gastrointestinal complications between NSAIDs.Citation29,Citation71,Citation72 Results of a 2012 meta-analysis of 28 observational studies showed a low risk (relative risk [RR] <2) of upper gastrointestinal complications for aceclofenac, celecoxib, and ibuprofen; intermediate risk (RR 2–4) for diclofenac, meloxicam, and ketoprofen, among others; high risk (RR 4–5) for tenoxicam, naproxen, indomethacin, and diflunisal; and the highest risk (RR >5) for piroxicam, ketorolac, and azapropazone.Citation71 In keeping with results for individual NSAIDs, drugs that have greater selectivity for COX-2 than COX-1 generally have been associated with a lower risk of upper gastrointestinal complications than other NSAIDs.Citation20,Citation29,Citation73 In the recent meta-analysis of data from more than 220,000 patients, the predicted absolute annual risk of upper gastrointestinal complications was lower for NSAIDs with greater COX-2 selectivity (coxibs and diclofenac) compared with non-selective NSAIDs ().Citation20
Figure 5 Predicted annual absolute risks of major vascular events or upper gastrointestinal complications with long-term, high-dose therapy.
Abbreviations: COX, cyclooxygenase; NSAIDs, non-steroidal anti-inflammatory drug; pa, per annum; SE, standard error.
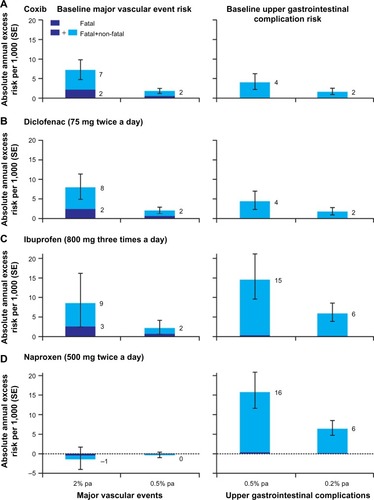
Drugs with a long plasma half-life and/or slow-release formulations have been associated with an increased risk of upper gastrointestinal bleeding, likely due to persistent exposure of the gastrointestinal tract to circulating NSAIDs.Citation29 In addition, the use of high daily doses of NSAIDs is also associated with a two- to three-fold increase in the risk of upper gastrointestinal complications relative to low daily doses,Citation71 possibly due to dose-related effects on the levels of COX-1 and COX-2 inhibition.Citation54,Citation74 Because both COX-1 and COX-2 are associated with the production of cytoprotective prostanoids, simultaneous and sustained inhibition of both COX isozymes by NSAIDs may translate into a profound suppression of these prostanoids and promote damage to the gastrointestinal tract.Citation23,Citation29
Cardiovascular safety and tolerability
Although aspirin confers cardioprotective effects, most other NSAIDs are associated with an increased risk of cardiovascular adverse events, including hypertension, myocardial infarction, stroke, and heart failure.Citation10,Citation11,Citation75–Citation77 In the meta-analysis of data from more than 220,000 patients, a significant increase in the risk of major vascular events was observed for high doses of coxibs and diclofenac, and was largely related to increased risk of major coronary events; in contrast, neither ibuprofen or naproxen were associated with an increased risk of major vascular events, although ibuprofen was associated with an increase in major coronary events comparable with that of coxibs and diclofenac ().Citation20 The cardiovascular safety findings for diclofenac may reflect the use of doses above the IC80 for COX-2 (typically 150 mg/day).Citation20 At lower doses, there is little evidence for an increased risk of cardiovascular events with diclofenac.Citation78 A recent meta-analysis of cardiovascular risk and total daily diclofenac dose from eleven studies demonstrated a linear relationship between diclofenac dose and risk of experiencing a cardiovascular event, with only a small (although significant) increase in risk observed with low doses of diclofenac compared with not taking an NSAID.Citation79
Like the risk of gastrointestinal complications, the risk of cardiovascular complications can be affected by drug exposure.Citation21,Citation77 In a 2008 observational study, the risk of myocardial infarction was increased with higher doses and with exposure to slow-release formulations of NSAIDs, even after adjusting for the dose.Citation21 Based on these and other safety findings, the American Heart Association recommends patients take the lowest effective dose of NSAIDs for the shortest duration of time.Citation25
The cardiovascular risk conferred by non-aspirin NSAIDs is likely associated with inhibition of COX-2–mediated production of PGI2, which has a cardioprotective role in the circulatory system, promoting vasodilation, preventing platelet activation and cell adhesion,Citation22,Citation23 and counteracting the action of TxA2, as well as several other stimuli.Citation22 The antiplatelet effects of aspirin are mediated by irreversible inhibition of COX-1, resulting in profound and persistent reduction of TxA2 production and TxA2-dependent platelet activation (for several days after aspirin administration).Citation38,Citation66 However, for most other traditional NSAIDs and coxibs, COX-1 inhibition is only transient and insufficient to translate into inhibition of atherothrombosis.Citation23 The exception is naproxen, which has a long half-life and potently inhibits platelet COX-1 activity sufficiently to prevent platelet aggregation at high doses;Citation44,Citation80 however, unlike aspirin, naproxen is a reversible inhibitor of COX-1 with variable effects across dosing intervals.Citation80 Moreover, naproxen profoundly affects systemic PGI2 biosynthesis, while aspirin (at a low dosage) has only a marginal effect on biosynthesis of PGI2.Citation80 Thus, as shown in the clinical data, naproxen is neutral with regard to cardiovascular risk, while aspirin is associated with a protective role.Citation19
Biomarkers to predict drug responses
The use of biochemical markers of COX inhibition may assist clinicians in the rational selection of an appropriate NSAID dose to achieve efficacy; this dose should be the lowest effective dose to limit the risk of adverse events.Citation25 However, differences in the efficacy and tolerability of NSAIDs have been observed between individuals,Citation21,Citation29,Citation30,Citation47 resulting from genetic factors that affect the pharmacokinetics and pharmacodynamics of NSAIDs ().Citation26 For example, cytochrome (CYP) enzymes are responsible for the metabolism of approximately 70%–80% of all drugs, including several NSAIDs,Citation81,Citation82 and interindividual differences in CYP450 expression can therefore result in altered metabolism of some NSAIDs.Citation26 A number of single-nucleotide polymorphisms in the genetic sequence of CYP2C9 have been described,Citation83 and the product of one of these altered genes, CYP2C9*2, is associated with reduced metabolism of celecoxib and an accompanying increase in CYP2C9*2 plasma concentration within 4 hours of administration in healthy individuals.Citation30
Figure 6 Determinants and sources of variability in the individual response to an NSAID.
Abbreviations: ASA, acetylsalicylic acid; COX, cyclooxygenase; CV, cardiovascular; CYP, cytochrome enzymes; GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug; PU, peptic ulcer; SNP, single-nucleotide polymorphism.
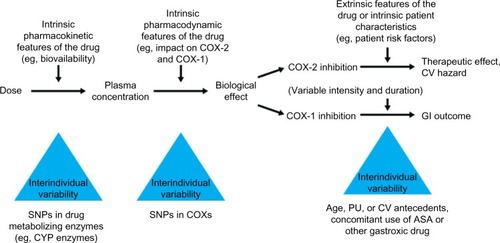
In addition to interindividual variability in COX inhibition, the individual’s clinical background, particularly gastrointestinal and cardiovascular risk factors, should also be considered as important predictors of the risk of adverse reactions due to NSAIDs. Together, these factors should be used to select an NSAID and dose that will be both effective and well tolerated,Citation26,Citation55 and to help guide individualized treatment strategies.
Studies are ongoing to verify the feasibility of using of biochemical markers of COX isozyme inhibition ex vivo and in vivo together with genetic biomarkers to identify individuals at accelerated risk of developing cardiovascular and gastrointestinal adverse events by NSAID administration.
Conclusion
NSAIDs are among the most commonly prescribed and used medications worldwide.Citation9,Citation10 However, they are associated with safety and tolerability concerns.Citation23 There are a number of factors that need to be considered in the selection of an NSAID, including its potency, selectivity for the COX-1 and COX-2 isoforms, pharmacokinetic properties, pharmacodynamic interactions, and the overall impact of these features on tolerability and safety. In addition to the gastrointestinal and cardiovascular safety of NSAIDs, the negative effects of inhibiting prostanoid production on other body systems should be considered. Emerging factors, including biomarkers, may assist clinicians in the selection of appropriate doses and types of NSAIDs. Finally, the individual’s clinical background, particularly gastrointestinal and cardiovascular risk factors, should be considered as important determinants that may exacerbate adverse reactions to NSAIDs. The risk factors for cardiovascular and gastrointestinal side effects of NSAIDs are summarized in . NSAIDs should be used with caution in patients with risk factors for atherothrombotic events. The long-term use of higher doses of NSAIDs represents an independent risk factor for cardiovascular and gastrointestinal adverse events;Citation25,Citation84 therefore, the lowest dose of an NSAID should be used, particularly in patients with other known risk factors.Citation25
Table 1 Risk factors for NSAID gastrointestinal and cardiovascular side effects
Current knowledge of factors affecting the safety and tolerability of NSAIDs, including the impact of selectivity, dose, and pharmacokinetics, is being used to guide the development of novel formulations of NSAIDs that address some of these tolerability concerns. For example, a novel immediate-release diclofenac formulation, which has a short half-life, acidic profile, and COX-2 selectivity, has been developed that allows for the use of lower doses (thus reducing systemic exposure and the potential for adverse events) together with rapid attainment of Cmax, providing sustained analgesia with a rapid onset.Citation85 Strategies to mitigate the risks and maximize the therapeutic benefits associated with NSAIDs should continue to be employed, such as use of the lowest effective dose for the shortest period of time, use of immediate-release formulations, avoidance of known drug interactions (eg, concomitant use with corticosteroids, low-dose aspirin, or other antiplatelet/anticoagulation events), and limited use of NSAIDs with high gastrointestinal toxicity.Citation23,Citation86,Citation87 Additional strategies for minimizing the risks associated with NSAIDs in individuals with gastrointestinal and/or cardiovascular risk factors are summarized in .
Table 2 Prevention strategies in patients with cardiovascular and/or gastrointestinal risk factors treated with NSAIDs
Author contributions
KB and PP developed the concepts for the article, provided evaluation of relevant literature information, revised the manuscript critically for intellectual content, and approved the final version of the manuscript for publication.
Disclosures
Editorial support for the writing of this manuscript was provided by Kimberly Brooks, PhD, of SciFluent, and was funded by Iroko Pharmaceuticals, LLC. The authors retained full editorial control over the content of the manuscript. KB has provided consulting services to and received honoraria from Iroko Pharmaceuticals. PP has received grants from Associazione Italiana per la Ricerca sul Cancro, Ministero dell’Istruzione, dell’Università e della Ricerca; has received speaker fees from Bayer and Iroko Pharmaceuticals; and has provided consulting services to and received honoraria from Iroko Pharmaceuticals.
References
- Committee on Advancing Pain Research, Care, and Education; Institute of MedicineRelieving Pain in AmericaWashington, DC, USANational Academies Press2011
- ReidKJHarkerJBalaMMEpidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impactCurr Med Res Opin201127244946221194394
- TsangAVon KorffMLeeSCommon chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disordersJ Pain200891088389118602869
- LeadleyRMArmstrongNLeeYCAllenAKleijnenJChronic diseases in the European Union: the prevalence and health cost implications of chronic painJ Pain Palliat Care Pharmacother201226431032523216170
- EadHImproving pain management for critically ill and injured patientsDynamics2005163263117725266
- McCarbergBHNicholsonBDToddKHPalmerTPenlesLThe impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet surveyAm J Ther200815431232018645331
- American Society of Anesthesiologists Task Force on Acute Pain ManagementPractice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain ManagementAnesthesiology2012116224827322227789
- American Society of Anesthesiologists Task Force on Chronic Pain ManagementAmerican Society of Regional Anesthesia and Pain MedicinePractice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain MedicineAnesthesiology2010112481083320124882
- BreivikHCollettBVentafriddaVCohenRGallacherDSurvey of chronic pain in Europe: prevalence, impact on daily life, and treatmentEur J Pain200610428733316095934
- ConaghanPGA turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicityRheumatol Int20123261491150222193214
- AtchisonJWHerndonCMRusieENSAIDs for musculoskeletal pain management: current perspectives and novel strategies to improve safetyJ Manag Care Pharm2013199 Suppl AS3S1924261788
- ChouRQaseemASnowVDiagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain SocietyAnn Intern Med2007147747849117909209
- HochbergMCAltmanRDAprilKTAmerican College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and kneeArthritis Care Res (Hoboken)201264446547422563589
- National Institute for Health and Care ExcellenceClinical guideline 88. Low back pain: early management of persistent non-specific low back pain2009 Available from: http://www.nice.org.uk/guidance/cg88Accessed November 29, 2014
- National Institute for Health and Care ExcellenceClinical guideline 177. Osteoarthritis: care and management in adults2014 Available from: http://www.nice.org.uk/guidance/cg59Accessed Novemeber 29, 2014
- WilliamsCMMaherCGLatimerJEfficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trialLancet201438499541586159625064594
- DohertyMHawkeyCGoulderMGibbIHillNAspleySReaderSA randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee painAnn Rheum Dis20117091534154121804100
- BruneKHinzBThe discovery and development of antiinflammatory drugsArthritis Rheum20045082391239915334450
- RicciottiEFitzgeraldGAProstaglandins and inflammationArterioscler Thromb Vasc Biol2011315986100021508345
- BhalaNEmbersonJMerhiAVascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trialsLancet2013382989476977923726390
- García RodríguezLATacconelliSPatrignaniPRole of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general populationJ Am Coll Cardiol200852201628163618992652
- GrosserTFriesSFitzgeraldGABiological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunitiesJ Clin Invest2006116141516395396
- PatrignaniPTacconelliSBrunoASostresCLanasAManaging the adverse effects of nonsteroidal anti-inflammatory drugsExpert Rev Clin Pharmacol20114560562122114888
- KorotkovaMJakobssonPJCharacterization of microsomal prostaglandin E synthase 1 inhibitorsBasic Clin Pharmacol Toxicol20141141646924138533
- AntmanEMBennettJSDaughertyAFurbergCRobertsHTaubertKAUse of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart AssociationCirculation2007115121634164217325246
- BrunoATacconelliSPatrignaniPVariability in the response to non-steroidal anti-inflammatory drugs: mechanisms and perspectivesBasic Clin Pharmacol Toxicol20141141566323953622
- PatrignaniPPanaraMRGrecoABiochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthasesJ Pharmacol Exp Ther19942713170517127996488
- PatronoCCiabattoniGPincaELow dose aspirin and inhibition of thromboxane B2 production in healthy subjectsThromb Res1980173–43173277368167
- Masso GonzalezELPatrignaniPTacconelliSGarcía RodríguezLAVariability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleedingArthritis Rheum20106261592160120178131
- FriesSGrosserTPriceTSMarked interindividual variability in the response to selective inhibitors of cyclooxygenase-2Gastroenterology20061301556416401468
- PatronoCPatrignaniPGarcía RodríguezLACyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outsJ Clin Invest2001108171311435450
- RaoPKnausEEEvolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyondJ Pharm Pharm Sci200811281s110s19203472
- FitzgeraldGAPatronoCThe coxibs, selective inhibitors of cyclooxygenase-2N Engl J Med2001345643344211496855
- SmythEMGrosserTWangMYuYFitzgeraldGAProstanoids in health and diseaseJ Lipid Res200950SupplS423S42819095631
- SimmonsDLBottingRMHlaTCyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibitionPharmacol Rev200456338743715317910
- MurataTUshikubiFMatsuokaTAltered pain perception and inflammatory response in mice lacking prostacyclin receptorNature199738866436786829262402
- VardehDWangDCostiganMCOX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in miceJ Clin Invest2009119228729419127021
- MeekILvan de LaarMAVonkemanHENon-steroidal anti-inflammatory drugs: an overview of cardiovascular risksPharmaceuticals2010321462162
- PatronoCBaigentCHirshJRothGAntiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th edChest20081336 Suppl199S233S18574266
- HinzBChereminaOBruneKAcetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in manFASEB J200822238339017884974
- AndersonBJParacetamol (acetaminophen): mechanisms of actionPaediatr Anaesth2008181091592118811827
- SciulliMGSetaFTacconelliSEffects of acetaminophen on constitutive and inducible prostanoid biosynthesis in human blood cellsBr J Pharmacol2003138463464112598417
- BlainHBoileauCLapicqueFLimitation of the in vitro whole blood assay for predicting the COX selectivity of NSAIDs in clinical useBr J Clin Pharmacol200253325526511874389
- ReillyIAFitzgeraldGAInhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugsBlood19876911801863790723
- CaponeMLTacconelliSSciulliMGHuman pharmacology of naproxen sodiumJ Pharmacol Exp Ther2007322245346017473175
- KearneyPMBaigentCGodwinJHallsHEmbersonJRPatronoCDo selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trialsBMJ200633275531302130816740558
- PanaraMRRendaGSciulliMGDose-dependent inhibition of platelet cyclooxygenase-1 and monocyte cyclooxygenase-2 by meloxicam in healthy subjectsJ Pharmacol Exp Ther1999290127628010381787
- DovizioMBrunoATacconelliSPatrignaniPMode of action of aspirin as a chemopreventive agentRecent Results Cancer Res2013191396522893199
- PanaraMRPadovanoRSciulliMGEffects of nimesulide on constitutive and inducible prostanoid biosynthesis in human beingsClin Pharmacol Ther19986366726819663182
- PatrignaniPPanaraMRSciulliMGSantiniGRendaGPatronoCDifferential inhibition of human prostaglandin endoperoxide synthase-1 and -2 by nonsteroidal anti-inflammatory drugsJ Physiol Pharmacol19974846236319444611
- TacconelliSCaponeMLSciulliMGRicciottiEPatrignaniPThe biochemical selectivity of novel COX-2 inhibitors in whole blood assays of COX-isozyme activityCurr Med Res Opin200218850351112564662
- PatrignaniPCampestriniJBransonJLumiracoxib is a selective inhibitor of cyclooxygenase-2 in patients with osteoarthritisAnn Rheum Dis2004631419142615020310
- CaponeMLTacconelliSDi FrancescoLSacchettiASciulliMGPatrignaniPPharmacodynamic of cyclooxygenase inhibitors in humansProstaglandins Other Lipid Mediat2007821–4859417164136
- Van HeckenASchwartzJIDepreMComparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteersJ Clin Pharmacol200040101109112011028250
- HuntjensDRDanhofMla PasquaOEPharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitorsRheumatology (Oxford)200544784685915855183
- BruneKRennerBHinzBUsing pharmacokinetic principles to optimize pain therapyNat Rev Rheumatol201061058959820820196
- BruneKFurstDECombining enzyme specificity and tissue selectivity of cyclooxygenase inhibitors: towards better tolerability?Rheumatology (Oxford)200746691191917459958
- BruneKPersistence of NSAIDs at effect sites and rapid disappearance from side-effect compartments contributes to tolerabilityCurr Med Res Opin200723122985299517949535
- RolfCEngstromBBeauchardCJacobsLDLe LibouxAIntra-articular absorption and distribution of ketoprofen after topical plaster application and oral intake in 100 patients undergoing knee arthroscopyRheumatology (Oxford)199938656456710402079
- BensonMDAldo-BensonMBrandtKDSynovial fluid concentrations of diclofenac in patients with rheumatoid arthritis or osteoarthritisSemin Arthritis Rheum1985152 Suppl 165674081792
- GoldsteinJLEisenGMLewisBGralnekIMZlotnickSFortJGVideo capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placeboClin Gastroenterol Hepatol20053213314115704047
- HawkeyCBurnettIGoldMSEndoscopic evaluation of the gastro-duodenal tolerance of short-term analgesic treatment with 25 mg diclofenac-K liquid capsulesAliment Pharmacol Ther201235781982722372517
- MortJRAparasuRRBaerRKInteraction between selective serotonin reuptake inhibitors and nonsteroidal antiinflammatory drugs: review of the literaturePharmacotherapy20062691307131316945053
- VerbeeckRKPharmacokinetic drug interactions with nonsteroidal anti-inflammatory drugsClin Pharmacokinet199019144662199127
- AnzellottiPCaponeMLJeyamALow-dose naproxen interferes with the antiplatelet effects of aspirin in healthy subjects: recommendations to minimize the functional consequencesArthritis Rheum201163385085921360514
- Catella-LawsonFReillyMPKapoorSCCyclooxygenase inhibitors and the antiplatelet effects of aspirinN Engl J Med2001345251809181711752357
- RendaGTacconelliSCaponeMLCelecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart diseaseClin Pharmacol Ther200680326427416952493
- MacDonaldTMWeiLEffect of ibuprofen on cardioprotective effect of aspirinLancet2003361935757357412598144
- Penning-van BeestFErkensJPetersenKUKoelzHRHeringsRMain comedications associated with major bleeding during anticoagulant therapy with coumarinsEur J Clin Pharmacol2005615–643944415947920
- DeviAMVargheseAKSriramSEvaluation of in vitro interactions of warfarin and duloxetine with selected coadministered nsaids in bovine serum albuminInt J Pharm Pharm Sci201131197199
- CastellsagueJRiera-GuardiaNCalingaertBIndividual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project)Drug Saf201235121127114623137151
- García RodríguezLAJickHRisk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugsLancet199434389007697727907735
- García RodríguezLABarrealesTLRisk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general populationGastroenterology2007132249850617258728
- HinzBBruneKCan drug removals involving cyclooxygenase-2 inhibitors be avoided? A plea for human pharmacologyTrends Pharmacol Sci200829839139718606461
- YuYRicciottiEGrosserTFitzgeraldGAThe translational therapeutics of prostaglandin inhibition in atherothrombosisJ Thromb Haemost20097Suppl 122222619630805
- McGettiganPHenryDCardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2JAMA2006296131633164416968831
- McGettiganPHenryDCardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studiesPLoS Med201189e100109821980265
- BruneKDiclofenac: increase of myocardial infarctions at low doses?Pharmacoepidemiol Drug Saf201423332632824596323
- OdomDMMladsiDMSaagKGRelationship between diclofenac dose and risk of gastrointestinal and cardiovascular events: meta-regression based on two systematic literature reviewsClin Ther201436690691724863260
- CaponeMLTacconelliSSciulliMGClinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjectsCirculation2004109121468147115037526
- GuzeyCSpigsetOGenotyping as a tool to predict adverse drug reactionsCurr Top Med Chem20044131411142115379654
- ZangerUMSchwabMCytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variationPharmacol Ther2013138110314123333322
- The Human Cytochrome P450 (CYP) Allele Nomenclature CommitteeThe human cytochrome P450 (CYP) allele nomenclature database2014 Available from: http://www.cypalleles.ki.se/Accessed November 29, 2014
- WolfeMMLichtensteinDRSinghGGastrointestinal toxicity of nonsteroidal antiinflammatory drugsN Engl J Med1999340241888189910369853
- GibofskyASilbersteinSArgoffCDanielsSJensenSYoungCLLower-dose diclofenac submicron particle capsules provide early and sustained acute patient pain relief in a phase 3 studyPostgrad Med2013125513013824113671
- American College of RheumatologyRecommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis GuidelinesArthritis Rheum20004391905191511014340
- BhattDLScheimanJAbrahamNSACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus DocumentsCirculation2008118181894190918836135
- JusticeECarruthersDMCardiovascular risk and COX-2 inhibition in rheumatological practiceJ Hum Hypertens20051911515385947
- DixonDABlancoFFBrunoAPatrignaniPMechanistic aspects of COX-2 expression in colorectal neoplasiaRecent Results Cancer Res201319173722893198
- MarnettLJThe COXIB experience: a look in the rearview mirrorAnnu Rev Pharmacol Toxicol20094926529018851701

