Abstract
Increasing evidence highlights bipolar disorder as being associated with impaired neurogenesis, cellular plasticity, and resiliency, as well as with cell atrophy or loss in specific brain regions. This has led most recent research to focus on the possible neuroprotective effects of medications, and particularly interesting findings have emerged for lithium. A growing body of evidence from preclinical in vitro and in vivo studies has in fact documented its neuroprotective effects from different insults acting on cellular signaling pathways, both preventing apoptosis and increasing neurotrophins and cell-survival molecules. Furthermore, positive effects of lithium on neurogenesis, brain remodeling, angiogenesis, mesenchymal stem cells functioning, and inflammation have been revealed, with a key role played through the inhibition of the glycogen synthase kinase-3, a serine/threonine kinase implicated in the pathogenesis of many neuropsychiatric disorders. These recent evidences suggest the potential utility of lithium in the treatment of neurodegenerative diseases, neurodevelopmental disorders, and hypoxic–ischemic/traumatic brain injury, with positive results at even lower lithium doses than those traditionally considered to be antimanic. The aim of this review is to briefly summarize the potential benefits of lithium salts on neuroprotection and neuroregeneration, emphasizing preclinical and clinical evidence suggesting new therapeutic potentials of this drug beyond its mood stabilizing properties.
Introduction
Lithium is still considered a first-line therapy for both the acute and long-term treatment of bipolar disorder (BD),Citation1 due to its well-documented antimanic,Citation2 antisuicidal,Citation3–Citation5 and prophylactic propertiesCitation6,Citation7 and the adjunctive treatment of major depression.Citation8 Although all potential mechanisms underlying lithium mood-stabilizing properties have not yet been completely clarified, a growing body of evidence has recently suggested the potential benefits on neuroprotection and neuroplasticity of this drug.
There is now evidence that BD is related to progressive impairment in neurocognitive functioning, as well as to regional impairments in neuroplasticity, cellular resilience, and adult neurogenesis.Citation9–Citation15 Morphometric brain-imaging studies, in fact, have documented cell atrophy or loss in several brain areas, especially prefrontal regions (eg, anterior cingulated and subgenual prefrontal cortex), as well as hippocampal volume decrease and smaller amygdalar volumes, in both adult and pediatric BD patients compared with healthy control subjects.Citation16–Citation20 Such regional brain abnormalities have been suggested to play a role in the pathophysiology of BD, giving rise to the debate as to whether they derive from neurodevelopmental, neurodegenerative, or combined deficits.Citation10,Citation21,Citation22
Consistently, most recent research studies have progressively focused on the possible neuroprotective effects of psychotropic medications for BD, and interesting results have particularly emerged for lithium salts.
This review aims to briefly summarize the most recent evidence on lithium benefits beyond its mood-stabilizing properties, with particular attention to its potential effects on neuroprotection and neuroregeneration, in both preclinical and clinical studies, highlighting possible new therapeutic potentials of this old drug.
Methodology
MEDLINE/PubMed (1985–2015) articles published in English were identified using the following keyword combinations: lithium, bipolar disorder, neuroprotection, neurogenesis, neurodegenerative disorders, neurodevelopmental disorders, autism, and glycogen synthase kinase-3 (GSK-3).
Neuroimaging outcomes of lithium treatment in BD
Several neuroimaging studies in BD patients have shown lithium treatment to increase cortical gray matter volumes, especially in the anterior cingulate and in the paralimbic cortices that are implicated in attention, motivation, and emotional modulation, as well as the hippocampus volume.Citation18,Citation19,Citation23–Citation30 The association between lithium treatment and gray matter volume increase has been demonstrated regardless of the mood state, the diagnostic subtype, and presence or absence of concomitant medications.Citation31
However, the nature and mechanisms of this positive association remain to be clarified in terms of whether these may depend on a direct effect of lithium on brain structures, through its action on the biochemical pathways involved in neurogenesis and apoptosis, or whether these are an epiphenomenon of the acute or prophylactic lithium efficacy. In a neuroimaging study using voxel-based morphometry, Hajek et alCitation32 recently demonstrated that the positive association between lithium treatment and increase in hippocampal volume seems to be independent of long-term treatment response, as occurred also in those BD patients on lithium who reported episodes of illness while on treatment. Additional evidence comes from studies using quantitative proton magnetic resonance spectroscopy to measure cortex levels of N-acetyl-aspartate (NAA), a marker of neuronal viability and/or functioning, in BD patients. Chronic lithium treatment was found to increase NAA levels in both BD patients and healthy controls.Citation33,Citation34 In a study comparing two groups of BD patients with a similar illness burden but differing lithium levels of exposure with respect to healthy control subjects, prefrontal NAA levels were found to be lower in patients without or with limited lifetime exposure to lithium compared with those under ongoing lithium treatment or controls.Citation35 Furthermore, these two latter groups of participants did not differ in terms of NAA levels; a negative correlation between prefrontal NAA and duration of illness was found only in the group of patients not exposed or with limited exposure to lithium. This lithium effect on NAA levels was suggested to be related to the expansion of neuropil content, accordingly with the evidence of lithium-induced increase in gray matter volumes.
Since the neuroprotective effects of lithium on brain structures have been shown even in healthy subjects and appear to be independent from prophylactic treatment response, Hajek and WeinerCitation31 recently hypothesized that lithium treatment might help maintain brain health even in patients without BD and could possibly demonstrate disease-modifying properties in neurodegenerative disorders. In this regard, Forlenza et alCitation36 evaluated whether the neurobiological properties of lithium reflect in increased regional brain glucose metabolism with the aid of 18 fluorine-fluoro-2-deoxy-d-glucose positron emission tomography in a sample of 19 older adults with amnestic mild cognitive impairment. The exposure to subtherapeutic levels of lithium carbonate (0.25–0.5 mEq/L) for 4 years in 12 of these patients was associated with a significant reduction in the glucose uptake in several clusters of the cerebellum and in both hippocampi, in comparison with matched controls. These results do not support the hypothesis that lithium might increase regional brain metabolism as a consequence of its neurotrophic effects. However, none of the patients in the sample reported any clinically relevant signs of lithium toxicity. Future studies with appropriate methodology are needed to address the clinical implications of these findings, particularly in light of the potential use of lithium as a disease-modifying treatment approach for certain neurodegenerative disorders.
Neuroprotective effects of lithium: preclinical evidence and molecular mechanisms
A number of preclinical in vitro and in vivo studies have documented a neuroprotective effect of lithium against neuronal injury caused by various noxious insults, mainly acted by preventing apoptosis and increasing neurotrophins excretion.Citation9,Citation13,Citation37–Citation40 Additional neuroprotective mechanisms include the modulation of autophagy and oxidative stress and the upregulation of mitochondrial function.Citation41 Furthermore, lithium appears to reduce proinflammatory status, by modulating inflammatory activity.Citation42–Citation46
In particular, lithium has been shown to inhibit GSK-3α and GSK-β; upregulate neurotrophins and cell survival molecules (eg, B-cell lymphoma 2 [Bcl-2], brain-derived neurotrophic factor [BDNF]/tropomyosin receptor kinase B [TrkB], cyclic adenosine monophosphate-responsive element-binding protein [CREB], heat shock protein 70 [Hsp70], and β-catenin); downregulate proapoptotic activities (eg, excitotoxicity, p53, Bcl-2-associated X protein, caspase, cytochrome c release, β-amyloid peptide production, and tau hyperphosphorylation); inactivate N-methyl-d-aspartate (NMDA) receptors; inhibit inositol monophosphatase (IMP); and activate the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) cell survival pathway.Citation38,Citation47–Citation56 Such a wide range of intracellular responses involved in the neuroprotective action of lithium may be secondary to its inhibitory effect on two key targets, namely, GSK-3β and IMP. The modification of these intracellular pathways is relevant to the understanding of the pathogenesis of certain neuropsychiatric and neurodegenerative diseases ().Citation41
Figure 1 A schematic illustration of neurotrophic and neuroprotective mechanisms targeted by lithium.
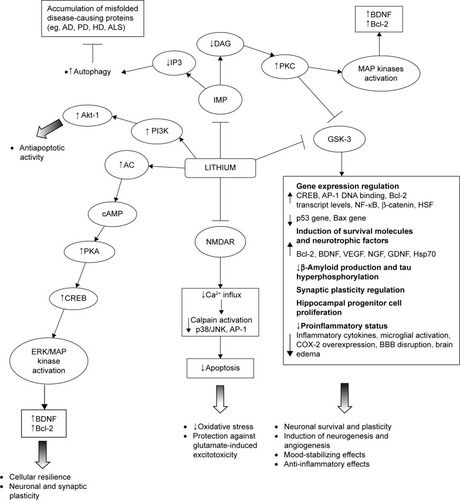
Lithium inhibition of the GSK-3, both direct binding to the magnesium-sensitive site of the enzyme and indirect, enhancing the phosphorylation of specific N-terminal serine residues, has been demonstrated to play a major role for lithium neuroprotective functions.Citation53,Citation57 There is also evidence of lithium modulation of GSK-3β expression at the transcriptional level in a dose-dependent manner, as a consequence of the inhibition of mRNA transcription.Citation58 The GSK-3 is a serine/threonine kinase that regulates several cellular processes, such as glycogen synthesis, gene transcription, synaptic plasticity, cell apoptosis, cellular structure and resilience, and the circadian cycle.Citation53 Its activation functionally inhibits CREB, β-catenin, and other survival-promoting transcription factors. Conversely, its inactivation has been suggested to promote neuronal survival and to improve cell structural stability directly influencing gene transcription.Citation50,Citation59–Citation61 In addition, GSK-3 directly regulates several neurotransmitter systems, including serotonergic, dopaminergic, cholinergic, and glutamatergic.Citation62,Citation63 Because of changes in these neurotransmitter systems, dysregulated GSK-3 has been linked to depression, BD, and schizophrenia.
The GSK-3 is also implicated in the pathogenesis of many neuropsychiatric disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), spinocerebellar ataxia type 1 (SCA1), multiple sclerosis (MS), fragile X syndrome (FXS), Down syndrome (DS), traumatic brain injury (TBI), and ischemic stroke.Citation40,Citation64–Citation72 It represents the predominant kinase in the brain responsible for the aberrantly hyperphosphorylation of the tau protein and also plays a major role in the amyloid deposition affecting the core pathological features of AD.Citation68,Citation73,Citation74 Therapeutic concentrations of lithium, through the inhibition of the GSK-3, have been demonstrated to significantly lower the levels of the tau phosphorylation in living cells and neurons, thus reducing the levels of aggregated, insoluble tau.Citation75,Citation76 Further, they have been demonstrated to block both the production of amyloid-beta peptides, by interfering with the amyloid precursor protein cleavage, and the accumulation of amyloid-beta peptides in the brain of mice that overproduce amyloid precursor protein.Citation76
Both the downregulation of GSK-3β and the elevated β-catenin, two components of the canonical Wnt signaling pathway, were implicated in adult hippocampal progenitor cell proliferation triggered by lithium treatment.Citation77 The GSK-3 may regulate cognitive functions by influencing the components of synaptic plasticity that are long-term depression (LTD) and long-term potentiation (LTP), involved in the regulation of learning and memory.Citation78,Citation79 This kinase is a key regulator of the balance between pro- and anti-inflammatory cytokine production in both the peripheral and the central nervous system and influences T-cell proliferation, differentiation and survival, so that its inhibition determines anti-inflammatory effects.Citation42 Administration of GSK-3 inhibitors in mice has been shown to control several inflammatory and immune conditions in both the periphery and the central nervous system.Citation42 The inhibition of GSK-3, as well as its genetic inactivation, has also produced antidepressive-like behaviors and strong anti-manic-like effects in a wide range of animal models.Citation80–Citation87 According to this, a deficient inhibition of GSK-3 has been linked to an increased susceptibility to mood disorders.Citation87 The finding that transgenic mice overexpressing β-catenin demonstrate behavioral changes comparable to those observed after lithium administration gives further support to the notion that the therapeutic effects of lithium are mediated by the inhibition of GSK-3 and the consequent increase in β-catenin levels.Citation88
As mentioned earlier, chronic inhibition of GSK-3 may mediate the neuroprotective effects of lithium in the long term by stimulating the gene and protein expression of antiapoptotic factors (eg, CREB, nuclear factor kappa-light-chain-enhancer of activated B-cells [NF-κB], Bcl-2, BDNF, β-catenin).Citation81,Citation89 In particular, preclinical studies in rat brains and human neurons showed chronic lithium administration to induce prominent neuroprotective and neurotrophic proteins, such as Bcl-2 and BDNF.Citation48,Citation51,Citation90,Citation91 The Bcl-2 not only is a major antiapoptotic protein but also stimulates axonal regeneration following injury.Citation55,Citation92 The upregulation of Bcl-2 by lithium has been postulated to account for the neuropil expansion manifesting as an increase in gray matter volume in the human brain.Citation18,Citation55 Interestingly, also the chronic administration of low lithium doses, producing plasma levels of ~0.35 mM, showed to robustly increase Bcl-2 levels in the frontal cortex and hippocampus of rats.Citation93 The BDNF is a neurotrophin playing a critical role in cortical development, synaptic plasticity, neuronal differentiation, and survival.Citation21,Citation94 It has been demonstrated to be involved in the pathophysiology of mental disorders, with decreased peripheral levels being reported during the acute manic, mixed and depressive episodes of BD,Citation95–Citation99 as well as in patients with chronic post-traumatic stress disorder (PTSD)Citation100 or schizophrenia.Citation101 Treatment with therapeutic concentrations of lithium and valproic acid is known to selectively increase the levels of exon IV-containing BDNF mRNA and the activity of BDNF promoter IV,Citation94 as well as to induce the subsequent activation of BDNF receptor (TrkB) in cultured rat cortical neurons.Citation38,Citation51 Consistently, elevated BDNF levels were reported in the hippocampus and in the frontal and temporal cortex of rat brains after chronic treatment with lithium and valproate.Citation90,Citation102–Citation104 Clinical studies showed lithium to restore and even increase BDNF levels in patients with BD,Citation105 and a relationship between lithium prophylactic efficacy and such levels was reported in a sample of euthymic bipolar patients.Citation106 Lithium has also been shown, in in vivo and in vitro preclinical studies, to increase the expression of other neurotrophins involved in neuronal survival and plasticity, such as the nerve growth factor, the glial cell line-derived neurotrophic factor, and the vascular endothelial growth factor (VEGF).Citation107–Citation112
Some evidence demonstrates that lithium produces neurotrophic effects affecting cyclic adenosine monophosphate-mediated signal transduction. This action is mainly exerted elevating basal adenylyl cyclase activity, but also reducing receptor-stimulated responses, as demonstrated in both preclinical and clinical studies.Citation60,Citation113,Citation114 The physiologic effects of cyclic adenosine monophosphate are primarily mediated by the activation of protein kinase A, one of whose major targets in the central nervous system is CREB, a transcription factor that plays a major role in mediating adaptive responses at glutamatergic synapses, as well as in the neuroprotective effects of neurotrophins and in long-term neuroplasticity.Citation60 Indeed, the activation of the extracellular-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway via CREB initiates the transcription of BDNF and also induces Bcl-2 expression. At therapeutically relevant concentrations, both lithium and valproate have been shown to activate the ERK/MAPK cascade in human neuroblastoma SH-SY5Y cells in vitro,Citation115 as well as in hippocampus and frontal cortex areas of the rodent brain,Citation103 thus producing neurotrophic effects.
Experimental studies also found chronic lithium administration to provide nearly complete protection against glutamate-induced, NMDA receptor (NMDAR)-mediated excitotoxicity in primary cerebellar, cerebral cortical, and hippocampal neuronal cultures.Citation52,Citation116,Citation117 Excessive NMDA throughput has been, in fact, hypothesized to be involved in stress-induced hippocampal atrophy and implicated in the pathogenesis of various neurodegenerative diseases.Citation38,Citation116,Citation118 This neuroprotective effect was obtained also with low lithium doses (0.1–0.6 mM) and was associated with the inhibition of NMDAR-mediated calcium influx.Citation38,Citation52 Additional evidence indicates that the lithium mechanism of action results from the attenuation of constitutive phosphorylation of the N-methyl D-aspartate receptor subtype 2B (NR2B) subunit of the NMDAR, which is catalyzed by Fyn, a member of the Src tyrosine kinase family.Citation52,Citation119 Brain ischemia is known to increase Src-mediated tyrosine phosphorylation of NR2A, as well as the interaction of NR2A with Src and Fyn, which is mediated by postsynaptic density protein 95.Citation120,Citation121 Lithium was shown to reduce NR2A phosphorylation and its postsynaptic density protein 95-mediated interactions with Src and Fyn in rat hippocampus following cerebral ischemia.Citation122 Furthermore, chronic lithium treatment antagonizes glutamate-induced activation of c-Jun-N-terminal kinase, p38 kinase, and activator protein-1 transcription factor binding, which has a major role in cytotoxicity, and suppresses glutamate-induced decrease of the CREB phosphorylation in cultured cerebellar granule neurons.Citation38,Citation54
According to the inositol depletion hypothesis,Citation123 the phosphatidylinositol (PI) pathway has also been proposed as a major target through which lithium could exert its therapeutic effects. As with GSK-3, lithium directly inhibits IMP and inositol polyphosphate-1 activity by the competitive displacement of Mg2+ from the catalytic site of the enzyme.Citation56 The inhibition of IMP and inositol polyphosphate-1 prevents the reuptake of inositol, leading to depletion of intracellular levels and subsequent inhibition of the phosphoinositol cycle.Citation41 The decrease in free-myoinositol levels and, subsequently in the production of diacylglycerol induced by the inhibition of IMP, has the downstream effect of decreasing protein kinase C levels and activity.Citation60 The reduction of protein kinase C levels and activity was demonstrated in cells as well as in rodents after chronic lithium treatment, with implication on neuroplastic events.Citation124 Moreover, the inhibition of protein kinase C has been suggested as a mechanism producing antimanic effects.Citation60,Citation125
More recently, the ability of lithium to inhibit the activity of IMP and subsequently decrease inositol 1,4,5-triphosphate levels was identified as a novel mechanism for inducing autophagy.Citation126–Citation129 Autophagy, a physiological process for degrading cytoplasmic proteins or organelles, has been recognized as a principal response to cellular stress and an important regulator of neuronal function and survival.Citation129 It is believed to be particularly beneficial as a “quality control” process in neurodegenerative disorders characterized by the accumulation of misfolded disease-causing proteins (eg, AD, PD, Huntington’s disease [HD], amyotrophic lateral sclerosis [ALS]).Citation130–Citation132
The inhibition of the two main targets of lithium, IMP, and GSK-3β, shows opposite effects on autophagy, that is modulated in a dose-dependent manner: lithium induces inhibition of IMP at lower doses (Ki ≈0.8 mM), enhancing autophagy,Citation126 while it induces inhibition of GSK-3β at higher doses (Ki ≈2 mM), downregulating autophagy via activation of mammalian target of rapamycin (mTOR).Citation118,Citation133
The inhibition of PI3K, a kinase involved in cell survival pathways, has been found to block the activity of the anti-apoptotic serine-threonine Akt-1 kinase causing neuronal death, and this apoptotic pathway was prevented by chronic lithium exposure increasing PI3K activity in primary cerebellar neurons.Citation47 Acute lithium exposure also protected cortical neurons via a PI3K-mediated increase in intracellular calcium through the phospholipase Cγ pathway.Citation134 Furthermore, in cultured human neuroblastoma SHSY5Y cells, lithium treatment ameliorated HIV-gp120-mediated toxicity via the PI3K/Akt pathway.Citation135
Recent evidence suggests that lithium treatment enhances the mitochondrial respiratory rate, reduces oxidative stress, protects DNA against damage from oxidative stress, and modulates calcium influx in the mitochondria.Citation60,Citation136–Citation139 Both lithium treatment of acute mania and clinical improvement of depressive symptoms after lithium administration were associated with a reduction of pro-oxidative stress markers.Citation12,Citation140 In addition, lithium showed to affect oxidative stress in healthy subjects increasing antioxidative and reducing pro-oxidative markers.Citation141 Nitric oxide (NO) is one of the most important intracellular second messenger activated by oxidative stress status. NMDAR activation can increase the activation of NO synthase, the enzyme responsible for NO production, and lithium treatment has recently been suggested to regulate this molecular mechanism.Citation142
Besides promoting neuronal survival after damage, lithium has been reported to promote hippocampal neurogenesis. In mammals, new neurons continue to be produced throughout adult life in the subventricular zone and the hippocampal dentate gyrus (DG).Citation143 Compared with preexisting mature dentate granule cells, newborn neurons exhibit peculiar electrophysiological features (ie, increased intrinsic excitability, enhanced synaptic plasticity, reduced sensitivity to GABAergic inhibition, and lower threshold for the induction of LTP) that showed to be essential for hippocampal function in the DG, an area primarily involved in cognitive processes.Citation144–Citation147 Impaired adult neurogenesis has been observed in various models relevant to neuropsychiatric disorders (ie, BD, major depression, schizophrenia, and AD) and neurodevelopmental disorders (ie, FXS and DS).Citation143,Citation148–Citation152 Recent data have correlated low proliferation and differentiation capacities of adult hippocampal stem cells with memory dysfunction,Citation152 suggesting adult neurogenesis might be a promising therapeutic target for disease-associated cognitive impairment. Several preclinical studies showed lithium to stimulate the proliferation and differentiation of neuronal progenitor cells, as well as their maturation and functioning.Citation153–Citation158
Lithium in neurodegenerative diseases
The risk of cognitive impairment and dementia has been reported to be higher in patients with BD than in the general population, correlating with the duration of illness, as well as with the number of illness episodes, especially the depressive ones, and the degree of impairment.Citation159,Citation160 Both preclinical and clinical studies have produced new evidence of lithium benefits in terms of preventing dementia through its action on GSK-3,Citation66,Citation161 leading to its investigation also in neurodegenerative disorders.
In a case–control study that compared AD prevalence rates among elderly euthymic bipolar patients on chronic lithium therapy with respect to those of similar patients who had not been treated with lithium recently, Nunes et alCitation162 showed lithium to be able to reduce AD prevalence to levels comparable with those of the general elderly population. Since the number of previous affective episodes was equivalent between the two patient groups, this putative neuroprotective effect was related to the intrinsic biological brain effects of lithium. Findings from two other observational studies corroborated the reduced risk of AD in BD patients on long-term lithium treatment.Citation163,Citation164 Conversely, this positive effect was not shown in patients taking anticonvulsants, antidepressants, or antipsychotics.Citation164
Evidence of benefits of lithium in preventing cognitive impairment in nonbipolar AD patients is mixed. A small, open-label study found no cognitive benefits of lithium carbonate administration for up to 1 year to 25 patients with mild to moderate AD.Citation165 In a randomized, single-blind, placebo-controlled, 10-week study of 71 patients with a mild form of AD, treatment with lithium at therapeutic levels (0.5–0.8 mmol/L) showed no significant benefits on either cognitive performance or cerebrospinal fluid concentrations of disease-related biomarkers.Citation166 On the contrary, in a subset of a greater sample recruited for a randomized, single-blind, placebo-controlled, parallel-group 10-week multicenter study, investigating the efficacy of lithium treatment in early AD patients, a significant increase of BDNF serum levels was shown.Citation167 In a 12-month, single-center, randomized, double-blind, placebo-controlled study, carried out on subjects with amnestic mild cognitive impairment,Citation168 chronic lithium administration was associated with a significant decrease in cerebrospinal fluid concentrations of P-tau and better performances on the cognitive subscale of the AD assessment scale and in attention tasks. The reduction of the cerebrospinal fluid P-tau was found in subjects with amnestic mild cognitive impairment who did not convert to AD, suggesting that this disease-modifying effect of lithium may be a useful parameter for predicting its property to prevent, or at least to delay, the progression from pre-dementia stage to clinical dementia. Lithium was administered at subtherapeutic levels (serum levels of 0.2–0.4 mmol/L), which resulted safe and well tolerated. In contrast with previous studies that failed to find a significant effect of lithium either on cognition or on AD-related biomarkers,Citation165,Citation166 this study included individuals without a manifest form of dementia. Finally, a more recent intriguing trial found beneficial effects of a lithium micro-dose of 300 μg administered once daily to AD patients for 15 months in preventing cognitive loss.Citation169 Overall, findings from clinical samples highlight preferential benefits of lithium in dementia prevention in patients with mood disorders and mild cognitive impairment, suggesting that long-term lithium treatment may have disease-modifying properties on the core pathophysiologic features of AD, mostly if started at the earlier stages of the disease process.Citation41,Citation168,Citation170
Lithium inhibition of the GSK-3 has also been hypothesized to produce neuroprotective effects in other neurodegenerative disease models, such as PD, tauopathies, HD, ALS, SCA1, and MS.Citation10,Citation40,Citation41,Citation65,Citation171–Citation173 Whether these pharmacodynamic properties of lithium can be translated into neurodegenerative diseases modifying effects in human subjects is a critical question.Citation174
Several preclinical studies have suggested that lithium can prevent neurodegeneration in vitro and in vivo models of PD.Citation67,Citation175–Citation177 Therapeutic concentrations of lithium have been shown to facilitate clearance of the mutant form of α-synuclein, one of the major components of Lewy bodies, inducing autophagic processes.Citation126,Citation177 Other preliminary data suggest that the antioxidant effects of lithium may be beneficial for PD by preventing free radical damage of dopaminergic neurons.Citation171 In a mouse model of PD, mice were fed with a diet containing 1.1, 2.2, 3.3, and 4.4 g/kg lithium chloride for 4 weeks prior to the injection of the parkinsonism neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).Citation178 The 3.3 g/kg lithium diet gave serum level value very similar to what is observed in lithium therapy in man and the 4.4 g/kg diet gave serum level values well above this. Results of this study showed chronic lithium diet to prevent the decrease of dopamine levels, as well as of tyrosine hydroxylase activity and protein levels in striatum. A direct relationship was established between serum lithium concentration and the prevention of MPTP-induced depletion of striatal dopamine. MPTP-treated mice showed significantly decreased striatal Bcl-2 levels, as opposed to increased Bcl-2-associated X protein levels, and lithium treatment was found to prevent such changes. Chen et alCitation179 demonstrated that 6-hydroxydopamine (6-OHDA), a PD mimetitc, activates GSK-3β in cultured human neuroblastoma SH-SY5Y cells, as well as in cultures of rat cerebellar granule neurons, inducing neuronal death. Lithium and other specific GSK-3β inhibitors showed to protect SH-SY5Y cells and cerebellar granule neurons from 6-OHDA-induced apoptosis. However, other studies have presented conflicting results, showing lithium to fail in the protection of dopaminergic neurons from 6-OHDA-induced degeneration in the substantia nigra of rats.Citation41,Citation180 To date, no clinical trials have been conducted in human patients to test these findings.
HD is a progressive, autosomal dominant neurodegenerative disorder caused by an expanded CAG repeats at exon 1 of the huntingtin (Htt) gene resulting in the aggregate formation of mutant huntingtin protein (mHtt).Citation181 Clinical presentation of HD includes psychiatric disturbances such as significant changes in personality or mood, cognitive decline that may gradually lead to dementia, and motor dysfunction characterized by chorea. Pathological processes and pathways involved in the development of HD include selective death/atrophy of medium-sized spiny neurons within the striatum and, to a lesser extent, loss of neuros in the cortex; dysregulation of cellular autophagy; mitochondrial dysfunction; decreased neurotrophic and growth factor levels; and transcriptional dysregulation and aberrant epigenetic modifications.Citation181 Presently no cure exists to treat or halt the progression of this devastating disease. On the basis of accumulating evidence indicating the promising role of lithium for the treatment of neurodegenerative diseases, its use has been investigated in cellular and animal HD models. Pre- and posttreatment with lithium chloride (2.5–5.0 mM) protected nonneuronal and neuronal cells transfected with mutant Htt exon 1 fragment containing 74 CAG repeats, increased cell survival, and decreased aggregate formation compared with nontreated group in both the cell types.Citation65 In a rat model of HD induced by quinolinic acid (QA) unilateral infusion into the striatum, subcutaneous injection of lithium chloride for 16 days prior to QA infusion significantly reduced the size of the excitotoxicity-induced striatal lesion. The lithium-elicited neuroprotective action was associated with an increase in Bcl-2 protein levels.Citation182 A subsequent study assessed the ability of lithium to reduce neurodegeneration and to stimulate cell proliferation in a rat model of HD induced by QA. LiCl (0.5–3.0 mEq/kg) was injected subcutaneously 24 hours before and 1 hour after QA infusion. Seven days after QA injection, lithium significantly diminished the loss of neurons, prevented apoptosis by attenuating QA-induced DNA damage and caspase-3 activation, upregulated Bcl-2 levels, and promoted neuronal and astroglial progenitor cell proliferation.Citation183 Combined treatment with therapeutic levels of lithium (1.0 mM) and valproate (0.5 mM) was found to be more effective than treatment with either drug alone in upregulating mRNA and protein levels of BDNF, as well as other neuroprotective proteins such as Hsp70 in the striatum and cortex, in two models of transgenic HD mice. Cotreatment with lithium and valproate also more effectively alleviated spontaneous locomotor deficits and depressive-like behaviors and markedly prolonged median survival of transgenic mice, compared with monotreatment.Citation65 Lithium use was also investigated in clinical trials of HD. Two recent clinical studies demonstrated lithium’s mood-stabilizing effects in HD patients of varying ages and duration of illness.Citation184,Citation185 The addition of lithium to tetrabenazine eliminated suicidal symptoms in all patients and improved depressive symptomts.Citation185 Moreover, it prevented the progression of chorea in three of these patients.Citation184 Despite these findings, clinical trials reported no benefits of lithium administration on improving choreiform movements.Citation181 However, it is important to notice that these studies may be inconclusive due to the methodological limitations and small sample size.
Evidence of the neuroprotective effects of lithium from ALS models is inconsistent.Citation186–Citation190 In mouse models of ALS, treatment with either lithium alone or in combination with an antioxidant has been shown to improve motor function and slow down disease progression.Citation189,Citation190 The mainly hypothesized mechanism for such improvement was the stimulation of autophagy by lithium.Citation186,Citation191 Combined treatment of ALS mice with lithium and valproate produced a greater and more consistent effect in delaying the onset of disease symptoms, prolonging the lifespan, and decreasing the neurological deficit scores, in comparison with monotreatment with either drug.Citation191 On the contrary, some preclinical studies reported no benefits of lithium administration in improving disease symptoms.Citation187,Citation188 In a small, 15-month pilot study on randomized ASL patients, a significant effect on disease progression and survival was reported in the lithium plus riluzole group (lithium plasma levels ranging from 0.4 to 0.8 mEq/L) compared with the riluzole only.Citation186 A subsequent randomized, double-blind, placebo-controlled trial showed no benefits of lithium (serum levels maintained between 0.4 and 0.8 mEq/L) plus riluzole combination in slowing down the progression of ALS comparing with riluzole alone.Citation192 More recently, a multicenter, double-blind, randomized, placebo-controlled trial (lithium carbonate in ALS trial) was designed to test the hypothesis that lithium may improve survival rates in ALS.Citation193 Oral doses of lithium carbonate (mean serum levels ranging from 0.4 to 0.8 mmol/L) or placebo were continuously administered for 18 months. Lithium treatment was shown to be safe, but study results did not support any evidence of lithium benefit on survival compared to placebo.Citation194,Citation195
Results from preclinical studies also suggest that lithium may be useful for therapeutic intervention in autoimmune and inflammatory diseases, such as MS, the most common inflammatory demyelinating disease of the central nervous system. Lithium pretreatment at therapeutic doses in experimental autoimmune encephalomyelitis models not only markedly suppressed the clinical symptoms of experimental autoimmune encephalomyelitis but also greatly reduced demyelination, microglia activation, and leukocyte infiltration in the spinal cord.Citation172
Finally, lithium efficacy was tested in a knock-in mouse model of SCA1 (Sca1[154Q/2Q] mice) that replicates many features of the human disease, a dominantly inherited neurodegenerative disorder characterized by progressive motor and cognitive dysfunction.Citation173 In this study, Sca1(154Q/2Q) mice and their wild-type littermates were fed either regular chow or chow that contained 0.2% lithium carbonate. Dietary lithium carbonate supplementation resulted in the improvement of motor coordination, learning, and memory in Sca1(154Q/2Q) mice, both presymptomatically and after symptom onset. Neuropathologically, lithium treatment improved hippocampal dendritic arborization in mutant hippocampal pyramidal neurons.
Lithium in neurodevelopmental disorders
Parallel to research on neurodegenerative disorders, lithium potential neuroprotective functions have been explored in neurodevelopmental disorders, with the strongest evidence emerging in preclinical studies of FXS, the most commonly inherited form of mental retardation and autism. FXS is caused by suppressed expression of the fragile X mental retardation protein,Citation196 an RNA-binding translational regulator important for establishing and maintaining functional neuronal networks.Citation197,Citation198 An altered synaptic plasticity, with pathologically enhanced metabotropic glutamate receptor (mGluR)-dependent LTD in the hippocampal CA1 neurons and deficits in NMDAR-dependent LTP at medial perforant path synapses in the DG, has been detected in mouse models of FXS (FX mice),Citation199–Citation201 suggesting enhanced mGluR signaling to be a major cause of this syndrome.Citation202 According to this, the inhibition of mGluR5 has been shown to reverse several behavioral and morphological phenotypes, including cognitive deficits, in both developing and adult FX mice.Citation203,Citation204 Overall, these findings established a clear link between synaptic dysfunction and learning deficits in FXS.
Furthermore, following the discovery that the GSK-3 activity and the mGluR5 signaling were coordinately elevated in the hippocampus, striatum, and cerebral cortices of FX mice, with the inhibition of mGluR5 leading to the inhibition of GSK-3, research studies investigated this kinase as a therapeutic target for the treatment of FXS.Citation205 According to this, GSK-3 has been identified as a bidirectional regulator of NMDAR-dependent synaptic plasticity, as LTP induction requiring the inhibition of GSK-3β, while GSK-3β activity is required for LTD induction.Citation206,Citation207
Lithium inhibition of GSK-3 was found to revert the altered phenotypes of FX mice, that is, to attenuate enhanced mGluR-mediated LTD, to ameliorate locomotor hyperactivity, audiogenic seizure hypersensitivity, increased spine density, excess protein synthesis, social behavior deficits, deficient passive avoidance learning and synaptic plasticity, and to normalize impaired hippocampus-dependent cognitive tasks.Citation205,Citation208–Citation214 To the best of our knowledge, only one small open-label trial has been conducted in FXS patients,Citation215 showing lithium treatment titrated to levels of 0.8–1.2 mEq/L to improve cognitive performance modifying the disrupted regulation of group 1 metabotropic glutamate receptor (mGluR and mGluR5)-dependent translation in dendrites.
Neuropathological alterations have also been described in autism spectrum disorders (ASDs). A preclinical study on neonatal rats isolated from their mother showed apparent autistic-like symptoms, such as social deficits, excessive repetitive self-grooming behavior, and increased anxiety-and depressive-like behaviors that were accompanied by impaired adult hippocampal neurogenesis, and enhanced basal inhibitory synaptic transmission in the hippocampal CA1 pyramidal neurons.Citation216 Chronic lithium administration was effective in ameliorating these pathological changes, completely overcoming autistic-like behaviors, and restoring adult hippocampal neurogenesis and the balance between excitatory and inhibitory synaptic activities to physiological levels, supporting the use of lithium as a potential therapeutic agent against ASDs. Interestingly, two recent case studies described the effects of lithium treatment in two patients diagnosed with ASDs in childhood and presented regression with progressive loss of verbal and motor skills, catatonic features, aggressive and impulsive behaviors, and sleep disturbances after a stressful event during adolescence.Citation217 They both presented a mutation/microdeletion of the SHANK3 gene on chromosome 22q13.3 that encodes a scaffold protein located at the glutamatergic synapses and involved in the regulation of the structural organization of dendritic spines and binding partner of neuroligins, accounting for approximately 2% of cases of intellectual disability in ASDs patients.Citation218 After deletion of SHANK3 gene, the nervous system is thought to become more susceptible to developmental problems and psychiatric disorders, less able to recover after psychiatric and somatic events and more vulnerable to degeneration in the long term.Citation219 Various psychotropic medications administered to the two patients (antipsychotics, benzodiazepines, antiepileptic drugs, antidepressants, and methylphenidate) failed to improve clinical symptoms and led to multiple side effects. In contrast, lithium therapy permitted reversibility of regression symptoms and catatonic features and the stabilization of behavioral disorders without significant side effects,Citation217 suggesting that SHANK3 patients with ASDs may represent a more vulnerable subgroup with an atypical form of BD,Citation219 in agreement with previous observations.Citation220,Citation221
Finally, lithium use has been explored in preclinical models of DS. DS is the leading genetic cause of intellectual disability and has been associated to impaired hippocampal neurogenesis. This latter plays a key role in establishing functional neuronal network and synaptic connectivity. The altered synaptic plasticity (eg, excitation/inhibition imbalance) discovered in mouse models of DS has been implicated in cognitive dysfunctions.Citation143,Citation222–Citation224 Recent studies showed that 1-month administration of lithium in the Ts65Dn mouse, a model of DS, was able to restore adult neurogenesis in the DG and subventricular zone to physiological levels by stimulating the proliferation of neuronal precursor cells through the pharmacological activation of the Wnt/β-catenin pathway.Citation143,Citation153 The restoration of adult neurogenesis completely rescued the synaptic plasticity of newborn neurons in the DG and led to the full recovery of behavioral performance in three distinct hippocampal-dependent cognitive tasks (contextual fear conditioning, object location, and novel object recognition).Citation143
Lithium in cerebral traumatic/ischemic injury
Emerging evidence from preclinical research suggests that lithium can mitigate neurological deficits incurred from traumatic or ischemic brain injury.Citation40,Citation70
The pathophysiology of TBI, after an initial mechanical injury that damages neurons, glia, and vascular structures, includes a cascade of secondary events that finally lead to neurodegeneration and loss of neurological functions: oxidative stress; excitotoxicity via excess glutamate release; increased NMDAR activation; neuroinflammation via proinflammatory cytokines, NO, or prostaglandins; mitochondrial disruption; failure of the blood–brain barrier (BBB) involving cerebral edema, hypoxia, and ischemia; and cellular death via necrosis and caspase-dependent (caspase-3) and/or caspase-independent apoptosis.Citation70,Citation225,Citation226 Neuropsychiatric sequelae such as depression, anxiety, and PTSD and behavioral and cognitive deficits accompany secondary injuries.Citation70 Patients afflicted by TBI show increased memory impairment, and increasing evidence suggests that TBI is a major risk factor for the development of AD.Citation227–Citation229
Given the neuroprotective effects of lithium, recent research has investigated its putative use in preclinical studies on TBI paradigms.Citation70 Lithium pretreatment was found to attenuate interleukin-1β expression, brain edema, hippocampal neurodegeneration, and loss of hemispheric tissues, improve memory and spatial learning, and alleviate depressive behaviors in mouse models of mild TBI.Citation230,Citation231 Postinjury injections with therapeutic doses of lithium also reduced lesion volume and attenuated TBI-induced neuroinflammation by inhibiting microglia activation and cyclooxygenase-2 induction, while BBB integrity was maintained through the inhibition of matrix metallopeptidase-9 expression. TBI-induced hyperlocomotor activity, anxiety-like behaviors, and impairments in motor coordination were all normalized by lithium treatment. In addition, GSK-3β phosphorylation was found to be robustly increased after postinjury administration of lithium, with subsequent β-catenin accumulation, and reduced neuronal loss in the hippocampal CA3 region, as well as decreased hippocampal-dependent deficits in learning and memory.Citation70,Citation232,Citation233
Similarly, GSK-3β has been strongly implicated in the pathogenesis of neuronal death caused by ischemic injury. While GSK-3β, in preclinical models of transient focal cerebral ischemia, was found to be activated inducing apoptotic cell death, its inactivation shortly after permanent focal or global cerebral ischemia was found to promote survival of vulnerable neurons, thus identifying GSK-3β inhibition as a major target for treatments.Citation49
Pretreatment with lithium and valproate was found to attenuate the hypoxia-induced serine dephosphorylation of GSK-3α and GSK-3β in mouse cerebral cortex, hippocampus, and striatum.Citation234 Consistently, in a model of neonatal rat hypoxic–ischemic brain injury, postinsult treatment with lithium therapeutic doses reduced the ischemia-induced dephosphorylation of GSK-3β and the extracellular signal-regulated kinase, the activation of calpain and caspase-3, the mitochondrial release of cytochrome c and apoptosis-inducing factor, as well as autophagy.Citation128 Lithium inhibition of GSK-3β is also accompanied by the upregulation of Bcl-2, increase in the DNA-binding activity of heat shock factor-1 to the heat-shock element, thus superinducing Hsp70, downregulation of p53, and decreased expression of Bcl-2-associated X protein in rat models of stroke.Citation235–Citation238 These findings highlight the prominent role of GSK-3β inhibition in the antiapoptotic effects of lithium under ischemic conditions.
Besides the inhibition of GSK-3, lithium showed to provide neuroprotection in rat hippocampus following cerebral ischemia through the downregulation of NMDAR function, overstimulated as a consequence of the glutamate extracellular accumulation.Citation122
Long-term lithium pretreatment at therapeutically relevant doses decreased brain infarct volume and apoptotic cell death and improved neurological deficits in a permanent rat model of brain focal ischemia.Citation238,Citation239 Moreover, it prevented ischemia-induced exploratory behavioral changes and memory impairments in an animal model of global cerebral ischemia.Citation235 These benefits were associated with an increase in the number of viable cells and a decrease in apoptotic cells in the CA1 hippocampal area of ischemic brain. Infarct volume, neurological deficits, and the number of neurons showing DNA damage in the ischemic brain were also reduced by postinsult subcutaneous injection of lithium at 0.5 mEq/kg in a rat ischemia/reperfusion model.Citation237
Lithium has been demonstrated to inhibit neuroinflammation resulting from ischemic brain injury by preventing inflammatory disorder of the hematoencephalic barrier and neurodegeneration.Citation233 Long-term lithium treatment of neonatal rats subjected to hypoxic–ischemic brain injury significantly reduced total tissue loss, at least partly by inhibiting microglia activation and attenuating proinflammatory cytokines (ie, interleukin-1β) or chemokine levels.Citation157 Overexpression of Hsp70, leading to the inactivation of the inflammatory transcription factor NF-κB,Citation240 has been proposed as a possible mechanism by which lithium exerts its anti-inflammatory activity in experimental stroke models.
The stimulation of hippocampal neurogenesis has also been implicated in the neuroprotective effect of lithium against brain ischemia.Citation157,Citation158 In addition, lithium treatment showed to improve stroke recovery in rats by augmenting neurovascular remodeling and vascular neoformation.Citation241 Specifically, it was found to increase the VEGF protein levels via a mechanism involving the PI3-kinase and GSK-3 signaling pathways in cultured rat brain endothelial cells.Citation109 Since VEGF has been linked to angiogenesis, neurogenesis, and neuroprotection,Citation242 this growth factor signaling mechanism may contribute to lithium’s reported ability to promote neurovascular remodeling after ischemic stroke.
Finally, studies on mesenchymal stem cells in rat models of brain ischemia showed that incubation with lithium chloride increased their migratory ability and homing to damaged tissues, reduced infarct volume, enhanced regenerative potential and angiogenesis in the infarcted penumbra regions, and improved functional recovery.Citation243,Citation244
Conclusion
Increasing evidence highlights BD, as well as other neuropsychiatric conditions, to be associated with morphological brain abnormalities, such as regional impairments in neuroplasticity, cellular resilience, and hippocampal neurogenesis, which highlight the role of neurodegeneration in BD. This new perspective has induced most recent research to focus on the possible neuroprotective effects of psychotropic medications, particularly those whose efficacy in the treatment of BD has been corroborated.Citation9–Citation11,Citation13–Citation15,Citation20,Citation245 Consistently, over the past few years, increasing promising results have emerged for lithium salts, a medication historically used to treat BD.
Since the GSK-3, the main target of lithium, plays a key role in the pathogenesis of many neuropsychiatric disorders, the therapeutic potentials of lithium have been investigated in various preclinical paradigms of different neurodegenerative diseases associated with cell atrophy/loss and impairment of cellular resilience, as well as, most recently, of neurodevelopmental disorders.Citation49,Citation117,Citation246–Citation248 There is now evidence that the neuroprotective benefits of this agent in the human brain are exerted through several complementary mechanisms leading to increased neuronal viability/function, enhanced angiogenesis and neurogenesis, BBB integrity maintenance, and anti-inflammation as well as disease-specific neuroprotective properties.Citation10 Thus, the findings reported in the present review provide intriguing support for the neurotrophic/neuroprotective effects of chronic treatment with lithium, suggesting possible implications for its use in other neuropsychiatric conditions, including those neurodevelopmental disorders, such as ASD, recently suggested as the possible common vulnerability factor for most mental disorders.Citation249,Citation250 The encouraging results reported so far suggest the need for further studies exploring the new therapeutic potentials of this old drug in clinical samples.
Acknowledgments
We thank Giulia Gray for the English language text revision.
Disclosure
The authors report no conflicts of interest in this work.
References
- YathamLNKennedySHParikhSVCanadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013Bipolar Disord201315114423237061
- YildizAVietaELeuchtSBaldessariniRJEfficacy of antimanic treatments: meta-analysis of randomized, controlled trialsNeuropsychopharmacology201136237538920980991
- BaldessariniRJTondoLDavisPPompiliMGoodwinFKHennenJDecreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic reviewBipolar Disord200685 Pt 262563917042835
- CiprianiAPrettyHHawtonKGeddesJRLithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trialsAm J Psychiatry2005162101805181916199826
- CiprianiAHawtonKStocktonSGeddesJRLithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysisBMC2013346f3646
- GeddesJRBurgessSHawtonKJamisonKGoodwinGMLong-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trialsAm J Psychiatry2004161221722214754766
- GrofPMüller-OerlinghausenBA critical appraisal of lithium’s efficacy and effectiveness: the last 60 yearsBipolar Disord200911Suppl 2S10S19
- NivoliAMColomFMurruANew treatment guidelines for acute bipolar depression: a systematic reviewJ Affect Disord20111291–3142620538341
- BauerMAldaMPrillerJYoungLTInternational Group for the Study of Lithium Treated Patients (IGSLI)Implications of the neuroprotective effects of lithium for the treatment of bipolar and neurodegenerative disordersPharmacopsychiatry200336Suppl 3S250S25414677087
- ChiuCTWangZHunsbergerJGChuangDMTherapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorderPharmacol Rev201365110514223300133
- ErikssonPSPerfilievaEBjörk-ErikssonTNeurogenesis in the adult human hippocampusNat Med1998411131313179809557
- DumanRSDepression: a case of neuronal life and death?Biol Psychiatry200456314014515271581
- Machado-VieiraRManjiHKZarateCAJrThe role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesisBipolar Disord200911Suppl 2S92S109
- Machado-VieiraRSoeiro-De-SouzaMGRichardsEMTeixeiraALZarateCAJrMultiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approachWorld J Biol Psychiatry2014152849523998912
- ZarateCAJrSinghJManjiHKCellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorderBiol Psychiatry200659111006102016487491
- ChangKKarchemskiyABarnea-GoralyNGarrettASimeonovaDIReissAReduced amygdalar gray matter volume in familial pediatric bipolar disorderJ Am Acad Child Adolesc Psychiatry200544656557315908839
- DrevetsWCPriceJLSimpsonJRSubgenual prefrontal cortex abnormalities in mood disordersNature199738666278248279126739
- MooreGJBebchukJMWildsIBChenGManjiHKLithium-induced increase in human brain grey matterLancet200035692371241124211072948
- SassiRBBrambillaPHatchJPReduced left anterior cingulate volumes in untreated bipolar patientsBiol Psychiatry200456746747515450781
- SelvarajSArnoneDJobDGrey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studiesBipolar Disord201214213514522420589
- AngelucciFBrenèSMathéAABDNF in schizophrenia, depression and corresponding animal modelsMol Psychiatry200510434535215655562
- MonkulESMalhiGSSoaresJCAnatomical MRI abnormalities in bipolar disorder: do they exist and do they progress?Aust N Z J Psychiatry200539422222615777357
- BeardenCEThompsonPMDalwaniMGreater cortical gray matter density in lithium-treated patients with bipolar disorderBiol Psychiatry200762171617240360
- DrevetsWCNeuroimaging and neuropathological studies of depression: implications for the cognitive emotional features of mood disordersCur Opin Neurobiol2001112240249
- HajekTKopecekMHöschlCAldaMSmaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysisJ Psychiatry Neurosci201237533334322498078
- HajekTCullisJNovakTHippocampal volumes in bipolar disorders: opposing effects of illness burden and lithium treatmentBipolar Disord201214326127022548899
- LyooIKDagerSRKimJELithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging studyNeuropsychopharmacology20103581743175020357761
- MooreGJCorteseBMGlitzDAA longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patientsJ Clin Psychiatry200970569970519389332
- YucelKMcKinnonMCTaylorVHBilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI studyPsychopharmacology (Berl)2007195335736717705060
- YucelKTaylorVHMcKinnonMCBilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatmentNeuropsychopharmacology200833236136717406649
- HajekTWeinerMWNeuroprotective effects of lithium in human brain? Food for thoughtCurr Alzheimer Res Epub2016218
- HajekTBauerMSimhandlCNeuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment responsePsychol Med201444350751723721695
- MooreGJBebchukJMHasanatKLithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects?Biol Psychiatry20004811810913502
- SilverstonePHWuRHO’DonnellTUlrichMAsgharSJHanstockCCChronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patientsInt Clin Psychopharmacol200382737912598817
- HajekTBauerMPfennigALarge positive effect of lithium on prefrontal cortex N-acetylaspartate in patients with bipolar disorder: 2-centre studyJ Psychiatry Neurosci201237318519222353634
- ForlenzaOVCoutinhoAMAprahamianILong-term lithium treatment reduces glucose metabolism in the cerebellum and hippocampus of nondemented older adults: an [¹8F]FDG-PET studyACS Chem Neurosci20145648448924730717
- CaminsAVerdaguerEJunyentFPotential mechanisms involved in the prevention of neurodegenerative diseases by lithiumCNS Neurosci Ther200915433334419889130
- ChuangDMNeuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases?Crit Rev Neurobiol2004161–2839015581403
- PlotnikovEYSilachevDNZorovaLDLithium salts – simple but magicBiochemistry (Mosc)201479874074925365484
- WadaAYokooHYanagitaTKobayashiHLithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseasesJ Pharmacol Sci200599430732116340157
- ForlenzaOVDe-PaulaVJDinizBSNeuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disordersACS Chem Neurosci20145644345024766396
- BeurelEMichalekSMJopeRSInnate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3)Trends Immunol2010311243119836308
- BosettiFRintalaJSeemannRChronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brainMol Psychiatry20027884585012232777
- GoldsteinBIKempDESoczynskaJKMcIntyreRSInflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literatureJ Clin Psychiatry20097081078109019497250
- NahmanSBelmakerRHAzabANEffects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cellsInnate Immun201218344745821994254
- RapaportMHManjiHKThe effects of lithium on ex vivo cytokine productionBiol Psychiatry200150321722411513821
- Chalecka-FranaszekEChuangDMLithium activates the serine/threonine kinase Akt-1 and suppresses glutamateinduced inhibition of Akt-1 activity in neuronsProc Natl Acad Sci U S A199996158745875010411946
- ChenRWChuangDMLong term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression: a prominent role in neuroprotection against excitotoxicityJ Biol Chem1999274106039604210037682
- ChuangDMWangZChiuCTGSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic strokeFront Mol Neurosci201141521886605
- GrimesCAJopeRSThe multifaceted roles of glycogen synthase kinase 3β in cellular signalingProg Neurobiol200165439142611527574
- HashimotoRTakeiNShimazuKChristLLuBChuangDMLithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicityNeuropharmacology20024371173117912504924
- HashimotoRHoughCNakazawaTYamamotoTChuangDMLithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylationJ Neurochem200280458959711841566
- JopeRSLithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomesTrends Biochem Sci2003249441443
- KopniskyKLChalecka-FranaszekEGonzalez-ZuluetaMChuangDMChronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activitiesNeuroscience2003116242543512559097
- ManjiHKMooreGJChenGLithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illnessJ Clin Psychiatry200061Suppl 9S82S96
- PatelSYenushLRodríguezPLSerranoRBlundellTLCrystal structure of an enzyme displaying both inositol-polyphosphate-1-phosphatase and 3′-phosphoadenosine-5′-phosphate phosphatase activities: a novel target of lithium therapyJ Mol Bio2002315467768511812139
- KleinPSMeltonDAA molecular mechanism for the effect of lithium on developmentProc Natl Acad Sci U S A19969316845584598710892
- MendesCTMuryFBde Sá MoreiraELithium reduces Gsk3b mRNA levels: implications for Alzheimer DiseaseEur Arch Psychiatry Clin Neurosci20092591162218932008
- LiangMHChuangDMRegulation and function of glycogen synthase kinase-3 isoforms in neuronal survivalJ Biol Chem200728263904391717148450
- QuirozJAMachado-VieiraRZarateCAJrManjiHKNovel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effectsNeuropsychobiology2010621506020453535
- ChinPCMajdzadehND’MelloSRInhibition of GSK3beta is a common event in neuroprotection by different survival factorsBrain Res Mol Brain Res20051371–219320115950778
- JopeRSRohMSGlycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventionsCurr Drug Targets20067111421143417100582
- BeaulieuJMGainetdinovRRCaronMGAkt/GSK3 signaling in the action of psychotropic drugsAnnu Rev Pharmacol Toxicol20094932734718928402
- AghdamSYBargerSWGlycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithiumCurr Alzheimer Res200741213117316163
- CarmichaelJSugarsKLBaoYPRubinszteinDCGlycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutationJ Biol Chem200227737337913379812097329
- DinizBSMachado-VieiraRForlenzaOVLithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disordersNeuropsychiatr Dis Treat2013949350023596350
- DukaTDukaVJoyceJNSidhuAAlpha-Synuclein contributes to GSK-3beta-catalyzed tau phosphorylation in Parkinson’s disease modelsFASEB J20092392820283019369384
- HooperCKillickRLovestoneSThe GSK3 hypothesis of Alzheimer’s diseaseJ Neurochem200810461433143918088381
- KohSHYooARChangDIHwangSJKimSHInhibition of GSK-3 reduces infarct volume and improves neurobehavioral functionsBiochem Biophys Res Commun2008371489489918477469
- LeedsPRYuFWangZA new avenue for lithium: intervention in traumatic brain injuryACS Chem Neurosci20145642243324697257
- LiDWLiuZQChenWYaoMLiGRAssociation of glycogen synthase kinase-3β with Parkinson’s disease (review)Mol Med Rep2014962043205024681994
- KingMKPardoMChengYDowneyKJopeRSBeurelEGlycogen synthase kinase-3 inhibitors: rescuers of cognitive impairmentsPharmacol Ther2014141111223916593
- MuyllaertDKremerAJaworskiTGlycogen synthase kinase-3b, or a link between amyloid and tau pathology?Genes Brain Behav20087Suppl 1S7S66
- PhielCJWilsonCALeeVMYKleinPSGSK-3α regulates production of Alzheimer’s disease amyloid-β peptidesNature2003423693843543912761548
- LovestoneSDavisDRWebsterMTLithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrationsBiol Psychiatry1999458995100310386182
- NobleWPlanelEZehrCInhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivoProc Natl Acad Sci U S A2005102196990699515867159
- WexlerEMGeschwindDHPalmerTDLithium regulates adult hippocampal progenitor development through canonical Wnt pathway activationMol Psychiatry200813328529217968353
- BradleyCAPeineauSTaghibiglouCA pivotal role of GSK-3 in synaptic plasticityFront Mol Neurosci201251322363262
- HooperCMarkevichVPlattnerFGlycogen synthase kinase-3 inhibition is integral to long-term potentiationEur J Neurosci2007251818617241269
- BeaulieuJMZhangXRodriguizRMRole of GSK3 beta in behavioral abnormalities induced by serotonin deficiencyProc Natl Acad Sci U S A200810541333133818212115
- Kaidanovich-BeilinOMilmanAWeizmanAPickCGEldar-FinkelmanHRapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on betacatenin in mouse hippocampusBiol Psychiatry200455878178415050857
- KalinichevMDawsonLAEvidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute maniaInt J Neuropsychopharmacol20111481051106721208504
- RoweMKWiestCChuangDMGSK-3 is a viable potential target for therapeutic intervention in bipolar disorderNeurosci Biobehav Rev200731692093117499358
- BeaulieuJMSotnikovaTDYaoWDLithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascadeProc Natl Acad Sci U S A2004101145099510415044694
- GouldTDEinatHBhatRManjiHKAR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim testInt J Neuropsychopharmacol20047438739015315719
- PrickaertsJMoecharsDCrynsKTransgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and maniaJ Neurosci200626359022902916943560
- Kaidanovich-BeilinOLipinaTVTakaoKAbnormalities in brain structure and behavior in GSK-3alpha mutant miceMol Brain200923519925672
- GouldTDEinatHO’DonnellKCBeta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviorsNeuropsychopharmacology200732102173218317299510
- GouldTDChenGManjiHKIn vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3Neuropsychopharmacology2004291323812942141
- FukumotoTMorinobuSOkamotoYKagayaAYamawakiSChronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brainPsychopharmacology2001158110010611685390
- ChenGZengWZYuanPXThe mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNSJ Neurochem19997228798829930766
- HuangXWuD-YChenGManjiHChenDFSupport of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2-dependent mechanismInvest Ophthalmol Vis Sci200344134735412506095
- GoodwinFKJamisonKRManic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders2nd edNew YorkOxford University Press2007
- YasudaSLiangMHMarinovaZYahyaviAChuangDMThe mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neuronsMol Psychiatry2009141515917925795
- Dell’OssoLDel DebbioAVeltriAAssociations between brain-derived neurotrophic factor plasma levels and severity of the illness, recurrence and symptoms in depressed patientsNeuropsychobiology201062420721220714169
- Machado-VieiraRDietrichMOLekeRDecreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episodeBiol Psychiatry200761214214416893527
- PiccinniAMarazzitiDCatenaMPlasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatmentsJ Affect Disord20081051–327928317553570
- PiccinniAVeltriACostanzoDDecreased plasma levels of brain-derived neurotrophic factor (BDNF) during mixed episodes of bipolar disorderJ Affect Disord201517116717025305432
- PostRMRole of BDNF in bipolar and unipolar disorder: clinical and theoretical implicationsJ Psychiatr Res2007411297999017239400
- Dell’OssoLCarmassiCDel DebbioABrain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorderProg Neuropsychopharmacol Biol Psychiatry200933589990219409951
- FernandesBSSteinerJBerkMPeripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implicationsMol Psychiatry20152091108111925266124
- FreyBNAndreazzaACCereserKMEffects of mood stabilizers on hippocampus BDNF levels in an animal model of maniaLife Sci200679328128616460767
- EinatHYuanPGouldTDThe role of the extracellular signal-regulated kinase signaling pathway in mood modulationJ Neurosci200323197311731612917364
- JacobsenJPMørkAThe effect of escitalopram, desipramine, electro-convulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levelsBrain Res200410241–218319215451381
- de SousaRTvan de BiltMTDinizBSLithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week studyNeurosci Lett20114941545621362460
- SuwalskaASobieskaMRybakowskiJKSerum brain-derived neurotrophic factor in euthymic bipolar patients on prophylactic lithium therapyNeuropsychobiology201062422923420714172
- AngelucciFAloeLJiménez-VasquezPMathéAALithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depressionInt J Neuropsychopharmacol20036322523112974988
- FreyBNAndreazzaACRosaARLithium increases nerve growth factor levels in the rat hippocampus in an animal model of maniaBehav Pharmacol200617431131816914949
- GuoSAraiKStrinsMFChuangDMLoEHLithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytesStroke200940265265518974377
- HellwegRLangUENagelMBaumgartnerASubchronic treatment with lithium increases nerve growth factor content in distinct brain regions of adult ratsMol Psychiatry20027660460812140783
- SilvaRMartinsLLongatto-FilhoALithium prevents stress-induced reduction of vascular endothelium growth factor levelsNeurosci Lett20074291333817980489
- Warner-SchmidtJLDumanRSVEGF as a potential target for therapeutic intervention in depressionCurr Opin Pharmacol200881141918061540
- JopeRSA bimodal model of the mechanism of action of lithiumMol Psychiatry199941212510089004
- GouldTDChenGManjiHKMood stabilizer psychopharmacologyClin Neurosci200223–4193212
- YuanPXHuangLDJiangYMGutkindJSManjiHKChenGThe mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growthJ Biol Chem200127634316743168311418608
- NonakaSHoughCJChuangDMChronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influxProc Natl Acad Sci U S A1998955264226479482940
- ChuangDMPrillerJPotential use of lithium in neurodegenerative disordersBauerMGrofPMuller-OerlinghausenBLithium in Neuropsychiatry: The Comprehensive GuideAbingdon, OxonInforma UK Ltd2006381398
- ChiuCTChuangDMMolecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disordersPharmacol Ther2010128228130420705090
- HashimotoRFujimakiKJeongMRChristLChuangDMLithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicityFEBS Lett20035381–314514812633868
- LiuYZhangGGaoCHouXNMDA receptor activation results in tyrosine phosphorylation of NMDA receptor subunit 2A(NR2A) and interaction of Pyk2 and Src with NR2A after transient cerebral ischemia and reperfusionBrain Res20019091–2515811478920
- HouXYZhangGYYanJZChenMLiuYActivation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemiaBrain Res20029551–212313212419528
- MaJZhangGYLithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemiaNeurosci Lett2003348318518912932824
- BerridgeMJDownesCPHanleyMRNeural and developmental actions of lithium: a unifying hypothesisCell19895934114192553271
- ManjiHKBersudskyYChenGBelmakerRHPotterWZModulation of protein kinase C isozymes and substrates by lithium: the role of myoinositolNeuropsychopharmacology19961543703818887991
- YildizAGuleryuzSAnkerstDPOngürDRenshawPFProtein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifenArch Gen Psychiatry200865325526318316672
- SarkarSFlotoRABergerZLithium induces autophagy by inhibiting inositol monophosphataseJ Cell Biol200517071101111116186256
- SarkarSRubinszteinDCInositol and IP3 levels regulate autophagy: biology and therapeutic speculationsAutophagy20062213213416874097
- LiQLiHRoughtonKLithium reduces apoptosis and autophagy after neonatal hypoxia-ischemiaCell Death Dis20101e5621364661
- ChiuCTChuangDMNeuroprotective action of lithium in disorders of the central nervous systemZhong Nan Da Xue Xue Bao Yi Xue Ban201136646147621743136
- WebbJLRavikumarBAtkinsJSkepperJNRubinszteinDCAlpha-Synuclein is degraded by both autophagy and the proteasomeJ Biol Chem200327827250092501312719433
- RavikumarBVacherCBergerZInhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington diseaseNat Genet200436658559515146184
- RubinszteinDCGestwickiJEMurphyLOKlionskyDJPotential therapeutic applications of autophagyNat Rev Drug Discov20076430431217396135
- SarkarSPerlsteinEOImarisioSSmall molecules enhance autophagy and reduce toxicity in Huntington’s disease modelsNat Chem Biol20073633133817486044
- KangHJNohJSBaeYSGwagBJCalcium-dependent prevention of neuronal apoptosis by lithium ion: essential role of phosphoinositide 3-kinase and phospholipase CgammaMol Pharmacol200364222823412869627
- EverallIPBellCMalloryMLithium ameliorates HIV-gp120-mediated neurotoxicityMol Cell Neurosci200221349350112498789
- ShalbuyevaNBrustovetskyTBrustovetskyNLithium desensitizes brain mitochondria to calcium, antagonizes permeability transition, and diminishes cytochrome C releaseJ Biol Chem200728225180571806817485418
- BachmannRFWangYYuanPCommon effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damageInt J Neuropsychopharmacol200912680582219149911
- BoscheBSchäferMGrafRHärtelFVSchäferUNollTLithium prevents early cytosolic calcium increase and secondary injurious calcium overload in glycolytically inhibited endothelial cellsBiochem Biophys Res Commun2013434226827223541580
- Ngok-NgamPWatcharasitPThiantanawatASatayavivadJPharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y cellsCell Mol Biol Lett2013181587423161404
- de SousaRTZarateCAJrZanettiMVOxidative stress in early stage bipolar disorder and the association with response to lithiumJ Psychiatr Res201450364124332923
- KhairovaRPawarRSalvadoreGEffects of lithium on oxidative stress parameters in healthy subjectsMol Med Rep20125368068222200861
- GhasemiMDehpourARThe NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithiumTrends Pharmacol Sci201132742043421492946
- ContestabileAGrecoBGhezziDTucciVBenfenatiFGaspariniLLithium rescues synaptic plasticity and memory in Down syndrome miceJ Clin Invest2013123134836123202733
- GeSYangCHHsuKSMingGLSongHA critical period for enhanced synaptic plasticity in newly generated neurons of the adult brainNeuron200754455956617521569
- Schmidt-HieberCJonasPBischofbergerJEnhanced synaptic plasticity in newly generated granule cells of the adult hippocampusNature2004429698818418715107864
- MongiatLAEspósitoMSLombardiGSchinderAFReliable activation of immature neurons in the adult hippocampusPLoS One200944e532019399173
- Marín-BurginAMongiatLAPardiMBSchinderAFUnique processing during a period of high excitation/inhibition balance in adult-born neuronsScience201233560731238124222282476
- CrewsLAdameAPatrickCIncreased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesisJ Neurosci20103037122521226220844121
- GarzaJCGuoMZhangWLuXYLeptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signalingMol Psychiatry201217879080822182938
- SnyderJSSoumierABrewerMPickelJCameronHAAdult hippocampal neurogenesis buffers stress responses and depressive behaviourNature2011476736145846121814201
- MaoYGeXFrankCLDisrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signalingCell200913661017103119303846
- CorasRSiebzehnrublFAPauliELow proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humansBrain2010133113359337220719879
- BianchiPCianiEContestabileAGuidiSBartesaghiRLithium restores neurogenesis in the subventricular zone of the Ts65Dn mouse, a model for Down syndromeBrain Pathol201020110611819298631
- ChenGRajkowskaGDuFSeraji-BozorgzadNManjiHKEnhancement of hippocampal neurogenesis by lithiumJ Neurochem20007541729173410987856
- HashimotoRSenatorovVKanaiHLeedsPChuangD-MLithium stimulates progenitor proliferation in cultured brain neuronsNeuroscience20031171556112605892
- KimJSChangMYYuITLithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivoJ Neurochem200489232433615056276
- LiHLiQDuXLithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cellsJ Cereb Blood Flow Metab201131102106211521587270
- YanXBHouHLWuLMLiuJZhouJNLithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemiaNeuropharmacology200753448749517686496
- PfennigAAldaMYoungTProphylactic lithium treatment and cognitive performance in patients with a long history of bipolar illness: no simple answers in complex disease-treatment interplayInt J Bipolar Disord20142125540718
- WingoAPHarveyPDBaldessariniRJNeurocognitive impairment in bipolar disorder patients: functional implicationsBipolar Disord200911211312519267694
- YoungAHMore good news about the magic ion: lithium may prevent dementiaBr J Psychiatry2011198533633721525515
- NunesPVForlenzaOVGattazWFLithium and risk for Alzheimer’s disease in elderly patients with bipolar disorderBr J Psychiatry200719035936017401045
- KessingLVSondergardLFormanJLAndersenPKLithium treatment and risk of dementiaArch Gen Psychiatry200865111331133518981345
- KessingLVFormanJLAndersenPKDoes lithium protect against dementia?Bipolar Disord2010121879420148870
- MacdonaldABriggsKPoppeMHigginsAVelayudhanLLovestoneSA feasibility and tolerability study of lithium in Alzheimer’s diseaseInt J Geriatr Psychiatry200823770471118181229
- HampelHEwersMBürgerKLithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week studyJ Clin Psychiatry200970692293119573486
- LeyheTEschweilerGWStranskyEIncrease of BDNF serum concentration in lithium treated patients with early Alzheimer’s diseaseJ Alzheimers Dis200916364965619276559
- ForlenzaOVDinizBSRadanovicMSantosFSTalibLLGattazWFDisease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trialBr J Psychiatry2011198535135621525519
- NunesMAVielTABuckHSMicrodose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s diseaseCurr Alzheimer Res201310110410722746245
- MauerSVergneDGhaemiSNStandard and trace-dose lithium: a systematic review of dementia prevention and other behavioral benefitsAust N Z J Psychiatry201448980981824919696
- VoTMPerryPEllerbyMBohnertKIs lithium a neuroprotective agent?Ann Clin Psychiatry2015271495425696782
- De SarnoPAxtellRCRamanCRothKAAlessiDRJopeRSLithium prevents and ameliorates experimental autoimmune encephalomyelitisJ Immunol2008181133834518566399
- WataseKGatchelJRSunYLithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse modelPLoS Med200745e18217535104
- ForlenzaOVAprahamianIde PaulaVJHajekTLithium, a therapy for AD: current evidence from clinical trials of neurodegenerative disordersCurr Alzheimer Res Epub2016218
- KingTDBijurGNJopeRSCaspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithiumBrain Res2001919110611411689167
- ArrafZAmitTYoudimMBFarahRLithium and oxidative stress lessons from the MPTP model of Parkinson’s diseaseNeurosci Lett20125161576122480690
- KimYHRaneALussierSAndersenJKLithium protects against oxidative stress-mediated cell death in α-synuclein-overexpressing in vitro and in vivo models of Parkinson’s diseaseJ Neurosci Res201189101666167521710541
- YoudimMBArrafZPrevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and BaxNeuropharmacology20044681130114015111020
- ChenGBowerKAMaCFangSThieleCJLuoJGlycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal deathFASEB J200418101162116415132987
- YongYDingHFanZLuoJKeZJLithium fails to protect dopaminergic neurons in the 6-OHDA model of Parkinson’s diseaseNeurochem Res201136336737421203835
- ScheuingLChiuCTLiaoHMLinaresGRChuangDMPreclinical and clinical investigations of mood stabilizers for Huntington’s disease: what have we learned?Int J Biol Sci20141091024103825285035
- WeiHQinZHSenatorovVVLithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s diseaseNeuroscience2001106360361211591460
- SenatorovVVRenMKanaiHWeiHChuangDMShort-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s diseaseMol Psychiatry20049437138514702090
- DanivasVMoilyNSThimmaiahROff label use of lithium in the treatment of Huntington’s disease: a case seriesIndian J Psychiatry2013551818323439971
- RajaMSoletiFBentivoglioARLithium treatment in patients with Huntington disease and suicidal behaviorJ Clin Psychopharmacol201333681982124018546
- FornaiFLongonePCafaroLLithium delays progression of amyotrophic lateral sclerosisProc Natl Acad Sci U S A200810562052205718250315
- GillAKiddJVieiraFThompsonKPerrinSNo benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALSPLoS One200948e648919649300
- PizzasegolaCCaronIDalenoCTreatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant miceAmyotroph Lateral Scler200910422122819308767
- FerrucciMSpalloniABartalucciAA systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithiumNeurobiol Dis201037237038319874893
- ShinJHChoSILimHRConcurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of amyotrophic lateral sclerosisMol Pharmacol200771496597517105868
- FengHLLengYMaCHZhangJRenMChuangDMCombined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse modelNeuroscience2008155356757218640245
- AggarwalSPZinmanLSimpsonESafety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trialLancet Neurol20109548148820363190
- Al-ChalabiAShawPJYoungCAUKMND-LiCALS. Protocol for a double-blind randomised placebo-controlled trial of lithium carbonate in patients with amyotrophic lateral sclerosis (LiCALS) [Eudract number: 2008-006891-31]BMC Neurol20111111121936930
- MorrisonKEDhariwalSHornabrookRfor UKMND-LiCALS Study GroupLithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trialLancet Neurol201312433934523453347
- ChiòAMoraGThe final chapter of the ALS lithium sagaLancet Neurol201312432432523453346
- BagniCTassoneFNeriGHagermanRFragile X syndrome: causes, diagnosis, mechanisms, and therapeuticsJ Clin Invest2012122124314432223202739
- BhakarALDolenGBearMFThe pathophysiology of fragile X (and what it teaches about synapses)Annu Rev Neurosci20123541743322483044
- GattoCLBroadieKThe fragile X mental retardation protein in circadian rhythmicity and memory consolidationMol Neurobiol200939210712919214804
- EadieBDCushmanJKannangaraTSFanselowMSChristieBRNMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout miceHippocampus201222224125421049485
- NosyrevaEDHuberKMMetabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndromeJ Neurophysiol20069553291329516452252
- YunSHTrommerBLFragile X mice: reduced long-term potentiation and N-methyl-D-aspartate receptor-mediated neurotransmission in dentate gyrusJ Neurosci Res201189217618221162125
- BearMFHuberKMWarrenSTThe mGluR theory of fragile X mental retardationTrends Neurosci200427737037715219735
- DolenGBearMFRole for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndromeJ Physiol2008586Pt 61503150818202092
- MichalonASidorovMBallardTMChronic pharmacological mGlu5 inhibition corrects fragile X in adult miceNeuron2012741495622500629
- MinWWYuskaitisCJYanQElevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potentialNeuropharmacology200956246347218952114
- MinesMaJopeRSGlycogen synthase kinase-3: a promising therapeutic target for fragile x syndromeFront Mol Neurosci201143522053151
- PeineauSTaghibiglouCBradleyCLTP inhibits LTD in the hippocampus via regulation of GSK3betaNeuron200753570371717329210
- ChoiCHSchoenfeldBPBellAJPharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatmentBrain Res2011138010611921078304
- FranklinAVKingMKPalomoVMartinezAMcMahonLLJopeRSGlycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X miceBiol Psychiatry201475319820624041505
- KingMKJopeRSLithium treatment alleviates impaired cognition in a mouse model of fragile X syndromeGenes Brain Behav201312772373123941202
- LiuZHChuangDMSmithCBLithium ameliorates phenotypic deficits in a mouse model of fragile X syndromeInt J Neuropsychopharmacol201114561863020497624
- LiuZHHuangTSmithCBLithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile X syndromeNeurobiol Dis20124531145115222227453
- MinesMAYuskaitisCJKingMKBeurelEJopeRSGSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autismPLoS One201053e970620300527
- YuskaitisCJMinesMAKingMKSweattJDMillerCAJopeRSLithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndromeBiochem Pharmacol201079463264619799873
- Berry-KravisESumisAHerveyCOpen-label treatment trial of lithium to target the underlying defect in fragile X syndromeJ Dev Behav Pediatr200829429330218698192
- WuXBaiYTanTLithium ameliorates autistic-like behaviors induced by neonatal isolation in ratsFront Behav Neurosci2014823425018711
- SerretSThümmlerSDorEVesperiniSSantosAAskenazyFLithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reportsBMC Psychiatry20151510725947967
- DurandCMBetancurCBoeckersTMMutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disordersNat Genet2007391252717173049
- DenayerAVan EschHde RavelTNeuropsychopathology in 7 patients with the 22q13 deletion syndrome: presence of bipolar disorder and progressive loss of skillsMol Syndromol201231142022855650
- VerhoevenWMEggerJIWillemsenMHde LeijerGJKleefstraTPhelan-McDermid syndrome in two adult brothers: atypical bipolar disorder as its psychopathological phenotype?Neuropsychiatr Dis Treat2012817517922570549
- VerhoevenWMEggerJICohen-SnuijfRKantSGde LeeuwNPhelan-McDermid syndrome: clinical report of a 70-year-old womanAm J Med Genet A2013161A115816123166010
- FernandezFMorishitaWZunigaEPharmacotherapy for cognitive impairment in a mouse model of Down syndromeNat Neurosci200710441141317322876
- ChakrabartiLGaldzickiZHaydarTFDefects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndromeJ Neurosci20072743114831149517959791
- ChakrabartiLBestTKCramerNPOlig1 and Olig2 triplication causes developmental brain defects in Down syndromeNat Neurosci201013892793420639873
- AlgattasHHuangJHTraumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injuryInt J Mol Sci201415130934124381049
- BlennowKHardyJZetterbergHThe neuropathology and neurobiology of traumatic brain injuryNeuron201276588689923217738
- OlssonACsajbokLOstMMarked increase of beta-amyloid (1–42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injuryJ Neurol2004251787087615258792
- SivanandamTMThakurMKTraumatic brain injury: a risk factor for Alzheimer’s diseaseNeurosci Biobehav Rev20123651376138122390915
- UryuKChenXHMartinezDMultiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humansExp Neurol2007208218519217826768
- ShapiraMLichtAMilmanAPickCGShohamiEEldar-FinkelmanHRole of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injuryMol Cell Neurosci200734457157717289399
- ZhuZFWangQGHanBJWilliamCPNeuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in miceBrain Res Bull201083527227720638460
- DashPKJohnsonDClarkJInvolvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcomePLoS One201169e2464821935433
- YuFWangZTchantchouFChiuCTZhangYChuangDMLithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injuryJ Neurotrauma201229236237421895523
- RohMSEomTYZmijewskaAADe SarnoPRothKAJopeRSHypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: protection by mood stabilizers and imipramineBiol Psychiatry200557327828615691529
- BianQShiTChuangDMQianYLithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbilsBrain Res2007118427027618028886
- BijurGNJopeRSOpposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3 beta in the regulation of HSF-1 activityJ Neurochem20007562401240811080191
- RenMSenatorovVVChenR-WChuangD-MPost-insult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion modelProc Natl Acad Sci U S A2003100106210621512732732
- XuJHCulmanJBlumeABrechtSGohlkePChronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic deathStroke20033451287129212677021
- NonakaSChuangDMNeuroprotective effects of chronic lithium on focal cerebral ischemia in ratsNeuroreport199899208120849674597
- ZhengZKimJYMaHLeeJEYenariMAAnti-inflammatory effects of the 70 kDa heat shock protein in experimental strokeJ Cereb Blood Flow Metab2008281536317473852
- KimYRvan MeerMPTejimaEFunctional MRI of delayed chronic lithium treatment in rat focal cerebral ischemiaStroke200839243944718187690
- FanYYangGYTherapeutic angiogenesis for brain ischemia: a brief reviewJ Neuroimmune Pharmacol20072328428918040863
- TsaiLKLengYWangZLeedsPChuangDMThe mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanismsNeuropsychopharmacology201035112225223720613717
- TsaiLKWangZMunasingheJLengYLeedsPChuangDMMesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke modelStroke201142102932293921836090
- GrayJDMcEwenBSLithium’s role in neural plasticity and its implications for mood disordersActa Psychiatr Scand2013128534736123617566
- BachmannRFSchloesserRJGouldTDManjiHKMood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeuticsMol Neurobiol200532217320216215281
- ChuangDMManjiHKIn search of the Holy Grail for the treatment of neurodegenerative disorders: has a simple cation been overlooked?Biol Psychiatry20076214617572175
- ManjiHKMooreGJChenGClinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illnessBiol Psychiatry200048874075411063971
- Dell’OssoLDalle LucheRMajMAdult autism spectrum as a transnosographic dimensionCNS Spectr201621213113326349624
- InselTRRethinking schizophreniaNature2010468732118719321068826

