Abstract
Objective
There are no direct comparisons between escitalopram and paroxetine controlled release in patients with major depressive disorder (MDD).
Methods
We conducted a 24-week, rater-masked, randomized trial of escitalopram (5–20 mg/day) versus paroxetine controlled release (12.5–50 mg/day) in patients with MDD (UMIN000011191). Patients with the diagnosis of moderate-to-severe MDD (a 17-item Hamilton Rating Scale for Depression [HAMD-17], with total score at baseline being ≥20) were recruited to participate in a parallel, randomized, controlled trial. The primary outcome for efficacy was an improvement in the 21-item HAMD (HAMD-21) total score at 24 weeks. The secondary outcomes were the response, remission, and discontinuation rates and the incidence of individual adverse events.
Results
A total of 88 patients with MDD (males, 61.4%; mean age, 40.8±13.4 years) were recruited. The discontinuation rate was 58.0% (escitalopram, 55.8%; paroxetine controlled release, 60.0%). Both escitalopram and paroxetine controlled-release treatment groups exhibited significant reduction in the HAMD-21 total score at 2, 4, 8, 12, and 24 weeks from the baseline. However, there were no significant differences in the HAMD-21 total score, response rate, remission rate, and discontinuation rate at any time point between the groups. In addition, there were no significant differences in the incidence of any individual adverse events (eg, nausea, vomiting, and somnolence) between the treatment groups.
Conclusion
Our results suggest that escitalopram and paroxetine controlled release had similar efficacy and safety profiles in patients with MDD. One of the primary limitations of this study is the small sample size.
Introduction
In 2009, the Meta-Analysis of New Generation Antidepressants StudyCitation2 reported that clinically important differences for both efficacy and acceptability exist among commonly prescribed antidepressants in favor of escitalopram and sertraline. In 2012, escitalopram was demonstrated to have the highest probability of remission and is the most effective and cost-effective pharmacological treatment in a primary care setting.Citation3 Escitalopram appears to be the best first-line antidepressant for treating major depressive disorder (MDD). In contrast, in 2010, paroxetine immediate release was the best-selling antidepressant in Japan.Citation4
There were three randomized trials of escitalopram versus paroxetine immediate release in patients with MDD. Boulenger et al’s study and Kasper et al’s study reported that escitalopram is more effective and safer than paroxetine immediate release in the long-term treatment of patients with MDD.Citation5,Citation6 Baldwin et al reported that significantly (P<0.01) more paroxetine immediate release was associated with a higher discontinuation rate compared with escitalopram.Citation7 As the National Institute for Health and Care Excellence guidelines indicated, a higher incidence of discontinuation symptoms is observed for paroxetine immediate release than for other selective serotonin reuptake inhibitors (SSRIs).Citation8 In order to overcome these drawbacks of paroxetine immediate release, paroxetine controlled release was developed in Japan in 2012 to improve general tolerability, particularly, gastrointestinal tolerability. Although there are no published data which demonstrate that paroxetine controlled release has a lower risk for producing discontinuation effects than paroxetine immediate release, one randomized trial showed that paroxetine controlled release is associated with low rates of early-onset nausea and dropout rates due to adverse events, which were comparable to those of placebo.Citation9 However, there are no direct comparisons between escitalopram and paroxetine controlled release in patients with MDD. Therefore, we conducted a 24-week, rater-masked, randomized trial of escitalopram versus paroxetine controlled release in Japanese patients with MDD.
Methods
Subjects
This study was conducted from July 2013 to December 2015 at the Fujita Health University Hospital, Jindai Clinic, Jindai Hospital, Toyota Memorial Hospital, Holy Cross Hospital, and Okehazama Hospital. The trial was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000011191). Patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria by the consensus of at least two experienced psychiatrists on the basis of structured interviews conducted using the Mini-International Neuropsychiatric Interview and a review of all medical records. All subjects met the following inclusion criteria: 1) age 20–70 years; 2) a 17-item Hamilton Rating Scale for Depression (HAMD-17),Citation1 with total score at baseline being ≥20; 3) no neurologic or systemic diseases, including disturbance of hematopoiesis; 4) no history of electroconvulsive therapy within 6 months before enrollment; 5) absence of pregnancy; and 6) no dependence on any addictive substances other than nicotine in the past 5 years before enrollment. All subjects underwent laboratory blood testing and electrocardiography at the time of enrollment, and no patients were excluded because of a medical condition.
The clinical trial was described in detail, and written informed consent was obtained from all participants and their guardians. This study was approved by the six ethics committees of the Fujita Health University Hospital, Jindai Clinic, Jindai Hospital, Toyota Memorial Hospital, Holy Cross Hospital, and Okehazama Hospital.
Procedures
All patients were randomly assigned to one of the two treatment groups by the central registration office. When the patients satisfied the inclusion and exclusion criteria for this multicenter trial, the randomization procedure was conducted by the authors in contact with a central registration office by telephone. The authors generated an allocation schedule before the start of the study using a random number table. The initial starting dose of escitalopram was 5–10 mg/day, with dose increases when needed in increments of 5–10 mg/day to a maximum of 20 mg/day. The initial starting dose of the paroxetine controlled release was 12.5 mg/day, with a dose increase of 12.5–50 mg/day. The subsequent antidepressant dosage was increased or decreased according to the patient’s tolerance and therapeutic response. Lorazepam was used as an anxiolytic, and the hypnotics brotizolam and eszopiclone were permitted during our clinical trial (by prescription and for valid clinical reasons).
Evaluation of psychopathology, tolerability, and safety
The 21-item HAMD (HAMD-21)Citation1 was completed at baseline and at 2, 4, 8, 12, and 24 weeks after starting administration or at discontinuation. Treatment-emergent adverse events were recorded at each time point, including immediately before the study, upon spontaneous complaints and through clinical observation using the Udvalg for Kliniske Undersogelser Side Effect Rating Scale.Citation10
The raters who administered the psychometric tests were masked to the treatment group allocation, but the patients and physicians were aware of the treatment that was received. Raters consisted of psychologists who were not involved in any treatment decisions or the evaluation of side effects. Therefore, they were not required to know the pharmacologic treatment. The following measures were taken to maintain the masking of the raters. Electronic data from the trial were password protected, all case report forms were securely stored, and discussions about patients among the research teams were restricted. Moreover, the participants were reminded to avoid open discussion of the treatment assignment with the raters.
The primary outcome measure was the improvement of the HAMD-21 total scores at week 24. The secondary outcomes included: the HAMD-21 scores at 2, 4, 8, and 12 weeks; the HAMD-17 scores at 2, 4, 8, 12, and 24 weeks; the response rate at 2, 4, 8, 12, and 24 weeks (clinical response defined as ≥50% reduction in the HAMD-17 or -21 total score from baseline to endpoint); the remission rate at 2, 4, 8, 12, and 24 weeks (clinical remission defined as the HAMD-17 total score ≤6 or HAMD-21 total score ≤7);Citation11 the discontinuation rate; and individual adverse events.
Statistical analysis
Modified intent-to-treat analysis was performed using the last observation carried forward method. Baseline continuous and categorical variables were compared between the treatment groups by an independent t-test and a chi-square test, respectively. Paired Student’s t-tests were used to assess the changes in the HAMD-21 and HAMD-17 total scores from the baseline to endpoint. The comparison between the escitalopram and paroxetine controlled-release treatment groups was made by determining the change in the HAMD-21 (or HAMD-17) total score at the endpoint (2, 4, 8, 12, and 24 weeks) from baseline, based on an analysis of covariance model using the baseline HAMD-21 (or HAMD-17) score as covariates. We also used multiple logistic regression analyses to examine which antidepressant was associated with a higher response rate or remission rate (dependent variable: response rate [HAMD-21 or HAMD-17] or remission rate [HAMD-21 or HAMD-17]; independent variable: treatment group; and covariates: baseline HAMD-21 or HAMD-17 score, respectively). A Kaplan–Meier analysis was used to estimate the time to discontinuation for the two treatment groups, and the results were compared using a log-rank test. We also compared the change in body weight from baseline to endpoint between the escitalopram and paroxetine controlled-release treatment groups based on an analysis of covariance model using the baseline value as a covariate. Incidences of individual adverse events during the study were compared between the treatment groups using a chi-square test. The final dose of antidepressants was evaluated based on the fluoxetine equivalent.Citation12 The statistical power regarding the primary outcome for efficacy was also calculated (alpha =0.5, http://www.biostat.ucsf.edu/sampsize.html).
Statistical analyses were performed using Statistical Package for the Social Sciences Statistics for Windows (Version 22.0; Corporation, Armonk, NY, USA) and JMP (JMP 12.2. 1J; SAS Japan Inc., Tokyo, Japan). A P-value of <0.05 was considered statistically significant for all tests.
Results
The demographics and other characteristics of the patients are presented in . A total of 88 patients were recruited, all of whom were diagnosed with MDD at enrollment. Of these, 77.3% were first-episode patients and 61.4% were male, and the mean age was 40.8±13.4 years. There were no significant differences in most variables between the treatment groups ().
Table 1 Baseline characteristics of the patients
The discontinuation rate was 58.0% (escitalopram, 55.8% and paroxetine controlled release, 60.0%; ). There was no significant difference in the mean times to discontinuation between the escitalopram and paroxetine controlled-release treatment groups (13.4±10.1 vs 13.2±9.92 weeks; χ2(1)=0.0836, P=0.773). All 88 patients were included in the efficacy and safety analyses. The mean escitalopram and paroxetine controlled-release doses at endpoint were 13.6±5.81 mg/day (fluoxetine equivalent, 30.2±12.9 mg/day) and 24.8±13.3 mg/day (fluoxetine equivalent, 29.2±15.7 mg/day), respectively (t(1)=0.0836, P=0.773).
Table 2 Discontinuation rate
Both escitalopram and paroxetine controlled-release treatments were associated with significant improvements in the HAMD-21 and HAMD-17 total scores at 2, 4, 8, 12, and 24 weeks (). However, there were no significant differences in the magnitude of the HAMD-21, and HAMD-17 total score decreases at any time between the escitalopram and paroxetine controlled-release treatment groups (). The statistical power with respect to the primary outcome was 43%. Also, there were no significant differences in the response rate at any time point between the treatment groups ( and ).
Figure 1 Response rate.
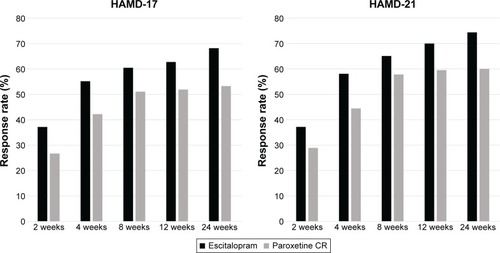
Figure 2 Remission rate.
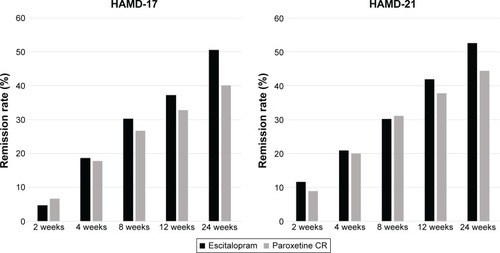
Table 3 Group difference in Hamilton Depression Rating Scale scores at 2, 4, 8, 12, and 24 weeks
No patients had serious adverse events such as death, suicide attempt, and serotonin syndrome, and there were no significant differences in the incidence of individual adverse events between the groups (). Moreover, there were no significant differences in body weight at the endpoint between the treatment groups ().
Table 4 Adverse events observed in the study (incidence ≥5%)
Discussion
This is the first randomized trial involving a comparison between escitalopram and paroxetine controlled release in Japanese patients with MDD. There were no significant differences in the efficacy and safety outcomes between the escitalopram and paroxetine controlled-release treatment groups in this study. However, our study found that discontinuation rates due to adverse events in both treatment groups were relatively high compared with other studies on SSRIs.Citation13 In a recent network meta-analysis, escitalopram emerged as the most efficacious agent among the SSRIs and was the best tolerated of the new-generation antidepressants (eg, agomelatine, duloxetine, escitalopram, fluvoxamine, fluoxetine, mirtazapine, paroxetine, sertraline, trazodone, and venlafaxine) that were analyzed.Citation13 We did not find significant differences in any efficacy outcome between the escitalopram and paroxetine controlled-release treatment groups in this study. The statistical power with respect to the primary outcome was 43%. Therefore, our results might be a statistical error due to an insufficient sample size.
Limitations
One of the primary limitations of this study is that this study was not double blinded. The physicians responsible for assessing the adverse events were aware of which treatment was received by which patient. Although the raters were masked to the nature of the antidepressant treatment and were not involved in any side effect ratings or management decisions, it is possible that they were inadvertently informed of the group allocation by the patients. The second limitation is that this study did not include a placebo arm. The third limitation is the small sample size, particularly in the subgroup analysis (the statistical power with respect to the primary outcome was 43%). Therefore, we did not perform an HAMD item analysis. The fourth limitation is that we did not correct for multiple comparisons (eg, using Bonferroni’s correction), because the application of a more stringent alpha level for secondary outcomes would have been too conservative in this small sample.Citation14,Citation15 The fifth one is that we did not count the number of patients who were screened for entry in the study. Finally, the intent-to-treat analysis using the last observation carried forward method may have influenced the results. The discontinuation rate was high, and thus, this method may yield a biased estimate of the treatment effect and underestimate the variability of the results.Citation16 It is not clearly justified why this analysis, in particular, is appropriate and valid. However, if we had selected a complete analysis, our sample size would have been too small.
Conclusion
In conclusion, our findings suggest that although escitalopram and paroxetine controlled release are effective in patients with MDD, discontinuation rates due to adverse events in both treatment groups were relatively high. However, because our study might be underpowered to detect significant differences in efficacy and safety outcomes between the escitalopram and paroxetine controlled-release treatment groups, further study using a larger sample size is required.
Acknowledgments
We thank the following colleagues who shared their data with us: Ms M Yamamoto, Ms R Nishioka, Ms Y Matsumoto, Ms M Tani, Ms S Isogai, Ms M Niwa, Ms M Miyako, Ms E Tsutajima, Ms N Soma, Ms A Hayashi, Mr W Shirota, Mr U Sato, Mr S Tsuboi, and Ms K Torii.
This study was supported by Grant-in-Aid for Young Scientists (B) (25861033) and Fujita Health University School of Medicine research grant.
Supplementary material
Table S1 Endpoint change in Hamilton Depression Rating Scale scores at 2, 4, 8, 12, and 24 weeks from the baseline
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Dr Kishi has received speaker’s honoraria from Abbvie, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Mochida, Shionogi, Tanabe-Mitsubishi, Tsumura, Novartis, and Pfizer and has a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B). Dr Matsuda has received speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, Otsuka, and Pfizer. Dr Matsunaga has received speaker’s honoraria from Eisai, Janssen, Novartis, Daiichi Sankyo, Ono, Eli Lilly, Takeda, and Otsuka and had a Fujita Health University School of Medicine research grant and Grant-in-Aid for Young Scientists (B). Dr Moriwaki has received speaker’s honoraria from Eli Lilly, Yoshitomi, and Novartis. Dr Otake has received speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, and Otsuka. Dr Akamatsu has received speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, and Otsuka. Dr Funahashi has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, and Pfizer. Dr Tabuse has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, and Pfizer. Dr Fujita has received speaker’s honoraria from Eli Lilly, Mochida, GlaxoSmithKline, Janssen, Yoshitomi, and Otsuka. Dr Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer. The authors report no other conflicts of interest in this work.
References
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- RamsbergJAsseburgCHenrikssonMEffectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness modelPLoS One201278e4200322876296
- Ai Report 2011 -chikenyakunenpou-[The sales amounts of antidepressants in Japan]CIMA Sci J2011
- BoulengerJPHuusomAKFloreaIBaekdalTSarchiaponeMA comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patientsCurr Med Res Opin20062271331134116834832
- KasperSBaldwinDSLarsson LonnSBoulengerJPSuperiority of escitalopram to paroxetine in the treatment of depressionEur Neuropsychopharmacol200919422923719185467
- BaldwinDSCooperJAHuusomAKHindmarchIA double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorderInt Clin Psychopharmacol200621315916916528138
- NICEDepression in Adults: Recognition and Management. [Last updated: April 2016]National Institute for Health and Care Excellence2009
- GoldenRNNemeroffCBMcSorleyPPittsCDDubeEMEfficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depressionJ Clin Psychiatry200263757758412143913
- JordanSKnightJPointonDMonitoring adverse drug reactions: scales, profiles, and checklistsInt Nurs Rev200451420822115530161
- RiedelMMollerHJObermeierMResponse and remission criteria in major depression – a validation of current practiceJ Psychiatr Res201044151063106820447651
- HayasakaYPurgatoMMagniLRDose equivalents of antidepressants: evidence-based recommendations from randomized controlled trialsJ Affect Disord201518017918425911132
- KhooALZhouHJTengMNetwork meta-analysis and cost-effectiveness analysis of new generation antidepressantsCNS Drugs201529869571226293743
- PernegerTVWhat’s wrong with Bonferroni adjustmentsBMJ19983167139123612389553006
- SankohAJHuqueMFDubeySDSome comments on frequently used multiple endpoint adjustment methods in clinical trialsStat Med19971622252925429403954
- MallinckrodtCHKaiserCJWatkinJGMolenberghsGCarrollRJThe effect of correlation structure on treatment contrasts estimated from incomplete clinical trial data with likelihood-based repeated measures compared with last observation carried forward ANOVAClin Trials20041647748916279288

