Abstract
Background:
Escitalopram is an allosteric selective serotonin reuptake inhibitor (SSRI) with some indication of superior efficacy in the treatment of major depressive disorder. In this systematic review, we critically evaluate the evidence for comparative efficacy and tolerability of escitalopram, focusing on pooled and meta-analysis studies.
Methods:
A literature search was conducted for escitalopram studies that quantitatively synthesized data from comparative randomized controlled trials in MDD. Studies were excluded if they did not focus on efficacy, involved primarily subgroups of patients, or synthesized data included in subsequent studies. Outcomes extracted from the included studies were weighted mean difference or standard mean difference, response and remission rates, and withdrawal rate owing to adverse events.
Results:
The search initially identified 24 eligible studies, of which 12 (six pooled analysis and six meta-analysis studies) met the criteria for review. The pooled and meta-analysis studies with citalopram showed significant but modest differences in favor of escitalopram, with weighted mean differences ranging from 1.13 to 1.73 points on the Montgomery Asberg Depression Rating Scale, response rate differences of 7.0%–8.3%, and remission rate differences of 5.1%–17.6%. Pooled analysis studies showed efficacy differences compared with duloxetine and with serotonin noradrenaline reuptake inhibitors combined, but meta-analysis studies did not. The effect sizes of the efficacy differences increased in the severely depressed patient subgroups.
Conclusion:
Based on pooled and meta-analysis studies, escitalopram demonstrates superior efficacy compared with citalopram and with SSRIs combined. Escitalopram shows similar efficacy to serotonin noradrenaline reuptake inhibitors but the number of trials in these comparisons is limited. Efficacy differences are modest but clinically relevant, especially in more severely depressed patients.
Introduction
Major depressive disorder (MDD) is a common and serious psychiatric condition with significant public health implications.Citation1 The World Health Organization estimates that, by 2030, MDD will be second only to ischemic heart disease as an overall cause of disability and disease burden.Citation2 The economic costs of depression and its treatment are estimated at C$6 billion in Canada,Citation3 US$83 billion in the US,Citation4 and €118 billion in Europe.Citation5
There are many evidence-based psychotherapy and pharmacotherapy treatments for MDD. Antidepressant medications remain a mainstay of treatment for MDD, especially for those with moderate to severe depression. Newer antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRIs), and novel mechanism agents offer fewer side effects and are safer in overdose compared with tricyclic antidepressants and monoamine oxidase inhibitors. Hence, most clinical guidelines consider the newer generation antidepressants to be first-line medications for MDD.Citation6–Citation8
Escitalopram, the S-enantiomer of racemic citalopram, is an SSRI that has an additional modulatory effect at an allosteric binding site on the serotonin transporter protein.Citation9 Escitalopram has been demonstrated in many placebo-controlled, randomized, controlled trials to be an efficacious antidepressant for MDD.Citation10,Citation11 Moreover, some randomized controlled trials have shown evidence for the superior efficacy and tolerability of escitalopram compared with other SSRIs and other agents.Citation12,Citation13
Unfortunately, the evaluation of comparative efficacy of antidepressants is complex. Placebo-controlled, randomized, controlled trials remain the gold standard for demonstrating the efficacy of treatments. However, most randomized controlled trials in MDD are designed to detect differences between an active antidepressant and placebo, and hence are not adequately powered to detect smaller, but still clinically relevant, differences between two active antidepressants.
Because of the limitations of randomized controlled trials, meta-analysis to investigate the comparative effectiveness of antidepressants is being increasingly used.Citation14 Meta-analysis is a statistical technique to synthesize results from many randomized controlled trials. It can be a powerful method to increase power to detect differences between agents even when individual randomized controlled trials cannot. There are two main types of meta-analysis, ie, those using pooled individual patient data (usually called pooled analysis studies) and those using summary data from individual trials (more typically known as meta-analyses).Citation15 Pooled analysis studies have the advantages of considerable power and the ability to examine subgroups, because all individual patient data are available for analysis. However, randomized controlled trials can only be pooled if they have very similar study designs (eg, use the same outcome measure) and if investigators agree to release of individual patient data. The latter is very difficult to arrange, hence many pooled analysis studies report on trials from a single sponsor. In contrast, standard meta-analyses can synthesize data from very different types of randomized controlled trials, because a standardized effect size can be calculated for any outcome measure and only summary data from a trial are necessary.
Regardless of the type of meta-analysis, the details of meta-analysis methodology are as important for interpretation of results as they are for randomized controlled trials. Selection criteria for inclusion of studies is perhaps the most important aspect of meta-analysis. Results may differ widely depending on these criteria, including whether published or unpublished trials are included.Citation15 Other important factors to consider include definitions of primary and secondary outcomes, duration of trials, dose comparability, and assessment of heterogeneity and publication bias.Citation14
The objective of this systematic review is to examine critically the evidence for the comparative efficacy and tolerability of escitalopram, focusing on studies using pooled analysis and meta-analysis to synthesize randomized controlled trial data.
Methods
A literature search was performed using PubMed with keywords including “escitalopram”, “depression”, “meta-analysis”, “pooled analysis”, and “systematic review”. We also scanned reference lists of review papers on escitalopram. Studies were included if they conducted analyses that synthesized data on randomized controlled trials using pooled analysis and meta-analysis. Studies were excluded if they did not primarily examine efficacy or if they only examined patient subgroups.
All of the randomized controlled trials represented within these studies used the Montgomery-Asberg Depression Rating Scale (MADRS) or the Hamilton Depression Rating Scale (Ham-D) as primary outcomes. The results of included studies were tabulated for the following outcomes: weighted mean difference (WMD) or standardized mean difference (SMD) from MADRS or Ham-D scores, response rate, remission rate, and withdrawal rate owing to adverse events (if not available, then all-cause withdrawal rate was used). Unless otherwise indicated, response is defined as a 50% or greater reduction in scale scores from baseline, while remission is defined as either MADRS ≤ 12 or Ham-D ≤ 7, depending on the scale used.
Results
The initial electronic search yielded 98 articles, of which 24 met the inclusion criteria as a pooled analysis or meta-analysis study (see ). Sixteen pooled analysis studies and six meta-analysis studies were identified. Two additional meta-analysis studies were “hybrid” studies, but were classified as pooled studies because they primarily reported pooled analyses of individual patient data, with only limited analyses of summary data.Citation16,Citation17
Figure 1 Flow diagram for identification of pooled and meta-analysis studies of comparative efficacy of escitalopram.
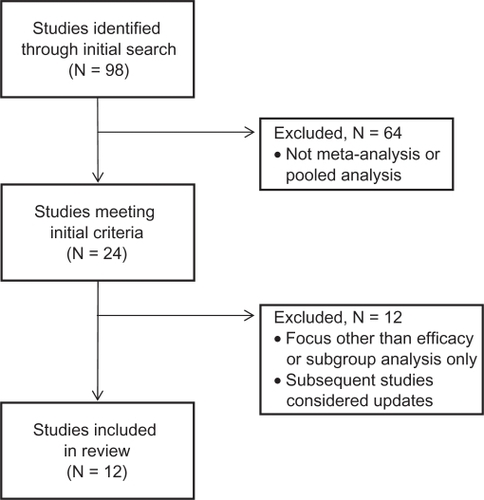
Of the 18 pooled analysis studies, we excluded five studiesCitation18–Citation22 primarily focusing on topics (symptom clusters, onset of action, predictors of response) other than efficacy, two studiesCitation17,Citation23 which were considered subsets of subsequent larger pooled studies, and five studiesCitation24–Citation28 focusing on severity analyses only. This left six pooled analysis studies for review (). Several of the pooled studies also analyzed outcomes for a severely depressed patient subgroup, defined as baseline MADRS ≥ 30 (). All studies except oneCitation29 used data from randomized controlled trials sponsored by the manufacturer or distributor of escitalopram (Lundbeck or Forest).
Table 1 Pooled analysis studies of comparative efficacy of escitalopram in all patients
Table 2 Pooled analysis studies of comparative efficacy of escitalopram: subgroups of severely depressed patients
Of the six meta-analysis (and two hybrid) studies, two studiesCitation17,Citation30 were excluded because subsequent studies were considered updates, but oneCitation16 of the hybrid studies included a direct meta-analysis, hence six meta-analysis studies were eligible for review ().
Table 3 Meta-analysis studies of comparative efficacy of escitalopram
An important consideration for systematic reviews and meta-analyses is the “universe” of randomized controlled trials from which trials are selected. Inspection of the included trials from these meta-analysis studies revealed a total of 22 comparative randomized controlled trials of escitalopram with citalopram (eight trials), fluoxetine (three trials), paroxetine (two trials), sertraline (two trials), bupropion XL (two trials), duloxetine (three trials), and venlafaxine XR (two trials). An updated electronic search of PubMed through to November 2010 found one additional trial with desvenlafaxine in postmenopausal women.Citation31
Pooled analysis studies
Two pooled analysis studies examined escitalopram compared with other SSRIs. Kennedy et alCitation16 pooled five trials with citalopram and found superiority for escitalopram in mean MADRS difference (1.2 points, P = 0.00094) and response rate difference (7.4%, P = 0.0043), but remission rate difference missed significance (5.1%, P = 0.0517). In addition, Kennedy et al also reported superiority for escitalopram over combined SSRIs (12 trials of citalopram, fluoxetine, paroxetine, sertraline), although this was largely explained by differences between escitalopram and citalopram. There was no difference in withdrawal rate owing to adverse events between escitalopram and SSRIs. Another study pooled data from two trials with paroxetine and found superiority for escitalopram in mean MADRS difference (2 points, P < 0.01), response difference (6.2%, P < 0.05), remission difference (6.4%, P < 0.05) and withdrawal rate difference (5.1%, P < 0.01).Citation32
Three pooled studies examined escitalopram compared with SNRIs. In the first, data from two randomized controlled trials with venlafaxine XR were pooled.Citation33 No significant differences were found in MADRS difference, response, or remission rates. However, the withdrawal rate for adverse events was lower for escitalopram (7.5% versus 11.2%, P < 0.05). The second study pooled data from two trials with duloxetine.Citation34 Escitalopram was superior in MADRS difference (2.6 points, P < 0.01), response difference (13.9%, P < 0.001) and remission difference (9.9%, P < 0.05). The withdrawal rate for adverse events also favored escitalopram (12.9% versus 24.3%, P < 0.001). The third study pooled results from the four randomized controlled trials of duloxetine and venlafaxine XR included in the previously described studies.Citation35 (Note that Kennedy et alCitation16 also reported a pooled analysis of these same four SNRI trials, but the results were identical.) At week 8, escitalopram was superior to the two SNRIs in MADRS difference (1.7 points, P < 0.01), and response and remission differences (9.3%, P < 0.01, and 7.2%, P < 0.05, respectively). The withdrawal rate for adverse events was also lower for escitalopram than the SNRIs (5.3% versus 12.0%, P < 0.0001).
A comprehensive pooled analysis compared 16 randomized controlled trials of escitalopram and six comparator antidepressants (citalopram, fluoxetine, paroxetine, sertraline, duloxetine, venlafaxine), which included all the trials in the previously described pooled studies.Citation16 Trials less than 8 weeks (two trials) and trials that did not include the MADRS (four trials) were excluded from this analysis. At week 8, escitalopram was superior to all comparators, with an estimated MADRS difference of 1.1 points (P < 0.0001), response difference of 5.4% (P < 0.0001), remission difference of 3.7% (P < 0.006), and withdrawal rate difference of 2.5% (P < 0.0007).
One final pooled analysisCitation29 involved two randomized controlled trials with bupropion XL, both sponsored by the manufacturer of bupropion (GlaxoSmithKline). No significant differences were found between escitalopram and bupropion XL in the efficacy outcomes or withdrawal rates.
Several of the pooled analysis studies also examined outcomes for a subgroup of patients who were severely depressed at baseline (). In these studies, when compared with the total group, the severely depressed subgroup showed increased differences between escitalopram and the comparator. In the Kennedy et alCitation16 pooled analysis of five citalopram trials, the MADRS difference at week 8 in patients with baseline MADRS ≥ 30 favored escitalopram by 2.0 points (P = 0.0013), as did the response rate difference (11.3%, P = 0.0012), although the remission rate difference of 6.3% was not statistically significant. Similarly, Kornstein et alCitation35 also found that the differences between escitalopram and SNRIs in the severely ill subgroup were greater than those overall, with a significant MADRS difference of 2.9 points and response/remission differences of 14.4%/13.4% (P < 0.001). Finally, the Kennedy et alCitation16 comprehensive pooled analysis of 16 trials found that the MADRS difference between escitalopram and all comparators in the severely depressed subgroup was 1.8 points, with a response difference of 8.6% and a remission difference of 6.1%, all of which were statistically significant.
Meta-analysis studies
A Cochrane systematic review of escitalopram was conducted as one of a series systematically evaluating the newer generation antidepressants. Cipriani et alCitation36 compared the efficacy and acceptability of escitalopram with other antidepressants in 20 published and unpublished trials, searched through to July 2008. In acute-phase treatment (6–12 weeks), escitalopram was shown to be significantly superior to citalopram (based on six trials) in SMD at endpoint (−0.17, P < 0.009) and in achieving response (60.7% versus 53.8%, P < 0.006) and remission (47.7% versus 38.5%, P < 0.02), and superior to fluoxetine (based on three trials) in SMD (−0.17, P < 0.02). There were no other significant differences in efficacy between escitalopram and paroxetine, sertraline, bupropion, duloxetine, and venlafaxine, but these analyses were limited to 2–3 trials per comparison.
Three other meta-analyses specifically focused on comparison of escitalopram versus citalopram. Although the included studies were similar for all three, each study used different analyses and reached different clinical conclusions. Gartlehner et alCitation37 included five randomized controlled trials, of which one was unpublished, all using the MADRS as primary outcome. At week 8, escitalopram was superior in WMD (1.13 MADRS points, P = 0.02) and response rate difference (about 7%, P < 0.05). TrkuljaCitation38 included seven randomized controlled trials (including all those in Gartlehner et alCitation37), but outcomes were analyzed for each weekly time point separately, and only those trials that reported data for that time point were included. At the week 8 time point, based on five trials, escitalopram was significantly superior, with a WMD of 1.23 (P = 0.012) and a response difference of 7% (P = 0.007). The week 6 time point results, based on four trials, showed a WMD of 1.73 (P = 0.004). Despite the statistically significant findings, the authors of these two studies concluded that the differences were not clinically relevant.
In contrast, Montgomery et alCitation39 meta-analyzed eight randomized controlled citalopram trials, including all those in the TrkuljaCitation38 review plus an additional small (n = 56) randomized controlled trial reported in a Chinese language journal. The outcomes included WMD (based on the six trials using the MADRS), response rates (based on all eight trials), and remission rates (based on the MADRS, reported in only four trials). The primary results showed superiority for escitalopram, with a WMD of 1.7 (P = 0.0002), a response difference of 8.3%, and a remission difference of 17.6%. These differences were regarded by the study authors as clinically significant.
Finally, Kennedy et alCitation16 included a limited meta-analysis of the five randomized controlled trials with the SNRIs, duloxetine and venlafaxine XR. In contrast with the pooled analysis results, this was conducted using Ham-D scores and mixed model repeated measures analyses of the primary trials. With these parameters, there were no significant differences in the Ham-D WMD between escitalopram and duloxetine (three trials) or between escitalopram and the combined SNRIs (five trials).
Multiple treatments meta-analysis studies
Two of the escitalopram meta-analyses used a newer statistical approach, multiple-treatments meta-analysis, also called network meta-analysis, to compare the efficacy and acceptability of 12 new-generation antidepressants. In contrast with a usual meta-analysis, where direct comparisons of two agents are analyzed, a multiple-treatments meta-analysis allows for the integration of data from both direct comparisons (when two agents are compared within one randomized controlled trial) and indirect comparisons (when two agents are compared by combining results based on randomized controlled trials with a common third agent).Citation40,Citation41
Gartlehner et alCitation37 included 114 randomized controlled trials (with 12 comparative escitalopram trials) searched through to April 2007, including unpublished trials. The primary efficacy outcomes were WMD and response rate. Direct comparison using meta-analysis was conducted if there were three or more trials, otherwise indirect comparisons of response rates were conducted using metaregression and modified network analysis. Only the direct meta-analyses found significant results in response rates: escitalopram superior to citalopram (described in the previous meta-analysis section), sertraline superior to fluoxetine, and venlafaxine superior to fluoxetine. However, the study authors questioned the clinical relevance of the small differences found.
Cipriani et alCitation42 conducted a multiple treatments meta-analysis that included 117 randomized controlled trials (with 19 escitalopram trials) from 1991 to November 2007, including 15 unpublished trials obtained from pharmaceutical company websites. The primary outcomes were efficacy, defined as the response rate, and acceptability, defined as the proportion of patients who withdrew from the study for all causes. Compared with the other antidepressants, the main efficacy results found superiority in response rates for escitalopram, mirtazapine, sertraline, and venlafaxine; inferiority was found only for reboxetine. A series of sensitivity analyses (examining dosing, imputation strategy, sponsorship, etc) did not change the results. Four antidepressants were also found to be superior in acceptability, ie, bupropion, citalopram, escitalopram, and sertraline. The authors concluded that these were clinically relevant differences in efficacy and acceptability.
Discussion
This systematic review identified a number of studies using pooled analyses of individual patient data and meta-analyses of summary trial data evaluating the comparative efficacy of escitalopram, but differences in criteria for inclusion of trials and statistical methodologies of these studies make direct comparisons difficult. In addition, the “universe” of known trials available for meta-analysis is a moving target, as new randomized controlled trials are added to the clinical trials database. For example, there were 22 comparative randomized controlled trials of escitalopram available for review, but none of the meta-analysis studies included all of them.
Overall, a comprehensive pooled analysisCitation16 and a network meta-analysisCitation42 both found evidence for superiority of escitalopram over other comparators, although one modified network meta-analysisCitation37 did not. However, there is consistent evidence that escitalopram is superior in efficacy to other SSRIs, especially citalopram. The pooled and meta-analysis studies with citalopram (with 3–8 trials included in each study) show consistent statistically significant findings in favor of escitalopram in WMD (1.13–1.73 MADRS points), response rate difference (7.0%–8.3%), and remission rate difference (5.1%–17.6%). A pooled analysis of two paroxetine trials also found superiority of escitalopram in these outcomes. Similarly, a pooled comparison of escitalopram with all SSRIs combined together (12 trials) also found significant differences in favor of escitalopram, although the effect sizes were smaller.Citation16
The comparative efficacy of escitalopram with SNRIs and other agents is less clear. Pooled analyses found significant superiority over duloxetine,Citation34 but no differences with venlafaxine XR,Citation33 while meta-analyses found no differences with either.Citation36,Citation37 A pooled analysisCitation35 (using MADRS scores) of the two SNRIs combined also favored escitalopram, while a meta-analysisCitation16 (using Ham-D scores) did not. The reason for the discrepancy between the pooled and meta-analysis studies of SNRIs may be owing to the small number of randomized controlled trials available, in that pooled analysis of individual patient data has greater power to detect differences than meta-analysis. The one comparison with bupropion (a pooled analysis of two trials) found no comparative differences, but in that analysis the bupropion group did not significantly differentiate from placebo in the primary outcome (WMD), whereas the escitalopram group did.Citation29
Despite the consistent evidence for superiority of escitalopram over SSRIs, there is still contention about the clinical importance of the differences. For example, very similar results were found in the pooled and meta-analysis studies with citalopram, but some authors interpreted their results as clinically relevant, while others did not. A major issue is that there is still no consensus about the definition for a minimal clinically important difference (MCID) for drug-placebo comparisons. Some suggested criteria for drug-placebo MCID with antidepressants include a MADRS difference of 2 points or a response rate difference of 10% (corresponding to a number needed to treat of 10).Citation43 Moreover, it is unclear whether active drug comparisons should use the same MCID as drug-placebo comparisons. If so, to be considered superior, an antidepressant would need to show an additional 2-point MADRS difference against a comparator, or 4 points relative to placebo; this seems to be an unreasonably difficult threshold to achieve. Hence, some investigators have suggested that the MCID between two active agents should be half the drug-placebo MCID, corresponding to at least 1 point MADRS difference, or 5% response rate difference (number needed to treat = 20).Citation44 Using these MCID criteria, the superiority of escitalopram over citalopram and other SSRIs would be considered clinically relevant.
Because of the high placebo response in clinical trials, some investigators have also suggested using methods to increase assay sensitivity for detecting clinically relevant differences between antidepressants. One such method is to examine subgroups of patients with higher baseline severity of symptoms. The more severely depressed subgroup, usually defined as MADRS ≥ 30 or Ham-D ≥ 25, may have better responses to medication and/or lower responses to placebo, either of which make it easier to detect specific effects of the active medications. In this review, the pooled analysis studies that examined severely depressed subgroups found larger efficacy differences for escitalopram, with WMDs ranging from 1.4 to 3.8 MADRS points and response rate differences ranging from 6.6% to 19.1%. These differences are well within any definition for clinical importance. The results are consistent with evidence from pooled analyses that the comparative effect sizes in favor of escitalopram increase with increasing baseline severity.Citation16,Citation24,Citation25 They are also consistent with those from head-to-head randomized controlled trials that prospectively enrolled patients with severe depression.Citation45,Citation46 Together, these studies provide some validation that the modest efficacy differences with escitalopram are clinically significant.
An important question is why should escitalopram have superior efficacy compared with racemic citalopram and other SSRIs? Biochemical studies have demonstrated that there are two distinct binding sites on the serotonin transporter protein, ie, a high-affinity, primary binding site that mediates the inhibition of serotonin reuptake, and a low affinity site that allosterically modulates the affinity of ligands at the primary site.Citation9,Citation47 Escitalopram uniquely binds to both the primary and allosteric sites,Citation48 leading to enhanced serotonergic neurotransmission and subsequent downstream effects on synaptic plasticity and neurogenesis.Citation49–Citation51 The R-enantiomer in racemic citalopram binds only to the allosteric site, which interferes with the effects of escitalopram and counteracts its allosteric modulatory action.Citation9,Citation52 The additional allosteric mechanism of escitalopram, which appears to be unique among SSRI antidepressants,Citation53 may explain its efficacy advantages in patients with MDD.
This systematic review also found evidence from the pooled analysis studies that escitalopram had lower withdrawal rates owing to adverse events compared with SNRIs, but not with citalopram or other SSRIs. Similarly, a multiple treatments meta-analysis found that escitalopram was one of four newer-generation antidepressants (along with bupropion, citalopram, and sertraline) that showed superior acceptability (based on all-cause withdrawals).Citation42 These results are also consistent with a pooled analysis from a clinical trial database of over 4000 patients showing that escitalopram demonstrated very good safety and tolerability for treatment of MDD and anxiety disorders.Citation13 Like other SSRIs, escitalopram is associated with sexual side effects, with pooled studies showing higher rates compared with bupropion.Citation29 However, a meta-analysis of studies using specific sexual functioning questionnaires suggested that escitalopram may have lower rates than other SSRIs.Citation54
The limitations of this systematic review must be considered. The meta-analysis and pooled studies were based on randomized controlled trials which mostly were eight weeks or less in duration. It is possible that any efficacy differences between escitalopram and comparators decrease over time. Similarly, doses may not have been optimized in the trials and results of randomized controlled trials may not be generalizable to more real-world conditions. Combining agents within a class (eg, all SSRIs, all SNRIs) as comparators may not be valid, especially because there is evidence that some agents within a class have greater efficacy than others. Finally, the total number of comparative randomized controlled trials of escitalopram (23 trials to November 2010) in MDD is still relatively low.
Conclusion
This systematic review of pooled analysis and meta-analysis studies found that escitalopram has superior efficacy compared with citalopram and SSRIs combined, and that the efficacy differences are modest but clinically relevant, especially in more severely depressed patients. Escitalopram also has at least similar efficacy to SNRIs and bupropion. In multiple-treatments (network) meta-analysis studies, escitalopram was one of four newer-generation antidepressants with evidence for superiority compared with the others. The efficacy differences of escitalopram may be related to its dual mechanism of action on the primary and allosteric binding sites on the serotonin transporter.
Many clinical factors, including efficacy, side effect profile, drug interactions, relapse prevention, simplicity of use, and cost-effectiveness must be considered together when making a clinical decision for a first-choice antidepressant.Citation7 This systematic review of the efficacy of escitalopram should add to the evidence database to help guide clinicians on the choice of an appropriate medication.
Disclosure
Dr Ali reports no disclosures. Dr Lam is on Speaker/Advisory Boards for, or has received research funds from, Advanced Neuromodulation Systems Inc (St Jude Medical), AstraZeneca, BrainCells Inc, Biovail, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Research Foundation, Eli Lilly, Janssen, Litebook Company Ltd., Lundbeck, Lundbeck Institute, Mathematics of Information Technology and Advanced Computing Systems, Michael Smith Foundation for Health Research, Servier, Takeda, UBC Institute of Mental Health/Coast Capital Savings, and Wyeth.
References
- PattenSBKennedySHLamRWCanadian Network for Mood and Anxiety Treatments (CANMAT) Clinical Guidelines for the Management of Major Depressive Disorder in Adults. I. Classification, burden and principles of managementJ Affect Disord2009117Suppl 1S5S1419674796
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med20063e44217132052
- StephensTJoubertNThe economic burden of mental health problems in CanadaChronic Dis Can200122182311397346
- GreenbergPEKesslerRCBirnbaumHGThe economic burden of depression in the United States: How did it change between 1990 and 2000?J Clin Psychiatry2003641465147514728109
- SobockiPJonssonBAngstJRehnbergCCost of depression in EuropeJ Ment Health Policy Econ20069879817007486
- DavidsonJRMajor depressive disorder treatment guidelines in America and EuropeJ Clin Psychiatry201071Suppl E1e0420371031
- LamRWKennedySHGrigoriadisSCanadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. PharmacotherapyJ Affect Disord2009117Suppl 1S26S4319674794
- AndersonIMFerrierINBaldwinRCEvidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2000 British Association for Psychopharmacology guidelinesJ Psychopharmacol20082234339618413657
- SanchezCThe pharmacology of citalopram enantiomers: The antagonism by R-citalopram on the effect of S-citalopramBasic Clin Pharmacol Toxicol200699919516918708
- ThaseMEManaging depressive and anxiety disorders with escitalopramExpert Opin Pharmacother2006742944016503815
- WaughJGoaKLEscitalopram: A review of its use in the management of major depressive and anxiety disordersCNS Drugs20031734336212665392
- MontgomerySABaldwinDSBlierPWhich antidepressants have demonstrated superior efficacy? A review of the evidenceInt Clin Psychopharmacol20072232332917917550
- BaldwinDSReinesEHGuitonCWeillerEEscitalopram therapy for major depression and anxiety disordersAnn Pharmacother2007411583159217848424
- LiebermanJAGreenhouseJHamerRMComparing the effects of antidepressants: Consensus guidelines for evaluating quantitative reviews of antidepressant efficacyNeuropsychopharmacology20053044546015647752
- LamRWKennedySHUsing metaanalysis to evaluate evidence: Practical tips and trapsCan J Psychiatry20055016717415830827
- KennedySHAndersenHFThaseMEEscitalopram in the treatment of major depressive disorder: A meta-analysisCurr Med Res Opin20092516117519210149
- KennedySHAndersenHFLamRWEfficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: A meta-analysisJ Psychiatry Neurosci20063112213116575428
- LamRWLonnSLDespiegelNEscitalopram versus serotonin noradrenaline reuptake inhibitors as second step treatment for patients with major depressive disorder: A pooled analysisInt Clin Psychopharmacol20102519920320357664
- WadeAGSchlaepferTEAndersenHFKiltsCDClinical milestones predict symptom remission over 6-month and choice of treatment of patients with major depressive disorder (MDD)J Psychiatr Res20094356857518954875
- KasperSSpadoneCVerpillatPAngstJOnset of action of escitalopram compared with other antidepressants: Results of a pooled analysisInt Clin Psychopharmacol20062110511016421462
- PapakostasGILarsenKTesting anxious depression as a predictor and moderator of symptom improvement in major depressive disorder during treatment with escitalopramEur Arch Psychiatry Clin Neurosci2010922 [Epub ahead of print]
- WadeAFriisAHThe onset of effect for escitalopram and its relevance for the clinical management of depressionCurr Med Res Opin2006222101211017076970
- GormanJMKorotzerASuGEfficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: Pooled analysis of placebo-controlled trialsCNS Spectr20027404415131492
- KiltsCDWadeAGAndersenHFSchlaepferTEBaseline severity of depression predicts antidepressant drug response relative to escitalopramExpert Opin Pharmacother20091092793619317630
- LamRWAndersenHFThe influence of baseline severity on efficacy of escitalopram and citalopram in the treatment of major depressive disorder: An extended analysisPharmacopsychiatry20063918018416944409
- BechPAndersenHFWadeAEffective dose of escitalopram in moderate versus severe DSM-IV major depressionPharmacopsychiatry20063912813416871468
- LlorcaPMAzorinJMDespiegelNVerpillatPEfficacy of escitalopram in patients with severe depression: A pooled analysisInt J Clin Pract20055926827515857321
- LepolaUWadeAAndersenHFDo equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorderInt Clin Psychopharmacol20041914915515107657
- ClaytonAHCroftHAHorriganJPBupropion extended release compared with escitalopram: Effects on sexual functioning and antidepressant efficacy in 2 randomized, double-blind, placebo-controlled studiesJ Clin Psychiatry20066773674616841623
- AuquierPRobitailSLlorcaPMRiveBComparison of escitalopram and citalopram efficacy: A meta-analysisInt J Psychiatry Clin Pract20037259268
- SoaresCNThaseMEClaytonADesvenlafaxine and escitalopram for the treatment of postmenopausal women with major depressive disorderMenopause20101770071120539246
- KasperSBaldwinDSLarssonLSBoulengerJPSuperiority of escitalopram to paroxetine in the treatment of depressionEur Neuropsychopharmacol20091922923719185467
- MontgomerySAAndersenHFEscitalopram versus venlafaxine XR in the treatment of depressionInt Clin Psychopharmacol20062129730916877901
- LamRWAndersenHFWadeAGEscitalopram and duloxetine in the treatment of major depressive disorder: A pooled analysis of two trialsInt Clin Psychopharmacol20082318118718545055
- KornsteinSGLiDMaoYLarssonSAndersenHFPapakostasGIEscitalopram versus SNRI antidepressants in the acute treatment of major depressive disorder: Integrative analysis of four double-blind, randomized clinical trialsCNS Spectr20091432633319668123
- CiprianiASantilliCFurukawaTAEscitalopram versus other antidepressive agents for depressionCochrane Database Syst Rev2009CD00653219370639
- GartlehnerGGaynesBNHansenRAComparative benefits and harms of second-generation antidepressants: Background paper for the American College of PhysiciansAnn Intern Med200814973475019017592
- TrkuljaVIs escitalopram really relevantly superior to citalopram in treatment of major depressive disorder? A meta-analysis of head-to-head randomized trialsCroat Med J201051617320162747
- MontgomerySHansenTKasperSEfficacy of escitalopram compared with citalopram: A meta-analysisInt J Neuropsychopharmacol201018
- SalantiGHigginsJPAdesAEIoannidisJPEvaluation of networks of randomized trialsStat Methods Med Res20081727930117925316
- LumleyTNetwork meta-analysis for indirect treatment comparisonsStat Med2002212313232412210616
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysisLancet200937374675819185342
- DuruGFantinoBThe clinical relevance of changes in the Montgomery-Asberg Depression Rating Scale using the minimum clinically important difference approachCurr Med Res Opin2008241329133518377706
- MontgomerySAMollerHJIs the significant superiority of escitalopram compared with other antidepressants clinically relevantInt Clin Psychopharmacol20092411111819357527
- BoulengerJPHuusomAKFloreaIBaekdalTSarchiaponeMA comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patientsCurr Med Res Opin2006221331134116834832
- MooreNVerdouxHFantinoBProspective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorderInt Clin Psychopharmacol20052013113715812262
- ChenFLarsenMBNeubauerHASanchezCPlengePWiborgOCharacterization of an allosteric citalopram-binding site at the serotonin transporterJ Neurochem200592212815606893
- SanchezCBogesoKPEbertBReinesEHBraestrupCEscitalopram versus citalopram: The surprising role of the R-enantiomerPsychopharmacology (Berl)200417416317615160261
- El MansariMSanchezCChouvetGRenaudBHaddjeriNEffects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: An in vivo electrophysiological study in rat brainNeuropsychopharmacology2005301269127715702136
- Mnie-FilaliOEl MansariMEspanaASanchezCHaddjeriNAllosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticityNeurosci Lett2006395232716330146
- Mnie-FilaliOFaureCMansariMER-citalopram prevents the neuronal adaptive changes induced by escitalopramNeuroreport2007181553155617885600
- StorustovuSSanchezCPorzgenPR-citalopram functionally antagonises escitalopram in vivo and in vitro: Evidence for kinetic interaction at the serotonin transporterBr J Pharmacol200414217218015037515
- ChenFLarsenMBSanchezCWiborgOThe S-enantiomer of R, S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitorsEur Neuropsychopharmacol20051519319815695064
- SerrettiAChiesaATreatment-emergent sexual dysfunction related to antidepressants: A meta-analysisJ Clin Psychopharmacol20092925926619440080
- LanconCSapinCNoteIFarisseJComparison of escitalopram and citalopram in outpatients with severe major depressive disorder: A prospective, naturalistic 8-week studyInt J Psychiatry Clin Pract200610131137
