Abstract
Auditory entrainment can influence gait performance in movement disorders. The entrainment can incite neurophysiological and musculoskeletal changes to enhance motor execution. However, a consensus as to its effects based on gait in people with cerebral palsy is still warranted. A systematic review and meta-analysis were carried out to analyze the effects of rhythmic auditory cueing on spatiotemporal and kinematic parameters of gait in people with cerebral palsy. Systematic identification of published literature was performed adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses and American Academy for Cerebral Palsy and Developmental Medicine guidelines, from inception until July 2017, on online databases: Web of Science, PEDro, EBSCO, Medline, Cochrane, Embase and ProQuest. Kinematic and spatiotemporal gait parameters were evaluated in a meta-analysis across studies. Of 547 records, nine studies involving 227 participants (108 children/119 adults) met our inclusion criteria. The qualitative review suggested beneficial effects of rhythmic auditory cueing on gait performance among all included studies. The meta-analysis revealed beneficial effects of rhythmic auditory cueing on gait dynamic index (Hedge’s g=0.9), gait velocity (1.1), cadence (0.3), and stride length (0.5). This review for the first time suggests a converging evidence toward application of rhythmic auditory cueing to enhance gait performance and stability in people with cerebral palsy. This article details underlying neurophysiological mechanisms and use of cueing as an efficient home-based intervention. It bridges gaps in the literature, and suggests translational approaches on how rhythmic auditory cueing can be incorporated in rehabilitation approaches to enhance gait performance in people with cerebral palsy.
Introduction
Cerebral palsy is a common developmental disorder.Citation1,Citation2 The global prevalence of cerebral palsy is approximately 1.5–3.5/1,000 children,Citation3,Citation4 and is supposedly growing in developing countries.Citation5 Cerebral palsy is primarily characterized by pre/postnatal damage to the brain,Citation3 often predisposing to grave neuromuscular and psychological disorders.Citation3,Citation6 The treatment of cerebral palsy inflicts substantial costsCitation7 and adversely impacts quality of life.Citation8,Citation9 Typically, motor dysfunction in cerebral palsy is characterized by spastic or extrapyramidal deficits.Citation10 These neuromuscular dysfunctions might cause dyskinesia, dystonia, ataxia, or hypotonia.Citation11,Citation12 Further, these might lead to increased fatigue, reduced dexterity/coordination, postural instability, muscle contracture, and joint subluxation. Also, these neuromuscular disorders progress with aging.Citation11 For instance, lack of mobility and hypertonia often lead to development of muscle and joint contractures and secondary bone deformities. These neuromuscular deficits among both children and older adults with cerebral palsy considerably impair kinetic and kinematic changes, impair locomotion, and predispose to falls. For instance, exaggerated anterior stooping posture associated with increased anterior tilt in the pelvis, hip flexion, adduction, and internal rotationCitation13–Citation15 adversely impact efficiency in energy expenditureCitation16 and spatiotemporal gait parameters.Citation17 Bourgeois et alCitation18 reported reduction in spatiotemporal gait parameters, such as cadence, stride length, and gait velocity associated with considerable enhancement in gait variability, which might predispose severely toward falls.Citation19
In addition to these musculoskeletal changes, Rosenbaum et alCitation2 suggested considerable discrepancies in sensory perceptions, cognition, and behavior. Neuroimaging studies report deficits in the dorsolateral prefrontal cortex, dorsal anterior cingulate gyrus,Citation20 somatosensory cortex,Citation21 and cerebellumCitation22 which might considerably impair intellectual and cognitive performance.Citation22–Citation24 Likewise, deficits in corticospinal, thalamocortical, superior occipitofrontal and superior longitudinal pathways have also been reported.Citation12,Citation20,Citation25 Together, these psychological constraints might also impair motor performance, such as in a dual-task scenario. For instance, Hung et alCitation26 reported drops in gait-performance measures in unilateral cerebral palsy patients while performing a dual task. Studies have suggested that this modification in gait patterns might happen due to an alleviation in “internal” conscious attention toward autonomic control that adversely impacts proprioception and autonomic functioning, possibly because of movement-specific reinvestment.Citation27–Citation29 The theory suggests that directing attention internally to control autonomic movements, such as gait, can have an adverse impact on performance,Citation29 especially in high-stress situations.Citation30 Common treatment strategies to curb motor dysfunctions in cerebral palsy include training with virtual reality,Citation31 biocueing,Citation32 physical/occupational therapy,Citation33 physical exercise,Citation34 treadmill,Citation35 and orthosis.Citation36,Citation37
Recently, several studies have tried to address the sensorimotor deficits in people with cerebral palsy by applying rhythmic auditory entrainment.Citation38–Citation41 Cueing aims to counteract sensory deficits, and has been shown to modulate neuromagnetic β-oscillations,Citation42 cortical reorganization, enhance biological motion perception,Citation43,Citation44 motor imagery,Citation45,Citation46 neural plasticity,Citation48 reduce shape variability in musculoskeletal-activation patterns,Citation47 and movement-specific reinvestment.Citation49 Moreover, as a cheapCitation50 and viableCitation51 treatment strategy, this approach can provide substantial benefits in developing countries, where prevalence of cerebral palsy due to socioeconomic factors is more prominent.Citation52,Citation53 We identified high-quality systematic reviews analyzing the effects of external auditory cueing on gait performance among healthy,Citation121 ParkinsonismCitation54–Citation56,Citation122 and stroke participants.Citation57–Citation59 However, to the best of our knowledge, no systematic or narrative analysis has been carried out to analyze the effects of auditory entrainment on gait in people with cerebral palsy. Therefore, we attempted to develop a state of knowledge for the use of cerebral palsy patients and medical practitioners, where both qualitative and quantitative data from high-quality studies can be interpreted.
Materials and methods
This review was conducted according to the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statementCitation60 and American Academy for Cerebral Palsy and Developmental Medicine (AACPDM) methodology for systematic reviews.Citation61
Data sources and search strategy
The academic databases Web of Science, PEDro, EBSCO, Medline, Cochrane Central Register of Controlled Trials, Embase and ProQuest were searched from inception until July 2017. A sample-search strategy is provided in .
Table 1 Sample search strategy – Embase
Data extraction
Upon selection for review, data extracted from each article were study aim, selection criteria, sample size, sample description (sex, age, health status), intervention, characteristics of auditory cueing, dual tasks, outcome measures, results, and conclusions. The data were then summarized and tabulated (). The inclusion criteria for the studies were: randomized controlled trials, cluster-randomized controlled trials, or controlled clinical trials; reporting reliable and valid spatiotemporal gait and kinematic parameters; including dynamic aspects of gait stability; use of PEDro methodological quality scale (score ≥4); conducted on human participants; published in a peer-reviewed academic journal; and published in English, German, or Korean.
Table 2 Studies analyzing the effects of RAC on gait
Quality and risk-of-bias assessment
The quality of the studies was assessed using the PEDro methodological quality scale.Citation62 The scale consists of eleven items addressing external validity, internal validity, and interpretability, and can detect potential bias with high reliability,Citation63 and validity.Citation62 A blinded rating of the methodological quality of the studies was carried out by the first (SG), second (IG) and third (AOE) reviewers. Ambiguous issues were discussed between reviewers and consensus was reached. Included studies were rated and interpreted according to scoring of 9–10, 6–8, and 4–5 for “excellent”, “good”, and “fair” quality,Citation64 respectively. Inadequate randomization, nonblinding of assessors, no intention-to-treat analysis, and no measurement of compliance were considered as major threats for biasing.Citation65
Data analysis
This systematic review also included a meta-analysis approach to develop a better understanding of the incorporated interventions.Citation66 Presence and lack of heterogeneity drove the use of either random- or fixed-effect meta-analysis.Citation67 A narrative synthesis of the findings structured according to intervention, population characteristics, methodological quality, and type of outcome is provided (). A meta-analysis was conducted between pooled studies using Comprehensive Meta-Analysis software (version 2.0; Biostat, Englewood, NJ, USA). Heterogeneity among the studies was assessed using I2 statistics. The data in this review were systematically distributed, and for each available variable pooled, dichotomous data were analyzed and forest plots with 95% CIs reported. Effect sizes were adjusted and reported as Hedge’s g.Citation68 Thresholds for interpretation of effect sizes were as follows: standard mean effect of 0 meant no change, negative effect meant a negative change, and a positive effect meant a positive change.
Mean effect of 0.2 was interpreted as a small effect, 0.5 a medium effect, and 0.8 a large effect.Citation69 Interpretation of heterogeneity via I2 statistics was 25%, 50%,75% as negligible, moderate and substantial heterogeneity, respectively. 75% as negligible, moderate, and substantial heterogeneity, respectively. Meta-analysis reports, including heterogeneity among studies, were evaluated to determine the reason for heterogeneity, and studies included were then pooled separately and analyzed again in a sub-group analysis. The α-level was set at 95%.
Results
Characteristics of studies included
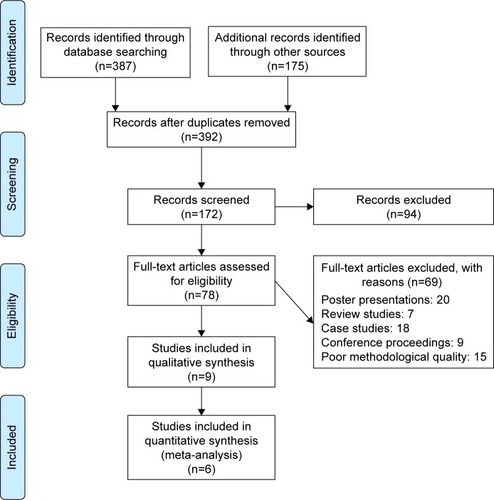
Our initial search across academic databases yielded a total of 387 studies; 175 studies were included from a personal library. After implementing our inclusion/exclusion criteria, nine studies were left (). Data from the studies included have been summarized in . Of the nine studies included, all were controlled clinical trials.
Participants
A total of 227 participants were analyzed in the incorporated studies. In the studies included, eight studies incorporated mixed-sex patients. Only one study included male participants.Citation70 The studies provided data on 227 participants (n=119 females/108 males). Moreover, in 108 children, the sex distribution was 57 females to 51 males, and for adults 62 females to 57 males. Descriptive statistics relating to the age (means ± SD) of the participants were tabulated across the studies ().
Risk of bias
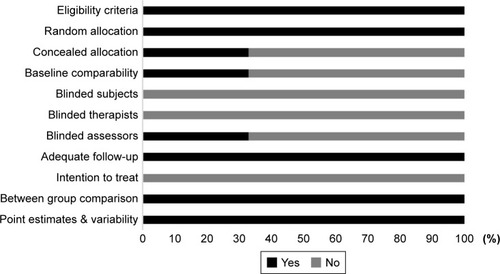
To reduce the risks of bias, studies scoring ≥4 on PEDro were included in the review. Moreover, research protocols to be included in the review were limited to gold-standard randomized controlled trials, cluster-randomized controlled trials, and controlled clinical trials. The individual scores attained by studies using the PEDro scale are reported in , and . The average PEDro score for the nine studies was 5 of 10, indicating fair quality for studies overall. Three studies scored 7, and six studies scored 4. Risk of bias across the studies is shown in .
Outcomes
The results provided evidence for a positive impact of rhythmic auditory cueing on spatiotemporal and kinematic gait parameters among adults and children with cerebral palsy. In all studies, significant enhancement in primary spatiotemporal and kinematic gait parameters were reported.
Meta-analyses
The evaluation of research studies via meta-analysis requires strict inclusion criteria to limit heterogeneity efficiently.Citation71 However, among the pooled group of studies after strict inclusion criteria, some unexplained heterogeneity was still observed. Subgroup analyses were then performed for identical studies to evaluate the cause of the heterogeneity. The parameters evaluated were spatiotemporal gait parameters, such as cadence, stride length, gait velocity, and kinematic the effects of rhythmic auditory cueing at preferred cadence on gait velocity in both adults and children separately. We included a generalized group analysis by first combining all the pooled studies. The studies excluded differed considerably in assessment methods or if descriptive statistics were not mentioned in the manuscript. However, attempts were made to contact the coauthors for the data.
Gait velocity
Gait velocity was analyzed in six studies. Here, two studies evaluated the effects of rhythmic auditory cueing on gait velocity in adultsCitation38,Citation72 and four in childrenCitation41,Citation73–Citation75 with cerebral palsy. One study included assessment of gait velocity while using patterned sensory enhancementCitation73 as the mode of auditory feedback. Analysis of studies revealed () a large positive effect (g=1.13, 95% CI 0.33–1.94). Substantial heterogeneity was observed between studies (I2=84%, P>0.01). Subgroup analyses were conducted to explore heterogeneity.
Figure 3 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on gait velocity in people with cerebral palsy.
Abbreviations: A, adults; C, children.
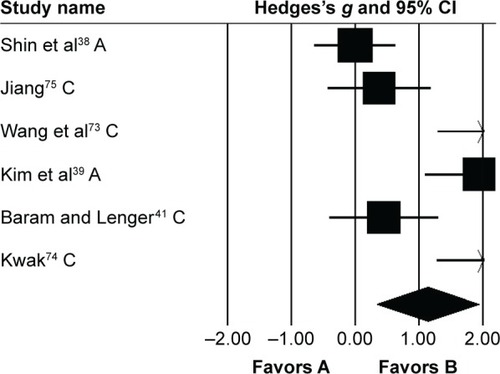
An analysis for effects of rhythmic auditory cueing on gait velocity in children revealed (), large positive effect with substantial heterogeneity (g=1.24, 95% CI 0.31–2.17, I2=81%; P<0.01). Here, the heterogeneity could possibly be attributed to different training regimes in the studies, ie, no training was included by one,Citation41 while othersCitation73–Citation75 had training regimes for ≥3 weeks. Subgroup analysis revealed () a large positive effect with substantial heterogeneity (g=1.53, 95% CI 1.07–1.98, I2=82%; P<0.01). Moreover, JiangCitation75 included only one training session per week. Whereas, others performed training for three (Wang et al.Citation73), and five times (KwakCitation74), per week. Subgroup analysis revealed a large positive effect with negligible heterogeneity (g=2.05, 95% CI 1.5–2.6, I2=0; P>0.05). Finally, subgroup analysis evaluating the effects of rhythmic auditory cueing on gait velocity in adults revealed a large positive effect with negligible heterogeneity (g=0.95, 95% CI −0.95 to 2.85, I2=0; P>0.05).
Stride length
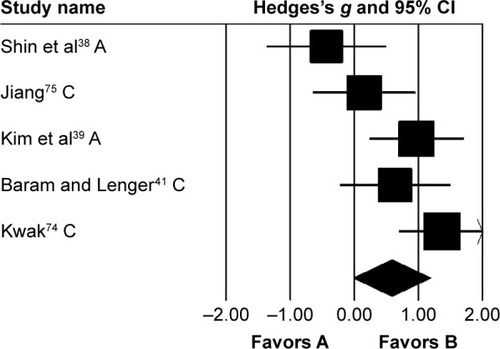
Stride length was analyzed in five studies. Two and three studies evaluated the effects of rhythmic auditory cueing on stride length in adultsCitation38,Citation72 and children,Citation41,Citation73–Citation75 respectively. Analysis revealed () a medium positive effect (g=0.58, 95% CI −0.02 to 1.19). Moderate heterogeneity was observed between studies (I2=65%, P>0.01). Subgroup analyses were conducted to explore the cause of heterogeneity. Analysis for effects of rhythmic auditory cueing on stride length in children revealed () a medium positive effect with negligible heterogeneity (g=0.75, 95% CI 0.01–1.48, I2=0; P>0.05). Subgroup analysis evaluating the effects of rhythmic auditory cueing on stride length in adults revealed a comparably smaller medium effect with negligible heterogeneity (g=0.3, 95% CI −1.07 to 1.67, I2=0; P>0.05).
Figure 4 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on stride length in people with cerebral palsy.
Abbreviations: A, adults; C, children.
Cadence
Cadence was analyzed in five studies, of which two evaluated the effects of rhythmic auditory cueing on cadence in adultsCitation38,Citation72 and three in childrenCitation41,Citation73–Citation75 with cerebral palsy. Analysis of studies revealed () a medium positive effect (g=0.33, 95% CI −0.41 to 1.07). Substantial heterogeneity was observed between studies (I2=79%, P>0.01). Subgroup analyses were conducted to explore heterogeneity. An analysis for effects of rhythmic auditory cueing on cadence in children revealed a small negative effect with negligible heterogeneity (g=−0.11, 95% CI −0.97 to 0.74, I2=0; P>0.05). Subgroup analysis evaluating the effects of rhythmic auditory cueing on cadence in adults revealed a large positive effect with negligible heterogeneity (g=1.04, 95% CI 0.44–1.64, I2=0; P>0.05).
Figure 5 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on cadence in people with cerebral palsy.
Abbreviations: A, adults; C, children.
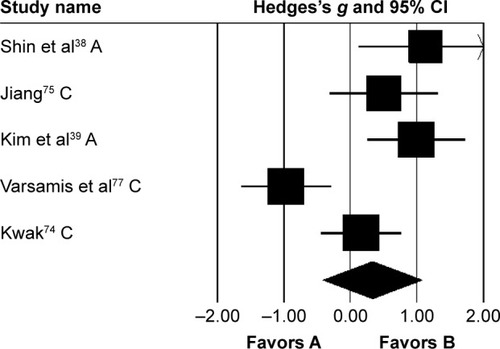
Kinematic parameters
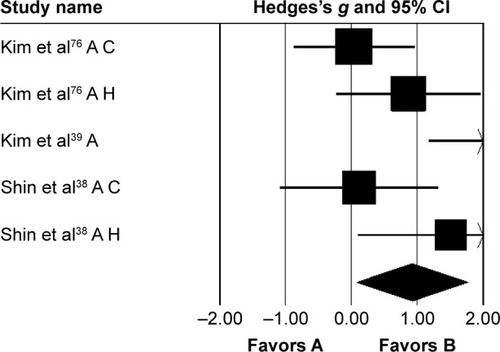
Three studies analyzed the effects of rhythmic auditory cueing on gait-dynamic index (a combined measure of lower-limb kinematic performance). Data for subgroup analysis on the gait dynamic index concerning community and household dwellers were extracted from two studies.Citation38,Citation76 Analysis revealed () a large positive effect (g=0.92, 95% CI 0.07–1.76, I2=0; P<0.01) with negligible heterogeneity. Further, an analysis of gait-dynamic index in community dwellers revealed a small positive effect with negligible heterogeneity (g=0.07, 95% CI −0.66 to 0.8, I2=0; P>0.05). Comparably, analysis of household dwellers revealed a large positive effect with negligible heterogeneity (g=1.11, 95% CI 0.24–1.98, I2=0; P>0.05). Subgroup analysis was also conducted on individual kinematic parameters to specify the magnitude of effects of rhythmic auditory cueing on specific joint kinematics.
Figure 6 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on gait-dynamic index in people with cerebral palsy.
Abbreviations: A, adults; C, children; H, household dwellers.
Subgroup analysis evaluating changes at the pelvis revealed () small negative effects with negligible heterogeneity (g=−0.23, 95% CI −0.68 to 0.21, I2=0; P>0.05). At the hip joint, medium negative effects with moderate heterogeneity (g=−0.43, 95% CI −0.89 to 0.01, I2=33.5%; P>0.01) were observed (). At the knee joint, medium positive effects with negligible heterogeneity (g=0.26, 95% CI −0.18 to 0.71, I2=0; P>0.05) were observed (). At the ankle joint, medium positive effects with moderate heterogeneity (g=0.36, 95% CI −0.09 to 0.81, I2=32.7%; P>0.01) were observed (). Finally, at the foot, small negative effects with moderate heterogeneity (g=−0.18, 95% CI −0.62 to 0.26, I2=0; P>0.05) were observed ().
Discussion
The primary objective of this systematic review and meta-analysis was to synthesize the current state of knowledge for the effects of rhythmic auditory cueing on gait in people with cerebral palsy. All nine studies reported beneficial effects of rhythmic auditory cueing on gait parameters in children and adults with cerebral palsy. Further, the meta-analysis found significant small–large standardized effects for the benefits of rhythmic auditory cueing on spatiotemporal and kinematic parameters of gait among patients affected with cerebral palsy.
Typically, spatiotemporal parameters of gait may worsen over time in those with cerebral palsy. Deficits in periventricular white matter,Citation12 gray matter,Citation78 cerebellum,Citation79 basal ganglia,Citation80 and thalamusCitation81 have been well documented.Citation12 These neural centers play an integral role in managing stabilization and performance during automated tasks, such as posture and gait.Citation82,Citation83 In addition, increasing psychological stress might be exerted on automated control for posture, gait, and cognitive processing by deficits reported in corticospinal, thalamocortical, superior occipitofrontal, and longitudinal pathways,Citation84–Citation86 possibly also explaining the loss of gait rhyth-micity.Citation87 Likewise, increased energy expenditure,Citation88 associated variability in muscle contraction, and force production add to the instability.Citation89 Rhythmic auditory cueing seems to counter these deficits efficiently. The current meta-analysis reported enhancements in gait velocity (1.24) and stride length (0.75) in children and gait velocity (0.95), stride length (0.3), and cadence (1.04) in adults. Beneficial effects were also observed in gross gait-dynamic index (a combined measure of kinematic variables during gait) for adult patients affected with cerebral palsy (0.92).
Several mechanisms have been suggested for the beneficial effects of rhythmic auditory cueing. For instance, auditory entrainment might aid in reducing errors while executing gait by guiding specific movement patterns.Citation90,Citation91 External entrainment might act as guidance for “heel-contact” and “push-off” timing and/or muscle contractions.Citation39 Likewise, such cross-sensory cueing might also reduce information overload in the native sensory modality by directing task-irrelevant information toward the underused sensory modality.Citation92 The application of auditory entrainment is believed to allow enhancement in gait performance by bypassing or facilitating the frontostriatal pathway via alternative pathways.Citation93–Citation95 Cunnington et alCitation96 reaffirmed and suggested that rhythmic cueing might directly serve as an input supplementary motor area, thereby reducing the onset of motor deficits and aiding in performance. Moreover, cueing has been shown to allow modulation of neuromagnetic β-oscillations in the auditory cortex, cerebellum, inferior frontal gyrus, somatosensory area, and sensorimotor cortexCitation42 and reduce hemispheric asymmetry.Citation97 Also, enhanced activation in inferior colliculi,Citation98 cerebellum, brain stem,Citation94,Citation99 and sensorimotor cortexCitation100,Citation101 have been reported. This might also suggest the facilitation of corticocerebellar network reorganization.Citation48 Finally, entrainment has also been shown to reduce variability in electromyographic activityCitation102 and optimize velocity/acceleration profiles of joint motions by scaling movement time,Citation103 thereby allowing stable pattern generation.
Studies have shown that rhythmic auditory cueing might also be an efficient tool to counteract dual-task-associated information-processing constraints.Citation121,Citation122 For instance, Lohnes and EarhartCitation104 suggested that rhythmic entrainment might allow alleviation in gait performance by possibly freeing up cognitive resources for dual-task execution. Although dual-task performance has been shown to reduce performance in people with cerebral palsy,Citation26 we did not identify any study analyzing the effects of rhythmic auditory cueing under higher information-processing constraints. We suggest future studies address this substantial gap in the literature. Moreover, recent studies evaluating the effects of rhythmic entrainment have revealed beneficial effects of action-relevant acoustic input on gait performanceCitation105,Citation122,Citation123 as compared to normal isosynchronous cueing.Citation106 Ecologically valid action-related sounds have been suggested to enhance salience of sensory information concerning spatiotemporal information, thereby aiding movement execution.Citation105–Citation107 Moreover, recent research has revealed the possibilities of including emotional,Citation108 motivational,Citation109 and expressivenessCitation110 components in auditory entrainment to portray differential effects on gait parameters. Unfortunately, a lack of pertinent literature concerning the specific type of modified auditory cueing in cerebral palsy limits our interpretation of the type of auditory cueing that might be beneficial in rehabilitation. Therefore, we suggest future studies address this gap.
Finally, we believe that auditory entrainment might be efficient because of its economical nature and high viability.Citation50,Citation51 The rhythmic entrainment factor could be utilized with music in rehabilitation and day-to-day lives. This could allow benefits in psychophysiological domains.Citation111,Citation112 Moreover, it is important to consider that the retention of enhancements in gait parameters relies not only on the training received in the clinic but also largely on how much the patient follows the treatment protocol at home. In the present meta-analyses, enhancements in kinematic gait parameters observed for household ambulators (1.11) were considerably larger compared to community ambulators (0.07). We believe that delivering this type of home-based intervention could be beneficial for people lacking access to medical interventions in developing countries.Citation113 The growing number of smartphone devices in developing countriesCitation114 can be used as a delivery tool while using a simple metronome app, such as WalkMateCitation115 or ListenMee,Citation116 which with proper medical guidance might allow curbing of motor deficits associated with aging.Citation117 We also suggest the use of rhythmic auditory cueing as an adjunct to other rehabilitation strategies, eg, assistive devices,Citation16,Citation124,Citation125 swimming, or other aquatic exercise regimes,Citation118 as it might enhance stability-associated quality of lifeCitation119,Citation120 and rehabilitation progress by focusing on psychophysiological components.
In conclusion, to the best of our knowledge, this review analyzes for the first time the effects of auditory entrainment on adults and children with cerebral palsy. The present findings are in agreement with systematic reviews and meta-analyses carried out to analyze auditory entrainment effects on healthy,Citation121 strokeCitation57 and parkinsonism population groups.Citation54,Citation56,Citation122 This review suggests the incorporation of rhythmic auditory cueing for enhancing gait performance and stability in people with cerebral palsy.
Author contributions
SG conceptualized the study, carried out the systematic review and statistical analysis, and wrote the paper. IG and AOE were involved in the systematic review process and reviewed the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
Publication of this article was funded by the Open Access Fund of Leibniz Universität, Hannover.
Supplementary materials
Figure S1 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on gait velocity in children with cerebral palsy.
Notes: Negative effects indicate reduction in gait velocity, positive effects enhancement in gait velocity. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
Abbreviation: C, children.
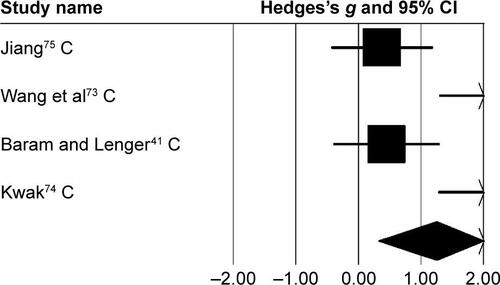
Figure S2 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on gait velocity in children with cerebral palsy posttraining.
Notes: Negative effects indicate reduction in gait velocity, positive effect sizes enhancement in gait velocity. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
Abbreviation: C, children.
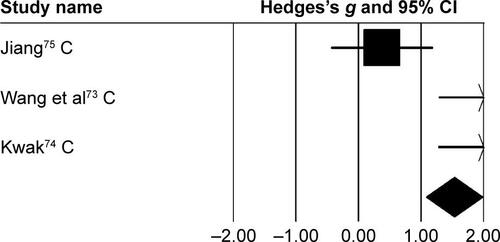
Figure S3 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on stride length in children with cerebral palsy.
Notes: Negative effects indicate reduction in stride length, positive effects enhancement in stride length. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean difference indicate favorable outcomes for control groups, positive mean difference favorable outcomes for experimental groups.
Abbreviation: C, children.
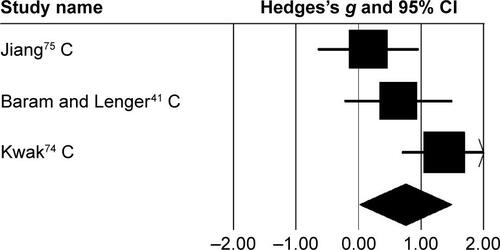
Figure S4 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on pelvic kinematics in adults with cerebral palsy.
Notes: Negative effects indicate reduction in pelvic kinematics, positive effects enhancement in pelvic kinematics. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicates favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
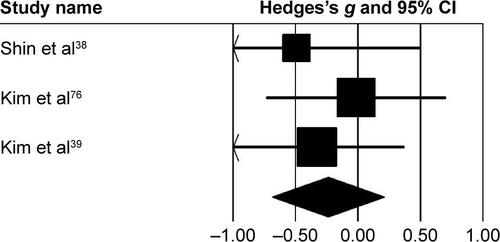
Figure S5 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on hip kinematics in adults with cerebral palsy.
Notes: Negative effects indicate reduction in hip kinematics, positive effects enhancement in hip kinematics. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
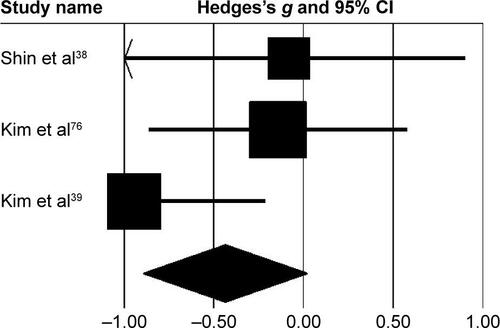
Figure S6 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on knee kinematics in adults with cerebral palsy.
Notes: Negative effects indicate reduction in knee kinematics, positive effects enhancement in knee kinematics. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
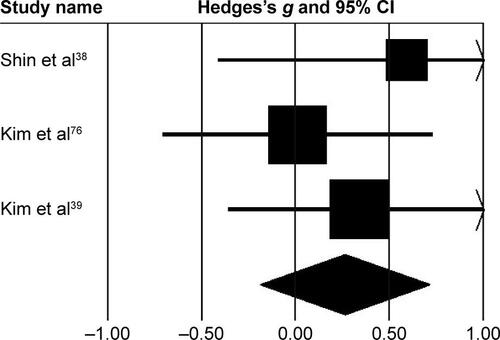
Figure S7 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on ankle kinematics in adults with cerebral palsy.
Notes: Negative effect sizes indicate reduction in ankle kinematics, positive effects enhancement in ankle kinematics. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrating repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean difference favorable outcomes for experimental groups.
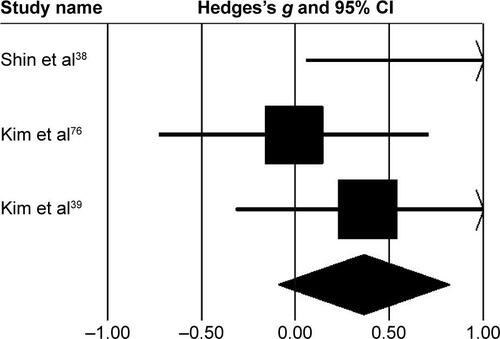
Figure S8 Forest plot illustrating individual studies evaluating the effects of rhythmic auditory cueing on foot kinematics in adults with cerebral palsy.
Notes: Negative effects indicated reduction in foot kinematics, positive effects enhancement in foot kinematics. Weighted-effect sizes – Hedge’s g (boxes) and 95% CI (whiskers) – demonstrate repositioning errors for individual studies. The diamond represents pooled effect sizes and 95% CI. Negative mean differences indicate favorable outcomes for control groups, positive mean differences favorable outcomes for experimental groups.
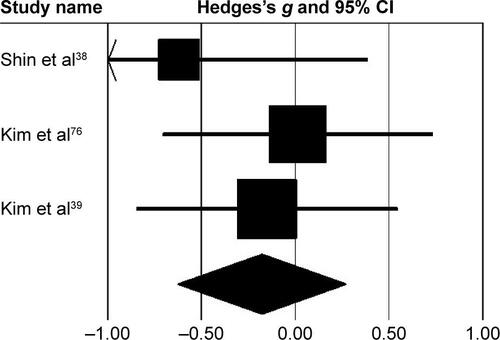
Table S1 Individual PEDro scores
Disclosure
The authors report no conflicts of interest in this work.
References
- KupermincMNStevensonRDGrowth and nutrition disorders in children with cerebral palsyDev Disabil Res Rev200814213714618646022
- RosenbaumPPanethNLevitonAA report: the definition and classification of cerebral palsy April 2006Dev Med Child Neurol Suppl200710981417370477
- EunsonPAetiology and epidemiology of cerebral palsyPaediatr Child Health2012229361366
- Yeargin-AllsoppMBraunKVDoernbergNSBenedictREKirbyRSDurkinMSPrevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaborationPediatrics2008121354755418310204
- GladstoneMA review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settingsAnn Trop Paediatr201030318119620828451
- AisenMLKerkovichDMastJCerebral palsy: clinical care and neurological rehabilitationLancet Neurol201110984485221849165
- KruseMMichelsenSIFlachsEMBrønnum-HansenHMadsenMUldallPLifetime costs of cerebral palsyDev Med Child Neurol200951862262819416329
- Vargus-AdamsJHealth-related quality of life in childhood cerebral palsyArch Phys Med Rehabil200586594094515895340
- MohammedFMAliSMMustafaMAQuality of life of cerebral palsy patients and their caregivers: a cross sectional study in a rehabilitation center Khartoum – Sudan (2014–2015)J Neurosci Rural Pract20167335536127365951
- TuguiRDAntonescuDCerebral palsy gait: clinical importanceMaedica (Buchar)20138438839324790675
- HaakPLenskiMHideckerMJLiMPanethNCerebral palsy and agingDev Med Child Neurol20095104162319740206
- HoonAHStashinkoEENagaeLMSensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathwaysDev Med Child Neurol200951969770419416315
- WintersTGageJHicksRGait patterns in spastic hemiplegia in children and young adultsJ Bone Joint Surg Am19876934374413818706
- SojkaAMStubergWAKnutsonLMKarstGMKinematic and electromyographic characteristics of children with cerebral palsy who exhibit genu recurvatumArch Phys Med Rehabil19957665585657763156
- AbelMFBlancoJSPavlovichLDamianoDLAsymmetric hip deformity and subluxation in cerebral palsy: an analysis of surgical treatmentJ Pediatr Orthop199919447948510412997
- El-ShamySMAbdelaalAAWalkAide efficacy on gait and energy expenditure in children with hemiplegic cerebral palsy: a randomized controlled trialAm J Phys Med Rehabil201695962963827149586
- GalliMCimolinVRigoldiCTenoreNAlbertiniGGait patterns in hemiplegic children with cerebral palsy: comparison of right and left hemiplegiaRes Dev Disabil20103161340134520674265
- BourgeoisABMarianiBAminianKZambelliPYNewmanCJSpatio-temporal gait analysis in children with cerebral palsy using, foot-worn inertial sensorsGait Posture201439143644224044970
- MorganPMurphyAOpheimAMcGinleyJGait characteristics, balance performance and falls in ambulant adults with cerebral palsy: an observational studyGait Posture20164824324827341531
- OkoshiYItohMTakashimaSCharacteristic neuropathology and plasticity in periventricular leukomalaciaPediatr Neurol200125322122611587877
- CooperJMajnemerARosenblattBBirnbaumRThe determination of sensory deficits in children with hemiplegic cerebral palsyJ Child Neurol19951043003097594266
- MackieSShawPLenrootRCerebellar development and clinical outcome in attention deficit hyperactivity disorderAm J Psychiatry2007164464765517403979
- ReillyDSWoollacottMHvan DonkelaarPSaavedraSThe interaction between executive attention and postural control in dual-task conditions: children with cerebral palsyArch Phys Med Rehabil200889583484218452729
- CourchesneETownsendJAkshoomoffNAImpairment in shifting attention in autistic and cerebellar patientsBehav Neurosci199410858488657826509
- JudasMRadosMJovanov-MilosevicNHrabacPStern-PadovanRKostovicIStructural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infantsAJNR Am J Neuroradiol200526102671268416286422
- HungYCMeredithGSInfluence of dual task constraints on gait performance and bimanual coordination during walking in children with unilateral cerebral palsyRes Dev Disabil201435475576024529863
- GhaiSDrillerMMastersRThe influence of below-knee compression garments on knee-joint proprioceptionGait Posture Epub201689
- MastersRSKnowledge, knerves and know-how: the role of explicit versus implicit knowledge in the breakdown of a complex motor skill under pressureBr J Psychol1992833343358
- MastersRSMaxwellJThe theory of reinvestmentInt Rev Sport Exerc Psychol200812160183
- GhaiSGhaiIEffenbergAOEffects of dual tasks and dual-task training on postural stability: a systematic review and meta-analysisClin Interv Aging20171255757728356727
- SniderLMajnemerADarsaklisVVirtual reality as a therapeutic modality for children with cerebral palsyDev Neurorehabil201013212012820222773
- MacIntoshAVignaisNBiddissEBiofeedback interventions for people with cerebral palsy: a systematic review protocolSyst Rev201761328086958
- MartinLBakerRHarveyAA systematic review of common physiotherapy interventions in school-aged children with cerebral palsyPhys Occup Ther Pediatr201030429431220735200
- VerschurenOKetelaarMTakkenTHeldersPJGorterJWExercise programs for children with cerebral palsy: a systematic review of the literatureAm J Phys Med Rehabil200887540441717993987
- WilloughbyKLDoddKJShieldsNA systematic review of the effectiveness of treadmill training for children with cerebral palsyDisabil Rehabil200931241971197919874075
- FigueiredoEMFerreiraGBMoreiraRCKirkwoodRNFettersLEfficacy of ankle-foot orthoses on gait of children with cerebral palsy: systematic review of literaturePediatr Phys Ther200820320722318703958
- NovakIMcIntyreSMorganCA systematic review of interventions for children with cerebral palsy: state of the evidenceDev Med Child Neurol2013551088591023962350
- ShinYKChongHJKimSJChoSREffect of rhythmic auditory stimulation on hemiplegic gait patternsYonsei Med J20155661703171326446657
- KimSJKwakEEParkESChoSRDifferential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: a randomized controlled trialClin Rehabil2012261090491422308559
- KimSJShinYKYooGEChongHJChoSRChanges in gait patterns induced by rhythmic auditory stimulation for adolescents with acquired brain injuryAnn N Y Acad Sci201613851536227918630
- BaramYLengerRGait improvement in patients with cerebral palsy by visual and auditory feedbackNeuromodulation2012151485222151772
- FujiokaTTrainorLJLargeEWRossBInternalized timing of isochronous sounds is represented in neuromagnetic β oscillationsJ Neurosci20123251791180222302818
- EffenbergAOFehseUSchmitzGKruegerBMechlingHMovement sonification: effects on motor learning beyond rhythmic adjustmentsFront Neurosci20161021927303255
- SchmitzGMohammadiBHammerAObservation of sonified movements engages a basal ganglia frontocortical networkBMC Neurosci2013143223496827
- HeremansENieuwboerASpildoorenJCued motor imagery in patients with multiple sclerosisNeuroscience201220611512122266343
- HeremansENieuwboerAFeysPExternal cueing improves motor imagery quality in patients with Parkinson diseaseNeurorehabil Neural Repair2012261273521778409
- MillerRAThautMHMcIntoshGCRiceRRComponents of EMG symmetry and variability in parkinsonian and healthy elderly gaitElectroencephalogr Clin Neurophysiol19961011178625872
- LuftARMcCombe-WallerSWhitallJRepetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trialJAMA2004292151853186115494583
- RochesterLBakerKNieuwboerABurnDTargeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: selective responses to internal and external cuesMov Dis2011263430435
- ZhaoYNonnekesJStorckenEJFeasibility of external rhythmic cueing with the Google Glass for improving gait in people with Parkinson’s diseaseJ Neurol201626361156116527113598
- RodgerMWCraigCMBeyond the metronome: auditory events and music may afford more than just interval durations as gait cues in Parkinson’s diseaseFront Neurosci20161027227378841
- WuYWXingGFuentes-AfflickEDanielsonBSmithLHGilbertWMRacial, ethnic, and socioeconomic disparities in the prevalence of cerebral palsyPediatrics20111273e674e68121339278
- OskouiMMesserlianCBlairAGamachePShevellMVariation in cerebral palsy profile by socio-economic statusDev Med Child Neurol201658216016626010819
- RochaPAPorfírioGMFerrazHBTrevisaniVFEffects of external cues on gait parameters of Parkinson’s disease patients: a systematic reviewClin Neurol Neurosurg201412412713425043443
- LimIvan WegenEde GoedeCEffects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic reviewClin Rehabil200519769571316250189
- SpauldingSJBarberBColbyMCormackBMickTJenkinsMECueing and gait improvement among people with Parkinson’s disease: a meta-analysisArch Phys Med Rehabil201394356257023127307
- NascimentoLRde OliveiraCQAdaLMichaelsenSMTeixeira-SalmelaLFWalking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic reviewJ Physiother2015611101525529836
- YooGEKimSJRhythmic auditory cueing in motor rehabilitation for stroke patients: systematic review and meta-analysisJ Music Ther201653214917727084833
- ZhangYCaiJZhangYRenTZhaoMZhaoQImprovement in stroke-induced motor dysfunction by music-supported therapy: a systematic review and meta-analysisSci Rep201663852127917945
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaborationAnn Intern Med20091514W65W9419622512
- WiartLKolaskiKButlerCInterrater reliability and convergent validity of the American Academy for Cerebral Palsy and Developmental Medicine methodology for conducting systematic reviewsDev Med Child Neurol201254760661122577944
- de MortonNAThe PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic studyAust J Physiother200955212913319463084
- MaherCGSherringtonCHerbertRDMoseleyAMElkinsMReliability of the PEDro scale for rating quality of randomized controlled trialsPhys Ther200383871372112882612
- TeasellREBRSR: evidence-based review of stroke rehabilitation2008 Available from: www.ebrsr.comAccessed November 2, 2017
- RamseyLWinderRJMcVeighJGThe effectiveness of working wrist splints in adults with rheumatoid arthritis: a mixed methods systematic reviewJ Rehabil Med201446648149224715196
- BorensteinMHedgesLVHigginsJRothsteinHRA basic introduction to fixed-effect and random-effects models for meta-analysisRes Synth Methods2010129711126061376
- HigginsJPGreenSCochrane Handbook for Systematic Reviews of InterventionsHoboken (NJ)John Wiley & Sons2011
- CummingGUnderstanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-analysisNew YorkRoutledge2013
- CohenJStatistical Power Analysis for the Behavioral Sciences2nd edHillsdale (NJ)Lawrence Erlbaum Associates1988
- EfraimidouVTsimarasVProiosMThe effect of a music and movement program on gait, balance and psychological parameters of adults with cerebral palsyJ Phys Educ Sport201616413571364
- BolierLHavermanMWesterhofGJRiperHSmitFBohlmeijerEPositive psychology interventions: a meta-analysis of randomized controlled studiesBMC Public Health20131311923390882
- KimJSOhDWHome-based auditory stimulation training for gait rehabilitation of chronic stroke patientsJ Phys Ther Sci2012248775777
- WangTHPengYCChenYLA home-based program using patterned sensory enhancement improves resistance exercise effects for children with cerebral palsy: a randomized controlled trialNeurorehabil Neural Repair201327868469423757295
- KwakEEEffect of rhythmic auditory stimulation on gait performance in children with spastic cerebral palsyJ Music Ther200744319821617645385
- JiangAThe Effect of Rhythmic Auditory Stimulation on Gait in Young Children with Spastic Cerebral Palsy [master’s thesis]MiamiUniversity of Miami2013
- KimSJKwakEEParkESChanges in gait patterns with rhythmic auditory stimulation in adults with cerebral palsyNeurorehabilitation201129323324122142756
- VarsamisPStaikopoulosKKartasidouLEffect of rhythmic auditory stimulation on controlling stepping cadence of individuals with mental retardation and cerebral palsyInt J Spec Educ20122736875
- InderTEWarfieldSKWangHHuppiPSVolpeJJAbnormal cerebral structure is present at term in premature infantsPediatrics2005115228629415687434
- MesserschmidtAFuikoRPrayerDDisrupted cerebellar development in preterm infants is associated with impaired neurodevelopmental outcomeEur J Pediatr2008167101141114718172680
- DyetLEKenneaNCounsellSJNatural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessmentPediatrics2006118253654816882805
- PiersonCRFolkerthRDBilliardsSSGray matter injury associated with periventricular leukomalacia in the premature infantActa Neuropathol2007114661963117912538
- CallisayaMLBeareRPhanTGBrain structural change and gait decline: a longitudinal population-based studyJ Am Geriatr Soc20136171074107923796055
- GrassoRPeppeAStrattaFBasal ganglia and gait control: apomorphine administration and internal pallidum stimulation in Parkinson’s diseaseExp Brain Res1999126213914810369137
- RazNRodrigueKMKennedyKMHeadDGunning-DixonFAckerJDDifferential aging of the human striatum: longitudinal evidenceAJNR Am J Neuroradiol20032491849185614561615
- WolpeNIngramJNTsvetanovKAAgeing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuitsNat Commun201671303427694879
- SeidlerRDBernardJABurutoluTBMotor control and aging: links to age-related brain structural, functional, and biochemical effectsNeurosci Biobehav Rev201034572173319850077
- NombelaCHughesLEOwenAMGrahnJAInto the groove: can rhythm influence Parkinson’s disease?Neurosci Biobehav Rev201337102564257024012774
- AboutorabiAArazpourMBahramizadehMHutchinsSWFadayevatanRThe effect of aging on gait parameters in able-bodied older subjects: a literature reviewAging Clin Exp Res201628339340526210370
- PerryMCCarvilleSFSmithICRutherfordOMNewhamDJStrength, power output and symmetry of leg muscles: effect of age and history of fallingEur J Appl Physiol2007100555356116847676
- SchmidtRAFrequent augmented feedback can degrade learning: evidence and interpretationsRequinRStelmachGETutorials in Motor NeurosciSpringer19915975
- WinsteinCJPohlPSLewthwaiteREffects of physical guidance and knowledge of results on motor learning: support for the guidance hypothesisRes Q Exerc Sport19946543163237886280
- HameedSFerrisTJayaramanSSarterNUsing informative peripheral visual and tactile cues to support task and interruption managementHum Factors200951212613519653478
- ElsingerCLRaoSMZimbelmanJLReynoldsNCBlindauerKAHoffmannRGNeural basis for impaired time reproduction in Parkinson’s disease: an fMRI studyJ Int Neuropsychol Soc2003971088109814738289
- HausdorffJMLowenthalJHermanTGruendlingerLPeretzCGiladiNRhythmic auditory stimulation modulates gait variability in Parkinson’s diseaseEur J Neurosci20072682369237517953624
- RubinsteinTCGiladiNHausdorffJMThe power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s diseaseMov Disord20021761148116012465051
- CunningtonRIansekRBradshawJLPhillipsJGMovement-related potentials in Parkinson’s diseaseBrain199511849359507655889
- CabezaRAndersonNDLocantoreJKMcIntoshARAging gracefully: compensatory brain activity in high-performing older adultsNeuroimage20021731394140212414279
- TierneyAKrausNThe ability to move to a beat is linked to the consistency of neural responses to soundJ Neurosci20133338149811498824048827
- DebaereFWenderothNSunaertSVan HeckePSwinnenSPInternal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedbackNeuroimage200319376477612880805
- AsanumaHKellerANeuronal mechanisms of motor learning in mammalsNeuroreport1991252172241912451
- SuhJHHanSJJeonSYEffect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patientsNeurorehabilitation201434119319924284453
- ThautMHMcIntoshGCRiceRRMillerRARathbunJBraultJMRhythmic auditory stimulation in gait training for Parkinson’s disease patientsMov Disord19961121932008684391
- ThautMHRhythm, Music, and the Brain: Scientific Foundations and Clinical ApplicationsNew YorkRoutledge2005
- LohnesCAEarhartGMThe impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson diseaseGait Posture201133347848321273075
- YoungWRRodgerMWCraigCMAuditory observation of stepping actions can cue both spatial and temporal components of gait in Parkinson’s disease patientsNeuropsychologia20145714015324680722
- DotovDBayardSde CockVCBiologically-variable rhythmic auditory cues are superior to isochronous cues in fostering natural gait variability in Parkinson’s diseaseGait Posture201751646927710836
- GaverWWHow do we hear in the world? Explorations in ecological acousticsEcol Psychol199354285313
- RizzoJRRaghavanPMcCreryJROh-ParkMVergheseJEffects of emotionally charged auditory stimulation on gait performance in the elderly: a preliminary studyArch Phys Med Rehabil201596469069625542677
- FraněkMvan NoordenLRežnýLTempo and walking speed with music in the urban contextFront Psychol20145136125520682
- LemanMMoelantsDVarewyckMStynsFvan NoordenLMartensJPActivating and relaxing music entrains the speed of beat synchronized walkingPLoS One201387e6793223874469
- FangRYeSHuangfuJCalimagDPMusic therapy is a potential intervention for cognition of Alzheimer’s disease: a mini-reviewTransl Neurodegener20176228149509
- StorkMJKwanMYGibalaMJGinisKAMusic enhances performance and perceived enjoyment of sprint interval exerciseMed Sci Sports Exerc20154751052106025202850
- RochesterLRaffertyDDotchinCMsuyaOMindeVWalkerRThe effect of cueing therapy on single and dual-task gait in a drug naïve population of people with Parkinson’s disease in northern TanzaniaMov Disord201025790691120175212
- GodaraBNikitaKSWireless Mobile Communication and Healthcare: Third International Conference, MobiHealth 2012, Paris, France, November 21–23, 2012 – Revised Selected PapersHeidelbergSpringer2013
- HoveMJSuzukiKUchitomiHOrimoSMiyakeYInteractive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patientsPLoS One201273e3260022396783
- LopezWOHigueraCAFonoffETSouzaCOAlbickerUMartinezJAListenMee and ListenMee smartphone application: synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s diseaseHum Mov Sci20143714715625215623
- PoushterJSmartphone ownership and Internet usage continues to climb in emerging economies2016 Available from: http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economiesAccessed November 2, 2017
- Fragala-PinkhamMASmithHJLombardKABarlowCO’NeilMEAquatic aerobic exercise for children with cerebral palsy: a pilot intervention studyPhysiother Theory Pract2014302697824328930
- CombsSADuganELPassmoreMBalance, balance confidence, and health-related quality of life in persons with chronic stroke after body weight-supported treadmill trainingArch Phys Med Rehabil201091121914191921112434
- KimDCorrelation between physical function, cognitive function, and health-related quality of life in elderly personsJ Phys Ther Sci20162861844184827390430
- GhaiSGhaiIEffenbergAOEffect of rhythmic auditory cueing on aging gait: a systematic review and meta-analysisA&DIn press2017
- GhaiSGhaiISchmitzGEffenbergAOEffect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysisSci repIn press2017
- GhaiSSchmitzGHwangTHEffenbergAOEffects of real-time auditory feedback on proprioceptive accuracythe 22nd Annual Congress of European College of Sports ScienceEssen, GermanyJuly 5–8, 2017 MetropolisRuhr – Germany
- GhaiSDrillerMGhaiIEffects of joint stabilizers on proprioception and stability: a systematic review and meta-analysisPhys Ther Sport201725657528262354
- GhaiSProprioception and performance: the role of below-knee compression garments and secondary tasks [dissertation]HamiltonUniversity of Waikato2016