Abstract
Background
Previous evidence indicated that efficacy of escitalopram (Esc) and duloxetine (Dul) was comparable in the treatment of major depressive disorder (MDD). Since such studies had small sample sizes, this study purposefully applied a systematic review to determine the efficacy, acceptability, and tolerability those antidepressants in treatment of MDD.
Participants and methods
The following primary databases were searched in July 2017: Scopus, PubMed, CINAHL, and Cochrane Controlled Trials Register. Any randomized controlled trials (RCTs) of Esc comparison with Dul in the treatment of MDD were included in this review. The primary efficacy of outcome was the pooled mean-changed scores of the rating scales for the standardized rating scales for depression.
Results
A total of 1,120 randomized subjects from 3 RCTs were collected for synthesis in the present meta-analysis. The mean-changed scores of the Hamilton Depression Rating Scale (HAMD) and Clinical Global Impression – Severity, overall response rate by the HAMD, and remission rate by the HAMD and Montgomery–Asberg Depression Rating Scale (MADRS) in the Esc- and Dul-treated groups showed no significant differences. However, the mean-changed score of the MARDS, mean-end scores of Clinical Global Impression – Improvement, and overall response by the MADRS in the Esc-treated group were greater than that of the Dul-treated group. Although the overall discontinuation rate had no significant differences between the 2 groups, the discontinuation rate due to adverse events in the Esc-treated group was greater than that of the Dul-treated group.
Limitations
This review had limited eligible studies.
Conclusion
This review indicated the efficacy in the acute treatment of Esc vs Dul varied relying on measurements across the studies. However, the tolerability of Esc was superior to Dul in acute MDD treatment. Therefore, selection between the 2 antidepressants may depend on the tolerability of MDD patients. Due to limited included studies in this review, more large-scale and well-defined RCTs in such patients should be carried out to determine these outcomes.
Background
Major depressive disorder (MDD), a recurrent, frequently chronic illness, negatively affects the functioning and quality of life and increases the risk of suicide.Citation1,Citation2 Although several studies have indicated the efficacy of several medications in the treatment in MDD, some patients with MDD do not respond to or tolerate some medications. For instance, previous evidence has shown that only 62%–63% and 68% of MDD patients responded to selective serotonin inhibitors and tricyclic antidepressants, respectively, while discontinuation rates for the 2 antidepressants were as high as 13% and 11%–17%, respectively.Citation3–Citation5 Therefore, information for alternative treatments of MDD is necessary for clinicians to make decisions in the choice of treatment for their patients in terms of efficacy and tolerability.
Some evidence has indicated that antidepressants acting on both serotonergic and noradrenergic receptors are more efficacious than those acting on only serotonergic receptors.Citation6,Citation7 As known, escitalopram (Esc) binds not only to the primary site on the serotonin transporter, but also to an allosteric site. The previous evidence suggests that efficacy of Esc in MDD is comparable to venlafaxine, which is a serotonin and noradrenaline reuptake inhibitor. However, Esc is more tolerable than venlafaxine. Duloxetine (Dul), another selective noradrenaline reuptake inhibitor, has shown its efficacy in the treatment for MDD patients. Similar to venlafaxine, Dul is likely less tolerable than other antidepressants such as Esc. Therefore, comparison of Esc and Dul in the treatment of MDD is important.
Although some randomized controlled trials (RCTs) compared the efficacy and tolerability of Esc and Dul in the treatment of MDD, the individual RCTs had varied outcomes.Citation8–Citation10 As a result, a powerful measurement in determining the true effect size, a meta-analysis, can help compare the efficacy, acceptability, and tolerability between Esc and Dul in the treatment of MDD.
The present systematic review was designed to evaluate the efficacy, acceptability, and tolerability of Esc vs Dul monotherapy for acute MDD. The measurement of efficacy was carried out by using the pooled mean-changed or mean-end scores of standardized rating scales for depressive symptoms, response rate, and remission rate, while acceptability and tolerability relied on the overall discontinuation rate and the discontinuation rate due to adverse events, respectively. Only relevant RCTs were eligible in this meta-analysis.
Participants and methods
Inclusion criteria
Types of included trials
The RCTs of Esc vs Dul that adhered inclusion criteria were included.
Types of participants
All participants diagnosed with MDD by using of any set of criteria, including the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases criteria, were eligible.
Type of interventions
Treatment of Esc vs Dul in any dose, form, and frequency were eligible.
Types of outcome measures
Primary efficacious outcome measures
The primary outcome measurements were obtained by the mean-changed scores of a standardized depressive rating scale and response rates.
Secondary efficacious outcome measures
The secondary outcome measures comprised the following:
Rates of remission that relied on individual studies
Clinical Global Impression–Severity Scale (CGI-S)
Clinical Global Impression–Improvement Scale (CGI-I).
Acceptability measures
Overall discontinuation rate.
Tolerability measures
Discontinuation rate due to adverse events.
Information sources
The databases EMBASE, PubMed, CINAHL, and Cochrane Controlled Trials Register were searched in July 2017. Citalopram and Dul studies were published in the PubMed since 2001 and 1988, respectively, and searches for those publications began from January 1988 to July 2017. Searching was confined to human studies. The ClinicalTrials.gov and EU Clinical Trials Register databases were also searched. The related references of any article derived from any method were evaluated. The relevant RCTs were considered. Limitation of language was not applied to individual studies.
Searches
For optimal sensitivity to identify the RCTs, search of the PubMed was strategically constricted to the following words and phrases: ([escitalopram] OR [Lexapro] OR [Cipralex]) AND ([duloxetine] OR [Cymbalta]) AND ([major depressive disorder] OR [major depression] OR [severe depressive episode] OR [MDD]). Based on the first publication of either Esc or Dul, the year of search was started from 1988. A similar method was applied for searching in the other databases.
Study selection
All abstracts and titles accumulated from the electronic databases were separately evaluated by 2 reviewers (NM and BM) to determine whether they met the eligibility criteria defined as above. After the full-text versions of the relevant articles were gathered, they were inspected separately by the 2 reviewers. If a disagreement between 2 reviewers arose, they conclusively solved the dispute by consensus.
Data collection process
The first reviewer (NM) extracted the data of the full-version eligible articles and filled in these data into the developed extraction form. Then, the second reviewer (BM) carefully reexamined this extracted data. Similarly, resolution of all arguments was also done by consensus between 2 reviewers. When the dispute was not able to be resolved, a third reviewer (MS) could decide.
Data items
The important data accumulated from the eligible studies consisted of the following: 1) essential details for evaluation of the study quality; 2) basic characteristic outcomes such as population, set of diagnostic criteria, study design, and inclusion/exclusion criteria; 3) forms, doses, and treatment duration of Esc vs Dul; 4) substantial results; and 5) intention-to-treat results.
Risk of bias in individual studies
The assessment of internal validity (quality) for each eligible trial was accomplished by 2 reviewers (NM and BM). Considered as the quality assessment of the Cochrane Collaboration handbook, evaluation of the risk of bias included the following: 1) sequence generation (randomization); 2) allocation concealment; 3) blinding of participants, personnel, and outcomes; 4) incomplete outcome data; 5) selective outcome reporting; and 6) other biases.Citation11
Summary measures
The efficacy, acceptability, and tolerability were the important outcomes. The primary efficacy was measured by using the end-point or mean-changed scores of the standardized depressive scale and the rate of response. Other efficacy outcomes were measured by remission rate, mean-changed scores of CGI-S and CGI-I scores. Similar to previous reviews, acceptability was determined by the overall discontinuation rate,Citation12 and tolerability was measured by the discontinuation rate due to adverse events.Citation13
Statistical analysis and synthesis of results
On a regular basis, synthesis of the continuous results was estimated by using the mean differences with 95% confidence interval (CI), either a weighted mean difference (WMD) or a standardized mean difference (SMD). The WMD or SMD with 95% CI was calculated by the mean difference between the compared groups divided by an estimate of the within-group standard deviation (SD). As known, a WMD is a direct comparison, or a combination of the study outcomes. Hence, this technique can apply if similar rating scales are applied across the studies. Conversely, when a measurement of the same outcomes is used, the various rating scales are unlikely to directly compare or combine such outcomes. Consequently, measuring compared or combined outcomes can be applied to the SMD since it has no units. As known, the SD of the mean-end or mean-changed scores may be not available. In this event, the SD may be calculated by using any of the statistical analyses or by direct substitution.Citation14 According to a statistical method of combination of outcomes, an inverse-variance, estimating a measure effect by weighing the influence of each study, was thoroughly applied to calculate the pooled mean-changed scores with 95% CIs.Citation11
As a rule, synthesis of dichotomous data was calculated by using the relative risk (RR), with the 95% CI. As known, the RR is exactly 1, indicating that the outcome had no difference between the intervention and the control groups. However, RR being more or less than 1 indicates that the intervention, respectively, increases or decreases the risk of the outcomes. For this reason, the RRs were used to compare all dichotomous outcomes, including response rates, remission rates, overall discontinuation rates, and discontinuation rates due to adverse events between the 2 groups. All pooled RRs of such outcomes with 95% CIs were estimated by using the Mantel–Haenszel technique.Citation11
Normally, synthesis of outcomes in systematic reviews can apply either the fixed- or the random-effect model. In the fixed-effect model, all included trials assume that the true effect size is the same across such studies, and the summary effect is the estimation of the common effect size. Actually, the assumption of 1 true effect size is less likely. Albeit when all eligible trials are rather homogenous, it is unlikely to determine that they are completely identical. Hence, synthesis of all outcomes in the present review used a random-effect model which supposes that the true effect size is different across eligible trials. In this meta-analysis, the RevMan 5.1 (The Cochrane Collaboration, London, UK) was used to synthesize all outcomes.
Risk of bias across studies
A funnel plot is a simple scatter plot of the treatment effect approximated from individually eligible clinical trials against a measure of each clinical trial’s size. If bias does not appear, the plot should look like a symmetrical inverted funnel.Citation15 If possible, a funnel plot can be applied to determine the reporting bias in this review.
Test of heterogeneity
Evaluation of the similarities in the clinical outcome can be carried out by using a test of heterogeneity. After the test was conducted in this meta-analysis, we hypothesized that the effect size was different owing to the differences in the quality of methodology in an individual study. The results of all trials were assessed to whether they were higher and had a difference from the anticipated results by chance alone. The outcomes, therefore, were determined by displaying as graphs and using the test of heterogeneity. In case of an I2 of 50% or more, it suggests that significant heterogeneity has occurred.
Results
Study selection
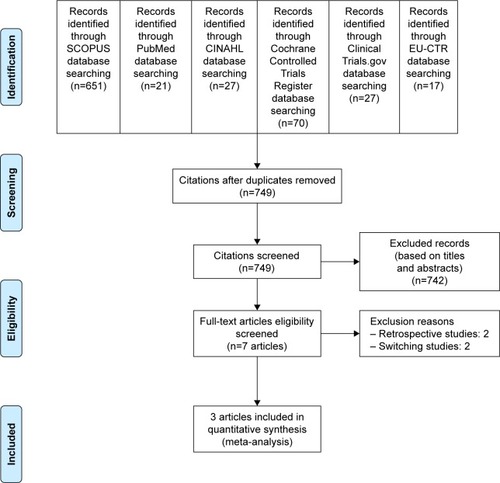
Based on the strategic search, a total of 813 citations (SCOPUS =651, PubMed =21, CINAHL =27, Cochrane Controlled Trials Register =70, ClinicalTrials.gov =27, and EU Clinical Trials Register =17) () were retrieved. When the duplicates were discarded, 749 citations remained. The titles and abstracts were, then, inspected and 9 citations were persistently eligible for the criteria. After full-version papers of 9 citations were evaluated, 5 citations were excluded from this review, 2 retrospective studiesCitation16,Citation17 and 2 switching studies.Citation18,Citation19 As a result, a total of 3 articles were included in review.Citation9,Citation10,Citation20 Unfortunately, a relevant or unpublished study meeting the eligibility criteria was not observed.
Study characteristics
All eligible participants of each included study were diagnosed with MDD by using the DSM-IV or DSM-IV-TR, with Montgomery–Asberg Depression Rating Scale (MADRS) total score ≥26 and CGI-S ≥4 for 2 studiesCitation9,Citation10 and MADRS total score ≥22 and CGI-S ≥4 for 1 study.Citation8 The study duration for all included studies was 8 weeks. The participants were randomly assigned to obtain either Esc or Dul. The doses of Esc and Dul were 10–20 mg/d and 60–120 mg/d, respectively (). The demographic and basic characteristics of the Esc- and Dul-treated groups were largely well matched across all included trials.
Table 1 Basic characteristic of RCTs of Esc vs Dul in MDD
A total of 1,120 randomized subjects were collected to synthesize in the present meta-analysis. The mean (SD) ages of the Esc- and Dul-treated groups were 41.8 (11.9) and 43.5 (12.6) years, respectively. The basic characteristics for all included clinical studies are displayed in . All eligible studies have reported the remission, response, and discontinuation rates.
Risk of bias within studies
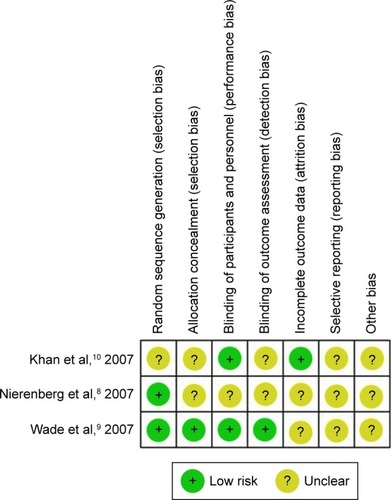
Risk of bias of each study is shown in the . The reporting bias was unclear in all studies. The remaining biases varied across the studies. All included trials applied intention-to treat analysis.
Synthesis of results
Efficacy
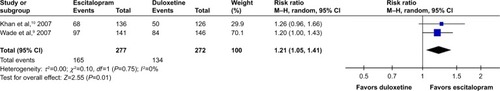
Considered in the primary efficacious outcomes, the significant heterogeneity was not illustrated in the WMDs for the pooled mean-changed scores for 17-item Hamilton Depression Rating Scale (HAMD-17) and MADRS and pooled response rates measured by the HAMD-17 and MADRS scales. The pooled mean-changed score of the HAMD-17 had no significant difference (WMD [95% CI] −0.38 [−1.88, 1.12], I2=37%), while the pooled mean-changed score of the MARDS in the Esc-treated group was greater than that of the Dul-treated group (WMD [95% CI] −2.10 [−3.65, −0.55], I2=0%) ( and ). The overall pooled response by the HAMD between 2 groups had no significant differences in terms of RRs (95% CI) 1.08 (0.92, 1.25), I2=33%, while the overall pooled response by the MADRS of the Esc-treated group was higher than that of Dul-treated group in terms of RR (95% CI) 1.21 (1.05, 1.41), I2=0% ( and ).
Figure 3 The forest plot of HAMD-17 mean-changed scores from baseline (95% CI) of escitalopram vs duloxetine in major depressive disorder.
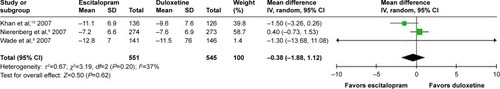
Figure 4 The forest plot of mean-changed scores from baseline of MADRS scores (95% CI) of escitalopram vs duloxetine in major depressive disorder.
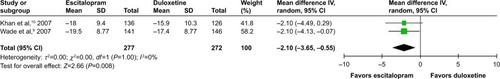
Figure 5 The forest plot of clinical response rate of HAMD relative risk (95% CI) of escitalopram vs duloxetine in major depressive disorder.
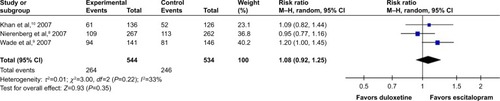
Figure 6 The forest plot of MADRS clinical response rate of relative risk (95% CI) in escitalopram vs duloxetine in major depressive disorder.
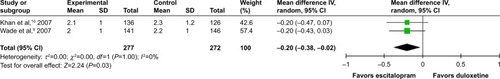
According to secondary efficacious outcomes, significant heterogeneity was not shown in the pooled remission rates measured by the HAMD-17 and MADRS scales, WMDs for pooled mean-changed scores for CGI-S, and for mean-end score for CGI-I. The overall pooled remission rates by the HAMD and MADRS between the 2 groups had no significant differences in terms of RRs (95% CI) 1.01 (0.83, 1.23), I2=32% and 1.14 (0.94, 1.38), I2=0%, respectively ( and ). The pooled mean-changed score of the CGI-S in the Esc- and Dul-treated groups had no significant differences (WMD [95% CI] −0.14 [−0.32, 0.04], I2=29%), while the pooled end score of CGI-I in the Esc-treated group was higher than that of Dul-treated group (WMD [95% CI] −0.20 [−0.38, −0.02], I2=0%) ( and ).
Figure 7 The forest plot of clinical remission rate by HAMMD-17 relative risk (95% CI) for escitalopram vs duloxetine in major depressive disorder.
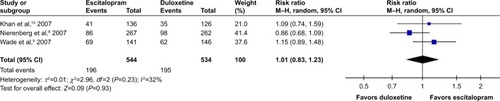
Figure 8 The forest plot of clinical remission rate by MADRS relative risk (95% CI) of escitalopram vs duloxetine in major depressive disorder.
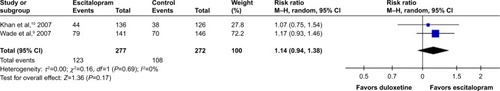
Figure 9 The forest plot of mean-changed scores from comparison of CGI-S (95% CI) of escitalopram vs duloxetine in major depressive disorder.
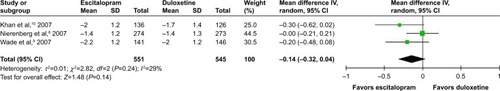
Figure 10 The forest plot of mean-end score from baseline comparing CGI-I scores (95% CI) of escitalopram vs duloxetine in major depressive disorder.
Acceptability
Heterogeneity was significantly observed in the overall discontinuation rate. Although the pooled overall discontinuation rate had no significant differences between 2 groups with RRs (95% CI) 0.69 (0.47, 1.00), I2=63%, acceptability of Esc tended to be better than Dul in MDD treatment.
Tolerability
The heterogeneity was significantly shown in the discontinuation rate due to adverse events between the 2 groups. Since the pooled discontinuation rate due to adverse events of the Esc-treated group was greater than that of the Dul-treated group, with RR (95% CI) 0.47 (0.25, 0.90), I2=51%, it suggests that Esc has a better tolerance than Dul in the treatment of MDD.
Risk of bias across studies
In cases where the eligible RCTs are <10 studies, a funnel plot to determine the publication bias in a systematic review possibly will not have enough power to detect the chances of real asymmetry occurring.Citation15 Since this review included only 3 RCTs, we decided to discard the test of funnel plot.
Discussion
The comparison of the efficacy between Esc and Dul in systematic review yielded varied efficacious outcomes on the measures of MDD. The mean-changed score of the MADRS and mean-end scores of the CGI-I indicated that Esc was more efficacious than Dul, while those differences were not found as measured by the HAMD-17 scale. Conversely, the rate of response measured by the MADRS illustrated that escitalopram was better than duloxetine, while the rate of response as measured by HAMD showed no differences between the 2 groups. However, the remission rate was not different between 2 active agents. Interestingly, patients with MDD tend to be more tolerant to Esc than Dul. Additionally, Esc also illustrated its tolerability as superior to Dul.
The various outcomes of efficacy between Esc and Dul in MDD were caused by the inconsistent outcomes across 3 included studies in the present review. Similar to a previous systematic review of venlafaxine and bupropion in MDD, the efficacy outcomes of the included studies were also varied.Citation21 Hence, more large-scale clinical studies could be more accurate for comparing such efficacious outcome treatments between such 2 antidepressants in MDD.
Previous evidence suggested that the acceptability of Dul was potentially less than other antidepressants, including Esc, which was similar to findings of the present review.Citation22 Again, tolerability of Dul is also less than other antidepressants such as paroxetineCitation22 and vortioxetine,Citation23 which was also compatible with our findings. This may explain the high dropout rate due to the adverse events of Dul. In comparisons between Esc and Dul, the former may more suitable than the latter in less-tolerable patients. Regarding acceptability and tolerability, Esc may be a better choice than Dul in the treatment of MDD. However, gradual dose titration of Dul may decrease the side effects and help in the maintenance of such MDD patients.
The present review had some limitations. Initially, owing to the limited number of eligible clinical trials, the pooled sample-size population could be affected in this review. Second, some included studies were sponsored by a patent holding company for either Esc or Dul, which may increase the potential overestimation of treatment effect owing to sponsorship bias. Those findings should be carefully interpreted. Finally, the test of funnel plot to assess an asymmetry could not be performed due to the limited numbers of studies.Citation15 Thus, exclusion of publication bias may be not possible.
Conclusion
This review indicated that the efficacy in acute treatment of Esc vs Dul is varied and relies on measurement across the studies. However, the tolerability of Esc is superior to Dul in acute MDD treatment. Based on this systematic review, selection between the 2 antidepressants may depend on the economic evaluation of each treatment as well as the tolerability of MDD patients. Due to a limited number of included studies in this review, more large-scale and well-defined RCTs in such patients should be carried out to determine these outcomes.
Author contributions
Benchalak Maneeton and Narong Maneeton should be regarded as joint first authors. All authors contributed toward data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This review received financial support from Chiang Mai University, Thailand. We are grateful for the manuscript editing provided by Ms Ruth Barnard Leatherman.
Disclosure
Benchalak Maneeton received honoraria and/or travel reimbursement from Lundbeck, Pfizer, and Servier. Narong Maneeton received travel reimbursement from Lundbeck, Pfizer, and Servier. Surinporn Likhitsathian received honoraria and/or travel reimbursement from Janssen-Cilag, Pfizer, Servier, Sanofi-Aventis, and Thai-Otsuka. Punjaree Wiriyacosol received travel reimbursement from Sanofi-Aventis, Ranbaxy, Servier, and GlaxoSmithKline. Pakapan Woottiluk, and Vudhichai Boonyanaruthee reported no potential conflicts of interest. Manit Srisurapanont received honoraria from Lundbeck, and Sumimoto Dainippon Pharma. The authors report no other conflicts of interest in this work.
References
- CohenRMGreenbergJMIsHakWWIncorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDDJAMA Psychiatry201370334335023303512
- BostwickJMPankratzVSAffective disorders and suicide risk: a reexaminationAm J Psychiatry2000157121925193211097952
- SteffensDCKrishnanKRHelmsMJAre SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysisDepress Anxiety19976110189394870
- PapakostasGIHombergerCHFavaMA meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorderJ Psychopharmacol200822884384818308801
- AndersonIMMeta-analytical studies on new antidepressantsBr Med Bull20015716117811719915
- SchmittABBauerMVolzHPDifferential effects of venlafaxine in the treatment of major depressive disorder according to baseline severityEur Arch Psychiatry Clin Neurosci2009259632933919255709
- SchuelerYBKoestersMWieselerBA systematic review of duloxetine and venlafaxine in major depression, including unpublished dataActa Psychiatr Scand2011123424726520831742
- NierenbergAAGreistJHMallinckrodtCHDuloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority studyCurr Med Res Opin200723240141617288694
- WadeAGembertKFloreaIA comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorderCurr Med Res Opin20072371605161417559755
- KhanABoseAAlexopoulosGSGommollCLiDGandhiCDouble-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorderClin Drug Investig2007277481492
- HigginsJPTAltmanDGAssessing risk of bias in included studiesHigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011)LondonThe Cochrane Collaboration2009
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- PapakostasGITolerability of modern antidepressantsJ Clin Psychiatry200869Suppl E181318494538
- WiebeNVandermeerBPlattRWKlassenTPMoherDBarrowmanNJA systematic review identifies a lack of standardization in methods for handling missing variance dataJ Clin Epidemiol200659434235316549255
- SterneJACEggerMMoherDAddressing reporting biasesHigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011)LondonThe Cochrane Collaboration2009 Available from: http://handbook-5-1.cochrane.org/Accessed July 19, 2018
- MallinckrodtCHPrakashAHoustonJPSwindleRDetkeMJFavaMDifferential antidepressant symptom efficacy: placebo-controlled comparisons of duloxetine and SSRIs (fluoxetine, paroxetine, escitalopram)Neuropsychobiology2007562–3738518037817
- WangJLiuXMullinsCDTreatment adherence and persistence with duloxetine, venlafaxine XR, and escitalopram among patients with major depressive disorder and chronic pain-related diseasesCurr Med Res Opin20112771303131321561393
- RaskinJGeorgeTGrangerREHussainNZhaoGWMarangellLBApathy in currently nondepressed patients treated with a SSRI for a major depressive episode: outcomes following randomized switch to either duloxetine or escitalopramJ Psychiatr Res201246566767422410206
- RomeraIPérezVMenchónJMEarly switch strategy in patients with major depressive disorder: a double-blind, randomized studyJ Clin Psychopharmacol201232447948622722513
- PigottTAPrakashAArnoldLMAaronsonSTMallinckrodtCHWohlreichMMDuloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorderCurr Med Res Opin20072361303131817559729
- ManeetonNManeetonBEurviriyanukulKSrisurapanontMEfficacy, tolerability, and acceptability of bupropion for major depressive disorder: a meta-analysis of randomized-controlled trials comparison with venlafaxineDrug Des Devel Ther2013710531062
- CiprianiAKoestersMFurukawaTADuloxetine versus other anti-depressive agents for depressionCochrane Database Syst Rev201710CD006533
- YılmazAEExamination of the metacognitive model of depression in a Turkish University student sampleTurk Psikiyatri Derg20162720 Turkish