Abstract
Objectives
To review current evidence on buprenorphine–naloxone (bup/nx) for the treatment of opioid-use disorders, with a focus on strategies for clinical management and office-based patient care.
Quality of evidence
Medline and the Cochrane Database of Systematic Reviews were searched. Consensus reports, guidelines published, and other authoritative sources were also included in this review. Apart from expert guidelines, data included in this review constitute level 1 evidence.
Findings
Bup/nx is a partial μ-opioid agonist combined with the opioid antagonist naloxone in a 4:1 ratio. It has a lower abuse potential, carries less stigma, and allows for more flexibility than methadone. Bup/nx is indicated for both inpatient and ambulatory medically assisted withdrawal (acute detoxification) and long-term substitution treatment (maintenance) of patients who have a mild-to-moderate physical dependence. A stepwise long-term substitution treatment with regular monitoring and follow-up assessment is usually preferred, as it has better outcomes in reducing illicit opioid use, minimizing concomitant risks such as human immunodeficiency virus and hepatitis C transmission, retaining patients in treatment and improving global functioning.
Conclusion
Bup/nx is safe and effective for opioid detoxification and substitution treatment. Its unique pharmaceutical properties make it particularly suitable for office-based maintenance treatment of opioid-use disorder.
Introduction
Dependence on opioids is a major health issue worldwide. The costs associated with opioid dependence are extensive, exacting an enormous toll in terms of health care, mental illness, quality of life, unemployment, and crime. Concomitant risks include the transmission of the human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus (HCV), and tuberculosis, as well as a high incidence of death due to respiratory depression and overdose.Citation1–Citation6 It is well recognized that the abuse of prescription opioids is on the rise in North America as well as Australia and New Zealand.Citation6–Citation9 In numerous street-drug populations in both the US and Canada, prescription opioids have replaced heroin as the main opioid of choice.Citation2,Citation10 Moreover, US medical emergencies related to opioid misuse increased by 183% between 2004 and 2011.Citation11
Given the high medical and psychiatric comorbidities associated with opioid abuse, both primary care physicians and mental health specialists are regularly confronted with the sequelae of prescription and illicit opioid dependence.Citation12,Citation13 Pharmacological substitution therapies, including buprenorphine and methadone, have been shown to be more effective than any other type of treatment for opioid dependence, particularly when used in combination with psychosocial interventions.Citation14 Methadone, which is often administered through specialized licensed opioid-treatment programs, has been the standard of care for illicit opioid dependence for the past 40 years, particularly in the US. However, the lack of global access to specialized methadone clinics, strict regulations, long waiting lists, and stigma often discourage patients from enrolling in substitution treatment.Citation15–Citation17 In Europe, buprenorphine (Subutex) was introduced widely in primary care facilities as an alternative to methadone for the treatment of opioid dependence, with demonstrated success.Citation18,Citation19 Prescription opioid-dependent patients in particular may be amenable to treatment in primary care or office-based practice rather than specialized addiction-treatment centers. They appear to be younger and of higher socioeconomic status, with a lower prevalence of HCV infection, fewer years of opioid use, and fewer prior addiction-treatment episodes compared to patients receiving treatment for illicit opioid dependence.Citation20
In the US, buprenorphine and buprenorphine–naloxone (bup/nx) were approved by the US Food and Drug Administration (FDA) in 2002, offering an office-based maintenance treatment for opioid dependence.Citation21–Citation23 Like methadone, buprenorphine and bup/nx substitution treatment have been shown to decrease hospital admissions, morbidity, and mortality.Citation24–Citation26 Moreover, buprenorphine is associated with lower risk of overdose and diversion, thereby offering more flexibility to both physicians and patients.Citation12,Citation27 The combination of buprenorphine with the opiate antagonist naloxone (bup/nx) demonstrates the same benefits as buprenorphine alone, with the added benefit of further reducing potential misuse.
Considering its safety profile, as well as its diminished abuse potential, bup/nx constitutes a promising alternative therapy for opioid dependence that could be used by general medical and mental health practitioners, thereby increasing access to substitution treatment. However, evidence is accumulating at a rapid pace, making it challenging for physicians unfamiliar with this topic to obtain the appropriate knowledge. In order to counteract barriers to the drug’s clinical use, this article was designed as a comprehensive review of the literature surrounding the use of bup/nx in the treatment of illicit and prescription-opioid dependence.
Quality of evidence
A Cochrane Database of Systematic Reviews and Medline search on Ovid of all published articles identified with the keyword “buprenorphine” was conducted as of June 2013, limiting the search to human studies published in English. It yielded a total of 2,160 potential articles. Studies related to pain control were eliminated. Subsequently, all other abstracts were reviewed, and relevant studies with adequate methodology, including randomized controlled trials, meta-analyses, and systematic reviews, were selected and reviewed in depth. Consensus reports and guidelines published by the Center for Substance Abuse Treatment, the Royal Australian College of Physicians, the Centre for Addiction and Mental Health, the Community Care Behavioral Health Organization, the American Society of Addiction Medicine, and the World Health Organization were also included in this review. Apart from expert guidelines, data included in this review constitute level 1 evidence.
Pharmacology/pharmacokinetics
Buprenorphine
Originally developed for pain treatment, buprenorphine is a semi-synthetic derivative of the opioid thebaine. Buprenorphine has a mixed agonist–antagonist action on opioid receptors: it acts as a partial agonist at the µ-receptor and as an antagonist at the κ-receptor.Citation28,Citation29 As a partial agonist with low intrinsic activity, it produces milder and less euphoric and sedating opioid effects while still occupying opioid receptors, thus preventing withdrawal in dependent patients.Citation30 There is also a “ceiling effect,” in which the agonist properties increase linearly up to a maximum of 16–32 mg daily, followed by a plateau, in which further increases in dosage produce no pharmacological effects.Citation29,Citation31 As a result, the intensity of the rewarding effects is milder than other full μ-opioid agonists at higher doses, and the risk of abuse as well as respiratory depression is lower.Citation30,Citation32
Buprenorphine has a higher affinity for the μ-receptor than other opioids, such as heroin, so it reduces the effects of full agonists and can in fact precipitate withdrawal symptoms in those actively using other opioids.Citation33–Citation35 This high affinity combined with a slow dissociation from the μ-receptor also results in milder withdrawal symptoms upon discontinuation compared to methadone and other opioids.Citation36–Citation38
Sublingual buprenorphine has a long half-life (24–60 hours, mean 37 hours), and it is highly bound to plasma proteins (96%). It has poor oral bioavailability, because it is inactivated by gastric acid and undergoes significant first-pass metabolism in the liver. It is metabolized by cytochrome P450 (CYP) 3A4 to various metabolites, including the active norbuprenorphine.Citation28,Citation32 Peak plasma concentrations are achieved in 90–150 minutes following administration, and the μ-receptor blockade can last for 3–5 days due to the slow dissociation from the receptor, allowing for the possibility of alternate-day dosing.Citation39
Buprenorphine–naloxone (Suboxone)
Suboxone® (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) is formulated as a combination of buprenorphine with the opiate antagonist naloxone in a 4:1 ratio. Naloxone binds tightly to the opioid receptor without producing a euphoric effect, thereby blocking or reversing the psychoactive effects of partial- or full-opioid agonists.Citation40 Naloxone has poor bioavailability in the sublingual form; therefore, it does not alter buprenorphine’s properties when the medication is taken as prescribed.Citation28,Citation41 If bup/nx is injected, however, sufficient naloxone is absorbed to precipitate acute withdrawal in opiate-dependent users, thus discouraging further abuse via the intravenous route of administration.Citation40,Citation42,Citation43
Dosing and formulation
Bup/nx is available sublingually in tablet (Zubsolv® [Orexo AB with AAIPharma, Wilmington, NC, USA] and generic) or film formulation (Suboxone). The Suboxone film is offered in bup/nx doses of 2/0.5 mg, 4/1 mg, 8/2 mg, and 12/3 mg, while the generic tablet only exists in doses of 2/0.5 mg and 8/2 mg. Zubsolv, a new sublingual tablet with higher bioavailability, received FDA approval in July 2013, and is distributed in 1.4/0.36 mg and 5.7/1.4 dosages.Citation44 All formulations and dosage strengths can be dispensed in the US for take-home use by prescription. Bup/nx is formulated as a combination of buprenorphine with the opiate antagonist naloxone in a 4:1 ratio. Bup/nx doses will be stated in terms of mg buprenorphine in the text below, as well as in the figures.
The target dose for maintenance therapy is usually 16 mg buprenorphine per day, although most recent guidelines favor an individualized approach with no specific dose or range recommendations. In both inpatient and outpatient settings, the therapeutic goal is to find the lowest dose to eliminate illicit opioid use, reduce withdrawal symptoms, and improve treatment retention.Citation34 The final maintenance dose is likely to be in the range of 4–24 mg buprenorphine per day.Citation45 Due to the ceiling effect, there is no added benefit of increasing the daily dose above 32 mg per day.Citation29,Citation31
Strategy for clinical management
There are two general approaches to the medical treatment of opioid addiction: medically supervised detoxification and opioid-substitution treatment (). Bup/nx is indicated for both acute detoxification and long-term substitution among patients who have a mild-to-moderate physical dependence on opioids.Citation45 All treatment options can be carried out in an inpatient or ambulatory setting.Citation34 In medical detoxification, bup/nx is used as replacement therapy to help support the transition from a physically dependent to a nondependent state. Although it can sometimes lead to total abstinence, it has a low success rate and relapse rates are high.Citation46–Citation48 Younger patients who are only dependent on oral opioids, with a short history of dependence, a good support system, and no significant psychiatric comorbidities, have a better prognosis with this approach.Citation33
Figure 1 Clinical management: opioid substitution versus acute detoxification.
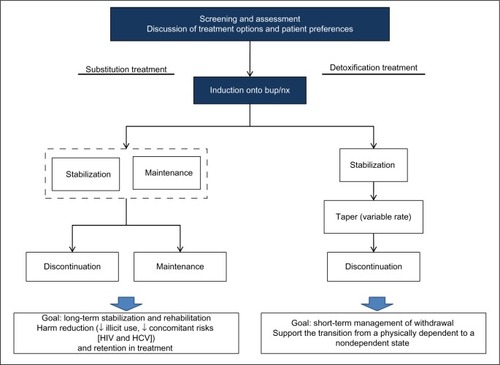
Among chronic intravenous drug users, opioid substitution is usually the preferred treatment approach. Substitution or maintenance treatment refers to the long-term use of a long-acting opioid (eg, bup/nx) with graduated take-home doses to manage opioid dependence. The goal is to reduce illicit opioid use and injection behavior, minimize concomitant risks such as HIV and HCV infection, and retain patients in treatment.Citation34,Citation49,Citation50 Long-term substitution treatment has better outcomes than medically supervised detoxification, and should be considered within the context of a harm-reduction approach.Citation51
As shown in , treatment is initiated with a thorough screening and assessment of opioid dependence, including a discussion of available treatment options and patient preferences. This is followed by an induction phase, in which patients are transferred from their opioid of abuse to bup/nx. Details related to assessment, induction, stabilization, maintenance, and detoxification are provided in the following sections.
Screening and assessment
The initial screening and assessment should include comprehensive medical, psychiatric, and psychosocial evaluations in order to confirm a diagnosis of opioid dependence.Citation45 The decision to initiate either medical detoxification or long-term substitution treatment should be guided by the severity of dependence, prior treatment history, and the patient’s preferences. Assessment of addiction severity is essential, as it will guide buprenorphine dosing and treatment needs, including potential risk of diversion. Consideration should be given to the time since the last opioid dose, the type of opioid used (long- versus short-acting), and the degree of physical dependence.Citation52 Due to opioid dependence, comorbidities and potential side effects of bup/nx, a pregnancy test, baseline liver enzymes, and HCV, Hepatitis B virus (HBV), and HIV testing are recommended prior to induction.Citation33
Induction phase
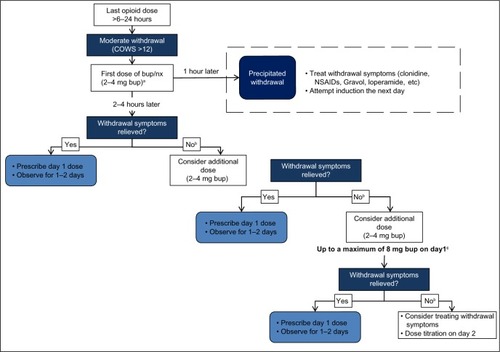
Bup/nx induction entails titration over 1–3 days until a comfortable level is reached. Patients can be started on bup/nx 6–24 hours after the last opiate dose, when they develop objective signs of spontaneous withdrawal.Citation28,Citation45,Citation53,Citation54 As shown in , cravings usually begin 4–6 hours after the last dose of a short-acting opioid, and the withdrawal pattern is delayed with ultralong-acting opioids, such as methadone. The main features of opioid withdrawal are nausea, vomiting, diaphoresis, yawning, fatigue, aches and pain, diarrhea, mydriasis, and piloerection.Citation57 Subjective symptoms are much greater than objective signs, and weak withdrawal discomfort is usually experienced after 36–72 hours and decreases thereafter.Citation57,Citation58 In theory (and practice), administration of a partial opioid agonist (bup/nx) exerts the least antagonistic effect when the patient is experiencing mild-to-moderate withdrawal symptoms.Citation32 If the patient is not in sufficient withdrawal when the first dose of bup/nx is administered, buprenorphine will displace the full-opioid agonist (eg, heroin) and intensify rather than relieve withdrawal symptoms (precipitated withdrawal). If precipitated withdrawal occurs, patients will experience intensified withdrawal symptoms after the first induction dose.Citation32–Citation35 Validated assessment tools, such as the Clinical Opioid Withdrawal Scale (scores >12) and the Clinical Institute Narcotic Assessment can be used to ensure that patients are in moderate withdrawal prior to bup/nx induction.Citation59
Table 1 Induction onto bup/nx for short-, long-, and ultralong-acting opioids
The initial dose is 2–4 mg buprenorphine, which is increased based on clinical symptoms up to a total first-day dose of 8 mg.Citation28 The goal is to reach the target dose of 12–16 mg buprenorphine during the first week.Citation34 A recent retrospective chart-review study (n=40) suggested that more rapid buprenorphine initiation up to 16 mg on the first day was well tolerated.Citation60 As indicated in , prior to induction onto bup/nx, it is possible to substitute long-acting opioids with an equivalent dose of a short-acting medication. provides an approximate equivalency among the various opioids and routes of administration.Citation61 However, clinicians must be aware that equivalency tables have not been developed for chronic (tolerant) opioid-dependent populations, and the information in must therefore be employed with clinical judgment. Extrapolating from Mattick et al, 6–12 mg buprenorphine would correspond roughly to 35–60 mg of methadone.Citation62
Table 2 Approximate opioid equivalencies compared with 10 mg of IV morphine
Induction is one of the most critical phases of bup/nx treatment. A detailed chart indicating the procedures and decision points during the first day of bup/nx induction is provided in . An association has been established between difficult induction (strong withdrawal symptoms and cravings) and poorer treatment retention and outcome.Citation63–Citation65 Evidence also suggests that increasing the induction dose more rapidly is associated with better treatment outcome.Citation66 Therefore, patients should be observed regularly during the induction period to exclude precipitated withdrawal, signs of overmedication (eg, sedation) or unwanted side effects, and to confirm the effectiveness of the dose at suppressing withdrawal symptoms.Citation34,Citation58 Current guidelines recommend direct observation of initial dosage of bup/nx for 2–4 hours, with a series of subsequent visits for dose adjustments.Citation34,Citation45,Citation67,Citation68 Observed induction can, however, present significant challenges to many office-based practitioners, and as an alternative, “home” or unobserved induction has been proposed. Evidence supporting this practice is accumulating, but further research is still needed to confirm the feasibility and safety of this innovative treatment approach.Citation13,Citation68–Citation71 To favor compliance, initial prescription of bup/nx can be written daily in order to provide supervised medication dispensing.Citation72
Figure 2 Bup/nx induction (day 1).
Abbreviations: Bup/nx, buprenorphine–naloxone; COWS, Clinical Opioid Withdrawal Scale; NSAIDs, nonsteroidal anti-inflammatory drugs; bup, buprenorphine.
Note that a bup/nx combination can be used for induction treatment in most patients. However, pregnant women and patients who are on ultralong-acting opioids (eg, methadone) should be induced using buprenorphine monotherapyCitation45,Citation73 instead of bup/nx. The transition from methadone to buprenorphine is gaining popularity, due to a more flexible outpatient setting, safer side-effect profile, and lower potential for misuse and abuse.Citation74–Citation77 However, concerns about the possibility of inducing significant withdrawal during the induction procedure limit the practice.Citation78,Citation79 Prior to the transition to buprenorphine, methadone dose should be gradually tapered to 30–40 mg per day and maintained at this dose or lower for at least 5–7 days. The patient should then discontinue methadone for 48–72 hours prior to receiving the first dose of buprenorphine.Citation45
Stabilization and maintenance
Stabilization begins when the patient is no longer having withdrawal symptoms or cravings and there are minimal side effects from bup/nx. The goal is to find the minimum dose that eliminates withdrawal symptoms, prevents other opioid use, decreases cravings, and improves psychosocial functioning (the maintenance dose).Citation80,Citation81 As each patient must be dosed individually, medication adjustment can take up to 1–2 months (7–14 days in acute detoxification), with slow increments/decrements every 5–7 days achieved through weekly contact with the patient during early stabilization. The Suboxone manufacturer recommends that dosing be observed for the first 2 months, even though this treatment protocol is not always possible in clinical practice. Relapse should be ruled out when a stable patient’s dosing requirements change suddenly.Citation45,Citation54
During the maintenance phase, moderate-to-high doses (8–16 mg buprenorphine) have been shown to be significantly more efficacious than low doses (1–4 mg buprenorphine).Citation62,Citation82,Citation83 Although the maximum recommended dose by the Suboxone manufacturer is 24 mg, doses of up to 32 mg have been used in some trials.Citation84 A recent observational retrospective chart-review study showed that using a flexible dosing schedule with the option of titrating the dose up to 32 mg offered better treatment outcomes, especially for patients who did not respond to lower doses.Citation85
The optimum duration of maintenance is unclear, but it may involve long-term or even lifetime medication use.Citation86 Patients who were induced with buprenorphine for the transition from methadone to bup/nx should be switched to bup/nx for maintenance once transition is complete.Citation34 An alternate-day dosing schedule of bup/nx is possible, and this may be preferred or better tolerated by some patients.Citation34,Citation45 The weekly dose can then be divided by the number of days of dosing (eg, double dose for alternate days). Routine urine toxicology screens can be utilized to assess bup/nx compliance and detect the use of other prescribed, undisclosed, or illicit substances (eg, alcohol, benzodiazepines, morphine, oxycodone, etc), although evidence for the cost-effectiveness of urine testing is weak.Citation72 On-site dispensing or observed taking of bup/nx is indicated until the patient has a negative urine screen or is compliant with treatment.Citation45
Discontinuation (detoxification)
In medically supervised withdrawal, reaching an opioid-free state is the ultimate goal of treatment, and discontinuation can also be considered for patients on long-term maintenance treatment. However, tapering off or cessation of bup/nx should only be considered for patients who are motivated to discontinue, have a stable income and housing arrangements, and have adequate psychosocial support.Citation34 Dose-reduction protocols range from 3 days (short), 10–14 days (moderate), to months (long).Citation34,Citation45,Citation73 Initial studies yielded conflicting results about the optimal duration of the taper.Citation87–Citation89 A recent large randomized trial demonstrated that after a month of stabilization on Suboxone treatment, a 7-day taper was equivalent to a 28-day taper in terms of the number of opioid-free urine samples.Citation90 Conversely, another comparative study suggested that a 30-day taper enhanced participation in longer-term treatment compared with a 5-day taper.Citation91 Recent guidelines suggest a gradual bup/nx discontinuation with a taper of no more than 2 mg every 5–7 days.Citation33,Citation34,Citation73 It is important to assess the patient regularly during the discontinuation process. If withdrawal symptoms emerge as the dose is decreased, the taper can be temporarily suspended.Citation34 Medications commonly used to alleviate withdrawal symptoms are presented in . Following completion of the taper, naltrexone, an opioid-receptor antagonist that blocks the euphoric effects of opioids, can also be used to minimize the risk of relapse to opioid abuse.Citation92
Table 3 Symptomatic treatment of opioid withdrawal
Integrated multidisciplinary approach
A purely pharmacological approach to medication-assisted recovery is rarely sufficient.Citation93 The goals of psychosocial interventions (counseling, self-help groups, and rehabilitation programs) are to engage patients in the process of change and retain them in treatment long enough to improve global functioning and quality of life. Contingency-management techniques that use motivational incentives to condition or influence behaviors (eg, receiving a “voucher” with monetary value for negative urine-toxicology screening), have been found to help in initiating and maintaining abstinence among addiction patients.Citation94 These resources are available in structured treatment programs as well as in the community.
The outcome of bup/nx treatment is best when combined with psychosocial counseling, prevention education, and recovery-support services.Citation67,Citation95–Citation97 The best evidence to support the value of a multidisciplinary approach in the treatment of opioid addiction comes from a Cochrane review by Amato et al.Citation14 This meta-analysis included eleven studies and evaluated five different psychosocial interventions and two pharmaceutical treatments (buprenorphine and methadone). The combination of pharmacological and psychosocial treatment was significantly superior in reducing dropout rates (relative risk [RR] 0.71), decreasing relapses or use of full agonist opioids (RR 0.82), and improving treatment compliance in terms of follow-up visits (RR 0.48). Currently, there are no data supporting a specific type of psychosocial approach. Despite limited evidence, most clinicians feel that bup/nx treatment should be accompanied by voluntary psychosocial counseling, participation in formal treatment programs and/or attendance at mutual aid groups, such as Narcotics Anonymous.Citation14,Citation86
Patient-focused perspectives
Bup/nx is usually well tolerated. It is a safe pharmacological treatment, with expected side effects of sedation, constipation, headache, nausea/vomiting, and dizziness. It has a lower risk of respiratory depression than full agonists, and is associated with less QTc prolongation than methadone.Citation98,Citation99 Liver-function tests should be periodically monitored.Citation28,Citation100
Many of the randomized trials on buprenorphine did not examine tolerability. In a 4-week randomized double-blind phase of a 52-week study comparing the efficacy of bup/nx with placebo, the most frequently reported adverse events were headache, withdrawal symptoms, pain, nausea, insomnia, and sweating. No serious treatment-related adverse events have been reported, except increases in hepatic alanine aminotransferase and aspartate aminotransferase.Citation101 These are all expected side effects of bup/nx.
Bup/nx is sometimes favored because it carries less stigma than methadone. Patients also appear to prefer bup/nx due to its more flexible administration schedule, which enables office-based treatment and allows lower clinic-attendance requirements for medication dispensing and carry-outs.Citation102 The main barrier to using bup/nx is its high cost, especially when compared to methadone.Citation24,Citation62,Citation103 Moreover, buprenorphine programs tailored for marginalized populations with unstable lifestyles and greater risk of comorbidities and infectious disease transmission are currently limited.Citation73
Special populations
Hepatitis and liver disease
Buprenorphine is contraindicated in patients suffering from severe liver disease only, with liver-function tests three to five times above the upper limit of normal.Citation73 It can be used safely in patients with hepatitis whose liver-function tests are below that range.
Concomitant drug dependence
The combination of bup/nx with sedative-hypnotics (eg, benzodiazepines, alcohol) has been associated with increased mortality, mainly due to respiratory depression.Citation28,Citation100 Sedative-hypnotic-dependent patients should be advised to reduce or discontinue their use prior to induction, and urine-toxicology testing should be performed regularly to assess concomitant use.Citation45 Active use of alcohol, benzodiazepines, and barbiturates is therefore a relative contraindication to buprenorphine outpatient treatment, and medically supervised withdrawal is suggested.Citation104–Citation106
Pregnancy and children
Bup/nx is contraindicated for pregnant women, as naloxone has not been approved for use in pregnancy. Buprenorphine monotherapy, like methadone, which has been the standard of care in obstetric care since the 1970s, has been shown to reduce illicit opiate use, enhance compliance, and improve neonatal outcomes, such as birth weight.Citation107–Citation110 Recently, the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study confirmed these conclusions and suggested the superiority of buprenorphine in terms of fetal outcomes, with less frequent and severe neonatal abstinence syndrome compared to methadone.Citation109,Citation110
The use of buprenorphine in women who are breastfeeding remains controversial. Both buprenorphine and its main metabolite (norbuprenorphine) appear at low concentrations in breast milk, levels that are unlikely to contribute to buprenorphine dependence in the infant. Due to limited data, the risks and benefits of buprenorphine exposure should be weighed before initiating or continuing substitution treatment among breastfeeding women.Citation110,Citation111
Intoxication with bup/nx in children is relatively safe.Citation84 In a retrospective study, the main clinical effects were drowsiness or lethargy (55%), vomiting (21%), miosis (21%), respiratory depression (7%), agitation or irritability (5%), pallor (3%), and coma (2%), with no reports of death. None of these severe effects occurred in children who ingested less than 4 mg buprenorphine.Citation112 Children should be referred to emergency for all ingestions of over 2 mg buprenorphine or any type of ingestion in patients under 2 years old.Citation33
Adolescents and youth
Few studies have evaluated buprenorphine and bup/nx in the treatment of adolescents with opioid dependence. There is good evidence suggesting that youth are at higher risk of mortality and morbidity from opioid-use disorders (overdose, HIV and hepatitis transmission, concurrent disorders, suicide, and death).Citation113–Citation116 The risk–benefit ratio of bup/nx treatment should therefore be carefully evaluated prior to buprenorphine initiation. If substitution treatment with opioid agonists is considered, bup/nx may be preferred over methadone. Bup/nx prescribing in adolescents and young adults follows the same steps and dosages as in adults.Citation33,Citation113
HIV patients
Bup/nx is associated with enhanced HIV outcomes, including increased antiretroviral therapy (ART) initiation, compliance and CD4+ count.Citation117 However, it should be used with caution in patients with HIV on ART due to the potential for drug interactions. In theory, several nonnucleoside reverse-transcriptase inhibitors and protease inhibitors are CYP3A4 inducers (efavirenz, nevirapine, nelfinavir, lopinavir/ritonavir) or inhibitors (delavirdine, ritonavir, atazanavir/ritonavir), and thereby the combination has the potential to alter the metabolism of buprenorphine, ART, or both. Some studies suggest that dose adjustments may be required, but no cases of opioid toxicity have been reported.Citation118
Conclusion
Opioid addiction is a serious public health problem that results in significant individual and social costs, including increased disability, criminal activity, death from overdose, and risk of infectious and blood-borne disease transmission.Citation1–Citation4,Citation6,Citation34 Bup/nx, a partial-opioid agonist with unique pharmacological properties, is the only office-based maintenance treatment offered for opioid-use disorders. It is an attractive option for opioid-substitution therapy because of its favorable side-effect profile, flexible dosing schedules, and low diversion and abuse potential. It has demonstrated efficacy in reducing opiate cravings, ameliorating withdrawal discomfort, and increasing periods of abstinence from illicit drug use. In most cases, treatment is required for long periods or even throughout life.Citation86,Citation92 Rather than being considered a treatment failure, long-term treatment, which is common to many chronic medical conditions, should be envisaged as a cost-effective way to prolong longevity and improve quality of life.
Although bup/nx has shown multiple benefits, the cost of the medication hinders its widespread use, mainly for marginalized populations and street-heroin users. Members of these populations are at greater risk of comorbidities and infectious disease transmission, and thus it is crucial to increase access to efficacious treatments.
Physicians who wish to prescribe bup/nx in the US and Canada must complete a training program. More exhaustive continuing medical education programs are available for physicians with less experience in treating opioid dependence.
Disclosure
The authors report no conflicts of interest in this work.
References
- DegenhardtLBucelloCCalabriaBWhat data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviewsDrug Alcohol Depend20111178510121377813
- FischerBArgentoEPrescription opioid related misuse, harms, diversion and interventions in Canada: a reviewPain Physician20125ES191ES20322786457
- FriedlandGInfectious disease comorbidities adversely affecting substance users with HIV: hepatitis C and tuberculosisJ Acquir Immune Defic Syndr201055S37S4221045598
- StockmanJKStrathdeeSAHIV among people who use drugs: a global perspective of populations at riskJ Acquir Immune Defic Syndr201055S17S2221045594
- Substance Abuse and Mental Health Services Administration (SAMHSA)State Estimates of Substance Use and Mental Disorders from the 2008–2009 National Surveys on Drug Use and HealthRockville, MDSAMHSA2011
- United Nations Office on Drugs and Crime (UNODC)World Drug Report 2013ViennaUN2013 Available from: http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdfAccessed November 18, 2013
- European Monitoring Center on Drugs and Drug Abuse (EMCDDA)Annual Report 2011: The State of the Drugs Problem in EuropeLuxembourgEMCDDA2011 Available from: http://www.emcdda.europa.eu/publications/annual-report/2011Accessed November 18, 2013
- HolmesDPrescription drug addiction: the treatment challengeLancet2012379171822232799
- Royal Australasian College of Physicians (RACP)Prescription Opioid Policy: Improving Management of Chronic Nonmalignant Pain and Prevention of Problems Associated with Prescription Opioid UseSydneyRACP2009 Available from: https://www.racp.edu.au/index.cfm?objectid=EA87198D-CA47-AB21-072D9B2F26FD4AA3Accessed November 18, 2013
- SigmonSCharacterizing the emerging population of prescription opioid abusersAm J Addict20061520821216923666
- Substance Abuse and Mental Health Services AdministrationDrug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits2013 Available from: http://www.Samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htmAccessed November 18, 2013
- Gwin MitchellSKellySMBrownBSUses of diverted methadone and buprenorphine by opioid-addicted individuals in Baltimore, MarylandAm J Addict20091834635519874152
- SohlerNLLiXKuninsHVHome- versus office-based buprenorphine induction for opioid-dependent patientsJ Subst Abuse Treat20103815315919801178
- AmatoLMinozziSDavoliMVecchiSPsychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxificationCochrane Database Syst Rev20119CD00503121901695
- FriedmanSRTempalskiBCooperHEstimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug usersJ Urban Health20048137740015273263
- PetersonJASchwartzRPMitchellSGWhy don’t out-of-treatment individuals enter methadone treatment programs?Int J Drug Policy201021364218805686
- PopovaSRehmJFischerBAn overview of illegal opioid use and health services utilization in CanadaPublic Health200612032032816476455
- AuriacombeMFatseasMDubernetJDaulouedeJPTignolJFrench field experience with buprenorphineAm J Addict200413S17S2815204673
- FatseasMAuriacombeMWhy buprenorphine is so successful in treating opiate addiction in FranceCurr Psychiatry Rep2007935836417915074
- MooreBFiellinDABarryDTPrimary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patientsJ Gen Intern Med20072252753017372805
- FiellinDAThe first three years of buprenorphine in the United States: experience to date and future directionsJ Addict Med20071626721768936
- KnudsenHJAbrahamAJJohnsonJARomanPMBuprenorphine adoption in the National Drug Abuse Treatment Clinical Trials NetworkJ Subst Abuse Treat20093730731219577406
- ParranTVAdelmanCAMerkinBLong-term outcomes of office-based buprenorphine/naloxone maintenance therapyDrug Alcohol Depend2010106566019717249
- BellJTrinhLButlerBRandallDRubinGComparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatmentAddiction20091041193120019563562
- JonesESMooreBASindelarJLO’ConnorPGSchottenfeldRSFiellinDACost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patientsDrug Alcohol Depend20099913214018804923
- NielsenSDietzePCantwellKLeeNTaylorDMethadone and buprenorphine related ambulance attendances: a population-based indicator of adverse eventsJ Subst Abuse Treat20083545746118295435
- SmithMYBaileyJEWoodyGEKleberHDAbuse of buprenorphine in the United States: 2003–2005Journal of Addictive Diseases20072610711118018814
- ViraniASBezchlibnyk-ButlerKZJeffriesJJProcyshynRMClinical Handbook of Psychotropic Drugs19th edBernHogrefe and Huber2012
- MelloNKMendelsonJBehavioral pharmacology of buprenorphineDrug Alcohol Depend1985142833033888577
- LutfyKCowanABuprenorphine: a unique drug with complex pharmacologyCurr Neuropharmacol2004239540218997874
- WalshSPrestonKStitzerMConeEBigelowGClinical pharmacology of buprenorphine: ceiling effects at high dosesClin Pharmacol Ther1994555695808181201
- StrainECPrestonKLiebsonIBigelowGBuprenorphine effects in methadone-maintained volunteers: effect at two hours after methadoneJ Pharmacol Exp Ther19952726286387853176
- Centre for Addiction and Mental HealthBuprenorphine/Naloxone for Opioid Dependence: Clinical Practice GuidelineTorontoCAMH2012 Available from: http://knowledgex.camh.net/primary_care/guidelines_materials/Documents/buprenorphine_naloxone_gdlns2012.pdfAccessed November 18, 2013
- Substance Abuse and Mental Health Services AdministrationClinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: A Treatment Improvement Protocol (TIP) 40Rockville, MDSAMHSA2004 Available from: http://buprenorphine.samhsa.gov/Bup_Guidelines.pdfAccessed November 18, 2013
- WessonDRBuprenorphine in the treatment of opiate dependence: its pharmacology and social context of use in the USJ Psychoactive Drugs2004Suppl 211912815279124
- AmassLLingWFreeseTEBringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA clinical trials network field experienceAm J Addict200413Suppl 1S42S6615204675
- CaldieroRParranTAdelmanCPicheBInpatient initiation of buprenorphine maintenance vs detoxification: can retention of opioid-dependent patients in outpatient counseling be improved?Am J Addict2006151716449087
- WhiteJBellJSaundersJOpen-label dose-finding trial of buprenorphine implants (Probuphine) for treatment of heroin dependenceDrug Alcohol Depend2009103374319403243
- SchuhKWalshSStitzerMOnset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humansPsychopharmacology199914516217410463317
- LingWJacobsPHillhouseMFrom research to the real world: buprenorphine in the decade of the Clinical Trials NetworkJ Subst Abuse Treat201038Suppl 1S53S6020307796
- ChiangCNHawksRLPharmacokinetics of the combination tablet of buprenorphine and naloxoneDrug Alcohol Depend200370Suppl 2S39S4712738349
- BigelowGEPrestonKLiebsonIAbuse liability assessment of buprenorphine-naloxone combinationsNIDA Res Monogr1987761451492449616
- PrestonKLBigelowGLiebsonIBuprenorphine and naloxone alone and in combination in opioid-dependent humansPsychopharmacology1988944844902453895
- US Food and Drug AdministrationDrugs@FDA: FDA-approved drug products Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfmAccessed November 18, 2013
- Community Care Behavioral Health OrganizationSupporting recovery from opioid addiction: community care best practice guidelines for buprenorphine and Suboxone2013 Available from: http://www.ct.gov/dmhas/lib/dmhas/publications/Community_Care_BP_Guidelines_for_Buprenorphine_and_Suboxone.pdfAccessed November 18, 2013
- BroersBGinerFDumontPMinoAInpatient opiate detoxification in Geneva: follow-up at 1 and 6 monthsDrug Alcohol Depend200058859210669058
- GossopMClonidine and the treatment of the opiate withdrawal syndromeDrug Alcohol Depend1988212532593048954
- VaillantGEWhat can long-term follow-up teach us about relapse and prevention of relapse in addiction?Br J Addict198883114711573191263
- CornishRMacleodJStrangJVickermanPHickmanMRisk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research DatabaseBMJ2010341c547520978062
- KimberJCopelandLHickmanMSurvival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatmentBMJ2010341c317220595255
- DoleVPNyswanderMEHeroin addiction – a metabolic diseaseArch Intern Med196712019246028693
- JohnsonREStrainECAmassLBuprenorphine: how to use it rightDrug Alcohol Depend200370S59S7712738351
- FiellinDKleberHTrumble-HejdukJMcLellanAKostenTConsensus statement on office-based treatment of opioid dependence using buprenorphineJ Subst Abuse Treat20042715315915450648
- Schering-Plough CanadaSuboxone Product MonographKirkland, QCSchering-Plough Canada2007
- KostenTRO’ConnorPGManagement of drug and alcohol withdrawalN Engl J Med20033481786179512724485
- DijkstraBAKrabbePFDe JongCAvan der StaakCPPrediction of withdrawal symptoms during opioid detoxificationJ Opioid Manag2008431131919070269
- FarrellMOpiate withdrawalAddiction199489147114757841858
- KleberHDetoxification from narcoticsLowinsonJHRuizPSubstance AbuseBaltimoreWilliams & Wilkins1981
- TompkinsDABigelowGEHarrisonJAJohnsonREFudalaPJStrainECConcurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrumentsDrug Alcohol Depend200910515415919647958
- GundersonEWLevinFRRomboneMMVosburgSKKleberHDImproving temporal efficiency of outpatient buprenorphine inductionAm J Addict20112039740421838837
- DucharmeSFraserRGillKUpdate on the clinical use of buprenorphine in opioid-related disordersCan Family Physician2012583741
- MattickRKimberJDavoliMBuprenorphine maintenance versus placebo or methadone maintenance for opioid dependenceCochrane Database Syst Rev20082CD00220718425880
- NielsenSHillhouseMThomasCHassonALingWA comparison of buprenorphine taper outcomes between prescription opioid and heroin usersJ Addict Med20137333823222095
- SoykaMZinggCKollerGKuefnerHRetention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized studyInt J Neuropsychopharmacol20081164165318205978
- WhitleySDSohlerNLKuninsHVFactors associated with complicated buprenorphine inductionsJ Subst Abuse Treat201039515720682186
- DoranCMBuprenorphine, buprenorphine/naloxone and methadone maintenance: a cost-effectiveness analysisExpert Rev Pharmacoecon Outcomes Res2005558359119807585
- BonhommeJShimRSGoodenRTyusDRustGOpioid addiction and abuse in primary care practice: a comparison of methadone and buprenorphine as treatment optionsJ Natl Med Assoc201210434235023092049
- LeeJDGrossmanEDiRoccoDGourevitchMNHome buprenorphine/naloxone induction in primary careJ Gen Intern Med20092422623219089508
- WalleyAYAlperenJKChengDMOffice-based management of opioid dependence with buprenorphine: clinical practices and barriersJ Gen Intern Med20082391393139818592319
- MintzerILEisenbergMTerraMMacVaneCHimmelsteinDUWoolhandlerSTreating opioid addiction with buprenorphine-naloxone in community-based primary care settingsAnn Fam Med20075214615017389539
- GundersonEWWangXQFiellinDABryanBLevinFRUnobserved versus observed office buprenorphine/naloxone induction: a pilot randomized clinical trialAddict Behav20103553754020106601
- FarrellMWodakAGowingLMaintenance drugs to treat opioid dependenceBMJ2012344e282322589497
- KrausMLAlfordDPKotzMMStatement of the American Society of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addictionJ Addict Med2011525426322042215
- EhretGBDesmeulesJABroersBMethadone-associated long QT syndrome: improving pharmacotherapy for dependence on illegal opioids and lessons learned for pharmacologyExpert Opin Drug Saf2007628930317480178
- FishmanMJWuLTWoodyGEBuprenorphine for prescription opioid addiction in a patient with depression and alcohol dependenceAm J Psychiatry201116867567921724673
- MartellBAArnstenJHKrantzMJGourevitchMNImpact of methadone treatment on cardiac repolarization and conduction in opioid usersAm J Cardiol20059591591815781034
- WhitleySDKuninsHVArnstenJHGourevitchMNColocating buprenorphine with methadone maintenance and outpatient chemical dependency servicesJ Subst Abuse Treat200733859017588493
- ManelliPPeindlKSLeeTBhatiaKSWuLTBuprenorphine-mediated transition from opioid agonist to antagonist treatment: state of the art and new perspectivesCurr Drug Abuse Rev20125526322280332
- KnudsenHKDucharmeLJRomanPMEarly adoption of buprenorphine in substance abuse treatment centers: data from the private and public sectorsJ Subst Abuse Treat20063036337316716852
- BaxterLEClinical Guidance on Methadone Induction and StabilizationChevy Chase (MD)American Society of Addiction Medicine2009
- JosephHStancliffSLagrodJMethadone maintenance treatment (MMT): a review of historical and clinical issues [review]Mt Sinai J Med20006734736411064485
- LingWCharuvastraCCollinsJBuprenorphine maintenance treatment of opiate dependence: a multicenter randomized clinical trialAddiction1998934754869684386
- AhmadiJMethadone versus buprenorphine maintenance for the treatment of heroin-dependent outpatientsJ Subst Abuse Treat20032421722012810142
- KakkoJGrönbladhLSvanborgKA stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trialAm J Psychiatry200716479780317475739
- FareedAVayalapalliSCasarellaJDexlerKTreatment outcome for flexible dosing buprenorphine maintenance treatmentAm J Drug Alcohol Abuse20123815516022175698
- World Health OrganizationGuidelines for the Psychosocially Assisted Pharmalogical Treatment of Opioid DependenceGenevaWHO2009 Available from: http://www.who.int/substance_abuse/publications/opioid_dependence_guidelines.pdfAccessed January 30, 2014
- WangRYoungLDouble-blind controlled detoxification from buprenorphineNIDA Res Monogr1996162114
- PychaCResnickRGalanterMBuprenorphine: rapid and slow dose-reduction for heroin detoxificationNIDA Res Monogr1994141453
- AmassLBickelWHigginsSHughesJA preliminary investigation of outcome following gradual or rapid buprenorphine detoxificationJ Addict Dis19941333457734458
- LingWHillhouseMDornierCBuprenorphine tapering schedule and illicit opioid useAddiction200910425626519149822
- KatzECSchwartzRPKingSBrief vs extended buprenorphine detoxification in a community treatment program: engagement and short term outcomesAm J Drug Alcohol Abuse200935636719199166
- KleberHDWeissRDAntonRFPractice guidelines for the treatment of patients with substance use disorderssecond editionArlington (VA)American Psychiatric Association (APA)82006 http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243188&PDFSource=6Accessed November 18, 2013
- DutraLStathopoulouGBasdenSLLeyroTMPowersMBOttoMWA meta-analytic review of psychosocial interventions for substance use disordersAm J Psychiatry200816517918718198270
- PendergastMPodusDFinneyJGreenwellLRollJContingency management for treatment of substance use disorders: a meta-analysisAddiction201010115461560
- CampbellBKFullerBELeeESFacilitating outpatient treatment entry following detoxificationPsychology of Addictive Behaviours2009232260270
- FiellinDAPantalonMChawarskiMCounselling plus buprenorphine-naloxone maintenance therapy for opioid dependenceN Engl J Med200635536537416870915
- WeissRDPotterJSProvostSEA multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodologyContemp Clin Trials20103118919920116457
- WedamEFBigelowGJohnsonRNuzzoPHaigneyMQT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trialArch Intern Med20071672469247518071169
- AnchersonKClausenTGossopMHansteenVWaalHPrevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: a mortality assessment studyAddiction200910499399919392907
- LangeWRFudalaPDaxEJohnsonRSafety and side-effects of buprenorphine in the clinical management of heroin addictionDrug Alcohol Depend19902619282209411
- FudalaPJBridgeTPHerbertSOffice-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxoneN Engl J Med200334994995812954743
- OrmanJSKeatingGMBuprenorphine/naloxone: a review of its use in the treatment of opioid dependenceDrugs20096957760719368419
- MattickRPBreenCKimberJDavoliMMethadone maintenance therapy versus no opioid replacement for opioid dependenceCochrane Database Syst Rev20093CD00220919588333
- FischerGOrtnerRRohrmeisterKMethadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison studyAddiction200610127528116445556
- LavieEFatseasMDenisCAuriacombeMBenzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependenceDrug Alcohol Depend20099933834418824311
- House of Delegates of the Federation of State Medical BoardsModel Policy on DATA 2000 and Treatment of Opioid Addiction in Medical OfficesUnited StatesFederation of State Medical Boards42013 Available from: http://www.fsmb.org/pdf/2013_model_policy_treatment_opioid_addiction.pdfAccessed January 2014
- KaltenbachKBerghellaVFinneganLOpioid dependence during pregnancy: effects and managementObstet Gynecol Clin North Am1998251391519547764
- Fajemirokun-OdudeyiOSinhaCTuttySPregnancy outcome in women who use opiatesEur J Obstet Gynecol Reprod Biol200612617017516202501
- JonesHEKaltenbachKHeilSHNeonatal abstinence syndrome after methadone or buprenorphine exposureN Engl J Med20103632320233121142534
- JonesHEFischerGHeilSHMaternal Opioid Treatment: Human Experimental Research (MOTHER) – approach, issues, and lessons learnedAddiction2012107Suppl 1283523106924
- Academy of Breastfeeding Medicine Protocol CommitteeJanssonLMABM Clinical Protocol #21: Guidelines for Breastfeeding and the Drug-Dependent WomanBreastfeed Med2009422522819835481
- HayesBDKlein-SchwartzWDoyonSToxicity of buprenorphine overdoses in childrenPediatrics2008121e782e78618381506
- BellJMutchCTreatment retention in adolescent patients treated with methadone or buprenorphine for opioid dependence: a file reviewDrug Alcohol Rev20062516717116627307
- WoodyGPooleSASubramaniamGExtended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trialJAMA20083002003201118984887
- FiellinDATreatment of adolescent opioid dependence: no quick fixJAMA20083002057205918984896
- LevySVaughanBLAnguloMKnightJRBuprenorphine replacement therapy for adolescents with opioid dependence: early experience from a children’s hospital-based outpatient treatment programJ Adolesc Health20074047748817448411
- AlticeFLBruceRDLucasGMHIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite studyJ Acquir Immune Defic Syndr201156Suppl 1S22S3221317590
- McCance-KatzEFSullivanLSNallaniSDrug interactions of clinical importance between the opioids, methadone and buprenorphine, and frequently prescribed medications: a reviewAm J Addict20091941620132117

