Abstract
Traumatic brain injury (TBI) is a growing health concern affecting civilians and military personnel. In this review, treatments for the chronic TBI patient are discussed, including pharmaceuticals, nutraceuticals, cognitive therapy, and hyperbaric oxygen therapy. All available literature suggests a marginal benefit with prolonged treatment courses. An emerging modality of treatment is near-infrared (NIR) light, which has benefit in animal models of stroke, spinal cord injury, optic nerve injury, and TBI, and in human trials for stroke and TBI. The extant literature is confounded by variable degrees of efficacy and a bewildering array of treatment parameters. Some data indicate that diodes emitting low-level NIR energy often have failed to demonstrate therapeutic efficacy, perhaps due to failing to deliver sufficient radiant energy to the necessary depth. As part of this review, we present a retrospective case series using high-power NIR laser phototherapy with a Class IV laser to treat TBI. We demonstrate greater clinical efficacy with higher fluence, in contrast to the bimodal model of efficacy previously proposed. In ten patients with chronic TBI (average time since injury 9.3 years) given ten treatments over the course of 2 months using a high-power NIR laser (13.2 W/0.89 cm2 at 810 nm or 9 W/0.89 cm2 at 810 nm and 980 nm), symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability improved. Symptoms were monitored by depression scales and a novel patient diary system specifically designed for this study. NIR light in the power range of 10–15 W at 810 nm and 980 nm can safely and effectively treat chronic symptoms of TBI. The clinical benefit and effects of infrared phototherapy on mitochondrial function and secondary molecular events are discussed in the context of adequate radiant energy penetration.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Traumatic brain injury (TBI) has recently moved into the limelight due to the recognition of its impact on professional athletes and military personnel. Yet, TBI is neither a new problem nor limited to those two populations. The Centers for Disease Control and Prevention estimated that 1.5 million Americans sustained TBI annually in 2000.Citation1 As of 2006, the estimates had risen to 1.7 million brain injuries annually.Citation2,Citation3 Undoubtedly, these point prevalence proportions will increase as military personnel return home,Citation4 and the problem of repeated mild TBI (mTBI) becomes more recognized in sports.Citation5 Current estimates of the prevalence of TBI among veterans range from 9.6%Citation6 to 20%,Citation7 with an estimated total of more than 300,000 cases of TBI among military personnel since 2000.Citation4 The current estimates of the combined number of sports-related concussions and brain injuries in the US are 1.6–3.8 million annually.Citation8–Citation10
TBI results in a wide spectrum of neurological, psychiatric, cognitive, and emotional consequences. In part, the variation is related to the severity of the injury (mild, moderate, severe TBI), which is stratified based on Glasgow Coma score, periods of unconsciousness, and degrees of amnesia. Furthermore, the diversity of sequalae can be related to the areas of the brain that are injured, the severity of the injury (highly variable within the classification of “mild” and “moderate”), and the evolution of the injury over time due to neuroinflammatory processes.Citation11,Citation12 Additional mechanisms thought to underlie the damage of TBI include decreased mitochondrial function, calcium and magnesium dysregulation, excitotoxicity, disruption of neural networks, free radical-induced damage, excessive nitric oxide, ischemia, and damage to the blood–brain barrier. Together, these can contribute to a progression of the damage over time.
Patients with TBI can experience headache, visual disturbances, dizziness, cognitive impairment, loss of executive skills, memory impairment, fatigue, impulsivity, impaired judgment, emotional outbursts, anxiety, and depression.Citation3,Citation13–Citation23 The situation can be further clouded by secondary and/or comorbid posttraumatic stress disorder (PTSD), depression, and anxiety,Citation17–Citation25 which can have symptoms that overlap with those described above and appear to be increasingly likely with repetitive concussive or subconcussive brain injury.Citation5,Citation24,Citation26
Treatments for TBI
Pharmacological treatments
Pharmacological treatment largely targets the neuropsychiatric sequalae of TBI, rather than providing any means of healing or repairing injury. In general, pharmacological treatment is focused on the modulation of major neurotransmitter systems – dopaminergic, serotonergic, noradrenergic, acetylcholinergic, and glutaminergic.Citation20 Disruption of the major neurotransmitter pathways may result from direct injury or excitotoxicity and other cytotoxic mechanisms.
The treatment of depression secondary to TBI is often approached with serotonin reuptake inhibitors. Several studies have examined the benefit of sertraline in post-TBI depression.Citation27–Citation29 Other serotonin reuptake inhibitors also have been examined. Tricyclic antidepressants appear to have some use in the treatment of post-TBI depression, although cautious dose titration is required. Patients with TBI are at greater vulnerability to sedation and cholinergic side effects of confusion and memory impairment. With serotonergic agents other than sertraline, cognitive effects also have been reported.Citation30 Similarly, lithium may be a less desirable agent in this population due to sedation and cognitive impairment. Patients with TBI may respond at lower doses and lower blood levels than expected.
Modulation of the dopaminergic system may improve alertness, attention, and cognitive processing speed. The stimulants are most commonly used for this purpose. Methylphenidate facilitates the release of dopamine and slows its reuptake. Dextroamphetamine strongly inhibits reuptake of dopamine, slows down the breakdown of dopamine by monoamine oxidase, and somewhat increases the release of dopamine. These subtle differences are sometimes imperceptible to the patient, but at other times, a patient will do best on one or the other stimulant. Increasing dopamine in the reticular activating system leads to enhanced arousal. Increasing dopamine within the frontal cortex and the striatum leads to enhanced processing speed and attention. Some evidence suggests that the stimulants may enhance neuronal recovery after injury.Citation31–Citation33 There are numerous potential side effects with stimulants, including abnormal heart rhythms, decreased seizure threshold, and death, but these severe side effects are extremely rare. The most common side effects with stimulants are decreased appetite, stomach upset, and headache. These are most severe at the beginning of treatment and improve over time for most patients. Insomnia is another common side effect, which may be more frequent in those with a TBI. Amantadine and bromocriptine may also increase dopamine. Studies of these agents have shown reduced abulia, anergia, and anhedonia in those with TBI.Citation34,Citation35 Amantadine may cause confusion, hallucinations, and hypotension. Small studies have suggested some benefits of bromocriptine in cognitive function.Citation36,Citation37
Arousal-enhancing agents also have found a use in the treatment of the neurocognitive sequalae of TBI. Modafinil is the oldest form of these medications, and armodafinil is an isomer of modafinil with longer activity and less side effects. These medications help to increase alertness and wakefulness. The precise mechanism of action of modafinil is unclear. It appears to increase histamine in parts of the brain involved in controlling the sleep–wake cycle; however, knock-out mice that lack histamine receptors still show increased wakefulness with modafinil.Citation38,Citation39 The picture is also murky for modafinil’s effect on orexins, which are wakefulness molecules in the hypothalamus.Citation40 Modafinil has been shown to weakly bind to the dopamine transporter – like the stimulants,Citation41 and dopamine transporter knock-out mice show no response to modafinil.Citation42 A number of research studies have examined the benefit of these agents in fatigue associated with multiple sclerosis, TBI, cancer, and other conditions.
Cognitive and memory impairments after TBI may reflect disruption of cholinergic function. The impact of anticholinergic agents on cognitive function of those with TBI supports this contention. Donepezil is the safest and most widely used of the cholinesterase inhibitors. Several reasonably large studies have shown improved memory and cognitive function.Citation43–Citation45 Donepezil has benefits in memory and cognition even several years after injury.Citation45,Citation46
Anticonvulsants are often prescribed initially after a TBI due to heightened risk for seizures. Post-TBI mania or mood lability may respond well to anticonvulsants, such as carbamazepine or sodium valproate. They are also often used to treat aggression after TBI. The anticonvulsant agent, topiramate, has been shown to adversely affect cognitive function in the TBI patients.Citation47
While insomnia is a significant issue for patients with TBI, affecting between 15% and 84% (mean of 40%),Citation3,Citation13,Citation19,Citation21,Citation23,Citation48,Citation49 little has been published on the treatment of this aspect of TBI. Benzodiazepines may be effective but carry a risk of disinhibition. Kemp et alCitation48 found that commonly used sleep aid, melatonin, was not effective. Antidepressants, including serotonin reuptake inhibitors and tricyclic antidepressants, are not effective in resolving insomnia in this population.Citation49 No single agent has emerged as a good solution for this symptom.
Cognitive rehabilitation
Cognitive rehabilitation now takes many forms and is often individualized to the particular needs of the patients. Protocols have been devised to remediate cognitive difficulties often encountered in those with TBI, such as impaired concentration, executive dysfunction, inattention, visual disturbances, memory dysfunction, and impaired language function. They range from simple strategies (using a planner to aid memory and organization) to specific protocols targeting particular cognitive functions (eg, short-term memory) that can be monitored with sequential neuropsychological testing. These interventions have been extensively reviewed elsewhere.Citation50,Citation51 Comprehensive programs which include psychotherapy and social skills components have been shown to have greater efficacy.Citation50,Citation52,Citation53 Overall, reports of benefits have been mixed.Citation54,Citation55
Behavioral therapies
Behavioral remediation strategies to eliminate problematic behaviors following TBI have met with mixed success, most often in terms of the poor generalization of specific skills to the outside world. Behavioral deficits that create difficulties for those with TBI and their families include poor hygiene, decline in tidying/cleaning habits, social withdrawal, reduced social comprehension, impaired memory, and poor organization. Behavioral excesses that create difficulties for those with TBI and their families include aggression, sleep disruption, and perseverations. These have been reviewed elsewhere.Citation56
Nutritional supplements
Nutritional supplements, herbs, and nootropics have been utilized for many years and are increasingly popular among the patient populations. There remains little clinical research on many of these agents, perhaps reflecting a lack of funding more than a lack of efficacy. Acetyl-l-carnitine is an ester of l-carnitine and is thought to protect brain cells after injury when glucose metabolic pathways are compromised. During this period, acetyl-l-carnitine supports alternative ketogenic pathways for metabolism.Citation57 It is also believed to enhance cholinergic function. While there are several clinical studies on patients with Alzheimer’s disease and preclinical data on animal models of TBI, the clinical literature on TBI remains sparse. Ginkgo biloba is a natural product of the tree by the same name. It has been shown to improve membrane fluidity and increase resistance to free-radical damage. It provides some subtle benefits to cognitive function in clinical studies of stroke, dementia, aging, and hypoxia damage.Citation58 It has not been systematically studied in TBI but is used extensively in clinic, often in combination with meclofenoxate which is an avid scavenger of free radicals.Citation59 S-Adenosylmethionine (SAMe) is a nutritional supplement which improves cell membrane fluidity and promotes the production of glutathione, an antioxidant. The benefit of SAMe has been assessed in a single clinical study of TBI.Citation60 Patients receiving SAMe had a 77% improvement in clinical scores of post-concussive symptoms. Citicholine provides a source of choline which can cross the blood–brain barrier. It has been used extensively in Europe and Japan as a treatment for TBI, stroke, and dementia. However, two large US studies failed to demonstrate significant benefit.Citation61,Citation62
Piracetam and the related oxiracetam and phenylpiracetam have shown some promise as nootropic agents. In one double-blind, placebo-controlled study, piracetam improved several symptoms of post-concussive syndrome, including headache and vertigo.Citation63 More recent clinical studies have shown marginal benefit.Citation64 Huperzine-A, an extract of Japanese club moss, is a natural acetylcholinesterase inhibitor. It may serve as a natural alternative to donepezil, rivastigmine, or galantamine. Galantamine warrants special mention as it appears to also modulate nicotinic receptors and appears to have more persistent benefit in the treatment of Alzheimer’s disease. It appears to modulate neuroimmune responses, in addition to its effects on acetylcholinesterase.Citation65 Cerebrolysin is a polypeptide that purportedly mimics the actions of neurotrophic factors.Citation66,Citation67 Studies have shown that it can reduce beta amyloid and phosphorylated tau protein accumulation. It may promote neurogenesis, synapse formation, and functional recovery.Citation66 In animal models of acute TBI, cerebrolysin-treated rats had more surviving neurons in the area of impact and showed greater functional recovery.Citation67 In a clinical trial of acute TBI, patients were recruited within 24 hours of injury and treated for 3 months with daily intravenous infusion of cerebrolysin. At 3 months, those receiving cerebrolysin performed significantly better on the Cognitive Abilities Screening Instrument.Citation68 It remains unclear if cerebrolysin provides long-term nootropic benefit.
The elevation of free radicals in TBI suggests that antioxidants should be beneficial. Clinical trials of pharmacological antioxidants over the past 30 years have not yielded a useful agent in acute TBI.Citation69 Agents, such as tirilazadCitation70 and polyethylene glycol-conjugated superoxide dismutase, have failed to show benefit in acute TBI. Omega-3 fatty acids may enhance brain repair and recovery, based on animal and clinical studies.Citation71 Similarly, vitamin D may offer neuroprotective and restorative benefitsCitation72 in the acute TBI setting. In chronic TBI, vitamin D and omega-3 fatty acids may work synergistically, as they both may reduce neuroinflammation, apoptosis, and oxidative stress.Citation73 Other nutritional supplements have been recommended, but prolonged therapy is necessary to possibly see benefits in TBI. A 6-month trial of ginkgo, vinpocetine, acetyl-l-carnitine, huperzine, alpha-lipoic acid, n-acetyl-cysteine, multivitamins, and over 5 g of omega-3 fatty acids daily yielded improved performance in cognitive testing and increased perfusion (function) in single-photon emission computed tomography (SPECT) scan.Citation74 Long-term use of dietary flavanols may improve cognition in mTBI.Citation75
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) has shown some promise in animal models of TBI.Citation76 However, a Cochrane review of the clinical application of TMS for depression noted no difference between repetitive TMS (rTMS) and sham rTMS using the Beck Depression Inventory (BDI) or the Hamilton Depression Rating Scale, except during the initial 2-week period.Citation77 The application of TMS in the post-TBI patients is limited by the risk of seizure induction.Citation78
Hyperbaric oxygen
Hyperbaric oxygen treatment has been explored as a treatment for TBI.Citation79–Citation91 Hyperbaric oxygen therapy is neither a benign treatment, given the concerns of oxygen toxicity,Citation79 nor a clear treatment in that the placebo condition of moderate hyperbaric room air also effectively improves cognitive function.Citation80,Citation81 The most carefully performed study compared a group in a cross-over design with an interval of both null treatment and hyperbaric oxygen at 100% oxygen and 1.5 atm.Citation82 The study described improvement in many of the symptoms associated with persistent TBI including headache, tinnitus, vision disturbance, memory dysfunction, and impaired cognitive function. Cognitive testing also showed improvement in attention, information processing speed, and a battery of cognitive tests. In an uncontrolled case series of 16 subjects, Harch et alCitation83 demonstrated that an abbreviated series of hyperbaric treatments using 100% oxygen at 1.5 atm could mitigate subjective symptoms of TBI (eg, headache, sleep disruption, irritability), improve cognitive testing scores, and improve cortical function based on SPECT imaging.Citation83 A study of a higher dose (2.4 atm) did not reveal any significant benefit of hyperbaric oxygen therapy compared to a sham-control group treated with 1.3 atm,Citation84 and this result has been extended and confirmed by a related group.Citation85 However, this may reflect an inverse dose–response curve, rather than an absence of benefit, in that the low-dose sham group demonstrated significant changes in cognitive testing and symptom frequency.Citation86 Hyperbaric oxygen remains a controversial area in both acute TBICitation86–Citation89 and chronic TBI.Citation82,Citation83,Citation85,Citation86,Citation90,Citation91
Physical exercise
High-energy activities and exercise programs completed through a health club facility or comprehensive rehabilitation program should focus on the same parameters of an age-adjusted and diagnosis-specific program for aerobic conditioning – flexibility, stabilization, and strength. Though it appears safe and is an accepted intervention for TBI, there is a need for further well-designed studies.Citation92 Exercise was a part of a 6-month study of lifestyle changes described above which yielded improved function based on cognitive testing and perfusion SPECT scans.Citation74
A new treatment for TBI
Unfortunately, little has been found to reverse the damage of TBI or repetitive concussion which is the root cause of residual cognitive and psychological impairment following TBI.Citation20,Citation93 One potential avenue of treatment for TBI is infrared light, which has shown promising data in a number of applications. Near-infrared (NIR) light has been investigated for its ability to modulate intracellular mechanisms related to healing. The application of NIR light by low-power laser or by light-emitting diode (LED) is also known as laser phototherapyCitation94 or near-infrared photobiomodulation.Citation92 NIR irradiation can facilitate wound healing,Citation95,Citation96 promote muscle repair,Citation95 and stimulate angiogenesis.Citation95,Citation96 NIR phototherapy has been studied and applied clinically in a wide array of ailments, including skin ulcers,Citation97 osteoarthritis,Citation98 peripheral nerve injury,Citation95,Citation96 low back pain,Citation99 myocardial infarction,Citation100 and stem cell induction.Citation101
The finding that NIR light passes relatively efficiently through bone has spurred interest in its application to treating disorders of the brain. Over the past decade, transcranial near-infrared light therapy (NILT)Citation102 has been studied in animal models to understand its ability to repair damaged or dysfunctional brain tissue resulting from stroke and TBI. The first published study of NILT for TBI in humans described two cases of chronic mTBI with significant disability.Citation103 Each patient was treated with an LED device delivering low-level low-level light therapy (LLLT) in the red and NIR range for 6–10 minutes per area daily for several months. Both patients had marked neuropsychological improvement after a minimum of 7–9 months of LLLT treatment.
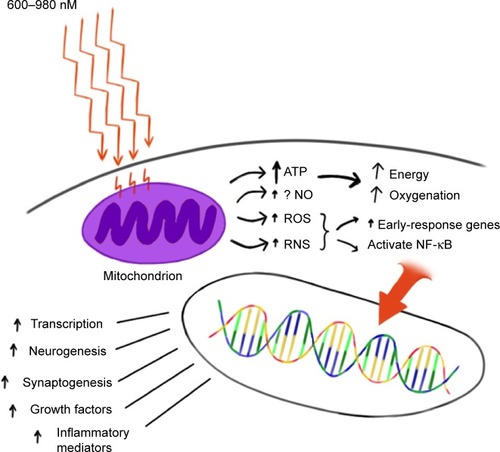
The precise mechanisms underlying photobiomodulation and its therapeutic benefits are not fully understood. The purported effects of NIR are illustrated in . Light in the wavelength range of 600–1,200 nm has significant photobiomodulation capability.Citation104 Current data most strongly support that absorption of NIR photons by cytochrome c oxidase in the mitochondrial respiratory chain is the key initiating event in photobiomodulation.Citation95,Citation96,Citation104,Citation105 This induces an increase in cytochrome c oxidase activity which in turn increases adenosine triphosphate (ATP) production. Such an increase in ATP in wounded or underperfused cells may be sufficient to activate cells in areas of injury or metabolic derangement.Citation106 Data from numerous tissue culture and animal studies point to the importance of several secondary molecular and cellular events. For example, NIR photonic energy can modulate reactive oxygen species,Citation95,Citation96,Citation102 activate mitochondrial DNA replication,Citation95,Citation96 increase early-response genes,Citation95 increase growth factor expression, induce cell proliferation, and alter nitric oxide levels.Citation95,Citation96,Citation102 These mechanisms are more fully described in the companion paper.Citation105
Figure 1 Hypothesized mechanism of action of NIR light therapy.
Abbreviations: NIR, near-infrared; ATP, adenosine triphosphate; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide; NF-κB, nuclear factor kappa B.
When examined in the specific model of neural tissue injury, NIR phototherapy can lead to demonstrable neural repair and recovery. For example, LLLT of a power density of 0.9–36 J/cm2 applied at 24 hours poststroke in a rodent model yielded a 32% reduction in neurological deficits, as well as histochemical evidence of neuron proliferation and migration.Citation106–Citation108 LLLT had similar benefits in a rodent model of TBI.Citation96,Citation109–Citation111 Interestingly, these cellular changes evolved over a period of days after light exposure and persisted for considerably longer than the interval of actual NIR exposure. These findings are consistent with a progressive regeneration cascade set in motion by the NIR light exposure.
NILT in stroke
NILT, predominately in the form of LLLT, has been investigated in laboratory models of stroke. LLLT applied in a single dose to an ischemic stroke model appeared to induce expression of the growth factor transforming growth factor-beta 1 and suppress the production of peroxynitrite.Citation112 In a rat model of middle cerebral artery occlusion, LLLT at a dose of 0.5–7.5 mW/cm2 using continuous wavelength light at 808 nm was administered at 24 hours after the acute stroke.Citation108,Citation113 This single application was estimated to deliver 1.8 J/cm2 in total to the cortex surface and resulted in demonstrable neurological improvement. Functional changes were not manifested until approximately 2 weeks after the single treatment. While there was no significant change in the size of the stroke lesion, histochemical evidence of neurogenesis and migrating neuronsCitation108 indicate that a cascade of secondary processes was initiated by NILT. A rabbit model of stroke utilizing injection of a blood clot embolus also demonstrated benefit from LLLT.Citation102,Citation114,Citation115 Herein, 808 nm light was applied with an LED delivering 7.5 mW/cm2 and an estimated 0.9–2.6 J/cm2 to the cortical surface. Cortical ATP levels were increased, indicative of increased mitochondrial activity.Citation114 Significant behavioral recovery was also noted; however, neither ATP increased nor neurological function changed at doses less than 0.3–0.7 J/cm2.Citation114,Citation115 At higher doses of 0.9–15 J/cm2, neurological improvement was seen.Citation114,Citation115
The clinical trials of NILT in acute stroke, the Neuro-Thera Effectiveness and Safety Trials 1, 2, and 3 (NEST-1, -2, -3), were conducted between 2006 and 2009. The Phase II clinical trial (NEST-1) involved 120 patients in a double-blind, placebo-controlled study of the effects of NILT within 24 hours of ischemic stroke.Citation116,Citation117 Approximately 60% of the patients experienced clinical benefit, and the safety profile was very good. Thus, NEST-2, a Phase III clinical trial, was undertaken in 2007. A total of 660 patients were enrolled.Citation118 Somewhat surprisingly, the study did not demonstrate statistical clinical improvement using a different outcome measure.Citation119 Post hoc analysis revealed that a portion of the patients who were moderately affected and/or had strokes limited to the cerebral cortex did realize clinically and statistically significant improvement.Citation102 The NEST-3 trial was halted midpoint when it failed to demonstrate statistical benefit on futility analysis.Citation120
A key factor in the interpretation of the results of NEST-3 is that, different from NEST-1, all types of stroke were included as opposed to just cortical strokes. Continuous laser light has a limited depth of penetration (≤1 cm into brain tissue) which likely prevents an effect on deeper brain matter. Therefore, the lack of significant benefits from NIR phototherapy in NEST-3 could be related to the fact that ischemic penumbra was not reached by the light (Luis DeTaboada, personal communication, January 2015). While pulsed NIR was not used in the NEST-3 study, it is estimated that pulsed NIR could penetrate up to 3 cm in depth from the cortical surface, therefore possibly extending the therapeutic target to deeper strokes (Luis DeTaboada, personal communication, January 2015).
NILT in TBI
Oron et alCitation109 conducted the first animal studies of NILT for TBI. They found that a single application of NIR light at 808 nm from a 200 mW emitter at 4 hours post-injury resulted in a significant reduction in lesion size by 5 days.Citation109 To date, several groups have studied NILT in animal models, and this material has previously been reviewed.Citation95,Citation121–Citation123 Single applications of 800–810 nm NIR light within 4 hours of injury have been shown to improve neurological function significantly.Citation110,Citation124–Citation126 The same dose of NIR light at 6 hours was less effectiveCitation125 and at 8 hours had no appreciable benefit.Citation125 NIR photonic energy at other wavelengths was less effective. Wu et alCitation110 examined red light (670 nm) at 4 hours and found a similar improvement in neurological function; however, 730 nm and 980 nm had no neurological benefit. Similar data for lesion volume have been reported. A single dose of 800–810 nm NIR light (fluence of 36 J/cm2) yielded an approximate 50% reduction in the volume of the lesion at 3–4 weeksCitation110,Citation111,Citation124–Citation126 and a possible reduction in the initial spread of neurological injury, based on the marked reduction in lesion volume found at 5 days post-injury.Citation109
Repeated NIR phototherapy treatments appear to have some benefit, but the frequency and number of treatments are critical factors. While a single NIR light application had benefit, daily applications for 3 days yielded much greater neurological benefitCitation126,Citation127 with smaller lesion size,Citation126 fewer degenerating neurons,Citation126 more proliferating cells,Citation126 and greater levels of brain-derived neurotrophic factor (BDNF)Citation127 compared to a single treatment in a mouse model. In contrast, daily treatment for 7 daysCitation128 or 14 daysCitation126 showed no difference from controls. NIR energy densities in the range of 0.9–36 J/cm2 resulted in significant biochemical and behavioral changes.Citation109–Citation111,Citation124–Citation127
Pulsing of NIR light appears to yield a greater neurological response but only within certain parameters. Pulsing at 10 Hz yielded greater neurological improvement and a significant reduction in lesion size compared to either continuous-wave or pulsed NIR at 100 Hz.Citation111 In the mouse model of moderate TBI, NILT (800–810 nm) improved learning and memory (Morris water maze performance),Citation128 as well as behaviors associated with depression and anxiety (immobility during tail suspension).Citation111,Citation124
The finding that NILT brought about a smaller lesion in the rodent model of TBI compared to untreated mice suggests that decreased apoptosis, reduced spreading lesion penumbra, and/or neurogenesis are induced by NILT. Indeed, NILT can decrease BAX expression, a pro-apoptosis gene,Citation129 increase expression of BCL-2, an anti-apoptosis gene,Citation129 increase nerve growth factor,Citation95 increase BDNF,Citation127 decrease inflammatory markers,Citation130 and decrease numbers of degenerating neurons.Citation126 Together, these mechanisms may reduce the enlargement of the initial lesion during the first day following the lesion.Citation109 Moreover, increased BDNF and nerve growth factor may contribute to synaptogenesis as shown by increased levels of synapsin-1,Citation127 and neurogenesis, as shown by increased numbers of proliferating cells.Citation127
In a double-blind study in healthy volunteers, NILT was beneficial – compared to sham – in memory and attention.Citation131 In this study, the authors shed only one application of NIR light to the right forehead, targeting the right frontal pole of the cerebral cortex (Brodmann’s area 9 and 10). The device was a Class IV laser CG-5000 (Cell Gen Therapeutics, Dallas, TX, USA), and the parameters were as follows: wavelength 1,064 nm, irradiance 250 mW/cm2, fluence 60 J/cm2, and time 4 minutes per site (two sites).Citation131 The subjects who received the NIR treatment had better attention after 2 weeks, measured by the psychomotor vigilance test. They also had better delayed visual memory at the Delayed Match-to-Sample test. This is the only published controlled trial assessing the impact of NILT on cognition; however, other reports have shown the therapeutic effects of NILT in small numbers of TBI patients.
In a two-case report in TBI patients,Citation103 NILT (870 nm) improved sustained attention, memory, and executive functions. Both patients were treated with an instrument with three separate LED cluster heads. The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 22.2–25.8 mW/cm2, fluence 13.3 J/cm2, and time 10 minutes per site.Citation103
The same group reported on a cohort of eleven subjects with persistent cognitive dysfunction and treated with a similar NILT protocol for chronic mTBI.Citation132 The eleven subjects received NILT with a device with three LED cluster heads (Model 1100; MedX Health, Toronto, ON, Canada). The parameters used for the treatment were the following: NIR wavelength 870 nm and 633 nm (red light), irradiance 22.2 mW/cm2, fluence 13 J/cm2, and approximate time 10 minutes per site. The NIR light was applied three times per week for 6 weeks (18 sessions), on eleven sites for 10 minutes per site (the total duration of each session was 20 minutes).Citation132 The sites on the skull were chosen on the midline, and bilaterally on frontal, parietal, and temporal areas. At the follow-up neuropsychological testing, NILT had a powerful effect on attention, inhibition, and inhibition switching in the Stroop task, and similarly improved verbal learning and memory, as well as enhanced long-delay free recall on the California Verbal Learning Test.
Eight subjects, from the same cohort, were identified as having mild, moderate, or severe depression based on the BDI-II total score (range: 15–34).Citation132 The three cases, who entered the study with only mild depression, remained the same after NILT treatment. Results for the five cases with moderate–severe depression were as follows: two moderate cases improved to mild/minimal depression 8 weeks after the end of NILT series, and one severe case improved to moderate depression. Two moderate or severe depression cases remained the same after 8 weeks of follow-up from the last NILT session.Citation132
Dose response and photonic penetration
A prevailing theory in photobiomodulation postulates that a bimodal response curve exists for the biological effects of NIR light.Citation95 The so-called Arndt–Schulz curve (a fundamental principle in homeopathic medicine) is frequently used to describe this biphasic dose response. Some data indicate that low levels of light have a much better effect on stimulating and repairing tissues than higher levels of light. Laboratory studies of cells in culture have demonstrated a bimodal dose response to light exposure in lymphocytesCitation133 and fibroblasts.Citation134,Citation135 For example, Chen et alCitation135 found that a range of 0.03–0.3 J/cm2 was beneficial in activating transcription factors in culture, while 3–30 J/cm2 inhibited the activation of these factors. In contrast, an order-of-magnitude greater dose (2 J/cm2) was best at activating fibroblasts in a superficial wound model.Citation136 Furthermore, an order-of-magnitude greater dose (30 J/cm2) proved to be best in a rodent joint inflammation model.Citation137 Thus, a dose-dependent effect for many biological responses to NIR light has been demonstrated,Citation95,Citation137–Citation139 but the critical parameter is dose at the level of the target tissue, rather than at the surface.Citation137,Citation140 The amount of energy that reaches a volume of tissue at depth is determined by the attenuation of the photonic energy as it passes through the overlying tissue. For example, only 2.45% of the energy from a 980 nm laser emitter penetrates to the level of the peroneal nerve.Citation140 Nevertheless, the biphasic dose response does not appear to be universally true. In primary microglial cell culture, a dose-dependent response to NIR was demonstrated with no detrimental effects at doses as high as 30 J/cm2.Citation141
So a critical question in the use of NILT is that of radiant energy penetration. In particular, some authors have challenged the efficacy of low-power LEDs used in LLLT.Citation142–Citation144 In laboratory studies, LLLT radiant energy is almost entirely absorbed in the first 1 mm of skin.Citation145,Citation146 In two unrelated studies, LLLT diode devices proved to be ineffective in the treatment of diabetic neuropathy,Citation142,Citation144 in contrast with prior reports.Citation147 Similarly, laboratory studies of NILT using LLLT transcranially have not consistently yielded positive results. For example, in a rat model of TBI, Giacci et alCitation148 found no benefit from daily 30-minute irradiation with either 670 nm or 830 nm 0.5 W LED emitters for a period of 7 days. Doses at the skin surface were 28.4 J/cm2 and 22.6 J/cm2, respectively.Citation148 Similarly, treatment of a rat model of contusive spinal cord injury with LLLT (830 nm at 22.6 J/cm2 or 670 nm at 28.4 J/cm2) for 30 minutes per day for 5 days resulted in no significant functional improvement and no reduction in lesion size, despite delivering 2.6 J/cm2 to the spinal cord.Citation148
LapchakCitation102 reported that the physical parameters of NILT in the clinical trials for the treatment of stroke utilized in the NEST-1 and NEST-2 trialsCitation116–Citation120 may have delivered insufficient energy to cortical tissues to be effective. Therein, NIR light of 808 nm wavelength with infrared energy densities of 0.9 J/cm2 was applied to the human scalp for a total of 40 minutes with applications at multiple sites during that time.Citation116,Citation118 Recall that animal models of both stroke and TBI suggest that NIR energy densities in the range of 0.9–36 J/cm2 resulted in significant biochemical and behavioral changes.Citation96,Citation106–Citation115,Citation125–Citation127 The concern raised from the NEST studiesCitation102 is that current clinical trials testing the effectiveness of low-energy NIR diodes to treat TBI may yield negative or inaccurate efficacy data, not because of a failure of infrared light to invoke a change but due to a dose error. Doses that are effective when directly applied to cells in a Petri dishCitation149,Citation150 or to 3–5 mm thick rodent brainsCitation96,Citation109–Citation111,Citation125,Citation126,Citation128 may be insufficient to penetrate 2–4 cm into the human brain.
In a companion paper, our own studies of photonic energy penetration are detailed.Citation105 To summarize, the laboratory tissue studies showed that 0.5 W LED emitters did not penetrate the 2 mm thickness of human skin. No detectable energy from 0.5 W LED NIR light emitters could be detected penetrating a similar thickness (1–2 mm) of sheep skin or 3 cm thick section containing sheep skin, skull, and brain. In contrast, 11% of the photonic energy from a 10 W 810/980 nm coherent NIR laser penetrated 2 mm of human skin. Similarly, 17% of the photonic energy from a 15 W 810 nm coherent NIR laser penetrated the same distance.Citation105 Energy from these more powerful NIR emitters could be detected penetrating 3 cm of sheep skin, skull, and brain with 0.4% of the 10 W 810/980 nm NIR laser’s energy reaching the depth of 3 cm and 2.9% of the 15 W 810 nm NIR laser’s energy traversing the same distance.Citation105 Anders also has demonstrated penetration of 808 nm light to 40 mm in the brain using a 5 W laser emitter (JJ Anders, personal communication, January 2015). Prompted by the mixed results in the literature and the observations by Lapchak,Citation102 Franzen-Korzendorfer et al,Citation144 Wan et alCitation151 and Lavery et alCitation142 we have been utilizing relatively high-power (10–15 W) lasers at the wavelengths of 810 nm and 980 nm in the clinic to treat patients with TBI. Clinically, the patients have shown excellent responses with resolution of many of their long-standing symptoms of TBI or post-concussive syndrome. Below is a retrospective series of such patients to illustrate the extent and character of response to this modality.
Methods
Patients in the case series were sequentially treated patients at a clinic which is engaged in ongoing NILT for a number of clinical conditions. The risks, benefits, and current state of research on the use of NILT were explained to each patient. Each patient consented to treatment. Institutional Review Board approval was obtained in a post hoc review, noting that the risk–benefit ratio was acceptable.
Between March 16, 2011 and February 20, 2013, sequential new referrals for chronic mild-to-moderate TBI were evaluated for treatment and selected for NILT using Class IV lasers, either the LT1000 (LiteCure, Newark, DE, USA), a 10 W adjustable NIR laser emitter with wavelengths of 810/980 nm capable of delivering continuous or pulsed NIR light, or the Diowave 810 (Diowave, Riviera Beach, FL, USA), an adjustable NIR emitter up to 15 W with a wavelength of 810 nm capable of delivering continuous or pulsed NIR energy. Demographics and laser treatment settings are detailed in . The fluence delivered to the skin of patients ranged from 55 J/cm2 to 81 J/cm2. No other treatment modalities (medications, exercise regimen, supplements) were added, discontinued, or changed while receiving NILT. Symptoms were monitored clinically. A baseline Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)Citation152 was completed for all patients, and the BDICitation153 was administered to seven of the ten patients before and after the course of treatment. In addition, each patient was instructed on how to create and maintain a patient and spousal diary of symptoms and subjective progress. Each of six patients received a single series of ten treatments with the LT1000 Class IV laser. Three additional patients each received a single series of 20 treatments with the LT1000 Class IV laser. One patient was treated with the Diowave 810 nm Class IV laser device in a series of 20 treatments. The patients and treating clinician wore protective eyewear. There were no incidents of burns or thermal discomfort ().
Figure 2 Treatment parameters per individual, based on area of the skull treated.
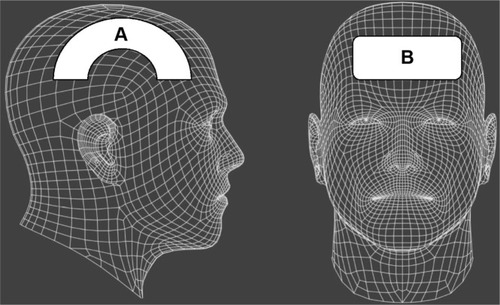
Table 1 Infrared light treatment parameters for each of the ten patients in the case series
The impact of high-watt NILT
While the patient group represented a diverse mix ( presents demographics), some notable commonalities of symptoms emerged. Over 90% of the patients had complaints of anxiety, depression, irritability, and insomnia. Other symptoms included headache (60%), suicidal ideation (50%), cognitive difficulties (50%), attention problems (50%), short-term memory problems (40%), loss of libido (30%), substance abuse (20%), fatigue (20%), and panic attacks (20%). Six of the patients were unemployed prior to treatment. Three of the patients were experiencing severe marital difficulties. All carried or had a confirmed diagnosis of TBI, but other comorbid diagnoses included PTSD, major depressive disorder, generalized anxiety disorder, bipolar disorder, and attention deficit/hyperactivity disorder. The patients’ baseline scores on the BDI were 25.3±12.1 (moderate depression range), and baseline scores on the QIDS-SR were 12.9±4.6 (moderate depression range).
During NILT treatments, skin temperature increased no more than 3°C with rapid cooling after removal of the NIR light. A continuous sweeping motion was utilized to minimize skin heating and cover a larger area. After a course of ten treatments of NILT (20 treatments in four patients), each patient experienced significant clinical improvement with resolution of many of their symptoms (). In addition, the BDI scores dropped to 12±6.5 (nondepressed range). This represented a significant decrease (P<0.01, Student’s t-test, one-tailed, Microsoft Excel). The QIDS-SR scores after treatment were 2.2±2.3 (nondepressed range), and the difference from baseline was highly significant (P<0.00001, Student’s t-test, one-tailed). Patients noted improvement in cognitive function, mood, anxiety, and sleep. None of the patients continued to have suicidal thoughts (50% at baseline). Other symptoms, such as anxiety and irritability, were markedly improved. Most notable were the nonquantifiable changes in patients’ lives. Patients reported improved cognitive ability and a desire to return to meaningful work. Five of the six unemployed patients have returned to work. The two patients who were Iraq/Afghanistan veterans have found new careers in highly skilled trades. The patients with marital difficulties have reconciled and were purchasing homes or otherwise solidifying their marriages. The clinical change can be attributed to NILT because no changes in medications, supplements, or exercise regimen were permitted during the course of NILT treatment.
Table 2 NILT case series with demographics, symptoms, and treatment response
All patients in the case series experienced significant clinical improvement which supports the conjecture that high-power NIR laser delivers sufficient energy to the human brain for photobiomodulation to occur. Insomnia and suicidal ideation, common symptoms in those with TBI or post-concussive syndromes,Citation3,Citation17–Citation20,Citation24,Citation25 resolved in 100% of cases. Headache, another common symptom for patients following a TBI,Citation6,Citation14,Citation15,Citation23 was reduced or resolved in the six patients so afflicted. Symptoms such as anxiety,Citation14,Citation15,Citation21,Citation24 depression,Citation21,Citation24,Citation25,Citation27–Citation29 and irritability resolved or were dramatically reduced in all patients. Cognitive function appeared to improve based on return to work or improved work performance, although cognitive tests were not performed. The quality of life dramatically improved in all cases, based on the observations of the patients, their family members, and the treating clinician.
At follow-up intervals of 6–7 months posttreatment, patients have reported continued improvements in symptoms. The precise areas of brain injury were not elucidated in the majority of these cases, so a correlation of symptoms changes and cortical function changes cannot be made; however, perfusion SPECT imaging in other patients has shown significant increases in perfusion in injured areas of the brain and overall improved cortical function following similar courses of high-watt NILT.Citation154
One concern that has been expressed about high-watt NIR lasers is the risk of tissue heating.Citation155 We explored this issue in our companion paper on NIR penetration.Citation105 Temperature change was 1°C–3°C at the skin surface using continuous-wave NIR lasers in the range of 10–15 W. Using pulsed settings, the high-powered lasers showed no significant temperature change in tissue samples. The temperature change on human skin was 1°C or less in the in vivo penetration studies while maintaining continuous movement of the laser probe head.Citation105 Clinically, patients in this case series reported only slight warming of the skin, but no discomfort, using the continuous motion technique.
Laboratory studies have largely focused on treatment of acute brain injury. The processes involved in the benefits of NIR light in chronic TBI as seen in this clinical case series may be quite distinct. Nevertheless, Schiffer et alCitation156 found that a single application of LLLT at 810 nm and 250 mW to the forehead over 8 minutes reduced depression and anxiety symptoms in ten patients for approximately 2 weeks. Similarly, the small case series by Naeser et alCitation103 demonstrated some benefit using NIR light, albeit at very low power levels over a prolonged course of several months with only transient benefit. Together with our clinical data, these findings suggest that at least some of the photobiomodulatory effects of NIR energy likely do occur in chronic neurological conditions.
Prior presentations on NILT for the treatment of TBI or stroke in humans have focused on getting photonic energy through the skull to the cortex surface which traverses a distance of about 6–10 mm; however, this model is flawed in that the distance to the areas of damage may be far greater. In other words, the cortex immediately subjacent to a portion of the skull may be 10 mm from the surface, but the NIR light energy may need to penetrate 3–7 cm to reach areas of damage. Much of the cortical surface is actually lining the walls and floors of sulci, rather than immediately subjacent to the skull. Analysis of NIR spectroscopy reveals that light propagation through varying media with irregular boundaries is subject to high levels of scatter.Citation157 In addition, review of the neuroimaging literature on TBI has revealed that the most common areas injured in TBI are the orbitofrontal cortex (at the ventral surface of the frontal lobe) and the anterior and medial temporal lobes.Citation158 It is not anatomically possible to position an NIR light emitter immediately exterior to the skull overlying these areas. Indeed, the orbitofrontal cortex positioned immediately above the eyes can only be reached from the forehead by angling the light emitter. Similarly, the temporal lobes are separated from the surface by epidermis, dermis, subcutaneous fat, subcutaneous blood vessels, accessory head of the temporalis muscle, connective tissue, temporalis muscle, skull, and dura mater.Citation159 Each of these structures has different absorption and refraction properties, and each interface between different materials also creates a barrier to transmission of photonic energy.Citation157 Blood flowing in the subcutaneous vessels is believed to create a unique barrier to transmission.Citation160 In summary, effectively targeting the areas most commonly injured in TBI with sufficient photonic energy to initiate reparative processes represents a significant challenge in NILT. This appears to have been overcome with the high-power laser protocol presented here and in a related paper.Citation154
As yet, the mechanism of action of NILT in treating TBI is not entirely clear. Moreover, the neurological benefits are not immediately apparent. Rather, a delay of 1–4 weeks was noted, consistent with a progressive regeneration cascade set in motion by the NILT.Citation96,Citation103,Citation105,Citation107,Citation109,Citation121,Citation122,Citation124,Citation127,Citation135 Similarly, most of the patients in the present case series did not notice benefits immediately or within the first few treatments. Instead, they reported benefits emerging over an interval of weeks, and in some cases, continuing after completion of the course of NILT. In addition, the clinical improvement reported by the patients in the above case series is more profound than that reported by patients treated with LLLT or low-powered lasers.Citation103 In fact, we observed that among seven subjects with documented moderate depression, per BDI scores, four had an antidepressant response (≥50% decrease of depression severity). In contrast, Naeser et alCitation132 reported that out of eight subjects with TBI and comorbid depression, only three had a significant improvement in their depressive symptoms (37.5%). Our results may be due to the greater penetration of more powerful, coherent, and pulsed NIR light from a laser source.
A unique outcome measure was developed for this protocol (Morries and Henderson, unpublished data, 2015). A patient diary and separate spousal diary provided a weekly update of patient’s response in his or her home environment. This novel approach to capturing the patient treatment experience provided the patient and family with tangible and pertinent documentation of the clinical response. While time consuming, the experiences recorded in these diaries proved to be valuable clinical tools to the treating clinicians.
Conclusion
To date, there has been little progress in developing effective treatments for chronic mild-to-moderate TBI or repetitive concussions. This area of need has become even more pressing with the return of veterans from military conflicts in Iraq and AfghanistanCitation4,Citation6,Citation7,Citation16,Citation17,Citation19,Citation161 and the recognition of the magnitude of sport-related TBI.Citation5,Citation8–Citation10 In addition, the dramatic growth in the geriatric population with attendant proprioceptive dysfunction has resulted in a rising incidence of fall-related TBI.Citation162
NILT has shown promise as a tool for the treatment of TBI. A critical issue is to assure that adequate photonic energy reaches the injured areas of the brain. The use of high-wattage lasers, as we have demonstrated, results in marked clinical improvement in patients with chronic TBI. Moreover, symptoms consistent with PTSD, anxiety, and/or depression also improved considerably or resolved in this group of patients. Further work in the use of high-wattage NILT in the treatment of TBI, depression, and other neurological disorders is encouraged.
Acknowledgments
The authors would like to acknowledge the technical assistance of Mr Charles Vorwaller (Diowave Corporation) and Lite Cure Corporation. The authors also acknowledge the contribution of Ms Taylor Tuteur in the artistic creation of .
Disclosure
Dr Larry D Morries is the CEO of Neuro-Laser Foundation, a nonprofit foundation. He has a private practice in Lakewood, CO. Theodore A Henderson is the president of The Synaptic Space, a medical consulting firm. He is the president of Dr Theodore Henderson, Inc., a clinical service firm. He is the co-owner of Neuro-Luminance, a clinical service organization. He is the president of the International Society of Applied Neuroimaging. He is the CFO of the Neuro-Laser Foundation, a nonprofit foundation. Dr Paolo Cassano received funding from the Brain and Behavior Research Foundation; Photothera Inc and from the Dupont Warren Fellowship (Harvard Medical School) to conduct research on NIR light for the treatment of major depressive disorder.
References
- BazarianJJMcClungJShahMNChengYTFlesherWKrausJMild traumatic brain injury in the United States 1998–2000Brain Inj2005192859115841752
- FaulMXuLWaldMCoronadoVGTraumatic Brain Injury in the United StatesU.S. Department of Health and Human Services Report2010 Available from: http://www.cdc.gov/TraumaticBrainInjury/Accessed March 2, 2014
- VaishnaviSRaoVFannJRNeuropsychiatric problems after traumatic brain injury: unraveling the silent epidemicPsychosomatics200950319820519567758
- DOD Worldwide Numbers for TBI [webpage on the Internet]Silver Spring, MDDefense and Veterans Brain Injury Center2015 Available from: http://dvbic.dcoe.mil/dod-worldwide-numbers-tbiAccessed January 25, 2015
- BailesJEPetragliaALOmaluBINaumanETalavageTRole of subconcussion in repetitive mild traumatic brain injuryJ Neurosurg201311951235124523971952
- CifuDXTaylorBCCarneWFTraumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veteransJ Rehabil Res Dev20145091169117624458958
- LoganBWGoldmanSZolaMMackeyAConcussive brain injury in the military: September 2001 to the presentBehav Sci Law201331680381324130079
- GilchristJThomasKEXuLMcGuireLCCoronadoVGNonfatal sports and recreation related traumatic brain injuries among children and adolescents treated in emergency departments in the United States, 2001–2009MMWR Morb Mortal Wkly Rep201160391337134221976115
- NobleJMHesdorfferDCSport-related concussions: a review of epidemiology, challenges in diagnosis, and potential risk factorsNeuropsychol Rev201323427328424242889
- SelassieAWWilsonDAPickelsimerEEVoroncaDCWilliamsNREdwardsJCIncidence of sport-related traumatic brain injury and risk factors of severity: a population-based epidemiologic studyAnn Epidemiol2013231275075624060276
- KumarALoaneDJNeuroinflammation after traumatic brain injury: opportunities for therapeutic interventionBrain Behav Immun20122681191120122728326
- ZiebellJMMorganti-KossmannMCInvolvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injuryNeurotherapeutics201071223020129494
- AndersonRJShell shock: an old injury with new weaponsMol Interv20088520421819015383
- KashlubaSHanksRACaseyJEMillisSRNeuropsychologic and functional outcome after complicated mild traumatic brain injuryAch Phys Med Rehabil2008895904911
- KennedyJEJaffeeMSLeskinGAStokesJWLealFOFitzpatrickPJPosttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injuryJ Rehabil Res Dev200744789592018075948
- LewHLRehabilitation needs of an increasing population of patients: traumatic brain injury, polytrauma, and blast-related injuriesJ Rehabil Res Dev2005424xiiixvi16320135
- LewHLVanderploegRDMooreDFOverlap of mild TBI and mental health conditions in returning OIF/OEF service members and veteransJ Rehabil Res Dev2008453xixvi18629743
- OkieSTraumatic brain injury in the war zoneN Engl J Med2005352202043204715901856
- VasterlingJJProctorSPAmorosoPKaneRHeerenTFranzMNeuropsychological outcomes of army personnel following deployment to the Iraq warJAMA2006296551952916882958
- Neurobehavioral Guidelines Working GroupGuidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injuryJ Neurotrauma200623101468150117020483
- FannJRBuringtonBLenonettiAJaffeKKatonWJThompsonRSPsychiatric illness following traumatic brain injury in an adult health maintenance organization populationArch Gen Psychiatry2004611536114706944
- BryanCJRepetitive traumatic brain injury (or concussion) increases severity of sleep disturbance among deployed military personnelSleep201336694194623729938
- TheelerBLucasSRiechersRG2ndRuffRLPost-traumatic headaches in civilians and military personnel: a comparative, clinical reviewHeadache201353688190023721236
- BryanCJClemansTARepetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnelJAMA Psychiatry201370768669123676987
- JorgeRERobinsonRGMoserDTatenoACrespo-FacorroBArndtSMajor depression following traumatic brain injuryArch Gen Psychiatry2004611425014706943
- PrinsMLAlexanderDGizaCCHovdaDARepeated mild traumatic brain injury: mechanisms of cerebral vulnerabilityJ Neurotrauma2013301303823025820
- FannJRUomotoJMKatonWJSertraline in the treatment of major depression following mild traumatic brain injuryJ Neuropsychiatry Clin Neurosci200012222623211001601
- FannJRUomotoJMKatonWJCognitive improvement with treatment of depression following mild traumatic brain injuryPsychosomatics2001421485411161121
- Turner-StokesLHassanNPierceKCleggFManaging depression in brain injury rehabilitation: the use of an integrated care pathway and preliminary report of response to sertralineClin Rehabil200216326126812017513
- SchmittJAKruizingaMJRiedelWJNon-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitorsJ Psychopharmacol200115317317911565624
- CrisostomoEADuncanPWPropstMDawsonDVDavisJNEvidence that amphetamine with physical therapy promotes recovery of motor function in stroke patientsAnn Neurol198823194973345072
- RauTFKothiwalASRovaARBrooksDMPoulsenDJTreatment with low-dose methamphetamine improves behavioral and cognitive function after severe traumatic brain injuryJ Trauma Acute Care Surg2012732 suppl 1S165S17222847088
- JohanssonBWentzelAPAndréllPOdenstedtJMannheimerCRönnbäckLEvaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and painBrain Inj201428330431024377326
- NickelsJLSchneiderWNDombovyMLWongTMClinical use of amantadine in brain injury rehabilitationBrain Inj1994887097187849690
- KrausMFMakiPThe combined use of amantadine and l-dopa/carbidopa in the treatment of chronic brain injuryBrain Inj19971164554609171930
- McDowellSWhyteJD’EspositoMDifferential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patientsBrain1998121pt 6115511649648550
- Catsman-BerrevoetsCEvon HarskampFCompulsive pre-sleep behavior and apathy due to bilateral thalamic stroke: response to bromocriptineNeurology19883846476493352928
- IshizukaTMurotaniTYamatodaniAModanifil activates the histaminergic system through the orexinergic neuronsNeurosci Lett2010483319319620696213
- GuoRXAnacletCRobertsJCDifferential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox−/− miceBr J Pharmacol2009157110411719413575
- ScammellTEEstabrookeIVMcCarthyMTHypothalamic arousal regions are activated during modafinil-induced wakefulnessJ Neurosci200020228620862811069971
- KimWTatenoAArakawaRIn vivo activity of modafinil on dopamine transporter measured with positron emission tomography and [18F]FE-PE2IInt J Neuropsychopharmacol201417569770324451483
- WisorJPNishinoSSoraIUhlGHMignotEEdgarDMDopaminergic role in stimulant-induced wakefulnessJ Neurosci20012151787179411222668
- TaverniJPSeligerGLichtmanSWDonepezil medicated memory improvement in traumatic brain injury during post acute rehabilitationBrain Inj199812177809483340
- WhelanFJWalkerMSSchultzSKDonepezil in the treatment of cognitive dysfunction associated with traumatic brain injuryAnn Clin Psychiatry200012313113510984001
- KayeNSTownsendJB3rdIvinsRAn open-label trial of donepezil (aricept) in the treatment of persons with mild traumatic brain injuryJ Neuropsychiatry Clin Neurosci200315338338412928519
- MoreyCECiloMBerryJCusickCThe effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot studyBrain Inj200317980981512850946
- SilverJMArciniegasDBYodofskySPsychopharmacologySilverJMMcAllisterTWYodofskySTextbook of Traumatic Brain InjuryArlington, VAAmerican Psychiatric Publishing2005609639
- KempSBiswasRNeumannVCoughlanAThe value of melatonin for sleep disorders occurring post-head injury: a pilot RCTBrain Inj200418991191915223743
- LeeHBLyketsosCGRaoVPharmacological management of the psychiatric aspects of traumatic brain injuryInt Rev Psychiatry200315435937015276957
- CiceroneKDDahlbergCKalmarKEvidence-based cognitive rehabilitation: recommendations for clinical practiceArch Phys Med Rehabil200081121596161511128897
- ParkNWInglesJLEffectiveness of attention rehabilitation after an acquired brain injury: a meta-analysisNeuropsychology200115219921011324863
- PrigatanoGPFordyceDJZeinerHKRouecheJRPeppingMWoodBCNeuropsychological rehabilitation after closed head injury in young adultsJ Neurol Neurosurg Psychiatry19844755055136736983
- Ben-YishayYDillerLCognitive remediation in traumatic brain injury: update and issuesArch Phys Med Rehabil19937422042138431107
- ChungCSYPollockACampbellTDurwardBRHagenSCognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damageCochrane Database Syst Rev20134CD00839123633354
- SooCTateRLPsychological treatment for anxiety in people with traumatic brain injuryCochrane Database Syst Rev20073Cd00523917636792
- CorriganPWBachPABehavioral treatmentSilverJMMcAllisterTWYodofskySPsychopharmacology in Textbook of Traumatic Brain InjuryArlington, VAAmerican Psychiatric Publishing2005661678
- PrinsMLMatsumotoJHThe collective therapeutic potential of cerebral ketone metabolism in traumatic brain injuryJ Lipid Res201455122450245724721741
- DiamondBJShiflettSCFeiwelNGinkgo biloba extract: mechanisms and clinical indicationsArch Phys Med Rehabil200081566867810807109
- al-ZuhairHAbd el-FattahAel-SayedMIThe effect of meclofenoxate with ginkgo biloba extract or zinc on lipid peroxide, some free radical scavengers and the cardiovascular system of aged ratsPharmacol Res199838165729697157
- Bacci BalleriniFLópez AngueraAAlcarazPHernández ReyesNTreatment of postconcussion syndrome with S-adenosylmethionineMed Clin (Barc)19838041611646345953
- BaşkayaMKDoğanARaoAMDempseyRJNeuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injuryJ Neurosurg200092344845210701532
- ZafonteRDBagiellaEAnselBMEffect of citicoline on functional and cognitive status among patients with traumatic brain injury: citicoline brain injury treatment trial (COBRIT)JAMA2012308191993200023168823
- HakkarainenHHakamiesLPiracetam in the treatment of post-concussional syndrome. A double-blind studyEur Neurol19781715055342247
- MalykhAGSadaieMRPiracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disordersDrugs201070328731220166767
- FurukawaSYangLSameshimaHGalantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn ratsInt J Dev Neurosci201437525724972037
- MasliahEDíez-TejedorEThe pharmacology of neurotrophic treatment with cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disordersDrugs Today (Barc)201248suppl A32422514792
- ZhangYChoppMMengYImprovement in functional recovery with administration of cerebrolysin after experimental closed head injuryJ Neurosurg201311861343135523581594
- ChenCCWeiSTTsaiaSCChenXXChoDYCerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized studyBr J Neurosurg201327680380723656173
- HallEDVaishnavRAMustafaAGAntioxidant therapies for traumatic brain injuryNeurotherapeutics201071516120129497
- HukkelhovenCWSteyerbergEWFaraceEHabbemaJDMarshallLFMaasAIRegional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trialsJ Neurosurg200297354955712296638
- HasadsriLWangBHLeeJVOmega-3 fatty acids as a putative treatment for traumatic brain injuryJ Neurotrauma2013301189790623363551
- AminmansourBNikbakhtHGhorbaniAComparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo groupAdv Biomed Res201215823326789
- ScrimgeourAGCondlinMLNutritional treatment for traumatic brain injuryJ Neurotrauma2014311198999924605947
- AmenDGWuJCTaylorDWilleumierKReversing brain damage in former NFL players: implications for traumatic brain injury and substance abuse rehabilitationJ Psychoactive Drugs20114311521615001
- TheadomAMahonSBarker-ColloSEnzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCTEur J Neurol20132081135114423384428
- PapeTLRosenowJLewisGTranscranial magnetic stimulation: a possible treatment for TBIJ Head Trauma Rehabil200621543745116983227
- CouturierJLEfficacy of rapid-rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta-analysisJ Psychiatry Neurosci2005302839015798783
- Castel-LacanalETarriMLoubinouxITranscranial magnetic stimulation in brain injuryAnn Fr Anesth Reanim2014332838724378049
- ClarkJMLambertsenCJGelfandREffects of prolonged oxygen exposure at 1.5, 2.0, or 2.5 ATA on pulmonary function in men (predictive studies V)J Appl Physiol (1985)19998612432599887137
- ColletJPVanasseMMaroisPHyperbaric oxygen for children with cerebral palsy: a randomised multicentre trial. HBO-CP Research GroupLancet2001357925658258611558483
- JamesPBHyperbaric oxygenation for cerebral palsyLancet20013572052205311441856
- Boussi-GrossRGolanHFishlevGHyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury – randomized prospective trialPLoS One2013811e7999524260334
- HarchPGAndrewsSRFogartyEFA phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorderJ Neurotrauma20122916818522026588
- WolfGCifuDBaughLCarneWProfennaLThe effect of hyperbaric oxygen on symptoms after mild traumatic brain injuryJ Neurotrauma201229172606261223031217
- MillerRSWeaverLKBahrainiNEffects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trialJAMA Intern Med20151751435225401463
- HarchPGHyperbaric oxygen therapy for post-concussion syndrome: contradictory conclusions from a study mischaracterized as sham-controlledJ Neurotrauma201330231995199924004322
- BennettMHTrytkoBJonkerBHyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injuryCochrane Database Syst Rev201212CD00460923235612
- RockswoldSBRockswoldGLZaunDAA prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injuryJ Neurosurg20101121080109419852540
- RockswoldSBRockswoldGLZaunDALiuJA prospective, randomized phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injuryJ Neurosurg201311861317132823510092
- GoldenZLNeubauerRGoldenCJGreeneLMarshJMlekoAImprovement in cerebral metabolism in chronic brain injury after hyperbaric oxygen therapyInt J Neurosci200211211913112325401
- WortzelHSArciniegasDBAndersonCAVanderploegRDBrennerLAA phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder: a neuropsychiatric perspectiveJ Neurotrauma201229142421242422519979
- MassettLMoseleyAMTateRHarmerARFitness training for cardiorespiratory conditioning after traumatic brain injuryCochrane Database Syst Rev20082CD00612318425937
- National Research CouncilCognitive Rehabilitation Therapy for Traumatic Brain Injury: Evaluating the EvidenceWashington, DCThe National Academies Press2011
- EnwemekaCSIntricacies of dose in laser phototherapy for tissue repair and pain reliefPhotomed Laser Surg200927338739319473073
- HuangYYChenACCarrollJDHamblinMRBiphasic dose response in low level light therapyDose Response20097435838320011653
- ChungHDaiTSharmaSKHuangYYCarrollJDHamblinMRThe nuts and bolts of low-level laser (light) therapyAnn Biomed Eng201240251653322045511
- MesterEMesterAFMesterAThe biomedical effects of laser applicationLasers Surg Med19855131393982191
- BjordalJMCouppéCChowRTTunérJLjunggrenEAA systematic review of low level laser therapy with location-specific doses for pain from chronic joint disordersAust J Physiother200349210711612775206
- BasfordJRSheffieldCGHarmsenWSLaser therapy: a randomized, controlled trial of the effects of low-intensity Nd:YAG laser irradiation on musculoskeletal back painArch Phys Med Rehabil199980664765210378490
- YangZWuYZhangHLow-level laser irradiation alters cardiac cytokine expression following acute myocardial infarction: a potential mechanism for laser therapyPhotomed Laser Surg201129639139821348574
- TubyHMaltzLOronUInduction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heartLasers Surg Med201143540140921674545
- LapchakPATaking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational scienceAnn Med201042857658621039081
- NaeserMASaltmarcheAKrengelMAHamblinMRKnightJAImproved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reportsPhotomed Laser Surg201129535135821182447
- KaruTIKolyakovSFExact action spectra for cellular responses relevant to phototherapyPhotomed Laser Surg200523435536116144476
- HendersonTAMorriesLDNear-infrared photonic energy penetration – can infrared phototherapy effectively reach the human brain?Neuropsychiatr Dis TreatIn press2015
- WuHMHuangSCVespaPHovdaDABergsneiderMRedefining the pericontusional penumbra following traumatic brain injury: evidence of deteriorating metabolic derangements based on positron emission tomographyJ Neurotrauma201330535236023461651
- YipKKLoSCLeungMCSoSKTangCYPoonDMThe effect of low-energy laser irradiation on apoptotic factors following experimentally induced transient cerebral ischemiaNeuroscience201119030130621712070
- OronAOronUChenJLow level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficitsStroke2006372620262416946145
- OronAOronUStreeterJLow-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficitsJ Neurotrauma20072465165617439348
- WuQHuangYYDhitalSLow level laser therapy for traumatic brain injury. Mechanisms for low-light therapy VProc SPIE20107552755206
- AndoTXuanWXuTComparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in micePLoS One2011610e2621222028832
- LeungMCLoSCSiuFKSoKFTreatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1Lasers Surg Med200231428328812355575
- DetaboadaLIlicSLeichliter-MarthaSOronUOronAStreeterJTranscranial application of low-energy laser irradiation improves neurological deficits in rats following acute strokeLasers Surg Med2006381707316444697
- LapchakPADe TaboadaLTranscranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbitsBrain Res2010130610010519837048
- LapchakPAWeiJZivinJATranscranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbitsStroke20043581985198815155955
- ZivinJAAlbersGWBornsteinNEffectiveness and safety of transcranial laser therapy for acute ischemic strokeStroke20094041359136419233936
- LamplYZivinJAFisherMInfrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1)Stroke20073861843184917463313
- HuisaBNStemerABWalkerMGTranscranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2Int J Stroke20138531532022299818
- StemerABHuisaBNZivinJAThe evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2Curr Cardiol Rep201012293320425181
- HackeWSchellingerPDAlbersGWTranscranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trialStroke201445113187319325293665
- RojasJCGonzalez-LimaFLow level light therapy of the eye and brainEye Brain201134967
- FitzgeraldMHodgettsSVan Den HeuvelCRed/near-infrared irradiation therapy for treatment of central nervous system injuries and disordersRev Neurosci201324220522623492552
- RojasJCGonzalez-LimaFNeurological and psychological applications of transcranial lasers and LEDsBiochem Pharmacol201386444745723806754
- WuQXuanWAndoTLow-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengthsLasers Surg Med20124421822622275301
- OronAOronUStreeterJNear infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficitsJ Neurotrauma201229240140722040267
- XuanWVatanseverFHuangLTranscranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimenPLoS One201381e5345423308226
- XuanWAgrawalTHuangLGuptaGKHamblinMRLow-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesisJ Biophotonics Epub201498
- KhumanJZhangJParkJCarrollJDDonahueCWhalenMJLow-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in miceJ Neurotrauma20122940841721851183
- QuirkBJTorbeyMBuchmannEVermaSWhelanHTNear-infrared photobiomodulation in an animal model of traumatic brain injury: improvements at the behavioral and biochemical levelsPhotomed Laser Surg201230952352922793787
- MoreiraMSVelascoITFerreiraLSEffect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in ratJ Photochem Photobiol B200997314515119800810
- BarrettDWGonzalez-LimaFTranscranial infrared laser stimulation produces beneficial cognitive and emotional effects in humansNeuroscience2013230132323200785
- NaeserMAZafonteRKrengelMHSignificant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol studyJ Neurotrauma201431111008101724568233
- MesterENagylucskaySWaidelichWEffects of direct laser radiation on human lymphocytesArch Dermatol Res19782633241245 German742904
- KreislerMChristoffersABWillershausenBd’HoedtBEffect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro studyJ Clin Periodontol200330435335812694435
- ChenACAranyPRHuangYYLow-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblastsPLoS One201167e2245321814580
- Demidova-RiceTNSalomatinaEVYaroslavskyANHermanIMHamblinMRLow-level light stimulates excisional wound healing in miceLasers Surg Med200739970671517960752
- CastanoAPDaiTYaroslavskyILow-level laser therapy for zymosan-induced arthritis in rats: importance of illumination timeLasers Surg Med200739654355017659584
- CorazzaAVJorgeJKurachiCBagnatoVSPhotobiomodulation on the angiogenesis of skin wounds in rats using different light sourcesPhotomed Laser Surg200725210210617508845
- DesmetKDPazDACorryJJClinical and experimental applications of NIR-LED photobiomodulationPhotomed Laser Surg200624212112816706690
- AndersJJMogesHWuXIn vitro and in vivo optimization of infrared laser treatment for injured peripheral nervesLasers Surg Med2014461344524338500
- von LedenRECooneySJFerraraTM808 nm wavelength light induces a dose-dependent alteration in microglial polarization and resultant microglial induced neurite growthLasers Surg Med201345425326323619903
- LaveryLAMurdochDPWilliamsJLaveryDCDoes anodyne light therapy improve peripheral neuropathy in diabetes? A double-blind, sham-controlled, randomized trial to evaluate monochromatic infrared photoenergyDiabetes Care200831231632117977931
- KolariPJPenetration of unfocused laser light into the skinArch Dermatol Res198527743423444004332
- Franzen-KorzendorferHBlackintonMRone-AdamsSMcCullochJThe effect of monochromatic infrared energy on transcutaneous oxygen measurements and protective sensation: results of a controlled, double-blind, randomized clinical studyOstomy Wound Manage2008546163118579924
- EsnoufAWrightPAMooreJCAhmedSDepth of penetration of an 850 nm wavelength low level laser in human skinAcupunct Electrother Res2007321–2818618077939
- BashkatovANGeninaEAKochubeyVITuchinVVOptical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2,000 nmJ Phys D Appl Phys20053825432555
- LeonardDRFarooqiMHMyersSRestoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatmentDiabetes Care200427116817214693984
- GiacciMKWheelerLLovettSDifferential effects of 670 and 830 nm red near infrared irradiation therapy: a comparative study of optic nerve injury, retinal degeneration, traumatic brain and spinal cord injuryPLoS One201498e10456525105800
- GrecoMVaccaRAMoroLHelium-Neon laser irradiation of hepatocytes can trigger increase of the mitochondrial membrane potential and can stimulate c-fos expression in a Ca2+-dependent mannerLasers Surg Med200129543344111891731
- FrankSOliverLLebreton-DeCCInfrared radiation affects the mitochondrial pathway of apoptosis in human fibroblastsJ Invest Dermatol2004123582383115482467
- WanSParrishJAAndersonRRMaddenMTransmittance of non-ionizing radiation in human tissuesPhotochem Photobiol19813466796816458827
- RushAJTrivediMHIbrahimHMThe 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depressionBiol Psychiatry200354557358312946886
- HomaifarBYBrennerLAGutierrezPMSensitivity and specificity of the beck depression inventory-II in persons with traumatic brain injuryArch Phys Med Rehabil200990465265619345782
- HendersonTAMorriesLDSPECT perfusion imaging demonstrates improvement of TBI with transcranial near-infrared laser phototherapyAdv Mind Body MedIn press2015
- JoensenJDemminkJHJohnsonMIIversenVVLopes-MartinsRÁBjordalJMThe thermal effects of therapeutic lasers with 810 and 904 nm wavelengths on human skinPhotomed Laser Surg201129314515321219241
- SchifferFJohnstonALRavichandranCPsychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxietyBehav Brain Funct200954619995444
- StrangmanGELiZZhangQDepth sensitivity and source-detector separations for near infrared spectroscopy based on the Colin27 brain templatePLoS One201388e6631923936292
- RajiCATarzwellRPavelDClinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: a systematic reviewPLoS One201493e9108824646878
- ClementeCAnatomy – A Regional Atlas of the Human Body2nd edBaltimoreUrban & Schwarzenburg Inc1981
- JagdeoJRAdamsLEBrodyNISiegelDMTranscranial red and near infrared light transmission in a cadaveric modelPLoS One2012710e4746023077622
- LangeRTBrickellTAIvinsBVanderploegRDFrenchLMVariable, not always persistent, postconcussion symptoms after mild TBI in U.S. military service members: a five-year cross-sectional outcome studyJ Neurotrauma2013301195896923205671
- CoronadoVGThomasKESattinRWJohnsonRLThe CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBIJ Head Trauma Rehabil200520321522815908822

