Abstract
Background
Approved doses of antidepressants in Japan are usually lower than those in the USA and European Union, but to date meta-analyses comparing antidepressants have all used the higher doses approved in the USA and European Union and often have used indirect comparisons. The purpose of this study was to conduct an integrated database analysis of patient level data to compare the effects of duloxetine with those of selective serotonin reuptake inhibitors (SSRIs) at the doses approved in Japan.
Methods
Pooled data were analyzed from four randomized, double-blind, placebo-controlled studies that compared duloxetine at the dose range approved in Japan (40–60 mg/day) with other SSRIs (paroxetine 20 mg/day or escitalopram 10 mg/day) and placebo in patients with major depressive disorder. In total, 1,694 patients were included in the analysis (duloxetine, n=688; selective serotonin reuptake inhibitors, n=690; placebo, n=316). The primary outcome measure was the mean change from baseline at week 8 in 17-item Hamilton Rating Scale for Depression (HAMD17) total and subscale scores.
Results
Duloxetine and both selective serotonin reuptake inhibitors were superior to placebo in HAMD17 total score at week 8 in both the all-randomized group and the more severe subgroup (HAMD17 total scores ≥19). Duloxetine was superior to SSRIs in improving the HAMD17 Retardation subscale score (least squares mean difference [95% confidence interval]): all-randomized group, −0.33 [−0.60, −0.07], P=0.015; severe subgroup, −0.45 [−0.83, −0.07], P=0.020).
Conclusion
Within the dose range approved in Japan for patients with major depressive disorder, duloxetine and selective serotonin reuptake inhibitors demonstrated comparable overall efficacy, with a possible advantage for duloxetine in improving loss of energy and interest. To the best of our knowledge, this analysis is unique not only in evaluating dosages specific to Japan, but also in using individual patient data and the same endpoint across studies to allow for strictly direct head-to-head data comparisons as opposed to pooling direct and indirect comparisons.
Introduction
Antidepressants are the mainstay of treatment for adult major depressive disorder (MDD). Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants are categorized as first-line treatments in many countries, including Japan.Citation1,Citation2 Thus, health care providers have the opportunity to choose an antidepressant from a variety of first-line treatment options. To select the most appropriate treatment for each patient, it is important for clinicians to be aware of the differences in efficacy between comparable antidepressants at the doses approved in their countries.
One long-standing approach to comparing efficacy has been meta-analysis of several randomized controlled trials that compare widely used treatments.Citation3–Citation10 However, results have been inconsistent, perhaps partly due to the use of different methodologies. Recent meta-analyses have used mixed treatment comparisons in which both direct and indirect comparisons were used.Citation11,Citation12
Duloxetine, a second-generation norepinephrine reuptake inhibitor that has demonstrated efficacy, safety, and tolerability in patients with MDD,Citation13–Citation16 has been the subject of several recent mixed treatment comparisons. A recent analysis by Cipriani et alCitation12 compared the effects of 12 new-generation antidepressants in adults with MDD, and Gartlehner et alCitation11 conducted an analysis of 234 studies that included placebo as a comparator. However, these results are not applicable to clinical practice in Japan, because they included data from patients taking duloxetine up to 120 mg/day, which is twice the maximum dosage (≤60 mg/day) approved in Japan. Another drawback was the inclusion of patients who took antidepressants not approved or not even available for use in Japan. Therefore, it is uncertain whether these results can be applied to daily clinical practice in countries (eg, Japan) where the approved dosage for duloxetine is 40–60 mg/day and SSRIs are limited to four compounds, ie, paroxetine, sertraline, escitalopram, and fluvoxamine. To the best of our knowledge, there are no meta-analyses that compare the efficacy of duloxetine ≤60 mg/day with these SSRIs. The primary purpose of the current study was to provide clinicians with efficacy results that would allow a comparison of duloxetine ≤60 mg/day with four approved SSRIs in Japan.
Materials and methods
Study selection and data collection
The Eli Lilly clinical trial database contains all clinical trials of duloxetine for patients with MDD that were conducted by Eli Lilly or its partners outside of Japan. We reviewed this database and selected randomized controlled trials that included duloxetine and SSRI for the acute treatment of MDD. For the SSRI selection, four SSRIs that were approved in Japan for the treatment of MDD (eg, paroxetine, sertraline, escitalopram, and fluvoxamine as of July 2014) were included. As a result, a total of four studies (three studies on duloxetine 40 or 80 mg/day versus paroxetine 20 mg/day and one study on duloxetine 60 mg/day versus escitalopram 10 mg/day) were selected (). As a next step, patients who received more than the approved daily dose ranges in Japan (duloxetine 80 mg/day) were excluded from the analysis. No studies meeting the criterion for comparison were excluded.
Table 1 Studies included in pooled analysis
Common inclusion criteria for the included studies (HMAT A, HMAT B, HMCV, HMCR) were as follows: male or female outpatients aged at least 18 years who met DSM-IV (Diagnostic and Statistical Manual for Mental Disorders, 4th Edition)Citation17 criteria for nonpsychotic MDD and had a Clinical Global Impression score ≥4, a 17-item Hamilton Rating Scale for Depression (HAMD17) total score ≥15, or a Montgomery–Åsberg Depression Rating Scale score ≥22.
The HMAT study (groups A and B) was a multicenter, parallel, double-blind, randomized, placebo-controlled and active comparator-controlled study with a blinded placebo lead-in and placebo lead-out. The primary objective was to assess mean changes in HAMD17 total score from baseline to endpoint (week 8). The treatments were duloxetine 40 or 80 mg/day, paroxetine 20 mg/day, or placebo.
The HMCV study was a multicenter, randomized, double-blind, double-dummy, parallel, active treatment-controlled Phase III trial comparing the efficacy and safety of duloxetine 60 mg/day and paroxetine 20 mg/day for inpatients and outpatients with MDD. The study included 8 weeks of active treatment followed by a one-week dose-tapering period. The primary objective of the study was to determine whether duloxetine 60 mg/day was noninferior to paroxetine 20 mg/day in the acute treatment of patients with MDD, as assessed by the baseline-to-endpoint change in HAMD17 total score over an 8-week period.Citation13
The HMCR study was a multicenter, randomized, 8-month, placebo-controlled, double-blind study that evaluated the comparative efficacy of duloxetine and escitalopram for patients with MDD.Citation14,Citation15 The primary objective was to compare the onset of antidepressant efficacy for patients taking duloxetine 60 mg/day or escitalopram 10 mg/day. This objective was evaluated by testing the hypothesis that the percentage of patients taking duloxetine who met onset criteria at week 2 was not inferior to (or at least as good as) the percentage of patients taking escitalopram.
Statistical analysis
Efficacy analyses included a comparison of duloxetine versus two SSRIs and placebo. The primary endpoint was mean change from baseline in HAMD17 total score at week 8. Secondary endpoints were mean change in HAMD17 subscale score, response rate, remission rate, and mean change in Hamilton Anxiety Rating Scale (HAMA) total score from baseline to week 8. Response was defined as a ≥50% reduction in HAMD17 total score from baseline at week 8. Remission was defined by a HAMD17 total score ≤7 at week 8.
Our analyses included two groups: all randomized patients who had a HAMD17 total score ≥15 and a subgroup of more severe patients who had a HAMD17 total score ≥19. This division was included because some studies suggest that patients with severe symptoms may have different treatment needs.Citation4,Citation18
HAMD17 items include (1) depressive mood; (2) feelings of guilt; (3) suicide; (4) early insomnia; (5) middle insomnia; (6) late insomnia; (7) work and activities; (8) psychomotor retardation; (9) agitation; (10) psychic anxiety; (11) somatic anxiety; (12) somatic symptoms gastrointestinal; (13) general somatic symptoms; (14) genital symptoms; (15) hypochondriasis; (16) weight loss; and (17) insight.Citation19
Five HAMD17 subscales were used for the subgroup analysis: Bech (items 1, 2, 7, 8, 10, 13) and Maier (1, 2, 7, 8, 9, 10), comprising items generally considered to be the core symptoms of depression; Retardation (items 1, 7, 8, 14), evaluating the degree of energy and interest levels; Sleep (items 4, 5, 6) assessing the degree of insomnia; and Anxiety/Somatization (items 10, 11, 12, 13, 15, 17) and HAMA total score, assessing anxiety.Citation19–Citation23
For the analysis of primary and secondary endpoints, missing data were imputed using the last observation carried forward approach. Continuous variables were calculated using analysis of covariance based on last observation carried forward values as follows: one analysis of covariance model was calculated for each study (counting the groups A and B of the HMAT study as two studies), with a fixed effect for investigator, treatment, and baseline score as covariates. Effect sizes in each model were calculated using least squares mean differences divided by the standard deviation of the residuals provided by the model of this study. Overall least squares mean estimates and effect sizes were calculated as a weighted mean of the corresponding estimates in all studies, with weights based on within-study variance, assuming a fixed study effect. The binary outcomes were analyzed using the Cochran-Mantel-Haenszel test adjusted for study. Relative risk is presented with 95% confidence intervals (CI) and P-values. The odds ratio (OR) was adjusted for the study by calculating the ORs within each study and then weighting these ORs across studies. Absolute numbers and averages were not adjusted for study and therefore cannot be directly compared with the ORs.
Safety and tolerability measures included patient-reported treatment-emergent adverse events and overall rates of study discontinuation due to adverse events. Reasons for discontinuation from the study were listed and treatment-emergent adverse events were reported using the Medical Dictionary for Regulatory Activities Terminology. Safety was analyzed by treatment descriptive statistics, and categorical safety measures were evaluated using Fisher’s Exact test.
Results
Patient demographics and disposition
At baseline, patients were mostly middle-aged (mean age 40.8–42.0 years), female (range 63.8%–67.4%), with mean HAMD17 total scores ranging from 17.6 to 19.0 and mean HAMA total scores ranging from 14.4 to 15.8. The majority of patients were Caucasian; patients from East Asia accounted for approximately 30% of duloxetine-treated and SSRI-treated patients (). Completion rates were approximately 70% (68.6% for duloxetine-treated patients, 72.3% for SSRI-treated patients, and 66.8% for placebo-treated patients). Adverse events were common reasons for discontinuation (duloxetine-treated patients, 8.9%; SSRI-treated patients, 7.1%; placebo-treated patients, 6.0%; see ). Discontinuation due to lack of efficacy was more likely in placebo-treated patients than in duloxetine-treated or SSRI-treated patients (13.3% versus 3.2% and 3.2%, respectively).
Table 2 Patient demographics
Table 3 Reasons for discontinuation
Efficacy comparison using HAMD17 and HAMA scores
A comparison of the mean changes in HAMD17 total, subscale scores and HAMA total score from baseline at week 8 (last observation carried forward) is shown in ; corresponding effect sizes are shown in . In the total population, no statistically significant differences were found in the mean change in HAMD17 total score at week 8 between duloxetine-treated and SSRI-treated patients (−10.08 and −9.69, respectively). However, the differences between duloxetine versus placebo and SSRI versus placebo were statistically significant (P<0.01). Duloxetine and SSRI were comparable on most other efficacy measures; however, duloxetine-treated patients showed a greater mean change from baseline in HAMD17 Retardation subscale score compared with SSRI-treated patients (least squares mean difference [95% CI]) (−0.33 [−0.60, −0.07]). For the more severe subgroup with HAMD17 total scores ≥19 at baseline, again statistically significant differences were found in mean change in HAMD17 total scores at week 8, but greater efficacy for duloxetine was seen with the Bech, Maier, and Retardation subscale scores compared with SSRIs: HAMD17 Bech (−0.62 [−1.16, −0.08]), HAMD17 Maier (−0.65 [−1.18, −0.12]), and HAMD17 Retardation (−0.45 [−0.83, −0.07]).
Figure 1 Effect sizes for duloxetine and SSRIs versus placebo in mean changes on HAMD17 total score, HAMD17 subscale score, and HAMA total score at week 8. Total population (HAMD17 ≥15) and more severe population (HAMD17 ≥19).
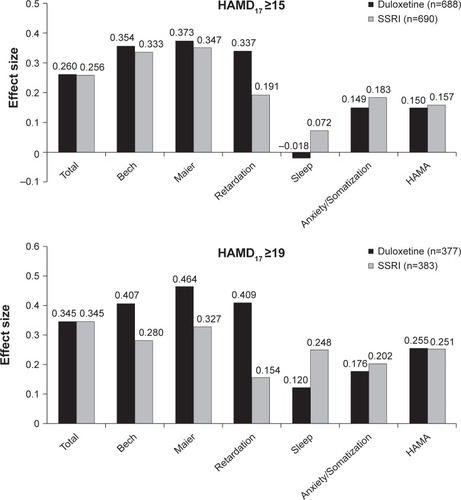
Table 4 Changes in HAMD17 total and subscale scores
In the total population, HAMD17 total score effect sizes for duloxetine and SSRI versus placebo were comparable (0.260 and 0.256, respectively). Differences in some HAMD17 subscale scores indicated an advantage for duloxetine over SSRIs. These were seen on the Retardation subscales for the total population (0.337 versus 0.191, respectively) in the more severe population (0.409 versus 0.154, respectively), and on the Bech and Maier subscales for the more severe population ().
Response
The response rates at week 8 were 42.0% for the duloxetine-treated patients, 44.5% (n=307) for the SSRI-treated patients, and 24.4% (n=77) for the placebo-treated patients. No statistically significant difference was found between the duloxetine-treated and SSRI-treated patients (OR 1.05; 95% CI 0.80–1.37). Statistically significant differences were shown for the duloxetine-treated versus placebo-treated patients (OR 1.61; 95% CI 1.11–2.33) and the SSRI-treated versus placebo-treated patients (OR 1.83; 95% CI 1.27–2.64).
Remission
The remission rates at week 8 were 35.2% for the duloxetine-treated patients, 36.1% (n=249) for the SSRI-treated patients, and 21.2% (n=67) for the placebo-treated patients. No statistically significant differences were found at week 8 for the duloxetine-treated versus SSRI-treated patients (OR 1.11; 95% CI 0.64–1.92), the duloxetine-treated versus placebo-treated patients (OR 1.53; 95% CI 0.74–3.18), or the SSRI-treated versus placebo-treated patients (OR 1.60; 95% CI 0.77–3.33).
Safety
The most common treatment-emergent adverse events are summarized in . Among duloxetine-treated patients, nausea (25.7%), dry mouth (17.3%), dizziness (12.6%), constipation (11.0%), and decreased appetite (11.9%) had a higher incidence than in placebo-treated patients, but the rates of these adverse events were similar between duloxetine-treated and SSRI-treated patients except for nausea and dry mouth.
Table 5 Adverse events with >5% incidence
Discussion
Our results show that duloxetine and SSRI treatment within their respective approved dosage in Japan demonstrated comparable efficacy with regard to overall depressive symptoms. However, certain HAMD17 subscale scores responded differently to duloxetine compared with SSRIs, and this may clarify the difference in clinical attributes among these antidepressants.
First, when assessing overall efficacy using the mean changes in HAMD17 total scores from baseline, both duloxetine and SSRI demonstrated greater efficacy than placebo, but did not indicate any significant difference between each other for either the total population (HAMD17 total score ≥15) or the more severe population (HAMD17 total score ≥19). On the one hand, this is consistent with a prior report by Thase et al,Citation4 where all randomized patients across any severity were analyzed and no significant difference in efficacy between duloxetine and SSRI was found. However, on the other hand, this is inconsistent with prior reports that duloxetine ≤120 mg/day showed greater efficacy than SSRIs in patients with at least moderately severe depression.Citation4,Citation16 Given this and the finding of a modest dose response trend for duloxetine efficacy by Mallinckrodt et alCitation16 this inconsistency may be explained by the upper limit on the dosage of duloxetine (≤60 mg/day) in this study.
Second, when assessing energy and interest using Retardation subscale scores, duloxetine showed greater efficacy regardless of baseline depression severity (HAMD17 total score ≥15 or ≥19). This is consistent with previous reports by Mallinckrodt et alCitation16 and Martinez et al.Citation24 Furthermore, Katz et alCitation25 reported that desipramine (a norepinephrine reuptake inhibitor) demonstrated better efficacy than paroxetine (an SSRI) on psychomotor retardation symptoms. These results may be explained by the effect of duloxetine on norepinephrine, which has been shown to be associated with arousal and activity.Citation26
The current results show some inconsistency with a report by Cipriani et al in which some SSRIs were significantly more efficacious than duloxetine. This is partly due to the different methodologies used in that study and in our current study. In Cipriani et al,Citation12 approximately one third of the comparisons were direct comparisons from randomized controlled trials and many of the pairwise comparisons were based on only one study. Furthermore, a “moderate” degree of heterogeneity and statistical incoherence (estimates based on the direct comparisons are not contained in the 95% CI for indirect comparisons) in the study network were detected. Study conclusions based on direct comparisons were much more conservative than those based on indirect ones. On the other hand, the strengths of this database analysis are that it analyzed individual patient data, used the same analysis method and endpoint across studies, and included similar patients. The adopted database analysis design allowed for a homogeneous patient population, with almost the same inclusion criteria and the exact same endpoint. Although our analysis resulted in a comparatively smaller number of studies and patients, it achieved high internal validity and reproducibility. In addition, direct comparisons of head-to-head data enabled us to avoid the uncertainty of how much the two different evidence levels are weighted when pooling direct and indirect comparisons.
Limitations
Several limitations of the current report warrant discussion. The integrated database only included studies that were conducted by Eli Lilly or its partners outside Japan; therefore, sponsorship bias cannot be fully mitigated. Using this database resulted in inclusion of fewer studies than publication-based meta-analyses; however, this methodology enabled us to analyze individual patient data that are not available in many publications. Similarly, using this global database resulted in inclusion of Caucasians as the majority (58.3%) of the patient population. Another limitation of this meta-analysis is that it only consisted of two SSRIs (paroxetine and escitalopram) although four SSRIs are available in Japan. Furthermore, SSRI doses used in this analysis (paroxetine 20 mg/day and escitalopram 10 mg/day) were at the lower approved dosage in Japan (20–40 mg/day for paroxetine, and 10–20 mg/day for escitalopram), whereas the duloxetine doses used in this analysis (40 mg/day or 60 mg/day) were the dosages approved in Japan. Further research that includes all available SSRIs using flexible dosing within their full therapeutic ranges will make it possible to generalize conclusions to the SSRI group as a whole. In addition, the exclusion of patients randomized to duloxetine >60 mg/day limits the ability to generalize conclusions in some countries with approved dosages above a maximum of 60 mg/day, but this was not within the scope of our analysis. Lastly, in these analyses, no correction for multiplicity was performed, so superiority based on P-values cannot be assessed.
Conclusion
Comparison between duloxetine and SSRI demonstrated comparable efficacy within the Japan-approved dose range in patients with MDD. Results of the HAMD17 subscale analysis indicated that duloxetine might be superior to SSRIs in improving loss of energy and interest.
Author contributions
EH, AS, and LM conceived and designed the study. AS conducted the data analysis and all authors were involved in interpretation of data. All authors critically revised the manuscript for important intellectual content and approved the final version.
Disclosure
This study was funded by Eli Lilly and Company and Shionogi & Co Ltd. EH, AS, LM, and RE are employees and stockholders in Eli Lilly and Company. TK has received honoraria from Astellas, Eli Lilly Japan, Meiji Seika, Mitsubishi Tanabe, MSD, Otsuka, and Pfizer, and has received research grants from Astellas, Dainippon Sumitomo, GlaxoSmithKline, and Pfizer. TK is also an advisory board member for GlaxoSmithKline, and has served as a consultant for Asahi-kasei. TT is a full-time employee and stockholder in Shionogi & Co Ltd. A part of this paper was presented at The International College of Neuropsychopharmacology Special Congress on Addiction and Mental Health 2013 (Kuala Lumpur City, Malaysia, October 1–4, 2013). Editorial assistance was provided by Angela Lorio and writing assistance was provided by Jody Arsenault, InVentiv Health Clinical, with financial support from Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
- TreuerTLiuCYSalazarGUse of antidepressants in the treatment of depression in Asia: guidelines, clinical evidence, and experience revisitedAsia Pac Psychiatry20135421923023857712
- Japanese Society of Mood DisordersTreatment Guideline II: Major Depressive Disorder, 2012 Ver. 1Japanese Society of Mood Disorders7262012 Japanese
- PapakostasGIThaseMEFavaMNelsonJCSheltonRCAre antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agentsBiol Psychiatry200762111217122717588546
- ThaseMEPritchettYLOssannaMJSwindleRWXuJDetkeMJEfficacy of duloxetine and selective serotonin reuptake inhibitors: comparisons as assessed by remission rates in patients with major depressive disorderJ Clin Psychopharmacol200727667267618004135
- ThaseMEOverview of antidepressant therapyManag Care2001108 Suppl6911729437
- HansenRAGartlehnerGLohrKNGaynesBNCareyTSEfficacy and safety of second-generation antidepressants in the treatment of major depressive disorderAnn Intern Med2005143641542616172440
- GartlehnerGGaynesBNHansenRAComparative benefits and harms of second-generation antidepressants: background paper for the American College of PhysiciansAnn Intern Med20081491073475019017592
- GartlehnerGThalerKHansenRAGaynesBNThe general and comparative efficacy and safety of duloxetine in major depressive disorder: a systematic review and meta-analysisDrug Saf200932121159117319916583
- SmithDDempsterCGlanvilleJFreemantleNAndersonIEfficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysisBr J Psychiatry200218039640411983635
- National Institute for Clinical ExcellenceClinical practice guideline No 23. Depression: management of depression in primary and secondary careLondon, UKNational Institute for Clinical Excellence2004 Available from: http://www.nice.org.uk/guidance/cg23Accessed November 19, 2014
- GartlehnerGHansenRAMorganLCComparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysisAnn Intern Med20111551177278522147715
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- LeePShuLXuXOnce-daily duloxetine 60 mg in the treatment of major depressive disorder: multicenter, double-blind, randomized, paroxetine-controlled, non-inferiority trial in China, Korea, Taiwan and BrazilPsychiatry Clin Neurosci200761329530717472599
- PigottTAPrakashAArnoldLMAaronsonSTMallinckrodtCHWohlreichMMDuloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorderCurr Med Res Opin20072361303131817559729
- NierenbergAAGreistJHMallinckrodtCHDuloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority studyCurr Med Res Opin200723340141617288694
- MallinckrodtCHPrakashAHoustonJPSwindleRDetkeMJFavaMDifferential antidepressant symptom efficacy: placebo-controlled comparisons of duloxetine and SSRIs (fluoxetine, paroxetine, escitalopram)Neuropsychobiology2007562–3738518037817
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders Text Revision (DSM-IV-TR)4th edWashington, DC, USAAmerican Psychiatric Association2000
- ThaseMESimonsADCahalaneJMcGearyJHardenTSeverity of depression and response to cognitive behavior therapyAm J Psychiatry199114867847892035722
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry1960231566214399272
- HamiltonMThe assessment of anxiety states by ratingBr J Med Psychol1959321505513638508
- MaierWPhilippMImproving the assessment of severity of depressive states: a reduction of the Hamilton Depression ScalePharmacopsychiatry1985181114115
- ClearyPGuyWFactor analysis of the Hamilton Depression ScaleDrugs Exp Clin Res197711–2115120
- BechPGramLFDeinEJacobsenOVitgerJBolwigTGQuantitative rating of depressive statesActa Psychiatr Scand19755131611701136841
- MartinezJMKatonWGreistJHA pragmatic 12-week, randomized trial of duloxetine versus generic selective serotonin-reuptake inhibitors in the treatment of adult outpatients in a moderate-to-severe depressive episodeInt Clin Psychopharmacol2012271172622027844
- KatzMMTekellJLBowdenCLOnset and early behavioral effects of pharmacologically different antidepressants and placebo in depressionNeuropsychopharmacology200429356657914627997
- MorilakDAFrazerAAntidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioral effects in depression and anxiety disordersInt J Neuropsychopharmacol20047219321815003145

