Abstract
Objective
To constitute the third revision of the guidelines for the treatment of bipolar disorder issued by the Korean Medication Algorithm Project for Bipolar Disorder (KMAP-BP 2014).
Methods
A 56-item questionnaire was used to obtain the consensus of experts regarding pharmacological treatment strategies for the various phases of bipolar disorder and for special populations. The review committee included 110 Korean psychiatrists and 38 experts for child and adolescent psychiatry. Of the committee members, 64 general psychiatrists and 23 child and adolescent psychiatrists responded to the survey.
Results
The treatment of choice (TOC) for euphoric, mixed, and psychotic mania was the combination of a mood stabilizer (MS) and an atypical antipsychotic (AAP); the TOC for acute mild depression was monotherapy with MS or AAP; and the TOC for moderate or severe depression was MS plus AAP/antidepressant. The first-line maintenance treatment following mania or depression was MS monotherapy or MS plus AAP; the first-line treatment after mania was AAP monotherapy; and the first-line treatment after depression was lamotrigine (LTG) monotherapy, LTG plus MS/AAP, or MS plus AAP plus LTG. The first-line treatment strategy for mania in children and adolescents was MS plus AAP or AAP monotherapy. For geriatric bipolar patients, the TOC for mania was AAP/MS monotherapy, and the TOC for depression was AAP plus MS or AAP monotherapy.
Conclusion
The expert consensus in the KMAP-BP 2014 differed from that in previous publications; most notably, the preference for AAP was increased in the treatment of acute mania, depression, and maintenance treatment. There was increased expert preference for the use of AAP and LTG. The major limitation of the present study is that it was based on the consensus of Korean experts rather than on experimental evidence.
Introduction
Recent advances in psychopharmacology and the development of novel psychotropic drugs have led to rapid changes in the pharmacological treatment strategies for bipolar disorder as well as the publication of various treatment algorithms and clinical practice guidelines.Citation1–Citation15 Because treatment strategies used for clinical practice vary widely from country to country due to diverse health insurance policies, economic situations, and ethnicities, several countries have initiated development of their own population-specific treatment guidelines. For example, the Korean Medication Algorithm Project for Bipolar Disorder (KMAP-BP) began in 2001, and the first set of guidelines, KMAP-BP 2002, was published 1 year later.Citation16 Feasibility studies of the KMAP-BP 2002 showed that its algorithm could be successfully implemented in clinical settings in Korea.Citation17–Citation19 And based on continuing progress in psychopharmacology, the KMAP-BP was further revised in 2006Citation20 and 2010.Citation21
Following the release of KMAP-BP 2010, novel clinical data and updated guidelines for the treatment of bipolar disorder became available, demonstrating that some medications were effective and some were not. Based on these studies, several medications were approved for the treatment of bipolar disorder, and various revised guidelines adopted these therapies as first-line or second-line options.Citation4,Citation12 Since 2010, further advances and novel findings have been reported and, thus, the Korean College of Neuropsychopharmacology and the Korean Society for Affective Disorders have undertaken a third revision of the KMAP-BP to reflect changes in expert opinion regarding the treatment of bipolar disorder. Here, an overview of the current consensus of Korean experts regarding pharmacological treatments for bipolar disorder is presented.
Methods
The detailed methods regarding the constitution of the review committee, preparation of the questionnaire, data analyses, and development of the treatment guidelines and algorithms were similar to those in previous KMAP-BP studies.Citation16–Citation22
Review committee
The review committee included 110 Korean psychiatrists who were life members of the Korean Society for Affective Disorders, had more than 15 years of clinical experience in the field of mood disorders, and had published at least one paper regarding mood disorders during the last year. The committee members worked in a wide variety of clinical settings including university hospitals (n=73), general/psychiatric hospitals (n=24), and private psychiatric clinics (n=13). Additionally, 38 experts from the field of child and adolescent psychiatry were included in the review committee to aid with the development of the child and adolescent section. Of the 110 committee members, 64 general psychiatrists (58.2%) and 23 of 38 child and adolescent psychiatrists (60.5%) responded to the survey.
Questionnaire
The KMAP-BP 2014 is a set of expert consensus guidelines modeled after the 2002, 2006, and 2010 KMAP-BP assessments. The questionnaire used in the KMAP-BP 2014 included a majority of the primary questions that were in the KMAP-BP 2010, which had 40 main questions divided into 311 sub-items with a total of 1,151 options, with some modifications. Based on the 2010 survey result, we identified key decision points in the treatment of bipolar disorder and feasible options for management. The major difference between the KMAP-BP 2010 and the KMAP-BP 2014 was the addition of a section for the treatment of children and adolescents with bipolar disorder. Additionally, the chapter that describes insufficient responses to treatment was divided into “partial response” and “nonresponse” sections for manic/hypomanic episodes, and a set of questions regarding mixed features (based on the changes in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5]) was included in the depressive episode chapter. The 2014 version of the revised KMAP-BP questionnaire had 56 main questions regarding important clinical situations; these were divided into 223 sub-items with a total of 1,799 response options organized into following sections: 1) acute manic episode; 2) acute hypomanic episode; 3) acute major depressive episode; 4) acute mixed episode; 5) rapid cycling; 6) maintenance treatment strategies for bipolar I disorder after acute manic/major depressive episode; 7) maintenance treatment strategies for bipolar II disorder after acute hypomanic/major depressive episode; 8) safety and tolerability issues; 9) treatment strategies for special situations including psychomotor agitation/retardation, violence, cognitive decline, and comorbid mental/physical disorder; 10) geriatric patients; and 11) pediatric patients. A written survey asked about the appropriateness of various treatment strategies and treatment agents commonly used by clinicians as the first-line. We also asked about next steps if there is an inadequate response to initial interventions for each clinical situation.
Rating scale
Of the 56 primary questions, 41 inquired about particular clinical cases and addressed the appropriateness of potential treatment options for these cases using a nine-point scale. This scale was based on the Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder 2000;Citation2 a score of 9 indicated “extremely appropriate”, a score of 7 or 8 indicated “usually appropriate” (first-line), a score of 4–6 indicated “equivocal appropriateness” (second-line), a score of 2 or 3 indicated “usually inappropriate” (a treatment you would rarely use), and a score of 1 indicated “extremely inappropriate” (a treatment you would never use). The remaining 15 questions were open-ended and inquired about the appropriate time period for treatment with a drug before switching strategies, the duration of treatment with antidepressants (ADs) and antipsychotics (APs), and other relevant issues. In their answers, the reviewers were asked to consider ideal treatment options rather than those actually practiced and to choose “q” if they had little experience or did not have available information for a particular question.
Medication categories
The medications were categorized as follows: typical APs (eg, haloperidol, chlorpromazine, molindone, perphenazine, pimozide, etc); atypical APs (AAPs) (eg, aripiprazole [ARI], olanzapine [OZP], quetiapine [QTP], risperidone [RIS], and ziprasidone [ZIP]); other AAPs that were not approved for the treatment of bipolar disorder by the Korean Ministry of Food and Drug Safety (eg, amisulpride, blonanserin, paliperidone, zotepine, and clozapine); mood stabilizers (MSs) (eg, lithium [LIT], valproic acid [VAL], and carbamazepine [CBZP]); and anticonvulsants (eg, gabapentin, levetiracetam, oxcarbazepine, pregabalin, phenytoin, retigabine, topiramate, zonisamide, and lamotrigine [LTG]).
Data analysis
For each option, we first defined the presence or absence of consensus. The rating scores on the nine-point scale were divided into three groups (1–3, 4–6, and 7–9), and a chi-square test was performed to test the distribution of scores across the three ranges of appropriateness. The consensus of the treatment options was defined as a nonrandom distribution of scores by chi-square test (P<0.05). Next, we calculated the means, standard deviation, and 95% confidence intervals (CI) for the score of each item, and each treatment option was divided into three categories based on the lowest score of the 95% CI: ≥6.5 for first-line/preferred treatments; <6.5 and ≥3.5 for second-line/reasonable treatments; and <3.5 for third-line/inappropriate treatments. For first-line treatments, the options rated as a 9 by 50% or more of the experts were defined as the treatment of choice (TOC); in other words, the most strongly recommended treatment. The SAS for Windows (version 9.2) was used for the preference ranking and multiple response analyses.
Development of the treatment guidelines and algorithms
Based on the preferred treatment strategies and medications identified by the present survey, the tables and algorithms of the guidelines were determined.
Ethics
The present study was conducted according to the Declaration of Helsinki, and the protocol was approved by the institutional review or ethics committee at each respective study site. The Institutional Review Boards waived the requirement for informed consent for this survey. All respondents received a predetermined fee for their participation.
Results
Acute manic episodes
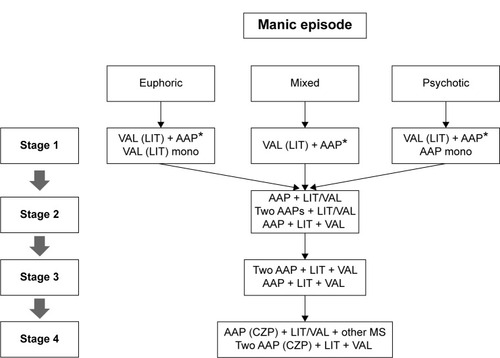
Manic episodes were categorized into three subtypes: euphoric, mixed, and psychotic. The TOC for all subtypes of mania, euphoric (95% CI: 7.8–8.4), mixed (95% CI: 8.3–8.7), and psychotic (95% CI: 8.7–8.9), was the combination of an MS and an AAP (MS plus AAP). The first-line treatments for euphoric and psychotic mania were MS monotherapy (95% CI: 6.8–7.6) and AAP monotherapy (95% CI: 6.7–7.4), respectively (). The preferred MSs were VAL for euphoric mania (95% CI: 8.0–8.5), mixed mania (95% CI: 8.1–8.6), and psychotic mania (95% CI: 8.0–8.5), and LIT for euphoric mania (95% CI: 7.7–8.3), mixed mania (95% CI: 6.6–7.3), and psychotic mania (95% CI: 7.3–7.9). VAL was the TOC for both euphoric and mixed mania. For all subtypes of mania, the recommended first-line APs were OZP (euphoric mania 95% CI: 7.8–8.3, mixed mania 95% CI: 8.0–8.5, and psychotic mania 95% CI: 8.3–8.7), QTP (euphoric mania 95% CI: 7.8–8.3, mixed mania 95% CI: 8.0–8.4, and psychotic mania 95% CI: 7.8–8.3), RIS (euphoric mania 95% CI: 6.9–7.6, mixed mania 95% CI: 6.8–7.5, and psychotic mania 95% CI: 7.7–8.3), and ARI (euphoric mania 95% CI: 6.7–7.4, mixed mania 95% CI: 6.7–7.4, and psychotic mania 95% CI: 6.8–7.5). OZP was the TOC for both mixed and psychotic mania.
Table 1 Initial treatment strategies for acute manic/hypomanic episode
In cases where patients responded poorly to initial MS monotherapy, augmentation with an additional AAP was the TOC for both partial response (95% CI: 8.3–8.7) and nonresponse (95% CI: 8.0–8.5) cases. Switching to an AAP was recommended as a first-line treatment option in a cases of nonresponse (95% CI: 6.6–7.3), and MS augmentation was the TOC for partial response to initial AAP monotherapy (95% CI: 8.2–8.7). MS augmentation (95% CI: 7.6–8.3) and switching to another AAP (95% CI: 6.9–7.6) were the first-line strategies for nonresponse to initial AAP monotherapy. In cases where the patients responded poorly to an initial combination of MS plus AAP, the addition of another MS (two MSs plus AAP; partial response 95% CI: 7.3–7.9 and nonresponse 95% CI: 7.2–7.9) or another AAP (two AAPs plus MS; partial response 95% CI: 6.8–7.5 and nonresponse 95% CI: 6.8–7.5), and switching from one AAP to another AAP (partial response 95% CI: 7.0–7.7 and nonresponse 95% CI: 7.7–8.2) were the first-line treatment strategies. If the patient did not show a sufficient response to a combination of two MSs plus AAP, the inclusion of an additional AAP (two MSs plus two AAPs) and switching to another AAP were the first-line treatment options regardless of the manic subtype and whether the case was a partial response or nonresponse case ().
Figure 1 Korean Medication Algorithm for Bipolar Disorder 2014: manic episode.
Abbreviations: AAP, atypical antipsychotics; CZP, clozapine; LIT, lithium; mono, monotherapy; MS, mood stabilizer; VAL, valproic acid.
Acute hypomanic episodes
The recommended first-line treatments for a hypomanic episode were MS monotherapy (95% CI: 7.8–8.3) and AAP monotherapy (95% CI: 7.2–7.8; ). The preferred MSs were LIT (95% CI: 7.8–8.3) and VAL (95% CI: 7.9–8.4), and the preferred AAPs were ARI (95% CI: 7.5–8.1), QTP (95% CI: 7.4–8.0), and OZP (95% CI: 7.1–7.7).
In cases where the patients responded poorly to initial MS monotherapy, augmentation with an AAP (partial response 95% CI: 7.5–8.1 and nonresponse 95% CI: 7.6–8.1), switching the MS (partial response 95% CI: 6.6–7.4 and nonresponse 95% CI: 7.3–8.0), a combination of two MSs (partial response 95% CI: 6.5–7.3 and nonresponse 95% CI: 6.6–7.4), and switching the AAP (partial response 95% CI: 6.6–7.2 and nonresponse 95% CI: 7.3–7.9) were recommended as first-line treatment options. If the patient was partially responsive to initial AAP monotherapy, then augmentation with an MS was the first-line treatment strategy (95% CI: 7.8–8.4), and the first-line treatment options for nonresponders were switching the MS (95% CI: 6.8–7.5) or AAP (95% CI: 7.4–8.0) and augmenting the MS (95% CI: 7.6–8.1).
Acute depressive episodes
Depressive episodes were categorized into three subtypes: mild, moderate to nonpsychotic severe, and psychotic (). The first-line treatment options for acute mild depressive episodes were MS monotherapy (95% CI: 6.8–7.5) and AAP monotherapy (95% CI: 6.5–7.2). For moderate and nonpsychotic severe depressive episodes, MS plus AAP (95% CI: 7.0–7.8) and MS plus AD (95% CI: 6.5–7.3) were recommended as first-line treatment options. For severe depressive episodes with psychotic features, AAP plus MS (95% CI: 7.6–8.2), AAP plus AD (95% CI: 6.5–7.1), AAP plus LTG (95% CI: 6.9–7.6), AAP plus MS plus LTG (95% CI: 6.5–7.2), AAP plus MS plus AD (95% CI: 7.0–7.6), and AAP plus LTG plus AD (95% CI: 7.0–7.6) were recommended as first-line treatment options ().
Figure 2 Korean Medication Algorithm for Bipolar Disorder 2014: depressive episode.
Abbreviations: AAP, atypical antipsychotics; AD, antidepressant; ECT, electroconvulsive therapy; LMT, lamotrigine; mono, monotherapy; MS, mood stabilizer (valproic acid, lithium, carbamazepine).
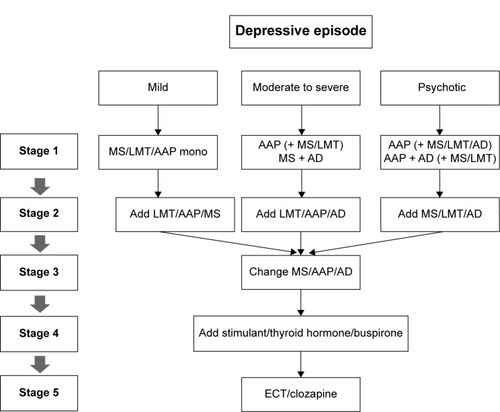
Table 2 Initial treatment strategies for acute depressive episode
The preferred first-line MSs were VAL (95% CI: 7.2–7.8), LIT (95% CI: 7.2–7.9), and LTG (95% CI: 6.9–7.7). There was not a specific TOC for MSs, but CBZP was a second-line treatment option (95% CI: 5.0–5.8). For severe depression with or without psychotic features, the first-line AAP treatment options were QTP (without psychotic features 95% CI: 7.6–8.1 and with psychotic features 95% CI: 7.5–8.1), ARI (without psychotic features 95% CI: 6.9–7.6 and with psychotic features 95% CI: 6.9–7.5), and OZP (without psychotic features 95% CI: 6.8–7.4 and with psychotic features 95% CI: 7.6–8.2). When an AD was necessary, citalopram or escitalopram (es) (95% CI: 6.8–7.6), bupropion (95% CI: 6.8–7.6), and sertraline (95% CI: 6.6–7.2) were the first-line ADs for moderate depression, and (es)citalopram (95% CI: 7.0–7.7), sertraline (95% CI: 6.8–7.4), bupropion (95% CI: 6.7–7.5), mirtazapine (95% CI: 6.7–7.4), and venlafaxine (95% CI: 6.6–7.3) were the first-line ADs for the treatment of acute depression. When considering efficacy and safety simultaneously, (es)citalopram, bupropion, and sertraline were the ADs recommended for the treatment of bipolar depression.
In cases where the patients responded poorly to initial MS monotherapy, the addition of an AAP (nonresponse 95% CI: 7.5–8.1 and partial response 95% CI: 7.4–8.0) or an LTG (nonresponse 95% CI: 6.8–7.6 and partial response 95% CI: 7.3–7.8) was preferred as the next-step strategy. The combination of two MSs was also recommended for a partial response to MS monotherapy (95% CI: 6.5–7.2). When there was no response to initial AAP monotherapy, the addition of an LTG (95% CI: 7.2–7.8) or another MS (95% CI: 7.1–7.9) and switching the initial AAP to another first-line AAP (95% CI: 6.7–7.3) were recommended. If there was a partial response to an AAP, the addition of an LTG (95% CI: 7.2–7.9) or another MS (95% CI: 7.4–8.0) was recommended as a next-step therapy.
The treatment options for cases where patients with nonpsychotic depression respond poorly to a combination of two medications are presented in . In most cases, the addition of another MS, AAP, or LTG was preferred for a partial response case, whereas the addition of another class of medication or switching the existing medication to another medication within same class was recommended for nonresponders. The second-step strategies for patients with psychotic depression who did not sufficiently respond to an initial combination of MS plus AAP were switching the AAP to another first-line AAP (95% CI: 7.1–7.7) or MS (95% CI: 6.7–7.4), the addition of another MS (95% CI: 6.6–7.3), or the addition of LTG (95% CI: 7.0–7.8) for nonresponsive patients, and the addition of an LTG (95% CI: 7.1–7.8) or another first-line AAP (95% CI: 7.0–7.5) or MS (95% CI: 6.8–7.4) for partial-response patients.
Table 3 Next step treatment strategies when initial treatment for bipolar depression was inadequate
Mixed features episodes
In the present version of the KMAP–BP, questions regarding mood episodes with mixed features and new material based on DSM-5 were added. Mixed-features episodes were categorized into three subtypes: mania with mixed features, depression with mixed features according to DSM-5 criteria, and mixed episodes defined by the DSM-IV. For all three subtypes of mixed features episodes, the combination of an MS and an AAP was recommended as the TOC (95% CI: 7.9–8.4), and AAP monotherapy was recommended as the first-line treatment option (95% CI: 6.5–7.1). The preferred medications for mania with mixed features were VAL (95% CI: 7.9–8.3), OZP (95% CI: 7.6–8.2), QTP (95% CI: 7.5–8.1), LIT (95% CI: 7.1–7.7), ARI (95% CI: 6.9–7.5), and RIS (95% CI: 6.7–7.3). The preferred medications for depression with mixed features were QTP (95% CI: 7.3–7.9), VAL (95% CI: 7.3–7.9), ARI (95% CI: 7.3–7.8), OZP (95% CI: 7.1–7.8), LIT (95% CI: 6.9–7.6), and LTG (95% CI: 6.8–7.5). The recommended first-line medications for mixed episodes were VAL (95% CI: 7.7–8.2), QTP (95% CI: 7.5–8.0), OZP (95% CI: 7.4–8.0), LIT (95% CI: 7.2–7.8), and ARI (95% CI: 7.1–7.6).
Rapid cycling
The recommended initial treatment strategies for patients not currently under medication were AAP monotherapy (95% CI: 6.5–7.2) or MS plus AAP (95% CI: 7.7–8.4) for those in a manic state, and AAP monotherapy (95% CI: 6.5–7.2), MS plus AAP (95% CI: 7.3–8.0), MS plus LTG (95% CI: 6.5–7.4), or AAP plus LTG (95% CI: 6.5–7.4) for those in a depressed state. For patients with rapid cycling, current manic episodes, or an insufficient response to MS monotherapy, the addition of an AAP was the TOC (95% CI: 7.8–8.4), and the addition of another first-line MS (95% CI: 7.1–7.7) was the first-line treatment option. If a patient was in a depressive state during rapid cycling and did not sufficiently respond to MS plus AD, the addition of an MS (95% CI: 7.0–7.6), an AAP (95% CI: 7.0–7.6), or LTG (95% CI: 6.9–7.7) and switching the MS to another first-line MS (95% CI: 6.6–7.2) were recommended. The recommended medications for a patient in a manic state during rapid cycling were VAL (95% CI: 7.8–8.3), QTP (95% CI: 7.5–8.0), OZP (95% CI: 7.3–7.8), LIT (95% CI: 6.8–7.4), and ARI (95% CI: 6.6–7.2). The recommended medications for a patient in a depressive state during rapid cycling were QTP (95% CI: 7.4–8.0), VAL (95% CI: 7.2–7.8), OZP (95% CI: 7.1–7.7), LTG (95% CI: 6.9–7.5), ARI (95% CI: 6.8–7.4), and LIT (95% CI: 6.8–7.4).
Maintenance treatment
The recommended first-line maintenance strategies for patients following a manic episode were MS plus AAP (95% CI: 7.7–8.2), MS monotherapy (95% CI: 7.2–7.8), and AAP monotherapy (95% CI: 7.0–7.6). If MS plus AAP was selected as the first-line treatment, then the recommended AAPs were QTP (95% CI: 7.6–8.1), ARI (95% CI: 7.5–8.0), and OZP (95% CI: 7.2–7.7), and the preferred AAP monotherapies were QTP (95% CI: 7.7–8.2), OZP (95% CI: 7.4–8.0), and ARI (95% CI: 7.4–8.0). The recommended AAP maintenance period following remission was 14–43 weeks, but 67.2% of the respondents recommended “maintaining the initial AAP as long as possible”.
The recommended first-line maintenance strategies for patients following a depressive episode were MS plus AAP (95% CI: 7.3–7.8), MS plus LTG (95% CI: 7.1–7.8), AAP plus LTG (95% CI: 7.0–7.6), MS monotherapy (95% CI: 6.8–7.4), LTG monotherapy (95% CI: 6.5–7.1), and MS plus AAP plus LTG (95% CI: 6.5–7.2). When an AD was necessary, bupropion (95% CI: 6.8–7.6), (es)citalopram (95% CI: 6.7–7.3), and sertraline (95% CI: 6.5–7.0) were the preferred ADs. The majority of experts recommended that initial AD treatment be stopped 8–19 weeks after a mild to moderate episode, 12–26 weeks after a severe episode without psychotic features, and 11–27 weeks after a severe episode with psychotic features. Of the respondents who suggested “maintaining the AD as long as possible”, 6.3% suggested this for a mild to moderate episode, 18.8% for a severe episode without psychotic features, and 28.1% for a severe episode with psychotic features.
If breakthrough mania occurred despite maintenance treatment with LIT, VAL, or LIT plus VAL, the addition of an AAP was the first-line treatment option (LIT monotherapy 95% CI: 7.9–8.4, VAL monotherapy 95% CI: 7.9–8.4, and LIT plus VAL 95% CI: 8.0–8.5). For cases of breakthrough mania that occurred during maintenance with AAP monotherapy, the addition of an MS was the TOC (95% CI: 8.0–8.4); the preferred MSs were LIT (95% CI: 7.7–8.3) and VAL (95% CI: 7.7–8.3), and the preferred AAPs were QTP (95% CI: 8.0–8.4), OZP (95% CI: 7.9–8.3), ARI (95% CI: 7.0–7.6), and RIS (95% CI: 6.7–7.4).
For maintenance treatment in patients with bipolar II disorder, the first-line treatment strategies following a hypomanic episode were MS monotherapy (95% CI: 7.6–8.0), AAP monotherapy (95% CI: 7.1–7.7), and MS plus AAP (95% CI: 7.1–7.8). The first-line maintenance strategies for patients with bipolar II disorder after a depressive episode were AAP plus LTG (95% CI: 7.2–7.8), MS plus LTG (95% CI: 7.1–7.7), MS plus AAP (95% CI: 7.0–7.7), and mono-therapy with MS (95% CI: 6.8–7.5), AAP (95% CI: 6.7–7.4), or LTG (95% CI: 6.7–7.3). The preferred medications were the same as those for patients with bipolar I disorder.
Safety, tolerability, and medical comorbidities
When the weight of a patient significantly increased due to pharmacotherapy, the primary recommendations included behavioral and diet modifications and switching to another AAP with a low risk of weight gain. If additional medications were needed to counteract weight gain, bupropion (95% CI: 4.5–5.6), topiramate (95% CI: 4.0–5.4), and metformin (95% CI: 4.0–5.4) were second-line treatment strategies. In obese or overweight patients, ARI (95% CI: 6.8–7.7) was the first-line treatment strategy, and LTG (95% CI: 5.9–6.6) and ZIP (95% CI: 6.0–7.0) were second-line options. In patients with signs or symptoms of hyperprolactinemia, including amenorrhea or galactorrhea, switching to an AAP with a low risk for hyperprolactinemia was the first-line treatment option, and a dose reduction of the current medication was the second-line treatment option. If benign skin rashes appeared during LTG treatment, reducing the dose and close monitoring were the first-line treatment options, and a discontinuation of LTG was the second-line treatment option.
In patients with bipolar disorder who also had cardiovascular, diabetic, or hepatic comorbidities, ARI was the first-line treatment strategy (cardiovascular disease 95% CI: 6.5–7.3, diabetes 95% CI: 7.1–7.8, and hepatic disease 95% CI: 6.7–7.3), whereas LIT was another first-line option for hepatic comorbidities (95% CI: 6.8–7.6). In cases of bipolar disorder with a renal comorbidity, ARI (95% CI: 6.7–7.4), VAL (95% CI: 6.6–7.3), and QTP (95% 95% CI: 6.5–7.2) were the preferred treatment options. VAL was recommended as the first-line treatment (95% CI: 7.2–7.8) for patients with bipolar disorder who had comorbid cerebrovascular diseases.
Children and adolescents
The first-line treatment strategy for manic episodes in children and adolescents with bipolar disorder was MS plus AAP (children 95% CI: 7.0–8.2 and adolescents 95% CI: 6.8–8.2). For children, AAP monotherapy was a first-line treatment strategy (95% CI: 6.6–7.7) but only a second-line option for adolescents (95% CI: 6.3–7.6; ). Among AAPs, ARI (children 95% CI: 7.5–8.3 and adolescents 95% CI: 7.5–8.3), RIS (children 95% CI: 7.4–8.1 and adolescents 95% CI: 7.8–8.4), and QTP (children 95% CI: 7.0–8.0 and adolescents 95% CI: 7.2–8.1) were first-line treatment options, and VAL (children 95% CI: 6.9–8.0 and adolescents 95% CI: 7.3–8.3) was the first-line MS for both children and adolescents. There was no consensus regarding first-line treatment for depressive episodes in children and adolescents with bipolar disorder (), but in cases where pharmacological treatment was necessary, ARI (95% CI: 7.6–8.5) and QTP (95% CI: 6.6–8.0) were recommended as first-line treatment options for children, and LTG (95% CI: 6.5–8.2), VAL (95% CI: 6.6–7.6), ARI (95% CI: 7.5–8.3), and QTP (95% CI: 6.8–8.0) were recommended as first-line options for adolescents. No consensus regarding a first-line treatment strategy for children and adolescents with depressive episodes and at high risk for bipolar disorder (eg, family history of bipolar disorder) was reached.
Table 4 Initial treatment strategies for bipolar disorder in children and adolescents
Elderly patients
For geriatric bipolar patients with acute manic episodes, AAP monotherapy (95% CI: 7.1–7.8), MS monotherapy (95% CI: 7.1–7.7), and MS plus AAP (95% CI: 7.0–7.8) were recommended as first-line treatment strategies. AAP plus MS (95% CI: 6.7–7.4) and AAP monotherapy (95% CI: 6.7–7.3) were the first-line treatment strategies for patients with acute depressive episodes (). The recommended first-line MSs for acute manic episodes were VAL (95% CI: 7.4–7.9) and LIT (95% CI: 6.6–7.3), and the recommended first-line AAPs were ARI (95% CI: 7.3–7.9), QTP (95% CI: 7.1–7.7), and OZP (95% CI: 7.4–7.6). The first-line MSs for patients with acute depressive episodes were LTG (95% CI: 6.8–7.5) and VAL (95% CI: 6.4–7.0); the first-line AAPs were ARI (95% CI: 7.2–7.9), QTP (95% CI: 7.2–7.8), and OZP (95% CI: 6.7–7.3); and the preferred ADs were (es) citalopram (95% CI: 7.2–7.8), bupropion (95% CI: 6.9–7.6), and sertraline (95% CI: 6.8–7.4). In cases where dementia was comorbid with bipolar disorder, ARI (95% CI: 7.0–7.7) and QTP (95% CI: 6.7–7.3) were recommended as first-line treatment options.
Table 5 Initial treatment strategies for geriatric bipolar disorder
Discussion
Treatment strategies for mania
The most preferred initial treatment strategy for mania, regardless of type, was MS plus AAP; the same recommendation was made in the KMAP-BP 2010.Citation21 However, while the KMAP-BP 2014 recommended AAP monotherapy as a first-line strategy for psychotic mania, AAPs were considered to be a second-line strategy in the KMAP-BP 2010. This finding reflects the recent accumulation of evidence and data supporting AAP monotherapy as a viable treatment strategy for bipolar patients with psychotic mania.Citation6
Because mania was categorized by symptom subtype in the KMAP-BP 2014, the present recommendations could not be directly compared with other guidelines that did not classify mania into subtypes. However, the present recommendation is in agreement with the Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines,Citation12 which suggest LIT, VAL, AAP monotherapy, or a combination of two of these drugs as first-line treatments. The guidelines for the biological treatment of bipolar disorder from the World Federation of Societies for Biological Psychiatry (WFSBP)Citation6 recommend monotherapy with ARI, RIS, ZIP, VAL, and LIT as first-line treatment options. When there is no response to monotherapy after 2 weeks or a partial response after 5 weeks following monotherapy in patients with severe mania, the WFSBP guidelines recommend the combination of two medications. Although the WFSBP guidelines stress that there is insufficient unambiguous evidence supporting combination therapy as a general first-line treatment for mania,Citation6 the present findings demonstrate greater efficacy of combination treatment. This result is supported by a previous meta-analysisCitation23 and the widespread use of combination treatment in clinical practice.Citation24,Citation25
According to the present guidelines, in the absence of a response to initial treatment, the appropriate next-step treatment strategies include AAP plus MS and switching to another first-line medication in the case of a partial response. If the response to treatment with MS plus AAP was inadequate, the addition of an AAP or MS, or switching from the original AAP to another AAP was the next-step strategy. This finding is different from that of the KMAP-BP 2010, which recommended the addition of another MS following an inadequate response to treatment with MS plus AAP. The KMAP-BP 2014 recommends replacing the current medication with another first-line medication, which is in agreement with the CANMAT guidelines.Citation12 Moreover, the KMAP-BP 2014 allows for a triple combination of pharmacological agents (two AAPs plus MS or AAP plus two MSs), although this treatment strategy is not supported by solid evidence at this stage. This finding may have been influenced by the Korean medical insurance system, which does not approve of the off-label use of pharmacotherapies such as paliperidone, oxcarbazepine, or tamoxifen that may be recommended in foreign guidelines.Citation6,Citation12
Treatment strategies for depression
The KMAP-BP 2010 did not identify a TOC for the initial treatment of acute bipolar depression, which underscores the difficulties and controversies involved in the selection of a treatment strategy for this type of bipolar episode. The KMAP-BP 2014 includes several changes from the previous version.Citation21 The KMAP-BP 2010 recommended monotherapy with MS or LTG for mild depression and combination therapy with MS plus AAP plus AD for severe depression without psychotic feature as first-line treatment options. However, in the KMAP-BP 2014, AAPs were included as an appropriate monotherapy for mild depression, and the combination of two medications from three types (AAP plus LTG) was added as a first-line treatment for moderate to severe depression. Additionally, LTG was added as a first-line option for the treatment of severe depression with psychotic features.
The most evident update in the KMAP-BP 2014 is the increased preference for the use of AAP monotherapy to treat moderate to severe depression, which is not surprising based on evidence from a number of recent studies,Citation26–Citation29 a recent meta-analysis,Citation30 and various treatment guidelines.Citation5,Citation12 Another notable change in the KMAP-BP 2014 is the strong preference for ARI and LTG for the treatment of moderate to severe depression. In recent meta-analyses, ARI monotherapyCitation30,Citation31 and LTG monotherapyCitation31 were not found to be superior to placebo. Furthermore, ARI was categorized as evidence category “E” in the WFSBP guidelines,Citation5 meaning that there was opposing evidence, and as “not recommended” in the CANMAT guidelines.Citation12 However, another meta-analysis suggested that ARI monotherapy could be effective for the treatment of acute depression because the combined data from two negative studies revealed a significant effect.Citation32,Citation33
The high preferences for ARI and LTG in the KMAP-BP 2014 are likely derived from tolerability issues. Clinically significant weight gain, metabolic disruption, and sedation are significant limitations of the use of OZP and QTP,Citation34 and the risk estimates for somnolence, sedation, and significant weight gain following treatment with OZP and QTP for bipolar depression were significantly higher than those with placebo.Citation35 Accordingly, these side effects may hamper the clinical use of these pharmacological agents.Citation36 Moreover, the consensus in the present study was that the combination of an AAP (QTP, OZP, or ARI) with an MS was appropriate for the treatment of moderate to severe depression. Although a small randomized controlled trial did not find a significant effect of this type of treatment compared with placebo,Citation37 some open-label trials have demonstrated the benefit of adjunctive ARI treatment for patients with bipolar depression.Citation38,Citation39 LTG was included in evidence category “B”, ie, limited positive evidence was available, in a previous controlled studyCitation5 and is a first-line treatment option according to CANMAT guidelinesCitation12 based on a meta-analysis of individual patient data that supported the efficacy of LTG treatment.Citation40
Treatment strategies for mixed-features episodes and rapid cycling
The first-line treatment options for mixed-features episodes and rapid cycling were MS plus AAP and AAP monotherapy. LTG was the preferred treatment for depressed states during rapid cycling and for depression with mixed features. Existing treatment guidelines typically do not recommend a specific treatment for mixed states or rapid cycling, and the pharmacological treatment options are limited and mostly based on findings from subanalyses or post hoc analyses. Therefore, there is no clear consensus regarding optimal pharmacological management for mixed states or rapid cycling, and the selection of a treatment strategy is usually based on individual factors such as safety and tolerability.Citation41 However, it is well known that patients with mixed-state episodes and/or rapid cycling exhibit a poorer pharmacological response than do patients with pure mood episodes, and that combination therapy is typically required.Citation42–Citation44 The KMAP-BP 2010Citation21 did not evaluate treatment strategies for mixed-features episodes, but the most-recommended treatment strategies for rapid cycling were MS plus AAP and the combination of two MSs. Because OZP, QTP, and ARI are effective for the treatment of acute bipolar episodesCitation45–Citation50 and ARI and OZP show promise for the maintenance of rapid cyclers,Citation46,Citation51 there was an increased preference for the use of AAPs in the KMAP-BP 2014.
Treatment strategies for maintenance therapy
Following a manic episode, maintenance therapy with MS plus AAP was the most-preferred strategy, although monotherapy with an MS or AAP was also regarded as a first-line treatment strategy for the prevention of a recurrent episode. Unlike the KMAP-BP 2010,Citation21 AAP monotherapy was included as a first-line treatment option in the KMAP-BP 2014. Moreover, the KMAP-BP 2014 recommends maintaining AAP monotherapy after remission from an acute episode, whereas the KMAP-BP 2010 recommended tapering the use of AAPs after recovery in conjunction with AAP or MS maintenance as a first-line treatment option. These changes from the KMAP-BP 2010 indicate the emerging preference for AAPs for maintenance treatment, as do other recent treatment guidelines.Citation4,Citation12
The KMAP-BP 2014 recommends MS plus LTG, AAP plus LTG, LTG monotherapy, and MS plus AAP plus LTG as first-line maintenance treatment options for depressive episodes. Thus, it appears that the preference of experts for the inclusion of LTG in maintenance therapies following a depressive episode is increasing compared with that seen in the KMAP-BP 2010. Recent Korean studies investigating bipolar disorder maintenance and safetyCitation52,Citation53 appear to have played a role in this change.
Special considerations
The KMAP-BP 2014 recommends ARI as a first-line treatment option and ZIP as a second-line treatment option for obese or overweight patients. Although the metabolic profile of ARI appears to be benign, this drug is not free of complications such as significant weight gain. A recent review found that the mean weight change following ARI treatment was not significantly different from that following placebo, but there was a clinically significant (≥7%) increase in weight.Citation54 On the other hand, ZIP is relatively weight neutral,Citation55 and, thus, the present findings of the KMAP-BP 2014 may be based on studies showing no significant difference between ZIP plus MS and placebo for the treatment of bipolar depression,Citation56 the inferiority of ZIP monotherapy compared with haloperidol,Citation57 and the absence of a significant difference between ZIP as an add-on therapy and placebo for patients with bipolar mania.Citation58 Although ZIP is recommended as a first-line treatment option for acute maniaCitation6,Citation12 and maintenance,Citation12 experts in the present study prefer ARI to ZIP for obese or overweight patients due to its overall effectiveness; this agrees with the WFSBP guidelines for long-term treatment.Citation4
The preferred treatment strategies for pediatric bipolar disorder with mania were MS plus AAP and AAP monotherapy. There was no consensus regarding a first-line treatment for depressive episodes. These findings make sense because most randomized controlled trials investigating pediatric bipolar patients assessed the acute treatment of manic symptoms. The Child and Adolescent Bipolar Foundation recommends LIT, VAL, CBZP, OZP, QTP, or RIS monotherapy for bipolar I manic/mixed episodes,Citation59 and The American Academy of Child and Adolescent Psychiatry recommends LIT, VAL, and AAPs for the treatment of bipolar I mania.Citation60 In the KMAP-BP 2014, the recommended medications for the treatment of mania in children are ARI, RIS, QTP, and VAL, whereas OZP and LIT were less preferred than for adult patients. The United States Food and Drug Administration approved ARI, RIS, and QTP for the treatment of acute mania in children and adolescents aged 10 years or older based on several trials.Citation61–Citation63 However, OZP was approved for children aged 13 years and older, and this may have affected the results. The risk of adverse metabolic events should also be considered as another possible cause of the low preference for OZP. Additionally, the presentation of mania in youths with irritability, rage, and labile moodCitation64 may also lead to a greater preference for VAL over LIT.
Limitations of the KMAP-BP 2014
A major limitation of the present study is that it was based on the consensus of Korean experts rather than on experimental evidence. Thus, some viable treatment strategies may not have been rated as first-line options despite evidence demonstrating their effectiveness. However, most of these experimental data are derived from randomized controlled trials and cannot reflect the complexity of various real-life clinical situations, which suggests that there may be some discrepancies between the findings of randomized controlled trials and the intricacies of real-world practice. Accordingly, the KMAP-BP 2014 suggests a variety of treatment options based on expert recommendations that reflect the unique characteristics of the Korean health care environment, clinical experience, and experimental evidence. The KMAP-BP assessments also reveal the updated recommendations of Korean experts for the treatment of bipolar patients with revisions every 4 years. As this project has proceeded, the expert consensus has changed for many treatment strategies, and the options and numbers of choices for treatment have increased. Additionally, because the KMAP-BP 2014 contains discussions regarding the safety of various medications and the consideration of special populations such as children, adolescents, and elderly patients, it will be effective for dealing with diverse challenging situations in real-world clinical practice.
Another limitation of the present study is that the review committee may have been too small to reach a valid consensus. Only 110 experts were engaged for the adult section, and only 38 experts for the children and adolescents section. However, because there are only 3,750 psychiatrists in South Korea and the membership of the Korean Society for Affective Disorders is 258, a sample of 148 psychiatrists may not be insufficient. Final, this algorithm does not include the dosage of medications or the nature of psychosocial interventions, which require further research. Moreover, novel pharmacological agents such as lurasidone, asenapine, and cariprazine, which are supported by increasing amounts of evidence and have been recommended in recent foreign guidelines,Citation4,Citation6,Citation12,Citation65 were excluded from this algorithm because they were not available in South Korea. Hence, this guideline could be limited in its use for clinicians in another countries. However, there might be countries in which newer agents are not available; therefore, this guideline could be informative in some countries.
In summary, the pharmacological treatment strategies supported by the KMAP-BP 2014 have markedly changed from previous versions. Most notably, the preferences for AAPs and LTG for the management of bipolar disorder have increased, and the current treatment options are in concordance with various other recent guidelines for bipolar disorder treatment. To our knowledge, the KMAP-BP 2014 is the only set of treatment guidelines in Asia that has been updated and revised every 4 years since 2002. Thus, despite the limitations of this expert consensus, it is expected that the KMAP-BP 2014 will provide clinicians with a wealth of information regarding appropriate strategies for treating patients with bipolar disorder.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The present manuscript is a secondary publication of our group’s papers which were already published in the Korean language. Though we have already published the papers in Korea, we decided to present and share the results with the experts who speak English according to conditions for acceptable secondary publications as stated in Uniform Requirements for Manuscripts Submitted to Biomedical Journals by International Committee of Medical Journal Editors. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/doSMpe.
Disclosure
The authors report no conflicts of interest in this work.
References
- No authors listedTreatment of bipolar disorder. The Expert Consensus Panel for Bipolar DisorderJ Clin Psychiatry199657Suppl 12A388
- SachsGSPrintzDJKahnDACarpenterDDochertyJPThe Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder 2000Postgrad Med2000Spec No110410895797
- American Psychiatric AssociationPractice guideline for the treatment of patients with bipolar disorder (revision)Am J Psychiatry20021594 Suppl150
- GrunzeHVietaEGoodwinGMThe World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorderWorld J Biol Psychiatry201314315421923480132
- GrunzeHVietaEGoodwinGMThe World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depressionWorld J Biol Psychiatry20101128110920148751
- GrunzeHVietaEGoodwinGMThe World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute maniaWorld J Biol Psychiatry20091028511619347775
- GrunzeHKasperSGoodwinGBowdenCMöllerHJWFSBP Task Force on Treatment Guidelines for Bipolar DisordersThe World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders, part III: maintenance treatmentWorld J Biol Psychiatry20045312013515346536
- GrunzeHKasperSGoodwinGThe World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders, Part II: Treatment of ManiaWorld J Biol Psychiatry20034151312582971
- GrunzeHKasperSGoodwinGWorld Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of bipolar disorders. Part I: Treatment of bipolar depressionWorld J Biol Psychiatry20023311512412478876
- GoodwinGMConsensus Group of the British Association for PsychopharmacologyEvidence-based guidelines for treating bipolar disorder: recommendations from the British Association for PsychopharmacologyJ Psychopharmacol2003172149173 discussion 14712870562
- GoodwinGMConsensus Group of the British Association for PsychopharmacologyEvidence-based guidelines for treating bipolar disorder: revised second edition – recommendations from the British Association for PsychopharmacologyJ Psychopharmacol200923434638819329543
- YathamLNKennedySHParikhSVCanadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013Bipolar Disord201315114423237061
- YathamLNKennedySHSchafferACanadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2009Bipolar Disord200911322525519419382
- YathamLNKennedySHO’DonovanCCanadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007Bipolar Disord20068672173917156158
- YathamLNKennedySHO’DonovanCCanadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversiesBipolar Disord20057Suppl 356915952957
- BahkWMShinYCJonDIKorean Medication Algorithm for Bipolar Disorder (I)Korean J Psychopharmacol200213205221
- KimCHMinKJShinYCFeasibility of Korean Medication Algorithm for Bipolar Disorder (I): Global assessmentKorean J Psychopharmacol200516225233
- ShinYCBahkWMKimCHFeasibility of Korean Medication Algorithm for Bipolar Disorder (II): Choice of medicationsKorean J Psychopharmacol200516285291
- JonDIBahkWMShinYCFeasibility of Korean Medication Algorithm for Bipolar Disorder (III): Treatment response and tolerabilityKorean J Psychopharmacol200516292300
- JonDIBahkWMYoonBHRevised Korean medication algorithm for bipolar disorderWorld J Biol Psychiatry2009104 Pt 384685518615367
- ShinYCMinKJYoonBHKorean medication algorithm for bipolar disorder: second revisionAsia Pac Psychiatry20135430130823857877
- Seok SeoJRim SongHBin LeeHThe Korean Medication Algorithm for Depressive Disorder: second revisionJ Affect Disord201416731232125010375
- SmithLACorneliusVWarnockATacchiMJTaylorDAcute bipolar mania: a systematic review and meta-analysis of co-therapy vs monotherapyActa Psychiatr Scand20071151122017201861
- LeeSMShimIHWooYSJunTYBahkWMA trend of medication prescription pattern for outpatients with bipolar disorder in a university hospital: focusing on atypical antipsychoticsKorean J Psychopharmacol2014253124133 Korean
- ChaJMShimIHWooYSJunTYBahkWMA trend of drug use in inpatients with bipolar disorder: comparing 2009–2012 with 1998–2001 in one university hospitalKorean J Psychopharmacol2014253114123
- TohenMVietaECalabreseJEfficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depressionArch Gen Psychiatry200360111079108814609883
- CalabreseJRKeckPEJrMacfaddenWA randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depressionAm J Psychiatry200516271351136015994719
- ThaseMEMacfaddenWWeislerRHEfficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study)J Clin Psychopharmacol200626660060917110817
- McElroySLWeislerRHChangWA double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II)J Clin Psychiatry201071216317420122366
- CruzNSanchez-MorenoJTorresFGoikoleaJMValentiMVietaEEfficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysisInt J Neuropsychopharmacol201013151419638254
- SelleVSchalkwijkSVázquezGHBaldessariniRJTreatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychoticsPharmacopsychiatry2014472435224549862
- FountoulakisKNVietaESchmidtFAripiprazole monotherapy in the treatment of bipolar disorder: a meta-analysisJ Affect Disord2011133336137021040979
- VietaEValentiMPharmacological management of bipolar depression: acute treatment, maintenance, and prophylaxisCNS Drugs201327751552923749421
- McIntyreRSChaDSKimRDMansurRBA review of FDA-approved treatment options in bipolar depressionCNS Spectr201318Suppl 1420 ; quiz 2124237641
- GaoKKempDEFeinENumber needed to treat to harm for discontinuation due to adverse events in the treatment of bipolar depression, major depressive disorder, and generalized anxiety disorder with atypical antipsychoticsJ Clin Psychiatry20117281063107121034695
- De FruytJDeschepperEAudenaertKSecond generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysisJ Psychopharmacol201226560361721940761
- QuanteAZeugmannSLuborzewskiAAripiprazole as adjunct to a mood stabilizer and citalopram in bipolar depression: a randomized placebo-controlled pilot studyHum Psychopharmacol201025212613220196183
- MazzaMSquillaciotiMRPecoraRDJaniriLBriaPBeneficial acute antidepressant effects of aripiprazole as an adjunctive treatment or monotherapy in bipolar patients unresponsive to mood stabilizers: results from a 16-week open-label trialExpert Opin Pharmacother20089183145314919040335
- DunnRTStanVAChrikiLSFilkowskiMMGhaemiSNA prospective, open-label study of Aripiprazole mono- and adjunctive treatment in acute bipolar depressionJ Affect Disord20081101–2707418272230
- GeddesJRCalabreseJRGoodwinGMLamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trialsBr J Psychiatry200919414919118318
- VietaEValentiMMixed states in DSM-5: implications for clinical care, education, and researchJ Affect Disord20131481283623561484
- YildizAVietaETohenMBaldessariniRJFactors modifying drug and placebo responses in randomized trials for bipolar maniaInt J Neuropsychopharmacol201114786387521299919
- González-PintoAAldamaAMosqueraFGonzález GómezCEpidemiology, diagnosis and management of mixed maniaCNS Drugs200721861162617630815
- FountoulakisKNKontisDGondaXYathamLNA systematic review of the evidence on the treatment of rapid cycling bipolar disorderBipolar Disord201315211513723437958
- BaldessariniRJHennenJWilsonMOlanzapine versus placebo in acute mania: treatment responses in subgroupsJ Clin Psychopharmacol200323437037612920413
- VietaECalabreseJRHennenJComparison of rapid-cycling and non-rapid-cycling bipolar I manic patients during treatment with olanzapine: analysis of pooled dataJ Clin Psychiatry200465101420142815491248
- ShiLSchuhLMTrzepaczPTHuangLXNamjoshiMATohenMImprovement of Positive and Negative Syndrome Scale cognitive score associated with olanzapine treatment of acute maniaCurr Med Res Opin20042091371137615383185
- SuppesTDattoCMinkwitzMNordenhemAWalkerCDarkoDEffectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depressionJ Affect Disord20101211–210611519903574
- SuppesTEudiconeJMcQuadeRPikalovA3rdCarlsonBEfficacy and safety of aripiprazole in subpopulations with acute manic or mixed episodes of bipolar I disorderJ Affect Disord20081071–314515417904226
- VietaECalabreseJRGoikoleaJMRainesSMacfaddenWBOLDER Study GroupQuetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled studyBipolar Disord20079441342517547587
- MuzinaDJMomahCEudiconeJMAripiprazole monotherapy in patients with rapid-cycling bipolar I disorder: an analysis from a long-term, double-blind, placebo-controlled studyInt J Clin Pract200862567968718373615
- WooYSBahkWMPaeCUObservational study to evaluate the clinical benefit of lamotrigine add-on therapy in bipolar patients in a naturalistic treatment settingAsia Pac Psychiatry20146333434124038834
- WooYSBahkWMJonDIRash in adult patients receiving lamotrigine to treat bipolar I disorder in Korea: a multicenter, prospective, naturalistic, open-label trialProg Neuropsychopharmacol Biol Psychiatry20093371147115219540298
- McIntyreRSAripiprazole for the maintenance treatment of bipolar I disorder: A reviewClin Ther201032Suppl 1S32S3820152551
- KempDEKarayalONCalabreseJRZiprasidone with adjunctive mood stabilizer in the maintenance treatment of bipolar I disorder: long-term changes in weight and metabolic profilesEur Neuropsychopharmacol201222212313121798721
- SachsGSIceKSChappellPBEfficacy and safety of adjunctive oral ziprasidone for acute treatment of depression in patients with bipolar I disorder: a randomized, double-blind, placebo-controlled trialJ Clin Psychiatry201172101413142221672493
- WarringtonLLombardoILoebelAIceKZiprasidone for the treatment of acute manic or mixed episodes associated with bipolar disorderCNS Drugs2007211083584917850172
- WeislerRWarringtonLDunnJAdjunctive ziprasidone in bipolar mania: Short- and long-term dataBiol Psychiatry20045543s43s
- KowatchRAFristadMBirmaherBTreatment guidelines for children and adolescents with bipolar disorderJ Am Acad Child Adolesc Psychiatry200544321323515725966
- McClellanJKowatchRFindlingRLWork Group on Quality IssuesPractice parameter for the assessment and treatment of children and adolescents with bipolar disorderJ Am Acad Child Adolesc Psychiatry200746110712517195735
- FindlingRLNyilasMForbesRAAcute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200970101441145119906348
- HaasMDelbelloMPPandinaGRisperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled studyBipolar Disord200911768770019839994
- PathakSFindlingRLEarleyWRAcevedoLDStankowskiJDelbelloMPEfficacy and safety of quetiapine in children and adolescents with mania associated with bipolar I disorder: a 3-week, double-blind, placebo-controlled trialJ Clin Psychiatry2013741e100e10923419231
- MillerLBarnettSMood lability and bipolar disorder in children and adolescentsInt Rev Psychiatry200820217117618386208
- WooYSWangHRBahkWMLurasidone as a potential therapy for bipolar disorderNeuropsychiatr Dis Treat201391521152924143101

