Abstract
Traumatic brain injury (TBI) is a growing health concern effecting civilians and military personnel. Research has yielded a better understanding of the pathophysiology of TBI, but effective treatments have not been forthcoming. Near-infrared light (NIR) has shown promise in animal models of both TBI and stroke. Yet, it remains unclear if sufficient photonic energy can be delivered to the human brain to yield a beneficial effect. This paper reviews the pathophysiology of TBI and elaborates the physiological effects of NIR in the context of this pathophysiology. Pertinent aspects of the physical properties of NIR, particularly in regards to its interactions with tissue, provide the background for understanding this critical issue of light penetration through tissue. Our recent tissue studies demonstrate no penetration of low level NIR energy through 2 mm of skin or 3 cm of skull and brain. However, at 10–15 W, 0.45%–2.90% of 810 nm light penetrated 3 cm of tissue. A 15 W 810 nm device (continuous or non-pulsed) NIR delivered 2.9% of the surface power density. Pulsing at 10 Hz reduced the dose of light delivered to the surface by 50%, but 2.4% of the surface energy reached the depth of 3 cm. Approximately 1.22% of the energy of 980 nm light at 10–15 W penetrated to 3 cm. These data are reviewed in the context of the literature on low-power NIR penetration, wherein less than half of 1% of the surface energy could reach a depth of 1 cm. NIR in the power range of 10–15 W at 810 and 980 nm can provide fluence within the range shown to be biologically beneficial at 3 cm depth. A companion paper reviews the clinical data on the treatment of patients with chronic TBI in the context of the current literature.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Traumatic brain injury (TBI) and stroke are major sources of morbidity and mortality worldwide. The World Health Organization has projected that TBI soon will be the third most frequent source of disability.Citation1 Stroke is the second leading cause of death worldwide.2 While attention to the pathophysiologic processes that underlie neurotrauma has yielded considerable information, efficacious treatment strategies have yet to emerge. Treatment of neurological trauma is largely limited to mitigating the symptoms (see Morries and colleaguesCitation3 for review). Over the past decade, near-infrared light (NIR) has gained attention as a potential treatment strategy for TBI and stroke in both the acute and chronic setting.
NIR has been investigated for its ability to modulate intracellular reparative mechanisms. NIR can facilitate wound healingCitation4,Citation5 and promote muscle repairCitation5 and angiogenesis.Citation4,Citation5 The application of NIR by low power laser or by light emitting diode (LED), referred to as either laser phototherapyCitation6 or near-infrared photobiomodulation,Citation5 has been studied and applied clinically in a wide array of ailments, including skin ulcers,Citation7 osteoarthritis,Citation8 peripheral nerve injury,Citation4,Citation5 low back pain,Citation9 myocardial infarction,Citation10 and stem-cell induction.Citation11 Since NIR passes relatively efficiently through bone, several studies of transcranial near-infrared light therapy (NILT)Citation12 in animal models of brain damage have been conducted by multiple laboratories. A large clinical trial of NILT for acute stroke showed clinical improvement – NeuroThera Effectiveness and Safety Trial (NEST)-1;Citation13 however, a subsequent Phase III clinical trial failed to show benefit at an interim futility analysis.Citation14 This and other findings of ineffective protocols for NIR in a variety of pathological conditionsCitation15,Citation16 raise an important question about the necessary elements of an effective therapy.
This review focuses on the relevant mechanisms of brain injury and NIR as it relates to the therapeutic use of NILT. To understand the ability of NILT to repair damaged or dysfunctional brain tissue resulting from stroke and TBI, it is first necessary to understand the relevant pathophysiological mechanisms of brain injury. Then the postulated mechanisms of NIR in mitigating these mechanisms will be reviewed. The physical properties of light and the manner in which it interacts with tissue are then elucidated. The ability of NIR to penetrate to sufficient depth with sufficient energy to exert a biological effect on the brain is key. Data on light penetration are provided and discussed in the context of the current literature on the topic.
Pathophysiological mechanisms of neurotrauma
TBI is a complex injury in which the nature of the sequelae depends on the region of the brain involved, injury severity, patient’s age, and the nature and delay in initial care. TBI disrupts membrane function and gives rise to early ionic and neurotransmitter perturbations.Citation17 Together with a substantial release of the excitatory neurotransmitter glutamate, these perturbations initiate a cascade of events that extensively disrupts normal cellular function, alters glucose metabolism, induces free radical production, and impairs mitochondrial function.Citation17–Citation20 An early event is increased release of potassium which is proportional to the severity of the injury.Citation19 This has a robust inhibitory effect on neuronal activity. Calcium (Ca2+) begins to accumulate within neurons and significantly impairs function. Increased intracellular Ca2+ activates mitochondrial uptake, leading to Ca2+ overload in mitochondria,Citation17 oxidative stress, and impaired mitochondrial function.Citation19–Citation21 Accumulations of Ca2+ can directly destroy portions of the diverse mitochondrial populationCitation22 and induce persistent damage in surviving mitochondriaCitation23 (although, see Pandya et alCitation24). Early excess glutamate can deplete metabolic pathways. Increased reactive oxygen and nitrogen species can indirectly deplete energy precursors and induce peroxidation of mitochondrial (and cellular) lipid membranes.Citation20,Citation25–Citation27 The byproducts of lipid peroxidation have been implicated in inactivation or disruption of mitochondrial (and cellular) proteins.Citation26 Mitochondrial DNA is particularly vulnerable to oxidative damage. As a result, all 37 mitochondrial DNA genes are disrupted, as well as 235 additional nuclear DNA genes.Citation28
Glucose is the primary energy source for neurons, but becomes considerably less available following TBI. Studies of cerebral glucose metabolism and fluorodeoxyglucose positron emission tomography have shown an initial brief increase in glucose metabolism, likely associated with glutamate flooding.Citation18 Decreased glucose metabolism follows within 1 hour of injury and appears to be proportional to the severity of the injury.Citation20,Citation29 During the first 3–4 days, cerebral perfusion can be increasedCitation18 and a spreading depression of neuronal function and metabolism can occur. This is followed by a prolonged depression of both cerebral perfusion and cerebral glucose metabolism which has been shown in both humansCitation30–Citation32 and animal models.Citation29,Citation33 In addition, glucose transport from blood vessels is disrupted.Citation30 Moreover, glucose appears to be shunted from mitochondrial pathways to the pentose phosphate pathway.Citation17–Citation20,Citation29 Overall these changes create an energy crisis inside the affected neurons.
This energy crisis promotes the increased concentration of free radicals, due to increased pentose phosphate metabolism, reduced mitochondrial function, and impaired free radical scavenger mechanisms.Citation19 The consequences of increased free radicals can be far-reaching including propagation of additional free radicals, breakdown of lipids within membranes,Citation34 edema, inflammation, and DNA damage.Citation18,Citation35
Neuroinflammation is an additional and incompletely understood mechanism in TBI. Recent evidence has shown that neural inflammation can persist for years following an injury.Citation36–Citation38 Using a positron emission tomography ligand for inflammation, one group demonstrated patients could have elevated inflammation 11 months to 17 years after a TBI.Citation39 Notably though, areas of accumulated inflammation marker did not correspond to areas damaged directly by TBI. Altogether, these mechanisms appear to contribute to a progression in the size of the initial injury to involve surrounding areas (penumbra).Citation36–Citation38
In contrast to acute TBI, neurophysiological dysfunction in chronic TBI is less well understood. It is clear that mechanisms activated in acute TBI,Citation18 such as neuronal injury and apoptosis, would have persistent consequences. Studies have shown that diffuse and Wallerian white matter degeneration occurs following TBI.Citation40,Citation41 In many ways, stroke and TBI share these long-term mechanisms.Citation42,Citation43 Long-term disruption of mitochondrial membranes by lipid peroxidation, disrupted Ca2+ regulation, loss of subpopulations of mitochondria, and reduced energy production can continue long past the acute phase of TBI.Citation43 In humans, impaired mitochondrial function may persist for months to years based on observations of decreased glucose metabolism in patients using fluorodeoxyglucose positron emission tomography.Citation31,Citation44 Decreased cerebral blood flow to the injured area also persists for many years based on perfusion single photon emission computed tomography scans.Citation32,Citation45 Injured and disrupted axons, as well as altered proteolytic pathways in injured neurons, can lead to the accumulation of amyloid precursor proteins and tau proteins.Citation46 Accumulations of these abnormal proteins can set in motion a sequence of pathophysiological processes leading to Alzheimer’s disease,Citation47,Citation48 chronic traumatic encephalopathy,Citation46,Citation49 and Parkinson’s disease.Citation48,Citation50,Citation51 Blast injury may be particularly harmful, resulting in persistent axonal abnormalities of varicosities and accumulated abnormal proteins.Citation52 Recent evidence has shown a strong correlation between persistent areas of disrupted white matter, shown by diffusion tensor imaging, and areas of decreased cerebral blood flow which were present at the time of injury.Citation41 In the next section, the mechanisms by which NIR can potentially restore, repair, or mitigate the pathophysiological processes involved in TBI are elaborated.Citation4,Citation53–Citation55
Mechanisms of photobiomodulation
Primary events
The precise mechanisms underlying photobiomodulation and its therapeutic benefits are not fully understood. The purported effects of NIR are illustrated in . Light in the wavelength range of 600–1,200 nm has significant photobiomodulation capability.Citation56 Current data most strongly support that absorption of NIR photons by cytochrome c oxidase (COX) in the mitochondrial respiratory chain is the key initiating event in photobiomodulation.Citation4,Citation54,Citation56 COX is a large transmembrane protein of the inner mitochondrial membrane. It contains two copper (Cu) centers and two heme-iron centers. These metal centers have different light absorption peaks. Reduction of CuA occurs with 620 nm, oxidation of CuA occurs with 825 nm, reduction of CuB occurs with 760 nm, and oxidation of CuB occurs at 680 nm.Citation54 These peaks correspond to the “optical window” associated with the biological effects of NIR. Irradiation of COX increases the activity of the entire electron transport chain producing more adenosine triphosphate (ATP). In addition, COX is auto-inducible and its gene expression is activity dependent, such that NIR irradiation may increase the amount of available COX over time.Citation57
Figure 1 Hypothesized mechanism of action of near-infrared light (NIR) photobiomodulation.
Abbreviation: ↑, increase.
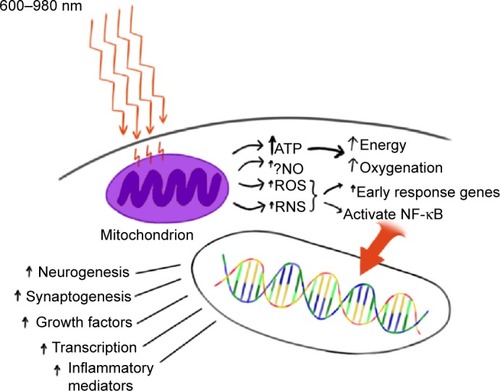
NIR’s effect has been studied in isolated mitochondria preparations. Irradiation with 632 nm light results in increased proton electrochemical potential and increased ATP production.Citation58 COX activity and oxygen consumption also increases.Citation59 In a more recent study, Yu et alCitation60 confirmed increased oxygen consumption and showed increased activation of several electron transport chain components. In the setting of acute neurotrauma, this increase in energy supply may be sufficient to reduce the consequences of injury. Neurons are often forced into anaerobic metabolism, which results in acidosis, insufficient energy to maintain ion pumps, and calcium overload. Later in the sequence of events following neurotrauma, more energy is required than at baseline due to the large energy requirements of repair. Increasing ATP during acute neurotrauma alone may be sufficient, but NIR appears to initiate a number of other events in the mitochondria.
Secondary events
In addition to the increase in ATP, the change in redox state leads to greater oxidation and activation of the mitochondrial permeability transition pore which alters intermembrane potentials within the mitochondria.Citation61 This may have a direct or indirect effect on gene transcription and protein synthesis.Citation62 For example, transcription factors, such as redox factor-1-dependent activator protein 1, activating transcription factor/cyclic adenosine monophosphate response element binding protein (ATF/CREB), and hypoxia-inducible factor alpha, are upregulated in response to changes in redox states. The change in redox state and NIR possibly directlyCitation63,Citation64 result in the production of reactive oxygen species (ROS), the most common of which is superoxide. Similarly, NIR (670 nm) modulates the effects of reactive nitrogen species in a model of multiple sclerosis.Citation65 ROS are potent second messenger molecules. ROS are involved in cell signaling, enzyme activation, nucleic acid synthesis, protein synthesis, and the activation of transcription factors.
The change in oxidation also likely contributes to the displacement of nitric oxide (NO) from the COX molecule. When bound, NO functions as an inhibitor by displacing oxygen from the binding site on COX. When NO is displaced, there is an increase in the activity of the electron transport chain.Citation66 In addition, NO serves as a vasodilator and thus increases local blood flow.Citation67 NO also functions as a second messenger. Numerous tissue culture and animal studies have demonstrated the effect of NIR on NO levels,Citation4,Citation68,Citation69 including the upregulation of NO synthase expression.Citation70
Nuclear factor kappa-B (NF-κB) is a redox sensitive transcription factor.Citation71 This pro-survival transcription factor modulates the expression of numerous genes, including ones involved in inflammation, early response (heat shock), anti-apoptosis, cellular migration, and cell survival.Citation71,Citation72 NIR (810 nm) activates NF-κB apparently via ROS in cultured fibroblasts.Citation72 NF-κB can be activated by ROS and indirectly by the effects of ROS on inflammatory cytokines (eg, tumor necrosis factor, interleukin-1).Citation73
Tertiary events
With NIR exposure, induction of mitochondrial RNA synthesis,Citation74 as well as protein synthesis, occurs.Citation75 More recent work has shown NIR induces the upregulation and downregulation of numerous genes both in the nucleus and in the mitochondria of various cell types. Kushibiki et alCitation76 have reviewed the mitochondrial RNA expression changes associated with photobiomodulation. Zhang et alCitation77 demonstrated changes in mRNA expression of over 100 genes in cultured fibroblasts exposed to 653 nm NIR. Genes involved in cell proliferation, such as mitogen-activated protein kinase 11 (MAPk11), and cell-cycle progression are increased.Citation77 Apoptosis-inhibiting genes are upregulated (eg, Janus kinase binding protein). Meanwhile, apoptosis-promoting genes, such as heat shock 70k Da protein 1A and caspase 6, are downregulated.Citation77 Expression of genes for antioxidants and inhibitors of the effects of ROS are increased. Similar work in the retina has shown that genes involved in cell survival, antioxidant production, transcription, and growth factor production are also upregulated in neural retina cells.Citation78
Data from tissue culture and animal studies of NIR reveal an increase in growth factor expressionCitation68,Citation72,Citation79,Citation80 and subsequent cell proliferation.Citation4,Citation12,Citation68,Citation69 Examples of these key growth factors include nerve growth factor, brain-derived neurotrophic factor, transforming growth factor-beta, and vascular endothelial growth factor, which may contribute to late brain remodeling after TBI.Citation4,Citation12,Citation54,Citation68,Citation73,Citation79–Citation86 For example, a fivefold increase in nerve growth factor mRNA transcription occurred after irradiation of skeletal muscle cell culture with 633 nm NIR light.Citation85
Recent data suggest that transcranial NIR phototherapy can increase the process of neurogenesis in adult mice with stroke or TBI. Increased numbers of neuroprogenitor cells have been demonstrated in both the dentate gyrus of the hippocampus and in the subventricular zone of the lateral ventricle of mouse models.Citation4,Citation87,Citation88 These cells also demonstrate increased expression of a microtubule protein associated with migrating neuroblasts.Citation88 Some studies provide evidence that NIR phototherapy may increase the process of synaptogenesis.Citation4,Citation88 Together, these processes may aid in the neuroplasticity responsible for neural repair and improved function in cases of chronic TBI.
These cellular changes appear to persist for considerably longer than the interval of light applicationCitation89 (when delivered at appropriate wavelengths and amplitudes).Citation4 For example, low level (red and) near-infrared light therapy (LLLT) of a power density of 0.9–36 J/cm2 applied in a single treatment at 24 hours post-stroke in animal models yielded a reduction in neurological deficits, as well as histochemical evidence of neuron proliferation and migration.Citation55,Citation88,Citation90,Citation91 A single application of LLLT in rodent models of TBI had similar benefits.Citation4,Citation87,Citation92–Citation94 Interestingly, these benefits were not immediately apparent. Rather, a delay of 1–4 weeks was noted, consistent with a progressive regeneration cascade set in motion by the NIR exposure.
Summary
During NIR phototherapy, absorption of red or NIR photons by COX in the mitochondrial respiratory chain causes secondary molecular and cellular events, including activation of second messenger pathways, changes in NO levels, and growth factor production. NILT leads to the reduction of excitotoxicity, the production of neurotrophic factors, the modulation of ROS, the transcription of new gene products with protective or pro-proliferative properties, and the release of numerous growth factors for neurons and other cells.Citation4,Citation64,Citation68,Citation73,Citation82–Citation84 NIR appears to initiate a cascade of subcellular events which can yield immediate, delayed, and persistent beneficial changes in the injured neuron or other cell.
Properties of NIR
Light has fundamental physical properties which are relevant to its clinical use. Light is a form of electromagnetic radiation which has properties of both waves and particles. Light is characterized by its wavelength (distance between two peaks), frequency, and amplitude. Light is also characterized by its energy content. This energy is quantified as joules (J). The amount of energy delivered per unit time constitutes the power of light in watts (W = J/second). For medical applications, light is typically reported in terms of wavelength (nm), energy (J), irradiance or power density (W/cm2), and radiant exposure or fluence or dose (J/cm2).Citation54,Citation95
NIR has a number of biological effects, but it is critical to understand the physical interactions between tissue and light. When light impinges on the surface, a portion (~10%) is reflected.Citation96 The energy that does penetrate the surface is refracted or bent toward a line perpendicular to the surface. This results from the particle property of light. These particles are, of course, photons. The photons entering the tissue can be transmitted through the tissue, scattered, or absorbed. Scatter increases the volume of tissue impacted by the light. Photons can change direction without loss of energy. Scattering is particularly likely at interfaces between different tissues. Reflection or refraction also occurs at such interfaces. These effects contribute to shortening the distance to which light will travel or penetrate into the tissue.Citation96–Citation98
Most tissues have the capacity to absorb light energy. Usually this is mediated by a molecule absorbing a photon. Molecules containing metal ions have a strong capacity for absorbing photonic energy, but DNA and water also can. The absorption of energy can induce a change in the confirmation and/or function of the molecule.
Penetration of NIR through tissues is determined by several factors: wavelength, energy, attenuation coefficient (composed of scatter, refraction, and absorption), area of irradiance, coherence, and pulsing. In general, longer wavelengths (up to 1,000 nm) will penetrate deeper; however, the absorption of water begins to predominate above 1,000 nm.Citation96 Increases in power density, in general, will lead to greater penetration. More photons will traverse the tissue. The area of surface irradiation also affects penetration due to scattering effects.
Coherence is a property of light waves in which waves of monochromatic light are aligned such that any point on the wave has the same amplitude and position as the equivalent point in an adjacent wave. Temporal coherence reflects the slight variations in the waveform over time. The more consistent the waveform is, the higher the temporal coherence. Monochromatic light typically has high temporal coherence. Spatial coherence results from the divergence of the light from the point of emission.Citation66 Laser has virtually no spatial divergence and creates a long narrow volume of coherent light. LEDs are not monochromatic, but emit light in a relatively narrow band over the peak wavelength. LEDs also have significant spatial divergence and therefore a wide volume of space is radiated; however, within that volume only a very small volume contains coherent light. As KaruCitation66 illustrates, the result is that noncoherent LED sources likely only provide coherent light in a thin volume, usually at surfaces. In contrast, laser generates a long narrow volume of coherent light which can penetrate deeper into tissues.
When coherent light enters a tissue, slight distortions in the timing and the shape of the waves occur. As a result, interference can occur between the waves. Polarization, the angle at which a wave is vibrating, also contributes to interference. On a single wave basis, interference results when the amplitude of the wave at a given point is different from that of an adjacent wave and of the population of coherent light waves. At the point of difference, the amplitudes can either cancel each other out {[+x] + [−x}], be additive {[+x] + [+x}], or any variation in between {[+x] + [−y}]. The result of these interactions is a field of randomly distributed points of increased and decreased light intensity, referred to as a speckle intensity pattern. Speckling can have a significant impact on the effective penetration depth.Citation99 As such, areas of high intensity will penetrate further or will have two to three orders of magnitude greater energy at a given depth.Citation99
Pulsing of NIR also increases the depth of penetration and the amount of energy delivered to any given point at the peak of a pulse. Yet, pulsing allows for troughs of energy output such that the overall energy delivered to the tissue can be equivalent or even lower than that delivered by a continuous emission. Pulsing is a property of lasers which cannot be duplicated by LEDs.
Limitations of NILT protocols
Prior clinical applications of NIR photomodulation have utilized LLLT emitters and prolonged courses of daily treatments often extending over months.Citation100,Citation101 For example, the first published study of NIR therapy for TBI in humans described two cases of chronic mild TBI with significant disability.Citation100 Each patient had marked neuropsychological improvement after a prolonged series of LLLT treatments using 870 and 633 nm LED arrays over 4–72 months. Yet, some clinical and laboratory studies of LLLT have failed to consistently demonstrate benefit.Citation15,Citation16,Citation102,Citation103 For example, Lavery et alCitation15 demonstrated that LLLT (890 nm LEDs delivering 1.3 J/cm2 for 40 minutes daily for 90 days) did not yield significant improvement in nerve conduction velocity in patients with diabetic neuropathy. Similarly, treatment of a rat model of contusive spinal cord injury with LLLT (830 nm at 22.6 J/cm2 or 670 nm at 28.4 J/cm2) for 30 minutes per day for 5 days resulted in no significant functional improvement and no reduction in lesion size.Citation16 The identical treatment regimen was applied to a rat model of TBI with no detectable improvement in motor or sensory function or change in lesion size.Citation16 In this animal model, Giacci et al calculated 2.6 J/cm2 reached the spinal cord with each treatment. This is within the range of reported beneficial doses; yet, it was not effective. Note that several studies have shown that LLLT radiant energy is almost completely absorbed in the first 1 mm of skin.Citation104,Citation105
A clinical example of this discrepancy has unfolded in the clinical trials for the treatment of stroke utilized in the NEST-1 and NEST-2 trials.Citation106,Citation107 LapchakCitation12 reported that the physical parameters of NILT in these studies may have delivered insufficient energy to cortical tissues to be effective. Therein, 808 nm NIR with energy densities of 0.9 J/cm2 was applied to the human scalp at multiple sites for a total of 40 minutes.Citation106,Citation107 Note that animal models of both stroke and TBI indicate NIR energy densities in the range of 0.9–36 J/cm2 yields significant biochemical and behavioral changes.Citation3,Citation4,Citation81,Citation87–Citation90,Citation93,Citation94 The concern raised from the NEST studiesCitation12 is that current clinical trials using LLLT to treat TBI may yield negative or inaccurate efficacy data, not because of the incapacity of NIR to invoke a change, but due to a dose error. Doses that are effective when directly applied to a monolayer of cellsCitation75,Citation82 or when penetrating 0.2 mm through the skull of a rodentCitation108 and the 5 mm through the full thickness of the mouse brain,Citation4,Citation81,Citation87,Citation88,Citation92–Citation94,Citation109 may be insufficient to penetrate to 20–30 mm into the human brain.
We have been utilizing relatively high power (10–15 W) lasers at the wavelengths of 810 and 980 nm in clinic to treat TBI with positive results.Citation3 The use of NIR in the treatment of stroke and of TBI are reviewed in a companion paper.Citation3 Skin is the first tissue encountered in the clinical application of NIR phototherapy and represents a barrier to effective penetration due to several factors. Human skin has multiple layers and, therefore, multiple interfaces. Each interface is a surface for scatter. In addition, each layer has different inherent optical properties.Citation97,Citation110 The epidermis, comprised in part of keratin, collagen, lipids, and melanin, has high absorption in the ultraviolet range, but also absorbs light in the infrared range of 600–1,100 nm.Citation110 The dermis, comprised in part of collagen, elastin, and proteoglycans, is of variable thickness and penetration varies as a result. Scattering is a predominate property of the dermis.Citation110 The dermis is also dense with blood vessels and the hemoglobin-rich blood therein. While hemoglobin has absorption peaks at 450, 550, and 600 nm,Citation97 it also absorbs photonic energy in the clinical NIR range of 800–1,100 nm.Citation110,Citation111 The NIR absorption of hemoglobin depends upon its oxygenation status, with carboxyhemoglobin having greater NIR absorption.Citation111 Altogether, NIR photonic energy must first overcome the hurdle of penetrating the skin to have an impact on deeper structures.
We have shown clinical improvement in patients with TBI utilizing high power NIR sources, but does high power NIR penetrate deeper and/or with greater fluence compared to LLLT? We have explored NIR penetration of tissues of clinical relevance or previously modeled to quantify the effective penetration at power densities ranging from the 50–200 mW levels used in LLLTCitation87,Citation88,Citation90–Citation94 to high power levels used in clinical studies of TBI treatment.Citation3 Specifically, NIR in the wavelengths often used in clinical studies of red and NIR photobiomodulation were utilized (650–670;Citation16,Citation100 810;Citation87,Citation93,Citation94 880;Citation100 980 nm112,Citation113). Power levels ranged from 50 to 200 mW to model LLLT and from 6 to 15 W to model high power NIR phototherapy. Specific tissues included sheep skin and human skin to model skin penetration based on issues raised in studies by Kolari and Airaksinen,Citation114 Bjordal et al,Citation8 and Esnouf et al.Citation104 Sheep head, with skin, skull, and brain intact, served as a model of human brain penetration.Citation3
Recent findings concerning NIR attenuation
Ethical considerations
Animal tissue was obtained from local slaughter facilities and no animals were sacrificed exclusively for these experiments. Human tissue was obtained from ScienceCare (Denver, CO, USA), a commercial agency which adheres to all federal and state legal requirements through the Uniform Anatomical Gift Act, and is accredited by the American Association of Tissue Banks. All guidelines for the ethical handling and disposal of human tissue were adhered to strictly. Ethics approval was not sought for the in vivo human tissue studies, because the authors themselves served as the subjects of the tissue experiments. Verbal informed consent was exchanged between the authors in the planning and preparation of these in vivo human tissue studies.
Ex vivo tissue studies
Sheep skin
Lamb heads were obtained within 12 hours of slaughter. Areas of skin were shaved and sections approximately 3×3 cm were excised. The thickness of the skin was measured with precision calipers. A recently calibrated light meter (Ophir-Spiricon LLC, North Logan, UT, USA) was positioned in a custom-made holder with a calibrated carriage to hold an NIR emitter at a fixed and measurable distance from the surface of the light meter. Several different NIR emitters were utilized in this phase of the study to explore the effects of frequency, power density, pulsing, and LED versus laser on the penetration of NIR. Each emitter was positioned in the carriage of the meter holder and set a fixed distance from the meter surface. The baseline light transmission through air at that distance was determined by five separate measurements. The skin sample was then interposed and NIR transmission through the skin sample was measured over five separate trials each lasting 10 seconds. Temperature readings were obtained using a laser digital sensor (Cen-Tec, Kunshan City, People’s Republic of China) from the surface facing the NIR emitter and the surface facing the light meter after each transmission trial. This procedure was repeated for all NIR emitters studied.
A 50 mW LED emitter at 810±20 nm was constructed to emulate commercially available NIR diode devices which are arranged in arrays and utilized in other studies of LLLT for the treatment of the human brain. This LED was constructed by arranging commercially available diodes in a concave metal faceplate to emit 50.4±5.0 mW. This custom device is referred to herein as “Custom LED”. Five commercially available NIR emitters were also evaluated. The In Light pad (In Light Wellness System, Albuquerque, NM, USA) has an array of LEDs emitting 650 and 880 nm light at 200 mW. The Eltech K-Laser 6D is a 6 W emitter (Eltech Srl, Treviso, Italy) with dual wavelengths of 670/970 nm. The LiteCure LT1000 (LiteCure LLC, Newark, DE, USA) is a 10 W adjustable laser NIR emitter with a dual wavelength of 810/980 nm and can be set to continuous or pulsed light emission. The Diowave 810 nm laser (Technological Medical Advancements, Inc, West Palm Beach, FL, USA) is adjustable up to 15 W, has a wavelength of 810 nm, and can deliver continuous or pulsed NIR. The Diowave 980 nm laser (Technological Medical Advancements, Inc) also is adjustable up to 15 W and can deliver continuous or pulsed NIR.
Penetration of sheep skin
Ex vivo studies of NIR penetration through fresh lamb skin revealed a marked decrease in power density through a skin thickness of only 2 mm (). The Custom 50 mW 810 nm LED did not appear to penetrate 2 mm of skin. Similarly, the commercial 0.2 W 650/800 nm LED system (In Light) did not show any detectable energy penetrating the 2 mm of sheep skin. When compared to the power density of penetration through 2 mm of air, the 6 W LED of wavelength 670/970 nm had an approximately 12%–20% penetration, predominately in the red light range of 670 nm. The 10 W combined 810/980 nm infrared laser (LiteCure) showed a power density drop of 91% across 2 mm of skin. The 15 W 810 nm laser (Diowave) demonstrated a 67% drop in power density, while the 15 W 980 nm laser (Diowave) demonstrated an 86% drop in power density across the same thickness of skin. All of these levels of penetration were statistically significant compared to the LED systems.
Table 1 Data on infrared light penetration of ex vivo skin samples
Using pulsed infrared light yielded much greater penetration. For example, 13.8% of the power density of a combined 810/980 nm infrared laser with a pulse frequency of 10 Hz penetrated 2 mm of skin compared to only 8.6% of the continuous wave light of similar parameters. The 15 W 810 nm laser with a pulse frequency of 10 Hz had a power density drop of 66% across 2 mm of skin. The 15 W 980 nm infrared laser with a pulse frequency of 10 Hz had only a 39% drop in power density across a similar skin thickness. This difference was not statistically significant with multiple comparisons, but a trend was evident.
One concern about higher-powered infrared light sources is the risk of tissue heating. Here, we observed the low power LEDs made no significant temperature change in the skin samples. The 10 W combined 810/980 nm and the 15 W 810 nm infrared lasers made no significant temperature change when keeping the emitter head in motion. Temperature increase with the 15 W 980 nm infrared laser was 1.33°C. Notably, temperature change using 10 Hz pulsing was not significant.
Animal skin serves as a useful model for human skin but differs from human skin in many ways. The density of hair follicles and melanin in the epidermis represent important differences which could be predicted to impact NIR penetration. We also studied NIR penetration in human skin samples obtained from a tissue bank.
Ex vivo human skin studies
The same protocol was used to measure light transmission through human skin. A full-thickness section of human skin measuring 15×15 cm square was prepared by removing all subcutaneous fat by dissection and isolating separate segments measuring 7×7 cm. The thickness of the human skin was measured with digital calipers. Different NIR emitters were again utilized. Each emitter was positioned in the carriage of the meter holder and set a fixed distance from the meter surface. The baseline light transmission through air at that distance was determined by five separate measurements. The skin sample was then interposed and light transmission through the skin sample was measured over five separate trials each lasting 10 seconds. As shown in , LEDs were positioned against the skin sample with an intervening sheet of clear plastic wrap. Temperature readings were obtained using the laser digital sensor from the surface facing the NIR emitter after each transmission trial. This procedure was repeated for all NIR emitters studied.
Figure 2 Ex vivo human skin studies illustrated.

Penetration of human skin
Ex vivo human skin was utilized to study transmission of NIR photonic energy (, ). The Custom 0.05 W 810 nm LED did not appear to penetrate 1.9 mm of human skin. The commercial 0.2 W 650/880 nm In Light LED system delivered 0.01±0.002 W across 2 mm of air. No energy could be detected penetrating the 1.9 mm thickness of human skin with this device. Energy from the 10 W combined 810/980 nm infrared laser could be detected penetrating 1.9 mm of human skin and a power density drop of 89% across 1.9 mm of human skin was noted with 0.994 W penetrating the tissue. The 15 W 810 nm laser demonstrated an 83% drop in power density across a similar thickness of human skin with 2.008 W penetrating the tissue. The 15 W 980 nm laser was not tested on human skin. Photonic energy penetration of the two laser devices was statistically different from that of any LED device (which showed no photonic energy transmission through human skin). Penetration of human skin also was statistically different between the two laser devices (P<0.000005).
Table 2 Data on infrared light penetration of ex vivo human skin samples
For NIR to penetrate to the brain and impact neurological injury (stroke or TBI) or disease, it must be able to reach the depths of the brain with sufficient fluence to trigger molecular events.Citation3 It is not sufficient to reach the cortical surface, as neurological disease involves areas beyond the cortical surface. Indeed, NIR photonic energy may need to reach depths of 3–7 cm. For example, TBI most frequently involves the ventral surface of the frontal lobe, and the anterior and medial temporal lobes.Citation32 Parkinson’s disease involves the substantia nigra and the striatum, both located 4–7 cm from the surface of the scalp. Stroke can often involve the cortical surface, but also impact deeper structures of the brain. In fact, this issue of penetration may have been at the root of the failure of NEST-3Citation13,Citation106,Citation115 concerning the clinical efficacy of NIR phototherapy for stroke. In that trial, the inclusion criteria concerning stroke locations were broadened to include strokes with deeper areas of involvement.Citation3
Skull and brain
Lamb heads were obtained within 12 hours of slaughter. Lamb heads were bisected with a coronal cut (ear to ear) and the portion of parietal and occipital skull and skin with underlying brain were utilized for NIR transmission studies. The same protocol was used to measure light transmission through the skull, overlying tissue, underlying tissue, and brain tissue of lamb heads. The distance of the linear trajectory though the partial lamb head was measured with digital calipers. The carriage of the meter holder was adjusted to allow sufficient clearance to interpose the large piece of tissue between the NIR emitter and the meter. The baseline light transmission through air at that distance was determined by five separate measurements. As shown in , the head sample was then interposed with the skull facing the NIR emitter and the exposed cut surface of brain in contact with the light meter. Light transmission through the sample was measured over five separate trials each lasting 10 seconds. Temperature readings were obtained with a laser digital sensor from the skin surface facing the NIR emitter and the brain surface facing the light meter after each transmission trial. This procedure was repeated for all NIR emitters studied, including the Custom 0.05 W LED device and five commercially available NIR emitters (the In Light 650 and 880 nm emitter, the Eltech K-Laser 6D 6W 670/980 nm emitter, the LiteCure LT1000 10 W adjustable laser NIR emitter with a dual wavelength of 810/980 nm, the Diowave laser adjustable up to 15 W with a wavelength of 810 nm, and the Diowave 980 nm laser adjustable up to 15 W).
Figure 3 Ex vivo brain tissue studies illustrated.
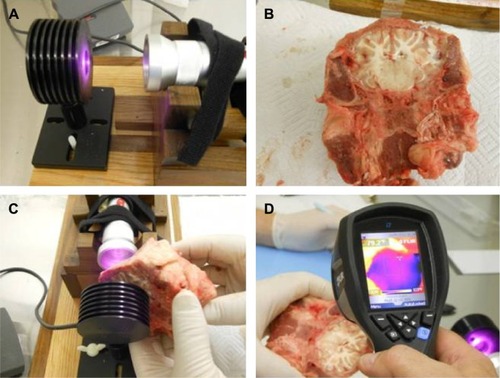
Penetration 3 cm into brain
Ex vivo studies of NIR penetration through 3 cm of lamb skull, tissue, and brain in a segmented head revealed a profound decrease in power density which was related to wavelength and wattage (, ). The Custom 0.05 W LED did not penetrate 3 cm through either air or brain tissue. No detectible energy from the 0.2 W 650/880 (In Light) LED system could be detected at 3 cm through air or tissue. The 6 W LED (Eltech K-Laser 6D) showed a 99.995% drop in power density across 3 cm of tissue. When compared to the power density of penetration through 3 cm of air, the 10 W 810/980 nm (LiteCure) device showed a 99.65% drop in power density across 3 cm of skin, skull, and brain tissue. The 15 W 810 nm (Diowave) device in continuous (non-pulsed) NIR delivered 2.9% of the surface power density, while only 1.22% of the surface power density at 980 nm reached the 3 cm depth through brain tissue indicating that wavelength is indeed an important parameter in reaching deep tissues. Photonic energy penetration with the laser devices was statistically different from the LED devices; however at this depth, the laser devices did not differ significantly from each other using continuous NIR delivery. Using pulsed NIR emission settings, lower total fluency and heat is delivered to the skin surface, while energy delivered to deeper structures at a pulse peak is greater. Pulsed energy penetration to 3 cm was significantly different between the three laser devices (P<0.01).
Table 3 Data on infrared light penetration of ex vivo lamb skull, tissue, and brain
Temperature changes at the skull surface ranged from 0.2°C to 3°C. Temperature change in the brain was less than 1°C except when using the 980 nm 15 W laser in continuous emission setting. In the latter case, the temperature variation was quite large. These data show less temperature change using the pulsed setting regardless of wavelength and power.
NIR transmission through living tissue is likely different than through postmortem tissue for a number of reasons. First, cross-linking of proteins is an early and progressive event in death. Second, changes in interstitial fluids occur within hours of death. Third, the perfusion of dermis and deeper tissues in vivo creates scatter and refraction of NIR. Fourth, the flow of blood also disperses heat from the site of NIR application. Lastly, some authors have suggested that NO created at the site of irradiation and carried throughout the body in the blood is responsible for the beneficial effects of NIR phototherapy.Citation116 This effect could account for the clinical benefits seen in TBI if NIR does not penetrate to the depths of 3 cm or greater in living tissue. Jagdeo et al modeled NIR penetration in living human tissue by serving as their own author/volunteers.Citation117 We have replicated some of their work using a higher-powered NIR source. Tissues included hand, cheek, ear, and triceps to model the complex mixture of skin, bone, connective tissues, and other tissues found in the pathway of NIR en route to the brain.
In vivo human tissue studies
The in vivo penetration of NIR through human tissue was measured compared to NIR penetration of air. The Diowave 810 nm high power laser was the only NIR emitter utilized in this portion of the study. The power output was set at 13.5 W for a duration of 5 seconds. First, the thickness of two different human hands was measured with digital calipers. The passage of NIR through 25 or 30 mm of air was repeatedly measured (). Then, the passage of NIR across the equivalent distance of the palm of a human hand was repeatedly measured. Intervening anatomical structures included skin, tendon, bone, muscle, blood, and connective tissue. In a similar fashion, the thickness of subcutaneous tissue at the level of the triceps muscle was measured with digital calipers. The penetration of NIR through skin, blood, and muscle was compared to NIR penetration through the equivalent distance (20 mm) of air. Measurements were collected on three separate trials.
The penetration of NIR through the 9 mm thickness of human cheek was determined by enclosing the laser light emitter in cellophane and inserting the light emitter into the mouth against the internal surface of the cheek and the sensor was positioned against the external surface of the cheek. The emitter was set at 13.5 W and activated for 5 seconds with a pulse setting of 10 Hz. The protocol was repeated three times. Human structures which were thinner than 9 mm were also measured in an identical fashion, including the web of the human hand (7 mm) and the human ear (5 mm). The latter structure allowed measurement of NIR penetration through anatomical structures of skin, cartilage, connective tissue, and blood. These measurements were replicated three times.
Penetration in human tissue
In vivo human tissue studies were conducted to assess the impact of blood flow and the absence of postmortem protein cross-linking and other possible changes to the manner in which NIR penetrated tissue. Several different tissues were examined (). Only the 810 nm laser at an output setting of 13.5 W was utilized in this portion of the study. All reported values were statistically different from transmission through an equal distance of air. Pulsed NIR appeared to have greater penetration in living tissue, unlike the finding in postmortem tissue. Only 0.6% of continuous wave NIR energy passed through 2.5–3.0 cm of human hand, while 0.8% of pulsed NIR penetrated the same distance. Penetration also appeared to be greater in living tissues that contained bone (human hand) compared to a similar thickness of subcutaneous flesh. Over 0.8% of the NIR energy passed through 25 mm of human hand, while only 0.3% of NIR penetrated a similar depth of tissue with no bones (subcutaneous flesh). Also, cartilage (ear) appeared to allow greater energy transmission compared to a similar thickness of skin (hand web). Temperature change after 5 seconds was 2°C or less at the skin surface and dropped quickly possibly related to blood flow.
Table 4 Data on infrared light penetration of in vivo human tissue
Attenuation of NIR energy
Given the much greater distance involved in delivering NIR in effective doses to the human brain, we examined NIR penetration to 3 cm depth through the skin, skull, and brain of ex vivo sheep head. As could be anticipated from the penetration studies of skin, the low power NIR emitters showed no evidence of penetration to this depth. The 6 W NIR emitter transmitted less than 1/10 of 1% of its surface energy through 3 cm of skull and brain. It did demonstrate that red light (670 nm) transmitted better – 22% of the red light energy that traversed 3 cm of air was able to penetrate 3 cm of tissue. High-powered NIR lasers delivered between 0.5% and 3.0% of their energy to the depth of the brain. Notably, penetration to this depth was significantly better using 810 nm NIR than it was when using 980 nm NIR. This is advantageous given the stronger evidence of 810 nm light being effective in photomodulation of neurological function.Citation87,Citation88,Citation91–Citation94
These tissue studies indicate NIR energy penetrates to depths of 3 cm whether in ex vivo or living tissue; however, sufficient energy must be delivered to the skin surface. The 0.2 and 0.5 W NIR diodes yielded no detectable energy at 3 cm depth. High-powered NIR lasers were able to deliver between 0.5% and 3.0% of their energy to the depth of 3 cm in living or postmortem tissue, with living tissue absorbing more energy over the intervening distance. Based on the power density of 55–81 J/cm2 delivered to the skin of patients in our clinical casesCitation3,Citation118 (using a 10–15 W NIR, 810/980 nm, or 810 nm laser), then the expected power density in the human brain at 3 cm depth would be in the range of 0.8–2.4 J/cm2 – precisely in the range shown to induce neurological benefit in animal models.Citation4,Citation81,Citation87,Citation88,Citation92–Citation94 The safety of NIR exposure at high doses has been explored in animal modelsCitation119–Citation121 and in humans.Citation3,Citation55,Citation115
Putting penetration in perspective
One of the earliest studies of NIR penetration and human skin examined transmittance of 633 nm light through progressively thicker sections of human skin.Citation102 Penetration through 0.4 mm of epidermis was 78% for 633 nm light and at 2 mm, the energy had dropped to approximately 5% of the incident 633 nm light. Later, the same group found no penetration of infrared light beyond 3 mm using the same light sources.Citation114 Bjordal et alCitation8 also concluded that 90% of the energy from 632 nm laser is lost in the skin. NIR at 820 nm has slightly greater skin penetration. Approximately 89% of 820 nm light will penetrate 0.4 mm of epidermis and about 13.5% traverses 2 mm of skin,Citation102 but 0% reaches a depth of 3 mm.Citation114 Others found 80% of the energy from an 820 nm source is lost in the skin.Citation8 Human skin also was examined by Esnouf et alCitation104 using an 850 nm continuous light source at 100 mW. They reported 34% of the light from the source could penetrate 0.784 mm.Citation104 The 904 nm laser may have greater skin penetration.Citation8 Using rat skin of an unspecified thickness, Joensen et alCitation113 found 20% of the 810 nm light from a 200 mW source penetrated skin. Our results show considerably less penetration by low level NIR. We found energy from a 50 mW 810 nm LED did not penetrate 2 mm of human or sheep skin. No energy could be detected penetrating either human skin or sheep skin from a commercially available 0.2 W LED (650+880 nm). In contrast, 9% of the energy from the 10 W combined 810/980 nm continuous wave infrared laser passed through 2 mm of skin (human or sheep). The 15 W 810 laser in continuous mode delivered 33% of its energy through 2 mm of skin. While longer wavelength is typically associated with greater penetration, we found that only 14% of the energy from a 15 W 980 nm laser in continuous mode penetrated 2 mm of (sheep) skin. These data are consistent with the transmission properties reported for skin, which has relatively good transmission in the range of 810 nm and somewhat less transmission at 980 nm.Citation122 Using pulsed infrared light yielded much greater penetration. For example, 41% of the power density of a combined 810/980 nm infrared laser with a pulse frequency of 10 Hz penetrated 1.9 mm of human skin compared to only 11% of the continuous wave light of similar parameters. The 15 W 810 nm laser with a pulse frequency of 10 Hz showed 69% of the energy penetrated 1.9 mm of human skin compared to only 17% of the continuous wave light of similar parameters.
Thicker segments of tissue have been studied by several groups. Penetration through mouse skull (0.2 mm)Citation108 and overlying skin with 800–810 nm light from an LLLT emitter ranges from 6.3%Citation95 to 46%.Citation109 Penetration through the entire depth of a mouse brain (3–5 mm) is reportedly 1%–4%.Citation109 Byrnes et alCitation122 examined penetration of 810 nm continuous light from a 150 mW source through skin, muscle, bone, and spinal cord of the rat (~24 mm). They found 6% of the energy penetrated these tissues. Similarly, Giacci et alCitation16 measured photonic energy transmission through an unspecified thickness of skin and muscle overlying the spinal column of the rat and found 6.6% of 670 nm light from a 0.5 W emitter penetrated this distance. Penetration of 830 nm light was slightly greater at 11.3%.Citation16 Penetration of these same wavelengths from the skin of the rat head to the optic nerve was estimated at 0.1%.Citation16 Hudson et alCitation112 precisely measured photonic energy at varying depths of bovine muscle using a 1 W 808/980 nm emitter. They found 808 nm light had greater penetration, despite the premise that longer wavelengths travel further. Indeed, the attenuation of 808 nm light at 3 cm was 99%, quite similar to the findings we describe here in both living human hand and the more heterogenous media of the ovine head.
In living human hand (25 mm), Jagdeo et alCitation117 found only 0.01%–0.09% of 830 nm light from a 0.5 W emitter penetrated this distance. We found that 0.6% of 810 nm light from a higher-powered source (10 W) was able to penetrate 25 mm of skin, bone, and soft tissue. This represents at least a sixfold increase in penetration compared to previous work with low power emitters.
Fitzgerald et alCitation111 modeled light penetration into the brain for a 670 nm and a 1,064 nm LED light source emitting 28 mW/cm2. Using a diffusion simulation computer model with assumptions of 10 mm of overlying scalp and skull, they derived that 2.5 mW/cm2 for 670 nm light and 13 mW/cm2 for 1,064 nm light would reach the surface of the cerebral cortex.Citation111 They further derived that at the center of the brain (10 mm skull +46 mm brain =56 mm), power density would be approximately 1.2×10−11 W/cm2 for 670 nm light and 1.4×10−7 W/cm2 for 1,064 nm light.Citation111
Jagdeo et al also examined LLLT penetration through isolated human skull from cadaver and found it was limited with only 7.4% of the light from an 830 nm 0.5 W continuous LED device (Omnilux) penetrating the bone.Citation117 Moreover, they found that no more than 0.5% of NIR at 830 nm penetrated approximately 10 mm of frontal skull and overlying tissue in a cadaver model. Also, no detectable NIR penetrated the temporal bone and overlying tissue.Citation117 These studies of LLLT and the computer model indicate that photonic energy from LLLT emitters does not appear to deliver significant fluence to the depths required to treat the human brain.
Our studies of 3 cm of sheep skull, brain, and overlying soft tissue are the closest model of the clinical practice of NILT for TBI. Again, NIR from devices generating less than 1 W could not be detected at this depth. The energy from a 6 W LED system showed a 99.995% drop across 3 cm of tissue. In contrast, 0.14% of the energy from a 10 W 810/980 nm device penetrated 3 cm of tissue. At 15 W, an 810 nm emitter in continuous mode delivered 1.26% of the surface power density and 0.80% of the 980 nm device emissions reached the 3 cm depth through brain tissue. Using pulsed NIR emission settings, a lower overall power density was delivered to the surface; however, similar penetration was achieved.
Some have suggested that NIR has a dosing window and have reported a bimodal response curve, such that high-dose NIR could be detrimental;Citation4,Citation5 however, others have shown a dose-dependent response to NIR with no detrimental effects at higher doses.Citation80
The penetration data mentioned and the data presented herein challenge the presumption that only LLLT protocols spanning weeks or months would possibly be clinically effective. For example, using a 0.5 W 830 nm LED, Jagdeo et alCitation117 estimated the power density reaching the cerebral cortex based on their model was 3 mW/cm2, which equates to a fluence of 0.0064 J/cm2 – 1/140th of the minimum thought to be necessary for ideal photobiomodulation.Citation5 Anders and colleagues noted NIR penetrates 4 cm into human cadaver skin, skull, and brain using a 5 W laser (JJ Anders, personal communication, January 13, 2015). From our data, we estimate that in our clinical applications of high-powered NIR lasers,Citation3 we are delivering 0.64–1.95 J/cm2 to a depth of 30 mm. This is 100-fold greater fluence than that delivered by an LED system, but within the range of fluence shown to have beneficial biological effects.
Conclusion
Extensive research has shown the fluence within the range of 0.9–15.0 J/cm2 is most effective in activating the biological processes involved in reversing or mitigating the pathophysiological effects of TBI. The attenuation of NIR energy as it passes through tissue has been examined in computer simulations, animal tissue, and human tissue. NIR penetration in the human brain is subject to attenuation by multiple tissues (skin, skull, dura, blood, cerebrospinal fluid) and multiple interfaces which scatter, absorb, and reflect the NIR to varying degrees. We have shown through the use of higher wattage NIR lasers that we can deliver fluence at therapeutic levels to the depths of the brain without tissue heating or damage. The protocols have been applied in our clinic with excellent clinical results and no side effects.
Acknowledgments
The authors would like to acknowledge the technical assistance of Phillip Hardinger, DC, DABCO, and Mr Charles Vorwaller (Diowave Corporation/LiteCure Corporation). Artistic creation : Ms Taylor Tuteur.
Disclosure
Dr Larry Morries is CEO of the Neuro-Laser Foundation, a nonprofit foundation. He has a private practice in Lakewood, CO, USA.
Theodore A Henderson is president of The Synaptic Space, a medical consulting firm and president of Dr Theodore Henderson, Inc, a clinical service firm. He is also co-owner of Neuro-Luminance, a clinical service organization, president of the International Society of Applied Neuroimaging, and CFO of the Neuro-Laser Foundation.
References
- HyderAAWunderlichCAPuvanachandraPGururajGKobusingyeOCThe impact of traumatic brain injuries: a global perspectiveNeuro Rehabilitation200722534135318162698
- FeiginVLForouzanfarMHKrishnamurthiRGlobal Burden of Diseases Injuries, and Risk Factors Study 2010 (GBD 2010)GBD Stroke Experts GroupGlobal and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010Lancet2014383991324525424449944
- MorriesLDCassanoPHendersonTATreatments for traumatic brain injury with emphasis on transcranial near infrared laser phototherapyNeuropsychiatr Dis Treat2015
- ChungHDaiTSharmaSKHuangYYCarrollJDHamblinMRThe nuts and bolts of low-level laser (light) therapyAnn Biomed Eng201240251653322045511
- HuangYYChenACCarrollJDHamblinMRBiphasic dose response in low level light therapyDose Response20097435838320011653
- EnwemekaCSIntricacies of dose in laser phototherapy for tissue repair and pain reliefPhotomed Laser Surg200927338739319473073
- MesterEMesterAFMesterAThe biomedical effects of laser applicationLasers Surg Med19855131393982191
- BjordalJMCouppéCChowRTTunérJLjunggrenEAA systematic review of low level laser therapy with location-specific doses for pain from chronic joint disordersAust J Physiother200349210711612775206
- BasfordJRSheffieldCGHarmsenWSLaser therapy: a randomized, controlled trial of the effects of low-intensity Nd:YAG laser irradiation on musculoskeletal back painArch Phys Med Rehabil199980664765210378490
- YangZWuYZhangHLow-level laser irradiation alters cardiac cytokine expression following acute myocardial infarction: a potential mechanism for laser therapyPhotomed Laser Surg201129639139821348574
- TubyHMaltzLOronUInduction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heartLasers Surg Med201143540140921674545
- LapchakPATaking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational scienceAnn Med201042857658621039081
- LamplYZivinJAFisherMInfrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1)Stroke20073861843184917463313
- HackeWSchellingerPDAlbersGWNEST 3Committees and InvestigatorsTranscranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trialStroke201445113187319325293665
- LaveryLAMurdochDPWilliamsJLaveryDCDoes anodyne light therapy improve peripheral neuropathy in diabetes? A double-blind, sham-controlled, randomized trial to evaluate monochromatic infrared photoenergyDiabetes Care200831231632117977931
- GiacciMKWheelerLLovettSDifferential effects of 670 and 830 nm red near infrared irradiation therapy: a comparative study of optic nerve injury, retinal degeneration, traumatic brain and spinal cord injuryPLoS One201498e10456525105800
- VeechRLValeriCRVanItallieTBThe mitochondrial permeability transition pore provides a key to the diagnosis and treatment of traumatic brain injuryIUBMB Life201264220320722241645
- BarkhoudarianGHovdaDAGizaCCThe molecular pathophysiology of concussive brain injuryClin Sports Med2011301334821074080
- PrinsMGrecoTAlexanderDGizaCCThe pathophysiology of traumatic brain injury at a glanceDis Model Mech2013661307131524046353
- ChengGKongRHZhangLMZhangJNMitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategiesBr J Pharmacol2012167469971923003569
- XiongYGuQPetersonPLMuizelaarJPLeeCPMitochondrial dysfunction and calcium perturbation induced by traumatic brain injuryJ Neurotrauma199714123349048308
- LifshitzJFribergHNeumarRWStructural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampusJ Cereb Blood Flow Metab200323221923112571453
- LifshitzJSullivanPGHovdaDAWielochTMcIntoshTKMitochondrial damage and dysfunction in traumatic brain injuryMitochondrion200445–670571316120426
- PandyaJDNukalaVNSullivanPGConcentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parametersFront Neuroenergetics20131851024385963
- VioliFMarinoRMiliteMTLoffredoLNitric oxide and its role in lipid peroxidationDiabetes Metab Res Rev199915428328810495477
- SinghINSullivanPGHallEDPeroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengersJ Neurosci Res200785102216222317510982
- MustafaAGSinghINWangJCarricoKMHallEDMitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicalsJ Neurochem2010114127128020403083
- SharmaPSuYABarryESGrunbergNELeiZMitochondrial targeted neuron focused genes in hippocampus of rats with traumatic brain injuryInt J Crit Illn Inj Sci20122317217923181213
- IpEYZanierERMooreAHLeeSMHovdaDAMetabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injuryJ Cereb Blood Flow Metab200323890091012902834
- HattoriNHuangSCWuHMAcute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injuryJ Nucl Med200445577578315136626
- ByrnesKRWilsonCMBrabazonFFDG-PET imaging in mild traumatic brain injury: a critical reviewFront Neuroenergetics201451324409143
- RajiCATarzwellRPavelDClinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: a systematic reviewPLoS One201493e9108824646878
- LiuYRCardamoneLHoganREProgressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the ratJ Nucl Med201051111788179521051651
- TokluHZHakanTBiberNSolakoğluSOğünçAVSenerGThe protective effect of alpha lipoic acid against traumatic brain injury in ratsFree Radic Res200943765866719468925
- CorneliusCCrupiRCalabreseVTraumatic brain injury: oxidative stress and neuroprotectionAntioxid Redox Signal201319883685323547621
- ZiebellJMMorganti-KossmannMCInvolvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injuryNeurotherapeutics201071223020129494
- KumarALoaneDJNeuroinflammation after traumatic brain injury: opportunities for therapeutic interventionBrain Behav Immun20122681191120122728326
- FinnieJWNeuroinflammation: beneficial and detrimental effects after traumatic brain injuryInflammopharmacology201321430932023296919
- RamlackhansinghAFBrooksDJGreenwoodRJInflammation after trauma: microglial activation and traumatic brain injuryAnn Neurol201170337438321710619
- HallEDBryantYDChoWSullivanPGEvolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methodsJ Neurotrauma200825323524718352837
- MettingZCerlianiLRödigerLAvan der NaaltJPathophysiological concepts in mild traumatic brain injury: diffusion tensor imaging related to acute perfusion CT imagingPLoS One201385e6446123704986
- LekerRRShohamiECerebral ischemia and trauma – different etiologies yet similar mechanisms: neuroprotective opportunitiesBrain Res Brain Res Rev2002391557312086708
- NaviauxRKMetabolic features of the cell danger responseMitochondrion20141671723981537
- LinAPLiaoHJMerugumalaSKPrabhuSPMeehanWP3rdRossBDMetabolic imaging of mild traumatic brain injuryBrain Imaging Behav20126220822322684770
- Boussi-GrossRGolanHFishlevGHyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury – randomized prospective trialPLoS One2013811e7999524260334
- DeKoskySTBlennowKIkonomovicMDGandySAcute and chronic traumatic encephalopathies: pathogenesis and biomarkersNat Rev Neurol20139419220023558985
- LyeTCShoresEATraumatic brain injury as a risk factor for Alzheimer’s disease: a reviewNeuropsychol Rev200010211512910937919
- SundmanMHHallEEChenNKExamining the relationship between head trauma and neurodegenerative disease: A review of epidemiology, pathology and neuroimaging techniquesJ Alzheimers Dis Parkinsonism20144pii137
- McKeeACCantuRCNowinskiCJChronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injuryJ Neuropathol Exp Neurol200968770973519535999
- HutsonCBLazoCRMortazaviFGizaCCHovdaDChesseletMFTraumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquatJ Neurotrauma20112891783180121644813
- WongJCHazratiLNParkinson’s disease, parkinsonism, and traumatic brain injuryCrit Rev Clin Lab Sci2013504–510310624156652
- RyuJHorkayne-SzakalyIXuLThe problem of axonal injury in the brains of veterans with histories of blast exposureActa Neuropathol Commun20142115325422066
- Mochizuki-OdaNKataokaYCuiYYamadaHHeyaMAwazuKEffects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissueNeurosci Lett2002323320721011959421
- RojasJCGonzalez-LimaFLow level light therapy of the eye and brainEye Brain201134967
- LapchakPAWeiJZivinJATranscranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbitsStroke20043581985198815155955
- KaruTIKolyakovSFExact action spectra for cellular responses relevant to phototherapyPhotomed Laser Surg200523435536116144476
- Wong-RileyMTLiangHLEellsJTPhotobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidaseJ Biol Chem200528064761477115557336
- PassarellaSHe-Ne laser irradiation of isolated mitochondriaJ Photochem Photobiol B1989346426432507765
- PastoreDGrecoMPassarellaSSpecific helium-neon laser sensitivity of the purified cytochrome c oxidaseInt J Radiat Biol200076686387010902741
- YuWNaimJOMcGowanMIppolitoKLanzafameRJPhotomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondriaPhotochem Photobiol19976668668719421973
- KaruTIPiatibratLVTiflovaOANikogosianDNSpecificity of the lethal and mutagenic actions of pico-second laser pulses of 532-nm wavelengthRadiobiologiia1988284499502 Russian3047771
- KaruTIMitochondrial signaling in mammalian cells activated by red and near-IR radiationPhotochem Photobiol20088451091109918651871
- FujimakiYShimoyamaTLiuQUmedaTNakajiSSugawaraKLow-level laser irradiation attenuates production of reactive oxygen species by human neutrophilsJ Clin Laser Med Surg200321316517012828853
- LiangHLWhelanHTEellsJTWong-RileyMTNear-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicityNeuroscience2008153496397418440709
- MuiliKAGopalakrishnanSEellsJTLyonsJAPhotobiomodulation induced by 670 nm light ameliorates MOG35–55 induced EAE in female C57BL/6 mice: a role for remediation of nitrosative stressPLoS One201386e6735823840675
- KaruTICellular mechanism of low power laser therapy: new questionsSimunovicFLasers in Medicine and Dentistry3RijekaZ Vitgraf200379100
- KobariMFukuuchiYTomitaMTanahashiNTakedaHRole of nitric oxide in regulation of cerebral microvascular tone and autoregulation of cerebral blood flow in catsBrain Res199466722552627697363
- LeungMCLoSCSiuFKSoKFTreatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1Lasers Surg Med200231428328812355575
- BrondonPStadlerILanzafameRJA study of the effects of phototherapy dose interval on photobiomodulation of cell culturesLasers Surg Med200536540941315880587
- MoriyamaYMoriyamaEHBlackmoreKAkensMKLilgeLIn vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imagingPhotochem Photobiol20058161351135516076245
- D’AngioCTFinkelsteinJNOxygen regulation of gene expression: a study in oppositesMol Genet Metab2000711–237138011001829
- ChenACAranyPRHuangYYLow-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblastsPLoS One201167e2245321814580
- HäckerHKarinMRegulation and function of IKK and IKK-related kinasesSci STKE20062006357re1317047224
- VaccaRAMarraEQuagliarielloEGrecoMActivation of mitochondrial DNA replication by He-Ne laser irradiationBiochem Biophys Res Commun199319527047098373408
- GrecoMVaccaRAMoroLHelium-Neon laser irradiation of hepatocytes can trigger increase of the mitochondrial membrane potential and can stimulate c-fos expression in a Ca2+-dependent mannerLasers Surg Med200129543344111891731
- KushibikiTHirasawaTOkawaSIshiharaMRegulation of miRNA Expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT)Int J Mol Sci2013147135421355823807510
- ZhangYSongSFongCCTsangCHYangZYangMcDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red lightJ Invest Dermatol2003120584985712713592
- EellsJTWong-RileyMTVerHoeveJMitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapyMitochondrion200445–655956716120414
- SzymanskaJGoralczykKKlaweJJPhototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretionJ Physiol Pharmacol201364338739123959736
- von LedenRECooneySJFerraraTM808 nm wavelength light induces a dose-dependent alteration in microglial polarization and resultant microglial induced neurite growthLasers Surg Med201345425326323619903
- XuanWAgrawalTHuangLGuptaGKHamblinMRLow-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesisJ Biophotonics20148650251125196192
- FrankSOliverLLebreton-De CosterCInfrared radiation affects the mitochondrial pathway of apoptosis in human fibroblastsJ Invest Dermatol2004123582383115482467
- LubartREichlerMLaviRFriedmanHShainbergALow-energy laser irradiation promotes cellular redox activityPhotomed Laser Surg20052313915782024
- MirskyNKrispelYShoshanyYMaltzLOronUPromotion of angiogenesis by low energy laser irradiationAntioxid Redox Signal20024578579012470506
- SchwartzFBrodieCAppelEKazimirskyGShainbergAEffect of helium/neon laser irradiation on nerve growth factor synthesis and secretion in skeletal muscle culturesJ Photochem Photobiol B200266319520011960729
- MengCHeZXingDLow-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s diseaseJ Neurosci20133333135051351723946409
- OronAOronUStreeterJLow-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficitsJ Neurotrauma200724465165617439348
- OronAOronUChenJLow level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficitsStroke200637102620262416946145
- LapchakPATranscranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseasesExpert Rev Med Devices201291718322145842
- YipKKLoSCLeungMCSoSKTangCYPoonDMThe effect of low-energy laser irradiation on apoptotic factors following experimentally induced transient cerebral ischemiaNeuroscience201119030130621712070
- WuHMHuangSCVespaPHovdaDABergsneiderMRedefining the pericontusional penumbra following traumatic brain injury: evidence of deteriorating metabolic derangements based on positron emission tomographyJ Neurotrauma201330535236023461651
- XuanWVatanseverFHuangLTranscranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimenPLoS One201381e5345423308226
- WuQXuanWAndoTLow-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengthsLasers Surg Med201244321822622275301
- AndoTXuanWXuTComparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in micePLoS One2011610e2621222028832
- JenkinsPACarrollJDHow to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studiesPhotomed Laser Surg2011291278578722107486
- SteinerRLaser-tissue interactionsRaulinCKarsaiSLaser and IPL Technology in Dermatology and Aesthetic MedicineBerlin and HeidelbergSpringer-Verlag20112336
- ListerTWrightPAChappellPHOptical properties of human skinJ Biomed Opt2012179090901
- WanSParrishJAAndersonRRMaddenMTransmittance of non-ionizing radiation in human tissuesPhotochem Photobiol19813466796816458827
- HodeLThe importance of the coherencyPhotomed Laser Surg200523443143416144489
- NaeserMASaltmarcheAKrengelMAHamblinMRKnightJAImproved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reportsPhotomed Laser Surg201129535135821182447
- NawashiroHWadaKNakaiKSatoSFocal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative statePhotomed Laser Surg201230423123322047598
- KolariPJPenetration of unfocused laser light into the skinArch Dermatol Res198527743423444004332
- Franzen-KorzendorferHBlackintonMRone-AdamsSMcCullochJThe effect of monochromatic infrared energy on transcutaneous oxygen measurements and protective sensation: results of a controlled, double-blind, randomized clinical studyOstomy Wound Manage2008546163118579924
- EsnoufAWrightPAMooreJCAhmedSDepth of penetration of an 850 nm wavelength low level laser in human skinAcupunct Electrother Res2007321–2818618077939
- BashkatovANGeninaEAKochubeyVITuchinVVOptical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2,000 nmJ Phys D Appl Phys2005381525432555
- ZivinJAAlbersGWBornsteinNNeuroThera Effectiveness and Safety Trial-2 InvestigatorsEffectiveness and safety of transcranial laser therapy for acute ischemic strokeStroke20094041359136419233936
- StemerABHuisaBNZivinJAThe evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2Curr Cardiol Rep2010121293320425181
- ChoiJJPernotMBrownTRSmallSAKonofagouEESpatiotemporal analysis of molecular delivery through the blood-brain barrier using focused ultrasoundPhys Med Biol200752185509553017804879
- KhumanJZhangJParkJCarrollJDDonahueCWhalenMJLow-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in miceJ Neurotrauma201229240841721851183
- AndersonRRParrishJAThe optics of human skinJ Invest Dermatol198177113197252245
- FitzgeraldMHodgettsSVan Den HeuvelCRed/near-infrared irradiation therapy for treatment of central nervous system injuries and disordersRev Neurosci201324220522623492552
- HudsonDEHudsonDOWiningerJMRichardsonBDPenetration of Laser Light at 808 nm and 980 nm in Bovine Tissue SamplesPhotomed Laser Surg201331416316823441909
- JoensenJOvsthusKReedRKSkin penetration time-profiles for continuous 810 nm and Superpulsed 904 nm lasers in a rat modelPhotomed Laser Surg2012301268869423025702
- KolariPJAiraksinenOPoor penetration of infra-red and helium neon low power laser light into the dermal tissueAcupunct Electrother Res199318117218098894
- HuisaBNStemerABWalkerMGRappKMeyerBCZivinJANEST-1 and -2 investigatorsTranscranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2Int J Stroke20138531532022299818
- SamoilovaKABogachevaONObolenskayaKDBlinovaMIKalmykovaNVKuzminikhEVEnhancement of the blood growth promoting activity after exposure of volunteers to visible and infrared polarized light. Part I: stimulation of human keratinocyte proliferation in vitroPhotochem Photobiol Sci2004319610114743286
- JagdeoJRAdamsLEBrodyNISiegelDMTranscranial red and near infrared light transmission in a cadaveric modelPLoS One2012710e4746023077622
- HendersonTAMorriesLDSPECT perfusion imaging demonstrates improvement of TBI with transcranial near infrared laser phototherapyAdv Mind Body Med2015
- LapchakPAHanMKSalgadoKFStreeterJZivinJASafety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbitsStroke200839113073307818687999
- IlicSLeichliterSStreeterJOronADeTaboadaLOronUEffects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brainPhotomed Laser Surg200624445846616942425
- McCarthyTJDe TaboadaLHildebrandtPKZiemerELRichieriSPStreeterJLong-term safety of single and multiple infrared transcranial laser treatments in Sprague-Dawley ratsPhotomed Laser Surg201028566366720961232
- ByrnesKRWaynantRWIlevIKLight promotes regeneration and functional recovery and alters the immune response after spinal cord injuryLasers Surg Med200536317118515704098

