Abstract
Introduction
Since its discovery several decades ago, nerve growth factor (NGF) has been found to play roles in different areas, such as neurology, endocrinology, and immunology. There is some evidence linking NGF and psychiatry, including the role of NGF in subjects’ response to stress, the alteration of NGF in different emotional states, and the penetration of NGF across the blood–brain barrier under specific conditions. There are many inconsistent findings regarding the differences in NGF in patients with major depressive disorder (MDD) at the present time. The aim of our study was to clarify whether NGF levels are different in MDD compared with healthy controls (HCs).
Methods
We conducted a thorough literature search and compared peripheral NGF levels between MDD and HC through meta-analysis, and investigated possible confounding variables through meta-regression.
Results
Seven studies were brought into the current meta-analysis comparing peripheral NGF in MDD and HCs. The main result was that the NGF levels were significantly lower in MDD than in HCs and that this had an inverse correlation with mean age and disease severity. In addition, meta-analysis of four articles found that the peripheral NGF levels did not change significantly before and after treatment.
Conclusion
Our study highlights the significant differences in peripheral NGF levels in patients with MDD. However, further exploration of the dynamic changes in peripheral NGF along with the disease course, and specific studies investigating the correlation of NGF in the peripheral and CNS environments are still needed.
Introduction
Nerve growth factor (NGF), a regulator of the development of the neurologic system, was initially discovered by Cohen and Levi-Montalcini, who won the 1986 Nobel Prize for this amazing discovery.Citation1–Citation4 Since first being discovered in 1979, NGF has been proved to play a variety of roles in different areas, such as neurology,Citation5,Citation6 angiogenesis,Citation7 immunology,Citation8 urology,Citation9 and others. In recent decades, some researchers have tried to investigate the possible role of NGF in the psychiatric area.
Major depressive disorder (MDD) is one of the most severe psychiatric diseases in the world. It leads to massive financial burdens, increases the risk of suicide, and is complicated with other physical comorbidities. The etiology of MDD is believed to be heterogeneous, and the pathophysiology of MDD remains unclear to this day. It is believed that neurobiological alteration and neurodegeneration may play a role in the pathophysiology of MDD.
In recent studies, some links between the neurobiological characteristics of NGF and MDD were found. The first link was the role of NGF in subjects’ response to stress – both emotional and physiologic stress. This could be explored in two aspects. The first line of evidence is derived from theoretical inference. NGF has been proved to be a modulatory factor in the hypothalamic–pituitary–adrenal (HPA) axis and has been shown to have an effect on the maintenance of the neuroendocrine and immune systems.Citation10 In addition, abnormality of HPA axis function in MDD has been reported in many studies.Citation11 The second line of evidence is derived from animal studies, which have revealed decreasing levels of NGF in specific brain areas of different mouse models, including anxiety vulnerability, stress-induced illness, learned helplessness, and threatening treatment.Citation12–Citation15 All of those mouse models are believed to represent forms of the depression models. Another link is the alteration of NGF in different emotional states. For example, Emanuele et al found higher levels of NGF in subjects “in love” who were intensely focusing attention on a preferred individual and who were emotionally dependent on and craving emotional union with this beloved person.Citation16 Similar alterations were seen in different emotional states.Citation17–Citation20 The third link is the correlation of NGF levels in the peripheral environment and the central nervous system (CNS). This link is especially important because it supports the rationale for researchers investigating peripheral NGF levels rather than directly investigating NGF levels in the CNS. Some studies have indicated that NGF could cross the blood–brain barrier (BBB) under special conditions, although not so readily.Citation21,Citation22 This is important because in clinical settings, it is difficult for clinicians to directly check NGF levels in the human CNS, but they can easily investigate peripheral NGF levels, through blood drawing.
As mentioned above, there are increasing studies and articles discussing the relationship between NGF and depressive disorders. Repeated studies have compared the differences in peripheral NGF levels in patients with MDD and healthy controls (HCs),Citation23,Citation24 and others have investigated the effect of different treatments on the peripheral NGF levels in patients.Citation25,Citation26 However, many of the results have been conflicting and have brought confusion to the direction of further study. Among studies investigating the differences in peripheral NGF levels in patients with MDD and HCs, some revealed that NGF protein levels were significantly lower in the patients than in HCs.Citation22,Citation23,Citation25,Citation27–Citation29 Other reports have suggested that NGF levels were significantly higher in patients than in HCsCitation30 or that there was no significant difference between these two groups in terms of NGF protein or messenger (m)RNA levels.Citation24,Citation31,Citation32 At the same time, some other studies have discussed the changes before and after adequate treatment in patients with MDD; however, almost all the results in these studies revealed no statistically significant difference before and after treatment.Citation25,Citation28,Citation31,Citation33 These inconsistent findings might have resulted from various confounding factors, such as differences in study design, ethnicity, physical illness, age, sample size, sex distribution, medical histories, length of illness, previous psychotropic exposure, severity of disease, or sample sources (plasma, serum, or whole blood). In addition, the correlation between peripheral NGF protein levels and clinical parameters still remains unclear.
In order to clarify the role of NGF in MDD, we performed a meta-analysis investigating whether peripheral NGF levels are different in patients with MDD and in HCs, and whether the peripheral NGF levels in patients change before and after adequate treatment.
Materials and methods
Literature search and screening
A systematic article search, using the platform of PubMed at the National Library of Medicine, was conducted by two independent psychiatrists (YSC and KYT). If there was an inconsistent selection and lack of agreement, another senior psychiatrist (PYL) made the final judgment and decision. The search was performed using the key words “depression” OR “affective disorder” OR “mood disorder” OR “major depressive disorder” AND “NGF”, for all articles available by January 13, 2015. The limitation was those written in English. As in our previous report (Tseng et al, unpublished data, 2015), the search process in this meta-analysis was divided into two subgroups. Initially, all articles that included the keywords and met the limitation mentioned above were collected, and the titles and abstracts of all these articles were screened by YSC and KYT to determine whether they were potentially eligible for inclusion in this meta-analysis. When there was disagreement on eligibility, we reached agreement through consensus. All reports that were not related to the topics of NGF and MDD in humans were excluded here. Later, we screened all the selected articles using the inclusion criteria: (1) articles discussing comparisons of peripheral NGF protein levels in patients with MDD, as either comparisons of those in patients with MDD or those in HCs, or comparisons of those before and after adequate treatment; (2) articles on clinical trials in humans; and (3) articles discussing quantitative peripheral NGF protein. The exclusion criteria were (1) case reports or series; (2) nonclinical trials; (3) studies not performed in humans; and (4) studies not discussing peripheral NGF protein.
Finally, we subdivided and regrouped the studies into two categories: (1) studies relevant to comparisons of peripheral NGF levels in patients with MDD and HC; and (2) studies comparing the changes in peripheral NGF levels pre- and post-adequate treatment in patients with MDD. In addition, we further researched the reference articles listed in the review studies. The screening and search protocol is depicted in .
Data extraction
The primary outcome was set as the peripheral NGF levels, which were extracted from all the studies recruited in the final stage; all other clinical variables were also extracted, if possible. When those data were not available in the articles, we tried to contact the authors to acquire the original data. The Hamilton Depression Rating Scale (HAM-D) 17-itemCitation34 scores were used as the first variables of disease severity because the 17 HAM-D items were used most frequently in the studies recruited for our meta-analysis. If the HAM-D scores were not available, we chose the other scales as variables of the severity of disease.
Meta-analytic methods and data extraction
The effect size (ES), expressing the differences in NGF levels in each meta-analysis recruited, was set as the standardized mean difference based on Hedges’ adjusted g. In the meta-analysis studies selected, values greater than “0” indicated that the NGF protein level was (1) higher in patients with MDD than in HCs or (2) increased after adequate treatment. Because of the extremely wide range of peripheral NGF levels in the studies, we did not choose the means and standard deviations (SD) of the NGF levels in each study; rather, we tried to derive the ES from other statistical parameters, such as the t-value or P-value with the sample sizes. All the ESs of individual studies were synthesized using the random effects model in every meta-analysis in the current study.
All the meta-analytic procedures were performed on Comprehensive Meta-Analysis software, version 2 (Biostat, Englewood, NJ, USA). Two-tailed P-value <0.05 was considered statistically significant. We performed sensitivity analysis, to make sure no single study strongly influenced the analysis, through removal of each study and reanalysis of the overall effect in the left studies.
Using Q statistics, their related P-value, and the I2 statistic, we investigated the heterogeneity of each study recruited in current meta-analysis. In addition, we used meta-regression to examine the possible source of heterogeneity, by using the unrestricted maximum likelihood method.
We investigated publication bias using the funnel plot. Egger’s regression analysis was used to statistically test for the significance of any possible publication bias.Citation35
The subgrouping meta-analytic strategy
In order to further clarify the possible cause of the bias, we used specific meta-analytic procedures in different subgroups. At first, we chose those studies using subjects who were “drug-free”, either those who were drug-naïve or those who had undergone an adequate washout period. Second, we subdivided those articles, based on age and HAM-D score. We chose, as a cutoff point, a HAM-D score of 20.
Results
Studies included in each meta-analysis
After the initial screening, a total of 21 articles reached screening stage. Three of the 21 articles were excluded because of the unavailability of detailed data,Citation24,Citation26,Citation36 seven because of a lack of relevance to MDD,Citation23,Citation37–Citation42 and another three because of the use of genetic analysis.Citation43–Citation45 After this selection process, a total of eight articles remained for the current meta-analysis; we subdivided these eight articles into two subgroups, as mentioned above: (1) those comparing the peripheral NGF protein levels in patients with MDD and HCsCitation22,Citation25,Citation27–Citation31 (); and (2) studies comparing the changes in peripheral NGF levels pre- and post-adequate treatment in patients with MDDCitation25,Citation28,Citation31,Citation33 ().
Table 1 Summary of the study characteristics in the current meta-analysis
The main results of meta-analysis (1) studies comparing peripheral NGF levels in patients with MDD and in HCs
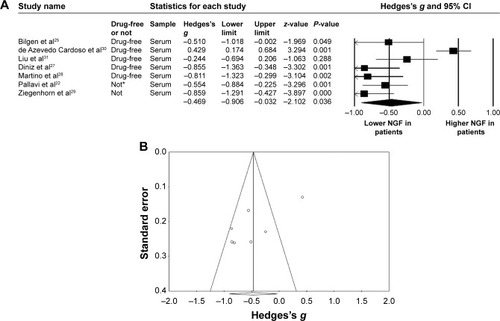
At this stage, we investigated the meta-analysis (1) studies, which compared only peripheral NGF levels in patients with MDD and in HCs. A total of 376 patients with MDD and 425 HCs were extracted from seven studies. The peripheral NGF protein levels were significantly lower in patients with MDD than in HCs (ES: −0.47, 95% confidence interval [CI]: −0.91 to −0.03, P=0.036) (). In addition, a significant heterogeneity within these studies was found (Q=49.1, df=6, I2=87.8%, P<0.001). Significant publication bias could also be detected, using Egger’s test (t=3.15, df=5, two-tailed, P=0.025) and visual examination of the funnel plot ().
Figure 2 (A) Forest plot of the meta-analysis of NGF in patients with MDD and in HCs. (B) Funnel plot of the meta-analysis of NGF in patients with MDD and in HCs.
Abbreviations: CI, confidence interval; HCs, healthy controls; MDD, major depressive disorder; NGF, nerve growth factor.
In this part of the meta-analysis, we used meta-regression to investigate any possible confounding factors within these studies. The meta-regression result revealed that mean age, proportion of females, and HAM-D score had confounding effects on peripheral NGF (slope =−0.01, P=0.0005; slope =0.02, P=0.0005; and slope =−0.05, P=0.00005, respectively).
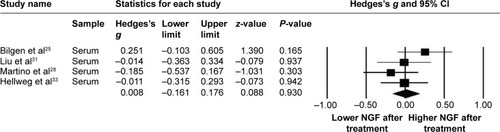
The main result of meta-analysis (2) studies comparing the changes in peripheral NGF levels pre- and post-adequate treatment in patients with MDD
Again, in meta-analysis (2), the procedures of treatment were divergent in the recruited studies, which included electroconvulsive therapy,Citation25 specific antidepressants,Citation28,Citation33 or unspecific antidepressants.Citation31 Within these studies, the duration of treatment ranged from 5 weeksCitation33 to 12 weeks.Citation28 We could not find a significant difference in peripheral NGF in patients before and after treatment (ES: 0.008, 95% CI: −0.16 to 0.18, P=0.93) ().
Meta-analysis of those drug-free or not
Next, our meta-analysis focused only on studies using subjects who were “drug-free”. Among these studies, that by Pallavi et alCitation22 contained some subjects who were in a “drug-free” state. Therefore, the data of the “drug-free” subjects were also extracted for our meta-analysis. The meta-analysis (1) result, when the patient selection was limited to “drug-free”,Citation22,Citation25,Citation27,Citation28,Citation30,Citation31 was statistically insignificant (ES: −0.36, 95% CI: −0.83 to 0.12, P=0.092).
The meta-analysis of different subgroups
Subgroups by age
Because of the heterogeneity of meta-analysis (1), as shown above, we subdivided the studies into subgroups of younger and older subjects, using mean ages <40 years and ≥40 years. We then ran the meta-analytic procedure again for comparison of peripheral NGF in patients with MDD and in HCs.
Here, we recruited three studies for the younger age subgroupCitation22,Citation25,Citation30 and three for the older age subgroup.Citation28,Citation29,Citation31 The peripheral NGF levels were significantly lower in MDD patients than in HCs in the older subgroup (ES: −0.63, 95% CI: −1.03 to −0.24, P=0.002) but not in the younger subgroup (ES: −0.20, 95% CI: −0.92 to 0.52, P=0.593). There was no significant publication bias for the older subgroup, using Egger’s test (P=0.897). The meta-regression of mean age revealed no significance in either subgroup (slope =0.03, P=0.09 [younger subgroup]; slope =−0.01, P=0.13 [older subgroup]).
Subgroups by HAM-D
In this part, we subdivided the studies recruited for the current meta-analysis into two subgroups: the nonsevere depression subgroup and the severe depression subgroup, using HAM-D score of <20 and ≥20, respectively.
We selected two articlesCitation27,Citation30 for the nonsevere depression subgroup and three for the severe depression subgroup.Citation25,Citation28,Citation31 In this part of the subgroup meta-analysis of comparisons of peripheral NGF in patients with MDD and HCs, there were significantly lower peripheral NGF levels in the MDD severe depression subgroup than in HCs (ES: −0.50, 95% CI: −0.83 to −0.18, P=0.002). However, the meta-analysis procedure could not be performed in the nonsevere depression subgroup because there were too few selected studies. No significant publication bias was detected using Egger’s test (P=0.297). Also, there was no significance detected in the meta-regression of the HAM-D scores in the severe depression subgroup (P=0.252).
Discussion
To our knowledge, this is the first study to conduct a thorough meta-analysis comparing peripheral NGF levels in patients with MDD and in HCs. We conducted a systematic literature search and reviewed all articles available through the PubMed online library. The main finding of the current meta-analysis was the significantly lower peripheral NGF levels in patients with MDD than in HCs. The meta-regression revealed a significant correlation between peripheral NGF levels and age and HAM-D scores. However, peripheral NGF levels did not change significantly before and after adequate treatment in these groups of patients.
In the current meta-analysis, we found significantly lower NGF levels in MDD patients than in HCs. This finding is similar to those for other neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF).Citation30,Citation46 Many studies have discussed the availability of peripheral biomarkers of mood disorder. However, all of those peripheral biomarkers, including BDNF and GDNF, encounter one problem – the BBB. Mood disorder is believed to be a disease of the CNS, which is isolated from the peripheral environment by the BBB. So, whether the differences in peripheral biomarkers could represent the actual changes in CNS biomarkers is a problem. However, as mentioned above, NGF has been believed to cross the BBB under special conditions, although not so easily.Citation21,Citation22 Therefore, it is reasonable for us to check NGF in the peripheral environment. Nevertheless, there is another problem to be resolved before concluding that the differences in peripheral NGF can represent the differences in NGF in the CNS environment, and this is our lack of direct evidence of the correlation of the dynamic changes in NGF levels in the peripheral and CNS environments. Without this, we could not conclude that the differences in peripheral NGF observed in our meta-analysis represented the differences in the CNS environment.
There was a controversial result in our study regarding the relationship between depression and NGF levels: in the meta-regression, the peripheral NGF levels had an inverse correlation with the HAM-D score; however, the peripheral NGF did not change significantly before and after adequate treatment. This phenomenon might be at least partially explained by the hypothesis of Liu et al who suggested that peripheral NGF levels might be more related to the stress of the mood state than a direct response to depressive moods.Citation31 As mentioned above, the NGF response to stress would increase in the acute state, through the compensatory effect of hyperactivities of the HPA axis and cortisol release;Citation47,Citation48 however, the related cortex atrophy under pathological chronic stress and chronic hyperactivity of the HPA axis, such as in depression, would induce long-term decreased NGF levels.Citation28,Citation31,Citation49,Citation50 This hypothesis could explain the main result of our meta-analysis: the peripheral NGF levels in pathological chronic stress, such as MDD, were decreased, but this was not reversed after adequate treatment. Therefore, decreased peripheral NGF might be the “result” of a reaction to stress in depression but not a pathophysiological or etiological factor of the depression itself.
Further, among the articles included in our meta-analysis, the report by de Azevedo Cardoso et al seemed to be an outlying result in the forest plot ().Citation30 We’ve tried to find out the possible cause of this inconsistent data. In fact, in the most of the other reports, the HCs were recruited through some physical surveillance to make sure they had no physical illness.Citation22,Citation25,Citation28,Citation29,Citation31 In addition, the blood samples were mostly drawn at early morning.Citation22,Citation25,Citation27,Citation28,Citation31 However, we could not retrieve such information from the report by de Azevedo Cardoso et al.Citation30 Therefore, we could not be sure that their HCs were both physically and psychiatrically healthy when their blood samples were drawn. In fact, repeated reports found that different physical illnesses could alter the NGF levels, such as metabolic disease or cardiovascular disease.Citation51,Citation52 Similarly, some evidence suggests that the presence of NGF plays an important role in the circadian rhythm.Citation48 There is one report showing that the peripheral NGF levels followed the ultradian rhythm.Citation53 At the same time, the mean disease severity of MDD patients in the report by de Azevedo Cardoso et al was milder than in others ().Citation30 One of the main results of our meta-analysis is the peripheral NGF levels have significantly inverse association with the disease severity.Citation30 All of the possible confounding factors, including disease severity, time of blood drawn, and physical health, would pose some possible confounding effect on the results of peripheral NGF levels.
Our study also had some limitations. First, there was significant publication bias in the studies recruited for the current meta-analysis, according to the funnel plot. Second, despite our efforts to investigate any possible confounding factors, there is still the risk of bias in the current study. Third, we did not take into consideration the possible effect of psychotropic agents, such as risperidone, citalopram, olanzapine, and haloperidol, on peripheral NGF – some studies have shown a possible effect of such psychotropic agents on the results.Citation54–Citation56 However, we could not perform meta-regression of the chlorpromazine equivalence or imipramine equivalence because only a few studies mentioned these data. We performed meta-analysis specifically of studies recruiting subjects that were “drug-free” and found statistical insignificance. However, this result might be related to small sample sizes. Also, as we mentioned in the discussion section, we did not take “acute versus chronic stress” into consideration in our meta-analysis. There was actually a lack of description of illness duration and recent psychosocial stress in our recruited studies, so we could not perform further statistical analysis of these clinical variables. Last, but not least, almost all the studies recruited in this meta-analysis were cross-sectional, so we could not clarify whether the NGF in MDD patients would change along with the disease course.
Conclusion
The current meta-analysis highlights the significantly lower peripheral NGF levels in patients with MDD than in HCs, with a significant inverse correlation between peripheral NGF levels and age and HAM-D scores. In addition, peripheral NGF in patients did not change significantly before and after adequate treatment. These findings add to the current knowledge of the role of NGF in MDD. However, we still need further exploration of the dynamic changes in peripheral NGF during the disease course, and specific studies investigating the correlation of NGF in the peripheral and CNS environments.
Disclosure
The authors report no conflicts of interest in this work.
References
- AloeLLevi-MontalciniRNerve growth factor induced overgrowth of axotomized superior cervical ganglia in neonatal rats. Similarities and differences with NGF effects in chemically axotomized sympathetic gangliaArch Ital Biol19791174287307550736
- Levi-MontalciniRCalissanoPThe nerve-growth factorSci Am197924066877472707
- AloeLLevi-MontalciniRNerve growth factor-induced transformation of immature chromaffin cells in vivo into sympathetic neurons: effect of antiserum to nerve growth factorProc Natl Acad Sci U S A197976312461250286308
- Levi-MontalciniRTrophic, tropic and transforming effects of the nerve growth factor on its target cellsBull Mem Acad R Med Belg19791344217228497507
- CovaceuszachSCapsoniSUgoliniGSpiritoFVignoneDCattaneoADevelopment of a non invasive NGF-based therapy for Alzheimer’s diseaseCurr Alzheimer Res20096215817019355851
- TuszynskiMHThalLPayMA phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer diseaseNat Med200511555155515852017
- ZacchignaSLambrechtsDCarmelietPNeurovascular signalling defects in neurodegenerationNat Rev Neurosci20089316918118253131
- BerryABindocciEAllevaENGF, brain and behavioral plasticityNeural Plast2012201278404022474604
- QuHCZhangWYanSLiuYLWangPUrinary nerve growth factor could be a biomarker for interstitial cystitis/painful bladder syndrome: a meta-analysisPLoS One201499e10632125181532
- AloeLAllevaEFioreMStress and nerve growth factor: findings in animal models and humansPharmacol Biochem Behav200273115916612076735
- HorowitzMAZunszainPAAnackerCMusaelyanKParianteCMGlucocorticoids and inflammation: a double-headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression?Mod Trends Pharmacopsychiatri20132812714325224896
- Schulte-HerbrüggenOChourbajiSMüllerHDifferential regulation of nerve growth factor and brain-derived neurotrophic factor in a mouse model of learned helplessnessExp Neurol2006202240440916914143
- Schulte-HerbrüggenOFuchsEAbumariaNEffects of escitalopram on the regulation of brain-derived neurotrophic factor and nerve growth factor protein levels in a rat model of chronic stressJ Neurosci Res200987112551256019360902
- DellaFPAbelairaHMRéusGZTreatment with tianeptine induces antidepressive-like effects and alters the neurotrophin levels, mitochondrial respiratory chain and cycle Krebs enzymes in the brain of maternally deprived adult ratsMetab Brain Dis20132819310523325329
- CirulliFAllevaEAntonelliAAloeLNGF expression in the developing rat brain: effects of maternal separationBrain Res Dev Brain Res20001232129134
- EmanueleEPolitiPBianchiMMinorettiPBertonaMGeroldiDRaised plasma nerve growth factor levels associated with early-stage romantic lovePsychoneuroendocrinology200631328829416289361
- SenSDumanRSanacoraGSerum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implicationsBiol Psychiatry200864652753218571629
- BersaniGIannitelliAFioreMAngelucciFAloeLData and hypotheses on the role of nerve growth factor and other neurotrophins in psychiatric disordersMed Hypotheses200055319920710985909
- KapczinskiFFreyBNKauer-Sant’AnnaMGrassi-OliveiraRBrain-derived neurotrophic factor and neuroplasticity in bipolar disorderExpert Rev Neurother2008871101111318590480
- HadjiconstantinouMMcGuireLDucheminAMLaskowskiBKiecolt-GlaserJGlaserRChanges in plasma nerve growth factor levels in older adults associated with chronic stressJ Neuroimmunol2001116110210611311335
- FridenPMWalusLRWatsonPBlood–brain barrier penetration and in vivo activity of an NGF conjugateScience199325950933733778420006
- PallaviPSagarRMehtaMSerum neurotrophic factors in adolescent depression: gender difference and correlation with clinical severityJ Affect Disord2013150241542323769609
- BarbosaIGHuguetRBNevesFSImpaired nerve growth factor homeostasis in patients with bipolar disorderWorld J Biol Psychiatry201112322823220923384
- XiongPZengYWuQCombining serum protein concentrations to diagnose schizophrenia: a preliminary explorationJ Clin Psychiatry2014758e794e80125191916
- BilgenAEBozkurt ZincirSZincirSEffects of electroconvulsive therapy on serum levels of brain-derived neurotrophic factor and nerve growth factor in treatment resistant major depressionBrain Res Bull2014104828724747833
- BrunoniARMachado-VieiraRZarateCAJrAssessment of non-BDNF neurotrophins and GDNF levels after depression treatment with sertraline and transcranial direct current stimulation in a factorial, randomized, sham-controlled trial (SELECT-TDCS): An exploratory analysisProg Neuropsychopharmacol Biol Psychiatry201556919625172025
- DinizBSTeixeiraALMachado-VieiraRTalibLLGattazWFForlenzaOVReduced serum nerve growth factor in patients with late-life depressionAm J Geriatr Psychiatry201321549349623570892
- MartinoMRocchiGEscelsiorANGF serum levels variations in major depressed patients receiving duloxetinePsychoneuroendocrinology20133891824182823507186
- ZiegenhornAASchulte-HerbrüggenODanker-HopfeHSerum neurotrophins – a study on the time course and influencing factors in a large old age sampleNeurobiol Aging20072891436144516879899
- de Azevedo CardosoTMondinTCWienerCDNeurotrophic factors, clinical features and gender differences in depressionNeurochem Res20143981571157824899094
- LiuXZhangTHeSNerve growth factor variations in patients with mood disorders: no changes in eight weeks of clinical treatmentNeuropsychiatr Dis Treat20141083584024868159
- OtsukiKUchidaSWatanukiTAltered expression of neurotrophic factors in patients with major depressionJ Psychiatr Res200842141145115318313696
- HellwegRZiegenhornAHeuserIDeuschleMSerum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatmentPharmacopsychiatry2008412667118311687
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- GrønliOStenslandGØWynnROlstadRNeurotrophic factors in serum following ECT: a pilot studyWorld J Biol Psychiatry200910429530119921971
- LiuXZhangTHeSElevated serum levels of FGF-2, NGF and IGF-1 in patients with manic episode of bipolar disorderPsychiatry Res20142181–2546024793757
- KandrataviciusLMonteiroMRAssiratiJACarlottiCGHallakJELeiteJPNeurotrophins in mesial temporal lobe epilepsy with and without psychiatric comorbiditiesJ Neuropathol Exp Neurol201372111029104224128677
- BanerjeeRGhoshAKGhoshBBhattacharyyaSMondalACDecreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: An analysis in human postmortem brainClin Med Insights Pathol2013611124031163
- KimYKNaKSHwangJAHigh insulin-like growth factor-1 in patients with bipolar I disorder: a trait marker?J Affect Disord2013151273874324012102
- FontenelleLFBarbosaIGLunaJVRochaNPSilva MirandaATeixeiraALNeurotrophic factors in obsessive-compulsive disorderPsychiatry Res2012199319520022494702
- RybakowskiJKPermoda-OsipASkibinskaMAdamskiRBartkowska-SniatkowskaASingle ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved?Hum Psychopharmacol2013281879023124710
- SecolinRBanzatoCEMellaLFSantosMLDalgalarrondoPLopes-CendesIRefinement of chromosome 3p22.3 region and identification of a susceptibility gene for bipolar affective disorderAm J Med Genet B Neuropsychiatr Genet2013162B216316823280964
- LesterKJHudsonJLTropeanoMNeurotrophic gene polymorphisms and response to psychological therapyTransl Psychiatry20122e10822832952
- CuiDZhangHYangBZVariation in NGFB is associated with primary affective disorders in womenAm J Med Genet B Neuropsychiatr Genet2011156B440141221294249
- Bocchio-ChiavettoLBagnardiVZanardiniRSerum and plasma BDNF levels in major depression: a replication study and meta-analysesWorld J Biol Psychiatry201011676377320334574
- FaureJUysJDMaraisLSteinDJDanielsWMEarly maternal separation followed by later stressors leads to dysregulation of the HPA-axis and increases in hippocampal NGF and NT-3 levels in a rat modelMetab Brain Dis2006212–318118816850259
- CirulliFAllevaEThe NGF saga: from animal models of psychosocial stress to stress-related psychopathologyFront Neuroendocrinol200930337939519442684
- ParianteCMMillerAHGlucocorticoid receptors in major depression: relevance to pathophysiology and treatmentBiol Psychiatry200149539140411274650
- AisaBGil-BeaFJMarcosBNeonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: involvement of the HPA axisPsychoneuroendocrinology200934101495150519505767
- ChaldakovGThe metabotrophic NGF and BDNF: an emerging conceptArch Ital Biol2011149225726321701997
- AliTKAl-GayyarMMMatragoonSDiabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injuryDiabetologia201154365766820957344
- BersaniGIannitelliAMassoniEUltradian variation of nerve growth factor plasma levels in healthy and schizophrenic subjectsInt J Immunopathol Pharmacol200417336737215461870
- HassanzadehPRahimpourSThe cannabinergic system is implicated in the upregulation of central NGF protein by psychotropic drugsPsychopharmacology (Berl)2011215112914121170518
- MartinottiGDi IorioGMariniSRicciVDe BerardisDDi GiannantonioMNerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: a reviewJ Biol Regul Homeost Agents201226334735623034254
- PillaiATerryAVJrMahadikSPDifferential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampusSchizophr Res20068219510616442781