Abstract
There are three mechanisms that may contribute to the health, performance, and safety problems associated with night-shift work: (1) circadian misalignment between the internal circadian clock and activities such as work, sleep, and eating, (2) chronic, partial sleep deprivation, and (3) melatonin suppression by light at night. The typical countermeasures, such as caffeine, naps, and melatonin (for its sleep-promoting effect), along with education about sleep and circadian rhythms, are the components of most fatigue risk-management plans. We contend that these, while better than nothing, are not enough because they do not address the underlying cause of the problems, which is circadian misalignment. We explain how to reset (phase-shift) the circadian clock to partially align with the night-work, day-sleep schedule, and thus reduce circadian misalignment while preserving sleep and functioning on days off. This involves controlling light and dark using outdoor light exposure, sunglasses, sleep in the dark, and a little bright light during night work. We present a diagram of a sleep-and-light schedule to reduce circadian misalignment in permanent night work, or a rotation between evenings and nights, and give practical advice on how to implement this type of plan.
Introduction
This review is intended for sleep and circadian-rhythms researchers, clinicians, and students. Others, such as shift workers, human resource managers, and professionals involved in shift-work health and safety issues, could skim most of it but concentrate on the sections “How to phase-shift human circadian rhythms” and “Recommended sleep-and-light schedule to reduce the circadian misalignment of night work.” We tried to make these sections stand alone to guide you to practical solutions.
Health problems associated with night-shift work
A cogent body of literature has established an association between night-shift work and diverse health problems. Relative to day workers, rotating or permanent night-shift workers have increased incidence of cardiovascular disease and its known risk factors;Citation1–Citation19 gastrointestinal,Citation15,Citation20–Citation24 metabolic,Citation5,Citation6,Citation8,Citation17,Citation25,Citation26 and reproductiveCitation27–Citation35 dysfunction; overweight and obesity;Citation2,Citation5,Citation14,Citation17,Citation36–Citation41 and cancer.Citation42–Citation48 In fact, the evidence in support of a causal link between shift work and cancer is sufficiently strong that the International Agency for Research on Cancer recently classified shift work as a “probable carcinogen.”Citation49 Data from some of these studies suggest that working as few as three night shifts per month for multiple years is associated with increased disease risk.Citation16,Citation26,Citation39,Citation42,Citation50
The mechanisms by which night-shift work contributes to these disorders are not well established, but there is accumulating evidence for at least three possible mechanisms: (1) circadian misalignment, (2) chronic partial sleep deprivation, and (3) light-induced melatonin suppression during night shifts.
Workers permanently on evening shifts get even more sleep than regular day workers.Citation51 Evening-shift workers usually have the same sleep schedule for work days and days off, and thus have even less circadian misalignment than regular day workers who go to bed and sleep later on weekends.Citation52 Although permanent evening-shift workers are not often studied, it appears that they do not have the health risks associated with shift work.Citation45 Nevertheless, the evening shift is not popular because it interferes with most family and social events.Citation53
Circadian misalignment
Circadian misalignment occurs when behaviors (eg, activity, sleeping, waking, eating, drinking, etc) are forced to occur at the wrong circadian phases relative to the internal physiological circadian rhythms. Animal models indicate that chronic shifts or inversion of the light/dark (LD) cycle, or the imposition of an LD cycle to which an animal cannot entrain, increases mortality,Citation54–Citation57 and the increased risk of mortality is most pronounced in older animals.Citation57 Chronic circadian misalignment in animals may increase mortality via accelerated progression of or susceptibility to disease states.Citation58–Citation68 In human studies, circadian misalignment is inevitably confounded with sleep deprivation, but data support the hypothesis that even brief circadian misalignment has adverse physiological sequelae.Citation69 There are currently no data to determine how the adverse health outcomes associated with shift work are related to the extent or duration of circadian misalignment.
Sleep deprivation
Chronic partial sleep deprivation is a well-established consequence of night-shift work. This occurs in part because sleep duration is dependent on the circadian phase at which it occurs.Citation70,Citation71 The longest sleep duration and the best sleep efficiency are obtained when sleep occurs around the temperature minimum (Tmin),Citation72 which is usually a few hours before waking. Because the circadian clocks of most night-shift workers do not align with daytime sleep,Citation73–Citation84 their Tmin usually remains in the nighttime (working) hours, and the duration of daytime sleep is curtailed because the circadian clock promotes wakefulness. Sleep duration when working night shifts is typically reduced by a few hours.Citation51,Citation85,Citation86 Experimental sleep restrictionCitation87–Citation90 as well as epidemiological data on habitual short sleepersCitation91–Citation109 indicate that short sleep duration increases risk for a number of the same diseases known to be associated with night-shift work. Recent data also suggest that several weeks of the combination of circadian misalignment and sleep restriction substantially impair normal human physiology.Citation110,Citation111
Melatonin suppression by light at night
A third mechanism by which night-shift work could increase disease risk is via light-induced suppression of endogenous melatonin while working night shifts. Melatonin is secreted at night, and its synthesis is suppressed in a dose-dependent manner by light; greater suppression is produced with brighter light.Citation112–Citation116 Because melatonin is an antioxidant and has oncostatic properties,Citation117,Citation118 and even normal room light (~200–300 lux) can suppress the synthesis of endogenous melatonin, shift workers could be at increased risk of cancer because their innate cancer defenses are compromised.Citation47,Citation119
There have been several studies showing that glasses with orange lenses that filter out short-wavelength light (blue and some green, to which the circadian system is most sensitive) can prevent light-induced melatonin suppression at night,Citation120–Citation123 and may also prevent suppression of circadian clock–gene rhythms.Citation123 This could be a way to reduce the cancer risk attributed to melatonin suppression and/or clock– gene disruption, although it cannot remove the risks due to circadian misalignment and partial chronic sleep deprivation. On the other hand, bright light during the night shift could preserve melatonin secretion by helping to delay circadian rhythms so that melatonin can be secreted during day sleep in a dark bedroom.
It is often taken for granted that melatonin levels will be suppressed during night work, because the circadian rhythms of most shift workers do not shift and most of their melatonin is secreted during the night shift. However, although some studies of real shift workers do report reduced levels of melatonin,Citation124–Citation126 many do not.Citation80,Citation81,Citation127–Citation129 This discrepancy could be due to methodological differences between studies (eg, spot urine samples versus 24-hour urine collection), lack of statistical power to detect a modest effect size, or other factors such as light-exposure history.
We found that melatonin suppression by light at night is affected by a person’s light history.Citation130 After a week that included about 4 hours of bright light daily (using sunlight and light boxes) there was less melatonin suppression from light at night than after a week in dim light (dark goggles when outside). Similarly, a study that included both indoor and outdoor workersCitation131 found less melatonin suppression from light at night if they had been exposed to more light during the prior 2 weeks. Another studyCitation132 found that light-induced melatonin suppression can be affected by light history over just the past 3 days.
Given that (1) the amount of melatonin suppressed by light at night depends on light intensity and (2) light history affects the amount of melatonin suppressed by light at night, melatonin suppression will be affected by workplace light, the weather, the photoperiod (season) and use of sunglasses. Thus, it is difficult to make predictions about the exact light level during night work that will suppress melatonin. In addition, as noted by Dumont et al,Citation129 because normal subjects show very large individual differences in nocturnal melatonin production,Citation133 it may be impractical to attempt to identify an absolute level of melatonin that could be considered “abnormally low.” Finally, ArendtCitation134 points out that the common adrenergic beta receptor-blocking drugs like atenolol and propranolol, which suppress melatonin, are not known to be carcinogenic.
Countermeasures for alertness, performance, and safety decrements during night-shift work
Nighttime decrements of alertness in shift workers, with concomitant performance impairment and subsequent safety hazards, are well documented and have been the subject of many previous review papers.Citation78,Citation86,Citation135–Citation140 Night work occurs when the circadian clock promotes sleep, and alertness reaches its circadian nadir. Daytime sleep duration is acutely reduced because the circadian clock promotes wakefulness. Chronic partial sleep deprivation, such as that which might occur over weeks or months in shift workers, may impair night-shift performance even when recent sleep has been sufficient, and this impairment is most pronounced during the nighttime.Citation141
There are a number of well-studied countermeasures that can be employed in an attempt to ameliorate some of the potentially dangerous side effects of shift work. These include improving night-shift alertness with stimulants, bright light, and naps, and improving daytime sleep duration with sedatives and exogenous melatonin. These countermeasures only provide some degree of symptomatic relief, because they do not address the underlying cause of the problem, which is circadian misalignment.
Stimulants
Caffeine is one of the most widely used stimulants in the world. Data from laboratoryCitation142–Citation152 and fieldCitation143 studies indicate that caffeine improves night-shift alertness and performance. However, despite these improvements, substantial impairments persist, and are pronounced late in the night shift near the circadian nadir of alertness. Another drawback of caffeine is that it disrupts daytime recovery sleep more than nighttime sleep,Citation153 and this sleep disruption is greater in older individuals.Citation154 Consequently, ingesting too much caffeine or consuming it too late in a night shift could further exacerbate daytime sleep difficulty.
The stimulants modafinil and the longer-acting armodafinil have been reported to attenuate night-shift sleepiness and improve alertness and performance both in healthy subjectsCitation155,Citation156 as well as real shift workers.Citation157,Citation158 However, sleepiness persists, especially late in the night shifts, about the time of the circadian nadir. For example, shift workers taking 150 mg armodafinil before night shifts were still pathologically sleepy (sleep latencies ≤ 5 minutes on the multiple sleep latency test) at 4:00, 6:00, and 8:00 am.Citation157 A further drawback of these medications is that they do not improve the duration or quality of daytime sleep. Healthy subjects taking modafinil prior to four consecutive night shifts had a daily daytime total sleep time (TST) that was similar to subjects taking placebo, which never exceeded 6 hours.Citation155 Shift workers taking modafinil had a very similar daytime TST of 5.9 hours at the conclusion of the study, while those taking armodafinil fared even worse, with an average daytime TST of only 5.4 hours and a sleep efficiency of 68%. This amount of daily sleep has been shown to produce degraded neurobehavioral performance with progressive worsening over several consecutive days.Citation159
Hypnotics and phase tolerance
Daytime sleep after night-shift work can be lengthened by the administration of sedatives or hypnotics.Citation160–Citation163 Despite this increase in daytime sleep, decrements in night-shift alertness and performance persist.Citation160–Citation163 It is a pervasive misconception that shift workers would be alert during night shifts if only they could get enough sleep during the day. However, obtaining adequate sleep during the day does not mean that night-shift alertness, performance, and safety will also be normalized. Millions of years of evolution have made us diurnal animals, programmed to be sleepy at night.
Some individuals are “phase tolerant”Citation164 in that after working at night they have the ability to sleep reasonably well during the daytime despite the fact that sleep occurs at the “wrong” circadian phase. In addition to individual differences in phase tolerance, both middle-agedCitation154,Citation165 and older adultsCitation166 are less phase tolerant than the young. However, being phase tolerant to sleeping during the day does not make one phase tolerant to being awake at night. In fact, the opposite may be true, since young subjects kept awake at night have decreased alertness and worse performance when compared to older subjects.Citation167,Citation168
Melatonin as a sleep promoter
Exogenous melatonin could benefit night-shift workers via two distinct mechanisms. Melatonin is a chronobiotic that can phase-shift the circadian clock.Citation169 Shift-work studies using melatonin for this purpose will be discussed later. Melatonin also makes people sleepy, especially at high doses and under some conditions, and thus could be used to promote daytime sleep.Citation170 Immediate-release oral melatonin,Citation171 sustained-release or multiple doses of oral melatonin,Citation172–Citation174 and daytime transdermal melatonin administrationCitation175 after a night shift have been reported to modestly increase sleep quantity and quality. This improvement is most pronounced late in the daytime sleep episodeCitation172,Citation175 and in subjects that would otherwise have poorer daytime sleep qualityCitation172 (eg, in subjects that are less phase tolerant). Results from several other studies are qualitatively similar, but did not reach statistical significance.Citation176–Citation178
Naps
Naps prior to or during night shifts have been shown to reduce night-shift sleepiness while improving alertness and performance.Citation144,Citation150,Citation179–Citation185 Despite this improvement, napping does not overcome the decrement in alertness that remains most pronounced at the circadian nadir.Citation144,Citation150,Citation180–Citation185 A study of unusually long naps (2 hours) during the night shift (1:00–3:00 or 3:00–5:00 am) made rapidly rotating factory workers slightly less sleepy at the end of the night shift and for a few hours after the end of work, but they were still very sleepy compared to ratings taken in the afternoon/ evening after their daytime sleep episodes.Citation186 There may not be an opportunity to nap so long, or at all, during a night shift due to the nature of the work itself (eg, too busy or stressful) or the work environment (eg, no comfortable and quiet place to nap).Citation187
In addition, napping during night shifts may be a concern because sleep inertia, the grogginess often felt upon awakening from sleep, is most severe at night.Citation188,Citation189 Studies of real shift workers on the job as well as in the laboratory have also shown that performance during a night shift is worse shortly after a nap compared to continued night-shift wakefulness.Citation179,Citation180 Therefore, time for recovery from sleep inertia after a nap (eg, 15–30 minutes) has to be built into the time taken away from work. Waking an individual (eg, resident physician or nurse) from a nighttime nap and expecting quickly made important decisions is a good recipe for a poor decision.
Bright light as a stimulant
A number of laboratory and field studies have shown that light exposure at night attenuates subjective and objective indices of sleepiness, while improving alertness and performance.Citation146,Citation152,Citation190–Citation202 Most of these studies used bright light of greater than 1000 lux, but the alerting effects of light may be present at room light levels of only 100–200 lux in subjects that have been adapted to dim light.Citation200 The alerting effects of light are more pronounced with blue light than longer-wavelength light.Citation203–Citation205 Although light exposure can acutely improve alertness at night, the trough of alertness at the circadian nadir is typically still present.Citation190,Citation199 The acute alerting effects of light are extinguished soon after light exposure ends.Citation192,Citation200 The alerting effects of light at night are not likely due to suppression of endogenous melatonin, because bright light augments alertness during the daytime, when circulating levels of melatonin are low and not altered by light exposure.Citation196,Citation206,Citation207
Summary of countermeasures
Combinations of countermeasures, including bright light and caffeine,Citation146,Citation152 as well as evening naps and caffeine,Citation143,Citation208 are generally more effective than an individual countermeasure for improving night-shift alertness. These countermeasures, along with education about sleep hygiene, circadian rhythms, and the importance of making sleep a high priority, are the basis of most fatigue-management plans, but they do not address the cause of the problem (ie, circadian misalignment). Although night-shift alertness can be improved to some extent by stimulants, this does not address chronic partial sleep deprivation or the impact of circadian misalignment. Likewise, daytime sleep duration can be moderately improved with sedatives and exogenous melatonin, but this does not improve night-shift alertness or attenuate circadian misalignment. Consequently, these countermeasures are most suitable for workers on rapidly rotating night-shift systems, or those working few and isolated night shifts, for whom phase-shifting the circadian clock is neither desirable nor achievable.
Phase-shifting the circadian clock for adaptation to night-shift work
How to phase-shift human circadian rhythms
The most reliable way to reduce the sleepiness, fatigue, impaired performance, safety problems, and, presumably, health risks of shift work is to reduce circadian misalignment. We have proposed the goal of phase-shifting (resetting) the circadian clock so that the sleepiest time of day, which usually occurs during nocturnal sleep and is conveniently marked by the temperature minimum (Tmin), delays at least as far to move out of the night shift and into the daytime sleep episode.Citation209 The Tmin typically occurs 2–3 hours before the end of sleep under normal, entrained conditions, but at the beginning of sleep when subjects free-run.Citation70,Citation210 Therefore, it appears that normal sleep quality and quantity can be obtained when the Tmin falls within this range, from the beginning of sleep to about 2 hours before the end of sleep.
In order to phase-shift the circadian clock, and therefore all the circadian rhythms (including the circadian rhythms of sleepiness, performance, temperature, and melatonin), we use two basic principles. One is that when the LD cycle is shifted, circadian rhythms gradually shift to realign or reentrain to the new LD cycle.Citation211 To phase-shift the circadian clock, it is important to create a strong shifted 24-hour LD cycle. This can be done by scheduling sleep in a very dark bedroom. The other principle is that the circadian clock can be shifted by pulses of light or by melatonin according to phase-response curves (PRCs). Thus, we can apply bright light and melatonin to enhance the phase shift produced by shifting the LD cycle using PRCs as guides for the optimal timing. For figures showing superimposed light and melatonin PRCs, see Revell and EastmanCitation212,Citation213 and Eastman and Burgess.Citation214
When shift workers choose to sleep before night work in the late afternoon and evening, their dark period is earlier than usual (advanced relative to the habitual sleep episode), creating an advanced LD cycle, which should reset the circadian clock earlier. When they choose to go to bed in the morning after night work, the dark period is later than usual, delayed, creating a delayed LD cycle, which should reset the circadian clock later. However, the circadian rhythms of real night-shift workers rarely shift to align with their new sleep schedules. One reason is that they do not maintain a consistently shifted LD cycle long enough. They are either rotated to a different shift and have to change their time of sleep or sleep at conventional times on days off. Most night-shift workers prefer to sleep after work in the morning and daytime hours, leaving the evening hours free for leisure activities with family and friends.Citation53 A few prefer to have the mornings free (eg, for farming or child care), and to sleep in the afternoon and evening before work. Many shift workers sleep at random times, napping and grabbing whatever sleep they can. This of course does not create the consistently shifted LD cycle necessary for phase-shifting the clock and reducing circadian misalignment.
Light PRCs show that light exposure in the evening and early night before about the time of the Tmin delays the circadian clock (ie, shifts it to a later time), while exposure late in the night and morning after the Tmin advances the circadian clock (shifts it earlier).
Controlling light and dark to reduce circadian misalignment
The circadian rhythms of shift workers who go to bed after night work do not usually delay to align with sleep because they are usually exposed to bright outdoor light on the way home from work, which “hits” the phase-advance portion of the light PRC. This outdoor light is a powerful phase shifter, because it is much more intense than indoor light, even on a cloudy day. Long ago, we proposed that shift workers wear dark sunglasses to attenuate outdoor light when it would oppose the desired phase shift.Citation215 In a simulated night-shift study we showed that wearing very dark sunglasses on the way home from the night shift can attenuate phase-advancing morning light and permit circadian rhythms to delay.Citation216
In another simulated night-shift study,Citation217 we tested 9-hour delays of the sleep (dark) episodes, which put sleep after night work, and 9-hour advances, which put sleep before night work. We used bright light exposure (3 hours of ~5000 lux), timed to facilitate or impede the phase shift by putting the light on the “right” or “wrong” portion of the PRC. For example, when sleep was advanced, facilitating bright light at the “right” time was timed to phase-advance the circadian clock, while conflicting bright light at the “wrong” was timed to phase-delay the circadian clock. With facilitating bright light, almost all of the subjects had their Tmin shift to within their sleep episodes. For the groups that were exposed to conflicting bright light, very few had Tmin that shifted that far. This study showed how bright light can inhibit the desired phase shift when it consistently hits the wrong part of the PRC.
In a subsequent simulated night-shift study,Citation218 we used facilitating light (3-hour exposures) during night shifts to promote delays and align the circadian clock with daytime sleep. We tested three light intensities: high (~5700 lux), medium (~1230 lux) and low (<250 lux). Subjects wore sunglasses on the way home and followed a regular daytime sleep schedule in very dark bedrooms. There was no significant difference between the groups who received high- and medium-intensity light; 100% of the subjects in the high-intensity group and 85% of those in the medium-intensity group delayed their Tmin into daytime sleep. In contrast, only 42% of those in the low-intensity group delayed that far. Therefore, very bright light was not necessary to reduce circadian misalignment significantly.
Boivin and JamesCitation219 set up light boxes in nursing stations and turned them on during the first 6 hours of 8-hour night shifts. Permanent night nurses were asked to spend as much time near the light boxes as their workload permitted, and were intermittently exposed to ~2000 lux. They wore sunglasses during the morning commute home, and had a fixed 8-hour daytime sleep schedule. The Tmin delayed to within the daytime sleep period for seven of the nine subjects; it delayed slightly too far for the other two subjects. Control subjects had to adhere to the same 8-hour daytime sleep schedule, but they were not exposed to the light boxes and did not wear sunglasses. Only three of the eight control subjects got their Tmin into day sleep. This study showed that (1) bright light can be used in a real shift-work situation and (2) adhering to a regular daytime sleep schedule was not enough for most subjects; bright light during the night shift and/or sunglasses for the commute home was necessary to ensure that their circadian clock delayed far enough.
Phase markers of the circadian clock
For many years, the circadian rhythm of body temperature, especially the lowest temperature each day – the Tmin – was the gold-standard marker used to infer the time of the circadian clock. More recently, the circadian rhythm of endogenous melatonin, especially the nocturnal rise – the dim-light melatonin onset (DLMO) – has become the preferred phase marker because of its reliability and ease of measurement from saliva samples.Citation220,Citation221 The DLMO usually occurs about 2–3 hours before bedtimeCitation222 and 6–8 hours before the Tmin.Citation200,Citation223–Citation230 In our lab, we routinely measure the DLMO and add 7 hours to estimate the time of the Tmin, because it is useful to visualize the phase position of the Tmin (the sleepiest time of day and an approximation for the crossover point on the light PRC).
Using melatonin to reduce circadian misalignment
The melatonin PRCs for 0.5- and 3.0-mg doses show that taking melatonin in the afternoon/evening, about 5–7 hours before natural fall-asleep time, can help phase-advance circadian rhythms, while taking melatonin at the end of the sleep episode and for a few hours after waking can help phase-delay rhythms. The optimal times depend on the dose.Citation231 For shift workers who want to go to bed in the morning after night work, and therefore need to phase-delay their circadian clocks, melatonin should be taken in the morning before daytime sleep. If they want to go to sleep before night work, and therefore need to phase-advance their circadian clocks, then melatonin should be taken in the afternoon/ evening before sleep.
We conducted a simulated night-shift study to test the phase-advancing and sleep-promoting effects of melatonin.Citation232 Subjects went to bed in the afternoon, before the night shifts, which was 7 hours before their usual baseline bedtime. They took melatonin or placebo before bed, which fell within the phase-advance portion of the melatonin PRCs. After 8 days with melatonin taken for the first 4 days, the DLMO advanced ~4 hours with 3.0 mg melatonin, ~3 hours with 0.5 mg melatonin and <2 hours with placebo. Thus, melatonin enhanced the phase shift produced by the advance of the LD cycle, but the circadian rhythms of many subjects did not advance enough to get their Tmin within sleep. Melatonin had a sleep-promoting effect and was able to keep sleep duration equivalent to baseline for the 4 days of administration. Since advancing human circadian rhythms is more difficult than delaying them,Citation209,Citation217 it is possible that a similar study with a delayed sleep schedule and melatonin in the morning before daytime sleep would have produced more circadian alignment.
Melatonin was tested on nurses and hospital personnel who worked seven consecutive 10-hour night shifts alternating with 7 days off.Citation233 During one 14-day block, they took 0.5 mg melatonin every day at bedtime, and during another they took placebo (double-blind crossover). Seven consecutive night shifts are unusual, and we might expect that the circadian rhythms of many subjects would shift substantially after that time, even on placebo. However, in this field study with uncontrolled sleep schedules and uncontrolled light and dark, most of these night workers did not phase-shift very much, even with melatonin.
Combinations of bright light and melatonin to reduce circadian misalignment
We designed a simulated night-shift study to assess the relative importance of different interventions that could promote phase delays during five consecutive night shifts (11:00 pm to 7:00 am).Citation234 The interventions were: (1) intermittent bright-light pulses during night shifts in the lab (~5000 lux, 20 minutes/hour, 4–5 pulses/night); (2) extremely dark (2% light transmission) or normal (15% transmission) sunglasses worn outside during daylight with the purpose of attenuating advancing morning light during the commute home (subjects were run in three summers in Chicago); (3) a strict, regular 7 hours for daytime sleep at home (8:30 am to 3:30 pm) in bedrooms that we made “darkroom dark”; and (4) melatonin (1.8 mg sustained release) ingested in the morning prior to daytime sleep (both for its phase-delaying and sleep-promoting effects). We used intermittent bright light, as in one of our previous studies,Citation235 because it is a more efficient light stimulus for phase-shifting the human circadian clock than continuous bright light,Citation236–Citation238 and because it is more practical for workers to receive in the workplace.
Subjects were free to sleep whenever they wanted during a baseline week, because we wanted them to start the night shifts with a range of baseline phase positions as would occur in real shift workers. The baseline Tmin (DLMO + 7 hours) ranged from about 3:00 to 10:00 am, and was the most important factor that determined whether subjects’ circadian clocks shifted to align with the day-sleep schedule. We divided subjects into those whose baseline Tmin was earlier than 7:00 am, and therefore fell within the time for night work, and those whose Tmin was later (see in Revell and EastmanCitation213). Bright outdoor light when traveling home from the night shift likely fell on the phase-advance portion of the light PRCs of the “earlier” subjects, whereas for the “later” subjects the same outdoor light occurring at an earlier circadian time was likely phase-delaying.
The circadian clocks of all of the “later” subjects (n = 23) became completely aligned with the day-sleep schedule, such that their Tmin after five night shifts occurred within the day-sleep episodes, even with minimal interventions (normal room light during night shifts [~150 lux], normal sunglasses [15%], and fixed daytime sleep [dark] episodes). In contrast, of the “earlier” subjects (n = 42) only 30% got their Tmin into day sleep with the same minimal interventions. All the subjects in the group given melatonin and extremely dark sunglasses (n = 13), including many “earlier” subjects got their Tmin into day sleep. We don’t know if melatonin would have worked as well combined with normal sunglasses, because that combination was not tested. As long as the combination included bright light during the night shifts (n = 26), all but one of the subjects delayed their Tmin into sleep; they did not need melatonin or extremely dark sunglasses.
This study showed that (1) complete realignment – complete reentrainment – of circadian rhythms to a daytime sleep schedule was possible after five night shifts, (2) bright light during the night shift was not necessary as long as subjects started out with relatively later circadian phases, wore sunglasses on the way home from the night shift, and maintained a regular daytime sleep schedule, (3) melatonin helped delay circadian rhythms as long as bright light was drastically attenuated, and (4) bright light during the night shifts was the most reliable way to ensure complete realignment of circadian rhythms with daytime sleep.
Importantly, when we compared subjects with partial realignment (Tmin into the first half of daytime sleep) to subjects with complete realignment (Tmin into the second half of daytime sleep) their sleepiness and performance during night shifts were similar to each other, and both were significantly better than for the subjects whose Tmin did not reach daytime sleep. Thus, complete realignment was not necessary to substantially improve night-shift alertness and performance.Citation239
Creating partial circadian alignment to accommodate days off
The simulated night-shift study discussed aboveCitation234,Citation239 showed we could reliably produce complete circadian alignment to night work and day sleep using only a total of 80–100 minutes of intermittent bright light per night shift. But if the circadian rhythms of real night workers delayed this far, then they would suffer from circadian misalignment on days off; they would have trouble sleeping at night and being alert during the day. We also showed that partial alignment to daytime sleep and night work was sufficient for improving night-shift alertness and performance. So in order to accommodate days off, we designed a sleep-and-light schedule to produce a compromise between complete alignment with a daytime sleep schedule and complete alignment with a normal nocturnal sleep schedule. We conducted a series of studies to test this schedule.Citation240–Citation243
We wanted to study only “earlier” people, because they have the most trouble adapting to night work, so we put subjects on an 11:00 pm to 7:00 am baseline sleep schedule for 3 weeks, with weekend bed and wake times allowed to be up to 1 hour later. Subjects were required to receive at least 15 minutes of outdoor light between 8:00 and 9:00 am. Thus, they began the study with a sleep schedule and morning light exposure typical of most day workers, and they all had “earlier” baseline phases (Tmin before 7:00 am) when the night shifts started, as shown in of Smith et al.Citation244
Figure 1 Sleep-and-light schedule for night-shift work that we tested to determine if it could align circadian rhythms with the sleep schedule enough to move the temperature minimum (Tmin) to within sleep.
Abbreviation: DLMO, dim-light melatonin onset.
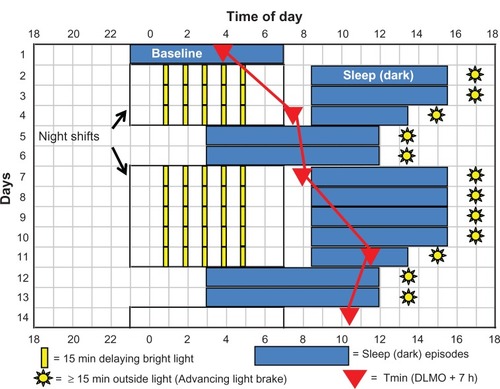
shows that after the 3 weeks of baseline, represented by the blue sleep rectangle on day 1, there were three night shifts, 2 days off, five more night shifts, and 2 days off. Experimental and control subjects had the same sequence of night shifts and days off, but experimental subjects were exposed to intermittent delaying bright light during the night shift, 15-minute light pulses (~4000 lux) separated by 45 minutes in room light (<50 lux), whereas control subjects remained in room light.
Experimental subjects followed the sleep schedule shown by the blue rectangles in , whereas control subjects were allowed to sleep whenever they chose after night shifts and on days off. Experimental subjects had to remain in bed in the dark for 7 hours after the night shifts (8:30 am to 3:30 pm) except following the last night shift in a series, when time in bed was 5 hours (8:30 am to 1:30 pm). The shorter sleep episodes before days off were designed to build up a small amount of homeostatic sleep pressure – a little sleep deprivation – to help subjects fall asleep earlier on the subsequent days off. Sleep on days off was from 3:00 am to noon.
Our goal was to delay the circadian clocks of experimental subjects so that the Tmin occurred during the hours that daytime sleep and days off sleep overlapped, so it would be in the beginning of sleep after night work and near the end of sleep on days off.
We conducted these studies in the summers, when conflicting, phase-advancing, bright light on the way home would be a powerful force to overcome. Experimental subjects wore Uvex (Smithfield, RI) sunglasses with Bandit frames and Espresso lenses (15% visual light transmission, increasing from 0% at 400 nm to about 22% at 650 nm). Control subjects wore the same frames with lighter lenses (36% light transmission, increasing from 0% at 400 nm to about 55% at 650 nm). Both spectral transmission curves are in Lee et al,Citation240 and can be seen at http://www.uvex.com. All subjects wore their sunglasses whenever outside during daylight. The purpose of the sunglasses was to attenuate phase-advancing bright light after night work, but we instructed subjects always to wear them when outside during daylight because we wanted to make it a habit.
Experimental subjects were required to go outside for 15 minutes of afternoon light exposure within 2 hours of their scheduled wake times (yellow suns in ). This advancing light exposure, the “light brake,” was intended to stop their circadian clocks from delaying too far. We wanted to avoid what happened in one of our previous studies,Citation218 when many subjects whose Tmin had delayed to daytime sleep continued to delay around the clock when they were abruptly placed back on their normal nocturnal sleep schedule.
Four separate studies were run, each with an experimental and control group progressing further through the schedule shown in . Each new study began with the 3-week baseline and ended with the final phase assessment on a later day in the sequence (a day marked by a red triangle). Thus, we acquired “snapshots” of the circadian phase that subjects obtained as they progressed through the schedule. The filled red triangle on day 1 illustrates the average Tmin determined after 2 weeks of baseline for all the subjects in the four experimental groups (n = 41). The triangle on day 4 shows the average final Tmin ± standard deviation for study 1 (7:36 ± 1.4 hours, n = 11),Citation240 day 7 for study 2 (7:59 ± 1.2 hours, n = 9),Citation241 day 11 for study 3 (11:34 ± 1.6 hours, n = 12),Citation242 and day 14 for study 4 (10:22 ± 2.0 hours, n = 9).Citation243
The red lines connecting the red Tmin triangles in are for illustrative purposes, and do not necessarily represent exactly how the circadian clocks shifted, which may not have been linear. Nonetheless, we can surmise that the Tmin reached the target zone (falling within sleep both after night work and on days off) by day 8 or 9 in , after 7 or 8 days on the sleep and light schedule. Because the final Tmin of the experimental group in study 3 (that ended on day 11) was later than necessary, we did not include the last light pulse from 4:45 to 5:00 am in study 4. Therefore, experimental subjects in study 4 followed the entire schedule with four rather than five light pulses, a total of only 1 hour of bright light per night shift, and still ended up with their Tmin in the target zone.
Around the time that these studies started, exciting discoveries showed that the human circadian system was most sensitive to blue light.Citation245–Citation248 To test whether the phase delays could be augmented by using lamps emitting additional blue light, we studied an additional group of subjects in study 1, which had two night shifts and then the final phase assessment. They received an identical number and pattern of intermittent light pulses, but were exposed to blue-enriched polychromatic light from fuorescent fixtures (17,000 K, ~3900 lux) rather than from “white” polychromatic fluorescent fixtures (5095 K, ~4000 lux).Citation249 The final Tmin for the blue-enriched group was not later than the white-light group (7:18 ± 1.3 hours vs 7:36 ± 1.4 hours).Citation249 We believe that the exact pattern, duration, and wavelength of light during the night shifts may not be crucial for practical purposes. The circadian clock will eventually delay, as long as the delayed sleep schedule is maintained and sunglasses are worn on the way home. If the rhythms delay too far, then the afternoon light brake should stop them.
On average, the circadian rhythms of the control subjects did not shift very much. Their Tmin stayed within the night shift or during the time of the commute home. The final Tmin of the control subjects in studies 1–4 were 4:48 ± 1.3 hours, 5:00 ± 1.8 hours, 7:39 ± 2.9 hours, and 6:24 ± 3.8 hours, respectively. However, there were large individual differences among the control subjects, which depended on the sleep times (and thus times of light exposure) that they self-selected. shows a control subject who adopted a sleep schedule that was similar to what we required of the experimental group; she went to bed relatively soon after the night shifts and slept until the afternoon, and went to bed and woke up late on days off. Her final Tmin fell within the target zone. In contrast, illustrates the sleep schedule of a control subject whose sleep pattern was similar to that of many night-shift workers. He went to bed and woke up relatively early on days off, often stayed awake during the morning hours after night shifts, and occasionally napped in the evening before the night shifts. Thus, he did not maintain a consistently delayed or advanced sleep (dark) period. His Tmin did not shift out of the night-shift hours, and he had the circadian misalignment typical of most night-shift workers.
Figure 2 Sleep times (blue rectangles) and baseline and final temperature minima (Tmin, filled red triangles) for a control subject in study 4.
Abbreviation: DLMO, dim-light melatonin onset.
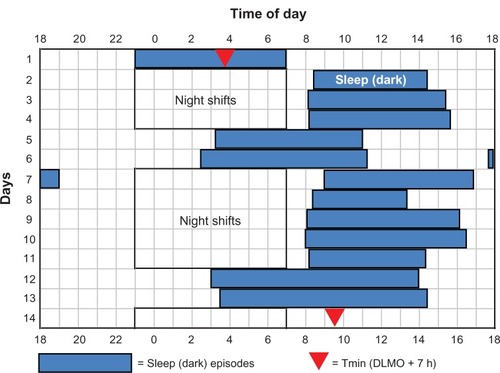
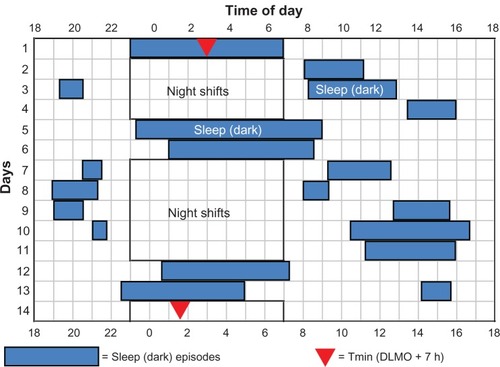
Figure 3 Sleep times for a control subject in study 4Citation243 that had short daytime sleep after night shifts, sometimes stayed awake for many hours in the morning after night work, napped before some night shifts, and went to sleep and woke up relatively early on days off.Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. J Biol Rhythms. 2009;24:161–172, Copyright © 2009 by the Journal of Biological Rhythms. Reprinted by Permission of SAGE Publications.Citation243
Abbreviation: DLMO, dim-light melatonin onset.
We previously reported that subjects who had either partial or complete realignment to a night-work and day-sleep schedule had improved night-shift alertness and performance compared to subjects whose rhythms did not shift.Citation239 However, we did not have measures during day work to judge the extent of this night-shift improvement. In the series of studies shown in , subjects completed computerized test batteries lasting ~25 minutes four times during their daytime baseline at 10:05 am, 12:05 pm, 2:05 pm, and 4:05 pm, and four times during the night shifts at 12:05, 2:05, 4:05, and 6:05 am. Each test bout included several measures of fatigue, mood, and objective performance. Subjects in studies 3 and 4 were classified as not realigned (not reen-trained) if their final Tmin was earlier than 8:30 am, putting it within the time corresponding to the night shift or during the travel home time, partially realigned if their final Tmin fell within the first half of the daytime sleep, between 8:30 am and noon, and completely realigned if their final Tmin fell within the second half of daytime sleep, after noon.Citation244
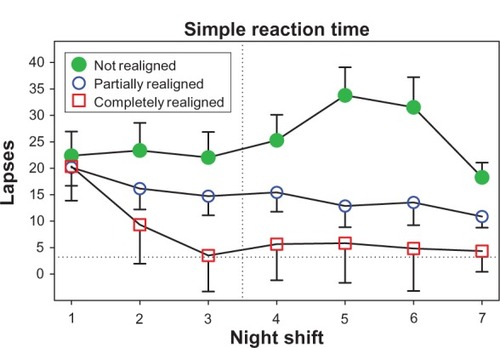
and show performance data, with average daytime scores depicted as horizontal dotted lines, and higher values on the y-axis depicting poorer performance. An example of how performance changed as the time of night progressed is shown with procedural reaction time (). The green filled circles show the typical pattern for shift workers whose circadian rhythms are not aligned with night work and day sleep: a steady slowing of reaction time as the night shift progressed. In contrast, performance of the partially and completely realigned groups was (1) significantly better than the not realigned group, (2) similar to each other, and (3) close to their daytime baseline level. To illustrate how performance changed across successive night shifts, shows simple reaction time. During the first night shift, as expected, all subjects had poorer performance (more lapses) than during the day shifts. The group that was not realigned (those with the most circadian misalignment) continued to have a large number of lapses during subsequent night shifts, whereas the partially and completely realigned groups improved across successive night shifts. There was a slight (but not statistically significant) difference between the partially and completely realigned groups, and by the end of the series of night shifts both groups were close to their daytime level of lapses. On all of the other tests of alertness, mood, and performance, the partially and completely realigned groups were more similar, as in . The simple reaction-time test is similar to the psychomotor vigilance task (PVT),Citation250 and is known to be very sensitive to sleep loss and circadian misalignment. On the other hand, the procedural reaction-time test required decision-making in addition to speed.
Figure 4 The number of slow responses on the procedural reaction-time task averaged over three night shifts (days 8–10 in ) for subjects whose circadian clocks were not realigned (n = 12), partially realigned (n = 21), or completely realigned (n = 6) to night work by the end of their series of night shifts and days off.
Copyright © 2009. Smith MR, Fogg LF, Eastman CI. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 2009;32:1481–1489.Citation244
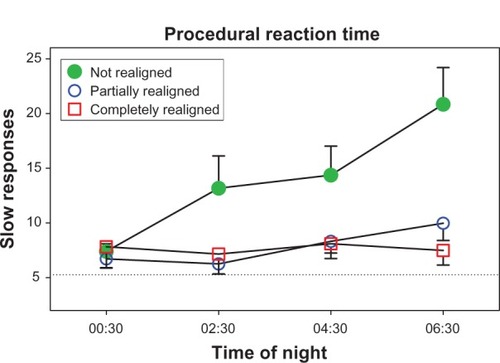
Figure 5 The number of lapses (reaction time > 500 milliseconds) at 6:30 am on the simple reaction-time task across successive night shifts for subjects whose circadian clocks were not realigned (n = 12), partially realigned (n = 21), or completely realigned (n = 6) to night work by the end of their series of night shifts and days off.
Copyright © 2009. Smith MR, Fogg LF, Eastman CI. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 2009;32:1481–1489.Citation244
The degree of improvement produced by our sleep and light schedule, with performance close to daytime levels and sustained over consecutive night shifts, is not obtained with conventional countermeasures that do not phase-shift circadian rhythms. Our subjects were young, mostly in their 20s, so it is important to test our sleep-and-light schedule on middle-aged and older adults, who may be less phase tolerant for obtaining daytime sleep with only partial circadian realignment.Citation165,Citation251 However, as mentioned earlier, older people are more phase tolerant for working at night; they have less severe decrements in response to sleep deprivation and circadian misalignment.Citation167,Citation168
We have shown that our sleep-and-light schedule produced partial realignment of circadian rhythms with daytime sleep, so that the Tmin, the sleepiest time of day, occurred within sleep. Furthermore, our experimental subjects were able to follow the prescribed sleep schedule, sleeping for nearly all of the allotted time in bed, both during daytime sleep and on days off.Citation242,Citation243 Finally, performance during the night shift was substantially improved, and was close to or at daytime levels. Given the success of our procedure, we think it is time to test it in real shift workers.
Recommended sleep-and-light schedule to reduce the circadian misalignment of night work
shows a sleep-and-light schedule that could be used to produce partial circadian alignment in permanent night-shift workers. The same type of schedule could be used for 12-hour shifts, except that the night shifts would start 4 hours earlier. This schedule () differs from the schedule we tested () in minor ways in order to make the procedure easier to understand and more practical for real shift workers. shows a sequence with blocks of five night shifts (11:00 pm to 7:00 am) alternating with 2 days off. The workers should be exposed to as much bright light as possible during their night shifts, especially between midnight and 4:00 am. We believe that the goal of having the sleepiest time of day – the temperature minimum (Tmin) – occur within sleep both after night work and on days off will eventually be reached regardless of the exact sequence of night shifts and days off. There will be a balance of forces that make the circadian clock later (phase-delaying bright light during the night shifts and the “pull” of the delayed sleep [dark] episodes) and forces that make the clock earlier (advancing light brake). Once this goal is reached, circadian phase should remain relatively stable for weeks or months until there is a vacation that changes the sleep schedule.
Figure 6 Sleep-and-light schedule designed to reduce the circadian misalignment produced by night-shift work, and thus to improve night-shift performance as well as sleep both after work and on days off.
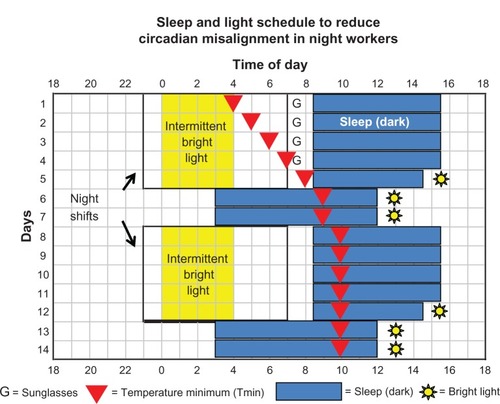
shows the expected path of the circadian clock (marked by the Tmin, red triangles) for a worker who starts the schedule after working days and thus has an early Tmin of 4:00 am. Most individuals who haven’t worked days or morning shifts will start out with Tmin that are later than 4:00 am, and will reach the target zone sooner. Based on our simulated night-shift studies,Citation234,Citation240–Citation243 we expect that the Tmin will delay by about 1 hour/day when there is bright light before the Tmin. When the Tmin moves too far away from the night-shift bright light, it will stop shifting because the delaying light exposures will no longer be occurring at a time that facilitates delays; they will be occurring at a less sensitive portion of the delay region of the light PRC. If the Tmin continues to drift later, it will be stopped by the phase-advancing light brake. If the light brake advances it too much, it will be again be nudged in a delaying direction by the bright light during the night shift.
Bright-light exposure during night work
The setup to deliver bright light during night shifts needs to be adapted to each unique night-work setting. This could be done by ceiling-mounted lights and/or light boxes around an employee’s work station. The worker does not have to look directly at the lights. A wide range of commercial light boxes with fluorescent tubes are available for helping to phase-shift the circadian clock. They are usually sold for the treatment of winter depression (seasonal affective disorder or SAD) and a list of some manufacturers is on the website http://www.sltbr.org. We recommend the larger light boxes,Citation252 at least about 50 cm wide, because they are more pleasant to sit in front of compared to very small, very bright white-light sources. Also, it is easier to stay within the range of a larger light box than a small light box, which can be easily, inadvertently, angled away from the face and therefore lose effectiveness. However, there are small light-emitting diode (LED) devices that are more practical if space is limited, with the added advantage that they could be more easily moved between work and home. Some light boxes are mounted on stands to aim the light from above, but this can substantially reduce the light level reaching the eyes when a person is looking down, such as while reading.
Some light boxes are enriched in the blue region of the spectrum to which the circadian system is most sensitive. However, we have shown that a blue-enriched polychromatic fluorescent light box (17,000 K) did not produce significantly greater circadian-rhythm phase shifts than a conventional “white” polychromatic fluorescent light box (4100 K).Citation253,Citation254 We have also tested the small goLITE P2 (Philips Consumer Lifestyle, Drachten, the Netherlands) made of blue LEDs in two subjects (unpublished observations) using the same 3-day advancing protocol with morning intermittent light as in some of our other studies.Citation238,Citation255 The phase-advance shifts with the GoLite were about 2 hours and fell in the middle of the range of phase shifts obtained with the much larger white fluorescent light box (~54 × 54 cm vs ~11 × 7 cm screen size). We have recently published a light PRC generated with the newer-model goLITE BLU, which shows phase shifts of up to about 2 hours.Citation256 The light stimulus was three 30-minute pulses of blue light (~185 lux) separated by 15 minutes in ordinary room light (~30 lux), administered at the same clock time for 3 consecutive days. Of interest for producing phase delays during night work, the phase-delay portion of this PRC started about 2–3 hours before bedtime and extended through most of the usual nocturnal sleep period. It should be noted that the light was administered in our lab with research assistants present to make sure the light box was aimed properly towards the eyes and at the correct distance (51 cm). If such tiny light boxes are used in the field, it might be better to use more than one of them. There is a twin-tower light box (Sunnex Biotechnologies, Winnipeg, Canada) with two tall (~45 cm high) thin green light boxes fastened together and made to go on each side of a computer screen. One study showed that after one night with the light box on from midnight to 2:00 am (~350 lux), the phase delay was about 45 minutes more than with dim light (<10 lux).Citation257
Sunglasses
The times to use sunglasses are shown in (as Gs) when bright light during the commute home occurs after the Tmin and thus would coincide with the phase-advance part of the light PRC and oppose the desired phase delay. Once the Tmin occurs during sleep episodes, sunglasses are no longer needed. Since it will be difficult to tell when an individual’s Tmin has delayed far enough, to be safe, the worker should continue to wear sunglasses. There are other advantages to wearing the sunglasses whenever outside in daylight. First, they block harmful ultraviolet (UV) light. Second, they could make the individual more sensitive to the bright light used during the night shift,Citation130 thus requiring lower-intensity light to achieve the same delaying effect. We recommend Uvex sunglasses with Bandit adjustable frames and Espresso lenses (15% light transmission). They are called “blue blockers,” but a more precise term would be “blue attenuators.” These lenses meet the American National Standards Institute traffic-signal color requirements and are recommended for driving. They are not darker than most sunglasses on the market. See http://www.uvex.com.
Driving home from the night shift is dangerous for night-shift workers, and it could be even more dangerous for workers who follow our sleep-and-light schedule if their Tmin starts out before the travel home time. When the Tmin (the sleepiest time of day) delays through the travel home time, driving performance could be even worse than before. But after the Tmin reaches the daytime-sleep episode, alertness and performance while driving home would be expected to improve. Whether driving performance and alertness is at an acceptable safety level when circadian rhythms are partially realigned, and how they compare to the driving performance and alertness of most real night-shift workers whose circadian clocks do not phase-shift, is unknown and should be studied. We recommend that shift workers who adopt our sleep-and-light schedule do not drive home for at least the first week of the schedule, or longer depending on their alertness levels after work. Employers could pay for transportation such as taxis to encourage night workers to attempt a schedule to reduce circadian misalignment.
Sleep (dark) schedule
In our studies, we used thick black plastic over bedroom windows secured with black masking tape over ordinary masking tape to make experimental subjects’ bedrooms completely dark. More professional room-darkening blackout shades could be used by real shift workers. They could also wear eye masks, but some have a tendency to come off during sleep.
has sleep starting 1.5 hours after the end of the night shift, but the sooner the worker can get to bed the better. An earlier bedtime means the Tmin does not have to delay as far to reach daytime sleep, and there is less time between the end of the night shift and bed for light to “hit” the phase-advance portion of the light PRC and inhibit the desired delay. The worker should avoid all activities that would delay bedtime, such as housework, shopping, child care, and even walking the dog. Overtime and morning meetings should not be tolerated by shift workers, and managerial and administration staff should not ask shift workers to remain on site after the night shift ends. Obviously, the worker will need the cooperation of family and employers in order to get to bed as soon as possible after night work. The sleep (dark) episodes after the night shifts are 7 hours in , except for the last sleep in a block, which is 6 hours. As in , the purpose of the shorter sleep is to build up a little sleep deprivation so that the worker can fall asleep earlier on days off. However, an individual should be able to cut it shorter or make it longer depending on their needs. The 7-hour sleep episodes could also be a little longer, but we do not recommend making them much shorter.
Sleeping late on days off is one of the most important parts of the schedule and may be the most difficult for workers to adhere to because of social pressures for morning activities. The worker needs to be willing to “give up” their mornings as a protected time for sleep, as long as s/he works permanent night shifts, except perhaps on vacations. It may also be necessary to plan late-night activities if others are sleeping. Again, the cooperation of family and friends is essential. With 12-hour shift systems, there are often more than 2 consecutive days off. The worker may not feel the need for 9-hour sleep opportunities on all days off (as in ), depending on previous sleep deprivation. However, it is still very important to sleep late so that the Tmin is within sleep to enable good sleep, and also to prevent morning light exposure.
A small proportion of the night-shift population already follows the basic sleep schedule we proposed for partial circadian alignment. Gamble et alCitation258 described 14% of permanent night nurses as “incomplete switchers,” because they went to bed and woke up late on days off rather than switching back to more normal earlier sleep times. This group had the lowest prevalence of nurses reporting that they were “not well adapted” to their work schedule (3%). In addition, although the incomplete switcher group consumed the fewest caffeinated beverages per day, they had the lowest proportion of nurses reporting a moderate or high likelihood of falling asleep during sedentary activities. Three other groups that adopted strategies that involved completely reverting to normally timed nighttime sleep on days off had a much higher prevalence of nurses reporting that they were not well adapted to working nights (9%–22%). Similarly, Gumenyuk et alCitation259 recently reported that a small sample of “asymptomatic” night workers (ie, good sleep quality and no excessive sleepiness) maintained a later sleep schedule on days off, compared to night workers with “shift-work disorder” (ie, insomnia and excessive sleepiness). The circadian rhythms of most of the asymptomatic night workers were delayed by several hours, while the rhythms of the workers with shift-work disorder were similar in phase to day workers.
Afternoon light brake
This phase-advancing light can be obtained, as in our studies,Citation240–Citation243 by going outside in the afternoon. Lighter sunglasses could be worn, as long as they block UV. A light box in workers’ homes could be used if outside light exposure was not practical (eg, because of inclement weather).
How much bright light?
You may be wondering: how much intermittent bright light is necessary during the night shifts? How intense does it have to be? How much bright light is needed for the afternoon light brake? These are difficult questions to answer because it depends on the individual’s light history, and thus their sensitivity to the phase-shifting effects of light, their circadian phase (Tmin) when starting the sequence of night shifts, and other individual differences. In study 4, which ended on day 14 in , the target zone was reached with only 1 hour of intermittent bright light (~4000 lux) during each of the night shifts. Once lighting systems are set up in actual shift-work environments, we speculate that getting 1 hour of intermittent bright-light exposure per night shift is actually not that daunting a task.
We know that bright light during the night shift is not absolutely necessary to achieve sufficient partial realignment. showed a control subject who did not get any bright light but delayed enough just by following the recommended sleep schedule and wearing light sunglasses (~36% transmission) on the way home. In one of our simulated night-shift studies,Citation234 all the subjects who had baseline Tmin later than the time that the night shift ended showed complete realignment of circadian rhythms after five night shifts, even if they did not get any bright light during the night shifts. But they all adhered to regular sleep (dark) episodes starting soon after work and wore sunglasses when outside. We have also shown that medium-intensity light (~1230 lux) during night shifts can have as much of a phase-shifting effect as much brighter light (~5700 lux).Citation218 Multiple large light boxes providing medium-intensity light would be more pleasant for the worker than a single tiny bright-white-light box. We believe that as long as the light during the night shift is brighter than indoor light (>~500 lux), then it should have a delaying effect. Nevertheless, we recommend the longest durations and brightest light that is practical for the night-work situation, rather than only relying on the other components of the sleep-and-light schedule, because the light should ensure than the rhythms delay as fast as possible.
One could argue that more research should be done to find out the exact timing, intensity, duration, and wavelengths of light that are needed during the night shift and for the afternoon light brake. While this type of basic research is important and ongoing, it can never provide exact parameters for real shift workers. Given the diversity of work places with different ambient-light levels, individual differences in light sensitivity and thus the magnitude of phase shifts from light, and individual differences in sleep need, there cannot be a one-size-fits-all sleep-and-light schedule. We hope that this review has explained the principles enough so that shift workers and employers can, perhaps with the help of circadian-rhythm experts, devise their own plans. The amount of delaying and advancing bright light may have to be titrated to find the right balance for an individual. This would require careful monitoring, at least keeping a log of light exposure, alertness during night work, alertness between night work and bed, sleep times and sleep quality.
We realize that adopting our sleep-and-light schedule comes at a social cost, and in some situations can be inconvenient. However, working night shifts and attempting to sleep in the daytime or evening without phase-shifting the circadian clock is already socially inconvenient, and carries with it none of the benefits of circadian adaptation. Light boxes, sunglasses, blackout shades and eye masks are much less expensive than the costs of severe circadian misalignment, such as absenteeism, employee turnover, reduced productivity, and impaired health and safety. Finally, developing a health problem (eg, cancer or cardiovascular disease) because of long-term exposure to shift work, or making a mistake on a night shift that results in injury or death, is also decidedly inconvenient and expensive for not only the shift worker but also those around them (eg, family, employer, coworkers) and for society.
The subjects in our studies were not allowed to have caffeine during their night shifts, which is in contrast to the majority of night-shift workers who regularly consume caffeine. As discussed earlier, caffeine improves night-shift alertness and performance, so the addition of caffeine to the sleep-and-light schedule could be beneficial, especially before the target zone has been reached. Caffeine ingested 3 hours before habitual evening bedtime has recently been reported to phase-delay the circadian clock.Citation260 The alerting and chronobiotic benefits of appropriately timed caffeine could be a practical adjunct intervention, but would need to be carefully balanced against disruptive effects that caffeine has on daytime sleep,Citation153 especially in older individuals.Citation154 A small dose of melatonin before daytime sleep could also be useful, especially for its sleep-promoting effect.
Rotating shift work
There is no way to reduce circadian misalignment for a rapid rotation that includes both night shifts and day shifts, because the circadian clock cannot phase-shift fast enough. This type of shift system is very common, but should be abolished because of the performance, safety, and health problems it creates. However, if the rotation is just between night shifts and evening shifts, then the sleep-and-light schedule shown in can be applied. Sleep times before evening shifts can be the same as on days off, and the afternoon light brake can be obtained on the way to work for the evening shift as well as in the afternoons on days off.
A rotation between day and evening shifts is another option that can produce less circadian misalignment than a rotation that includes both day shifts and night shifts. In this case, the worker’s sleep schedule would be like that adopted by most high school students, college students, and the young day-working population who go to bed and wake up 2–3 hours later on weekends (days off) than on work or school days, producing social jet lag.Citation52,Citation261–Citation263 The shift worker would go to bed and wake up earlier before day shifts, and go to bed and wake up later before evening shifts and days off. Although social jet lag is a serious problem, it is not as extreme as the circadian misalignment that accompanies night work.
Finally, it is possible to practically eliminate circadian misalignment with a three-shift system if there is a slowly rotating schedule that rotates in the delaying direction (eg, 2 weeks of days, 2 weeks of evenings, 2 weeks of nights, and so on). Then it is possible to delay both the sleep schedule and the circadian rhythms so that they remain fairly well aligned. See in EastmanCitation264 for the general idea. However, the worker would need to get delaying bright light or sunlight before bed on a few of the days in each cycle in order to keep the circadian clock delaying as fast as necessary. Scheduling social and family events would require careful planning, but some workers might prefer it to permanent night shifts.
It is time to take creative, bold steps towards solving the serious problems produced by night-shift work.
Acknowledgments
The studies referred to in this review as ours were performed in the Biological Rhythms Research Laboratory of Rush University Medical Center in Chicago, and were primarily supported by grant R01OH003954 from the National Institute of Occupational Safety and Health (NIOSH) of the Centers for Disease Control and Prevention (CDC) and also by grants from the National Institutes of Health (R01NS23421, R01NS35695, R01NR07677, R01HL086934) to Charmane Eastman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institutions: the National Institute of Occupational Safety and Health and the National Institutes of Health. These institutes had no involvement in designing the studies, data collection, data analysis and interpretation, or the writing of this manuscript. The authors have no conflicts of interest and did not receive any funds or salary support for writing this manuscript. We thank Daniel L Johnson, MD, for plodding through an earlier draft and providing tips for writing science for a lay audience.
Disclosure
The authors report no conflicts of interest in this work.
References
- HaMParkJShiftwork and metabolic risk factors of cardiovascular diseaseJ Occup Health200547899515824472
- BiggiNConsonniDGalluzzoVSoglianiMCostaGMetabolic syndrome in permanent night workersChronobiol Int20082544345418484373
- GhiasvandMHeshmatRGolpiraRShift working and risk of lipid disorders: a cross-sectional studyLipids Health Dis20065916606444
- KarlssonBHKnutssonAKLindahlBOAlfiredssonLSMetabolic disturbances in male workers with rotating three-shift work. Results of the WOLF studyInt Arch Occup Environ Health720037642443012783235
- KarlssonBKnutssonALindahlBIs there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 peopleOccup Environ Med20015874775211600731
- De BacquerDVan RisseghemMClaysEKittelFDe BackerGBraeckmanLRotating shift work and the metabolic syndrome: a prospective studyInt J Epidemiol20093884885419129266
- KnutssonAAkerstedtTJonssonBGPrevalence of risk factors for coronary artery disease among day and shift workersScand J Work Environ Health1988143173213201192
- NagayaTYoshidaHTakahashiHKawaiMMarkers of insulin resistance in day and shift workers aged 30–59 yearsInt Arch Occup Environ Health20027556256812373318
- RomonMNuttensMCFievetCIncreased triglyceride levels in shift workersAm J Med1992932592621524076
- SuwazonoYDochiMSakataKShift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort studyHypertension20085258158618625889
- OishiMSuwazonoYSakataKA longitudinal study on the relationship between shift work and the progression of hypertension in male Japanese workersJ Hypertens2005232173217816269958
- OhiraTTanigawaTIsoHEffects of shift work on 24-hour ambulatory blood pressure and its variability among Japanese workersScand J Work Environ Health20002642142611103841
- YamasakiFSchwartzJEGerberLMWarrenKPickeringTGImpact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholaminesHypertension1998324174239740605
- LinYCHsiaoTJChenPCPersistent rotating shift-work exposure accelerates development of metabolic syndrome among middleaged female employees: a five-year follow-upChronobiol Int20092674075519444753
- DrakeCLRoehrsTRichardsonGWalshJKRothTShift work sleep disorder: prevalence and consequences beyond that of symptomatic day workersSleep2004271453146215683134
- BrownDLFeskanichDSanchezBNRexrodeKMSchernhammerESLisabethLDRotating night shift work and the risk of ischemic strokeAm J Epidemiol20091691370137719357324
- LiYSatoYYamaguchiNShift work and the risk of metabolic syndrome: a nested case-control studyInt J Occup Environ Health20111715416021618947
- FujinoYIsoHTamakoshiAA prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workersAm J Epidemiol200616412813516707650
- LieuSJCurhanGCSchernhammerESFormanJPRotating night shift work and disparate hypertension risk in African-AmericansJ Hypertens201230616622134389
- KnutssonABoggildHGastrointestinal disorders among shift workersScand J Work Environ Health201036859520101379
- NojkovBRubensteinJHCheyWDHoogerwerfWAThe impact of rotating shift work on the prevalence of irritable bowel syndrome in nursesAm J Gastroenterol201010584284720160712
- LuWGweeKHoKFunctional bowel disorders in rotating shift nurses may be related to sleep disturbancesEur J Gastroenterol Hepatol20061862362716702851
- SegawaKNakazawaSTsukamotoYPeptic ulcer is prevalent among shift workersDig Dis Sci1987324494533568932
- SaberiHRMoravvejiARGastrointestinal complaints in shift-working and day-working nurses in IranJ Circadian Rhythms20108920929565
- LundJArendtJHamptonSMEnglishJMorganLMPostprandial hormone and metabolic responses amongst shift workers in AntarcticaJ Endocrinol200117155756411739022
- PanASchernhammerESSunQHuFBRotating night shift work and risk of type 2 diabetes: two prospective cohort studies in womenPLoS Med20118e100114122162955
- BisantiLOlsenJBassoOThonneauPKarmausWShift work and subfecundity: a European multicenter studyJ Occup Environ Med1996383523588925318
- UehataTSasakawaNThe fatigue and maternity disturbances of night workwomenJ Hum Ergol (Tokyo)198211Suppl4654747188479
- XuXDingMLiBChristianiDCAssociation of rotating shiftwork with preterm births and low birth weight among never smoking women textile workers in ChinaOccup Environ Med1994514704748044246
- AhlborgGJrAxelssonGBodinLShift work, nitrous oxide exposure and subfertility among Swedish midwivesInt J Epidemiol1996257837908921457
- AxelssonGLutzCRylanderRExposure to solvents and outcome of pregnancy in university laboratory employeesBr J Ind Med1984413053126743577
- AxelssonGAhlborgGJrBodinLShift work, nitrous oxide exposure, and spontaneous abortion among Swedish midwivesOccup Environ Med1996533743788758031
- AxelssonGRylandferRMolinIOutcome of pregnancy in relation to irregular and inconvenient work schedulesBr J Ind Med1989463933982818973
- McDonaldADMcDonaldJCArmstrongBFetal death and work in pregnancyBr J Ind Med1988451481573348991
- MarinoJLHoltVLChenCDavisSShift work, hCLOCK T3111C polymorphism, and endometriosis riskEpidemiology20081947748418379422
- SuwazonoYDochiMSakataKA longitudinal study on the effect of shift work on weight gain in male Japanese workersObesity (Silver Spring)2008161887189318535539
- Di LorenzoLDe PergolaGZocchettiCEffect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industryInt J Obes Relat Metab Disord2003271353135814574346
- MorikawaYNakagawaHMiuraKEffect of shift work on body mass index and metabolic parametersScand J Work Environ Health200733455017353964
- KawachiIColditzGAStampferMJProspective study of shift work and risk of coronary heart disease in womenCirculation199592317831827586301
- van AmelsvoortLGSchoutenEGKokFJDuration of shiftwork related to body mass index and waist to hip ratioInt J Obes Relat Metab Disord19992397397810490804
- MacagnanJPattussiMPCanutoRHennRLFassaAGOlintoMTImpact of nightshift work on overweight and abdominal obesity among workers of a poultry processing plant in southern BrazilChronobiol Int20122933634322390246
- ViswanathanANHankinsonSESchernhammerESNight shift work and the risk of endometrial cancerCancer Res200767106181062217975006
- SchernhammerESLadenFSpeizerFENight-shift work and risk of colorectal cancer in the nurses’ health studyJ Natl Cancer Inst20039582582812783938
- KuboTOzasaKMikamiKProspective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort studyAm J Epidemiol200616454955516829554
- HansenJStevensRGCase-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systemsEur J Cancer2011481722172921852111
- SchernhammerESKroenkeCHLadenFHankinsonSENight work and risk of breast cancerEpidemiology20061710811116357603
- DavisSMirickDKStevensRGNight shift work, light at night, and risk of breast cancerJ Natl Cancer Inst2001931557156211604479
- HansenJIncreased breast cancer risk among women who work predominantly at nightEpidemiology200112747711138824
- StraifKBaanRGrosseYCarcinogenicity of shift-work, painting, and fire-fightingLancet Oncol200781065106619271347
- SchernhammerESLadenFSpeizerFERotating night shifts and risk of breast cancer in women participating in the nurses’ health studyJ Natl Cancer Inst2001931563156811604480
- PilcherJJLambertBJHuffcuttAIDifferential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic reviewSleep20002315516310737332
- RoennebergTAllebrandtKVMerrowMVetterCSocial jetlag and obesityCurr Biol20122293994322578422
- MonkTHWhat can the chronobiologist do to help the shift worker?J Biol Rhythms200015869410762026
- AschoffJvon Saint PaulUWeverRALifetime of fies under influence of time displacementNaturwissenschaften197158574 German5139293
- PittendrighCSMinisDHCircadian systems: longevity as a function of circadian resonance in Drosophila melanogasterProc Natl Acad Sci USA197269153715394624759
- von Saint PaulUAschoffJLongevity among blowfies Phormia terraenovae R. D. kept in non-24-hour light-dark cyclesJ Comp Physiol A Neuroethol Sens Neural Behav Physiol1978127191195
- DavidsonAJSellixMTDanielJYamazakiSMenakerMBlockGDChronic jet-lag increases mortality in aged miceCurr Biol200616R914R91617084685
- KortWJWeijmaJMEffect of chronic light-dark shift stress on the immune response of the ratPhysiol Behav198229108310877163387
- FilipskiEDelaunayFKingVMEffects of chronic jet lag on tumor progression in miceCancer Res2004647879788515520194
- PreussFTangYLaposkyADArbleDKeshavarzianATurekFWAdverse effects of chronic circadian desynchronization in animals in a “challenging” environmentAm J Physiol Regul Integr Comp Physiol2008295R2034R204018843092
- MartinoTATataNBelshamDDDisturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronizationHypertension2007491104111317339537
- MartinoTAOuditGYHerzenbergAMCircadian rhythm disorganization produces profound cardiovascular and renal disease in hamstersAm J Physiol Regul Integr Comp Physiol2008294R1675R168318272659
- PenevPDKolkerDEZeePCTurekFWChronic circadian desyn-chronization decreases the survival of animals with cardiomyopathic heart diseaseAm J Physiol1998275H2334H23379843836
- KaratsoreosINBhagatSBlossEBMorrisonJHMcEwenBSDisruption of circadian clocks has ramifications for metabolism, brain, and behaviorProc Natl Acad Sci USA20111081657166221220317
- VarcoeTJWightNVoultsiosASalkeldMDKennawayDJChronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the ratPLoS One20116e1850421494686
- FonkenLKWorkmanJLWaltonJCLight at night increases body mass by shifting the time of food intakeProc Natl Acad Sci USA2010107186641866920937863
- LeeSDonehowerLAHerronAJMooreDDFuLDisrupting circadian homeostasis of sympathetic signaling promotes tumor development in micePLoS One20105e1099520539819
- LoganRWZhangCMuruganSChronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in ratsJ Immunol20121882583259122308312
- ScheerFAHiltonMFMantzorosCSSheaSAAdverse metabolic and cardiovascular consequences of circadian misalignmentProc Natl Acad Sci USA20091064453445819255424
- ZulleyJWeverRAschoffJThe dependence of onset and duration of sleep on the circadian rhythm of rectal temperaturePflugers Arch19813913143187312563
- StrogatzSHKronauerRECzeislerCACircadian regulation dominates homeostatic control of sleep length and prior wake length in humansSleep198693533643505735
- WyattJKRitz-De CeccoACzeislerCADijkDJCircadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h dayAm J Physiol1999277R1152R116310516257
- DumontMBenhaberou-BrunDPaquetJProfile of 24-h light exposure and circadian phase of melatonin secretion in night workersJ Biol Rhythms20011650251111669423
- KollerMHarmaMLaitinenJTKundiMPieglerBHaiderMDifferent patterns of light exposure in relation to melatonin and cortisol rhythms and sleep of night workersJ Pineal Res1994161271357932035
- RodenMKollerMPirichKVierhapperHWaldhauserFThe circadian melatonin and cortisol secretion pattern in permanent night shift workersAm J Physiol1993265R261R2678342696
- SackRLBloodMLLewyAJMelatonin rhythms in night shift workersSleep1992154344411455127
- WeibelLSpiegelKGronferCFolleniusMBrandenbergerGTwenty-four-hour melatonin and core body temperature rhythms: their adaptation in night workersAm J Physiol1997272R948R9549087659
- EastmanCIBoulosZTermanMCampbellSSDijkDJLewyAJLight treatment for sleep disorders: consensus report. VI. Shift workJ Biol Rhythms1995101571647632989
- FergusonSAKennawayDJBakerALamondNDawsonDSleep and circadian rhythms in mining operators: limited evidence of adaptation to night shiftsAppl Ergon20114369570122133975
- GrundyASanchezMRichardsonHLight intensity exposure, sleep duration, physical activity, and biomarkers of melatonin among rotating shift nursesChronobiol Int2009261443146119916841
- GrundyATranmerJRichardsonHGrahamCHAronsonKJThe influence of light at night exposure on melatonin levels among Canadian rotating shift nursesCancer Epidemiol Biomarkers Prev2011202404241221953114
- BurchJBYostMGJohnsonWAllenEMelatonin, sleep, and shift work adaptationJ Occup Environ Med20054789390116155474
- Quera-SalvaMADefranceRClaustratBDe LattreJGuilleminaultCRapid shift in sleep time and acrophase of melatonin secretion in short shift work scheduleSleep1996195395438899932
- FolkardSDo permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythmChronobiol Int20082521522418533325
- AkerstedtTShift work and disturbed sleep/wakefulnessOccup Med (Lond)200353899412637592
- AkerstedtTWrightKSleep loss and fatigue in shift work and shift work disorderSleep Med Clin2009425727120640236
- SpiegelKSheridanJFVan CauterEEffect of sleep deprivation on response to immunizationJAMA20022881471147212243633
- SpiegelKLeproultRVan CauterEImpact of sleep debt on metabolic and endocrine functionLancet19993541435143910543671
- BuxtonOMPavlovaMReidEWWangWSimonsonDCAdlerGKSleep restriction for 1 week reduces insulin sensitivity in healthy menDiabetes2010592126213320585000
- SchmidSMHallschmidMJauch-CharaKDisturbed glucoregulatory response to food intake after moderate sleep restrictionSleep20113437137721358855
- GottliebDJPunjabiNMNewmanABAssociation of sleep time with diabetes mellitus and impaired glucose toleranceArch Intern Med200516586386715851636
- CappuccioFPTaggartFMKandalaNBMeta-analysis of short sleep duration and obesity in children and adultsSleep20083161962618517032
- GottliebDJRedlineSNietoFJAssociation of usual sleep duration with hypertension: the Sleep Heart Health StudySleep2006291009101416944668
- WolffBVolzkeHSchwahnCRobinsonDKesslerCJohnURelation of self-reported sleep duration with carotid intima-media thickness in a general population sampleAtherosclerosis200819672773217289054
- KnutsonKLVan CauterERathouzPJAssociation between sleep and blood pressure in midlife: the CARDIA sleep studyArch Intern Med20091691055106119506175
- KingCRKnutsonKLRathouzPJSidneySLiuKLauderdaleDSShort sleep duration and incident coronary artery calcificationJAMA20083002859286619109114
- CappuccioFPStrangesSKandalaNBGender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II StudyHypertension20075069370017785629
- StrangesSDornJMCappuccioFPA population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal womenJ Hypertens20102889690220040890
- CappuccioFPCooperDD’EliaLStrazzulloPMillerMASleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studiesEur Heart J2011321484149221300732
- CappuccioFPD’EliaLStrazzulloPMillerMAQuantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysisDiabetes Care20103341442019910503
- von KriesRToschkeAMWurmserHSauerwaldTKoletzkoBReduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep – a cross-sectional studyInt J Obes Relat Metab Disord20022671071612032757
- RuttersFGerverWJNieuwenhuizenAGVerhoefSPWesterterp-PlantengaMSSleep duration and body-weight development during puberty in a Dutch children cohortInt J Obes (Lond)2010341508151420714331
- AyasNTWhiteDPMansonJEA prospective study of sleep duration and coronary heart disease in womenArch Intern Med200316320520912546611
- AyasNTWhiteDPAl-DelaimyWKA prospective study of self-reported sleep duration and incident diabetes in womenDiabetes Care20032638038412547866
- YaggiHKAraujoABMcKinlayJBSleep duration as a risk factor for the development of type 2 diabetesDiabetes Care20062965766116505522
- MallonLBromanJEHettaJHigh incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged populationDiabetes Care2005282762276716249553
- ChaputJPDespresJPBouchardCAstrupATremblayASleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family StudySleep Med20091091992419332380
- ChaputJPDespresJPBouchardCTremblayAAssociation of sleep duration with type 2 diabetes and impaired glucose toleranceDiabetologia2007502298230417717644
- Hoevenaar-BlomMPSpijkermanAMKromhoutDvan den BergJFVerschurenWMSleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN studySleep2011341487149222043119
- BuxtonOMCainSWO’ConnorSPAdverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruptionSci Transl Med20124129ra143
- NguyenJWrightKInfluence of weeks of circadian misalignment on leptin levelsNat Sci Sleep20102918
- LewyAJWehrTAGoodwinFKNewsomeDAMarkeySPLight suppresses melatonin secretion in humansScience1980210126712697434030
- BojkowskiCJAldhousMEEnglishJSuppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in manHorm Metab Res1987194374403692439
- TrinderJArmstrongSMO’BrienCLukeDMartinMJInhibition of melatonin secretion onset by low levels of illuminationJ Sleep Res1996577828795807
- AokiHYamadaNOzekiYYamaneHKatoNMinimum light intensity required to suppress nocturnal melatonin concentration in human salivaNeurosci Lett199825291949756329
- ZeitzerJMDijkDJKronauerREBrownENCzeislerCASensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppressionJ Physiol200052669570210922269
- ReiterRJMechanisms of cancer inhibition by melatoninJ Pineal Res20043721321415357667
- ViswanathanANSchernhammerESCirculating melatonin and the risk of breast and endometrial cancer in womenCancer Lett20092811719070424
- SchernhammerESSchulmeisterKMelatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels?Br J Cancer20049094194314997186
- SassevilleAPaquetNSevignyJHebertMBlue blocker glasses impede the capacity of bright light to suppress melatonin productionJ Pineal Res200641737816842544
- KayumovLCasperRFHawaRJBlocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift workJ Clin Endocrinol Metab2005902755276115713707
- KayumovLLoweARahmanSACasperRFShapiroCMPrevention of melatonin suppression by nocturnal lighting: relevance to cancerEur J Cancer Prev20071635736217554209
- RahmanSAMarcuSShapiroCMBrownTJCasperRFSpectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposureAm J Physiol Endocrinol Metab2011300E518E52721177289
- DavisSMirickDKChenCStanczykFZNight shift work and hormone levels in womenCancer Epidemiol Biomarkers Prev20122160961822315366
- Marie HansenAHelene GardeAHansenJDiurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure timeChronobiol Int2006231203121517190706
- YamauchiHEffects of night work on urinary excretion rates of 6-sulfatoxymelatonin, norepinephrine and estriol in pregnant womenInd Health20044226827615128179
- PeplonskaBBukowskaAGromadzinskaJNight shift work characteristics and 6-sulfatoxymelatonin (MT6s) in rotating night shift nurses and midwivesOccup Environ Med20126933934622368032
- SchernhammerESKroenkeCHDowsettMFolkerdEHankinsonSEUrinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levelsJ Pineal Res20064011612416441548
- DumontMLanctotVCadieux-ViauRPaquetJMelatonin production and light exposure of rotating night workersChronobiol Int20122920321022324558
- HebertMMartinSKLeeCEastmanCIThe effects of prior light history on the suppression of melatonin by light in humansJ Pineal Res20023319820312390501
- RufiangeMBeaulieuCLachapellePDumontMCircadian light sensitivity and rate of retinal dark adaptation in indoor and outdoor workersJ Biol Rhythms20072245445717876066
- SmithKASchoenMWCzeislerCAAdaptation of human pineal melatonin suppression by recent photic historyJ Clin Endocrinol Metab2004893610361415240654
- BurgessHJFoggLFIndividual differences in the amount and timing of salivary melatonin secretionPLoS One20083e305518725972
- ArendtJShift work: coping with the biological clockOccup Med (Lond)201060102020051441
- AkerstedtTSleepiness as a consequence of shift workSleep19881117343283910
- AkerstedtTWork hours, sleepiness and the underlying mechanismsJ Sleep Res19954152210607206
- FolkardSAkerstedtTTrends in the risk of accidents and injuries and their implications for models of fatigue and performanceAviat Space Environ Med200475A161A16715018280
- AkerstedtTTorsvallLGillbergMSleepiness and shift work: field studiesSleep19825S95S1066760335
- AkerstedtTCzeislerCADingesDFHorneJAAccidents and sleepiness: a consensus statement from the International Conference on Work Hours, Sleepiness and Accidents, Stockholm, September 8–10, 1994J Sleep Res19943195
- BargerLKLockleySWRajaratnamSMLandriganCPNeurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professionsCurr Neurol Neurosci Rep2009915516419268039
- CohenDAWangWWyattJKUncovering residual effects of chronic sleep loss on human performanceSci Transl Med2010214ra13
- WalshJKMuehlbachMJHummTMDickinsQSSugermanJLSchweitzerPKEffect of caffeine on physiological sleep tendency and ability to sustain wakefulness at nightPsychopharmacology19901012712732349369
- SchweitzerPKRandazzoACStoneKErmanMWalshJKLaboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night workSleep200629395016453980
- SchweitzerPKMuehlbachMJWalshJKCountermeasures for night work performance deficits: the effect of napping or caffeine on continuous performance at nightWork Stress19926355365
- MuehlbachMJWalshJKThe effects of caffeine on simulated night-shift work and subsequent daytime sleepSleep19951822297761739
- WrightKPBadiaPMyersBLPlenzlerSCCombination of bright light and caffeine as a countermeasure for impaired alertness and performance during extended sleep deprivationJ. Sleep Res1997626359125696
- BorlandRGRogersASNicholsonANPascoePASpencerMBPerformance overnight in shiftworkers operating a day-night scheduleAviat Space Environ Med1986572412493964153
- BonnetMHGomezSWirthOArandDLThe use of caffeine versus prophylactic naps in sustained performanceSleep199518971047792499
- KerKEdwardsPJFelixLMBlackhallKRobertsICaffeine for the prevention of injuries and errors in shift workersCochrane Database Syst Rev20105CD00850820464765
- RogersASSpencerMBStoneBMNicholsonANThe influence of a 1h nap on performance overnightErgonomics198932119312052598905
- SmithAPBrockmanPFlynnRMabenAThomasMInvestigation of the effects of coffee on alertness and performance during the day and nightNeuropsychobiology1993272172238232842
- BabkoffHFrenchJWhitmoreJSutherlinRSingle-dose bright light and/or caffeine effect on nocturnal performanceAviat Space Environ Med20027334135011952054
- CarrierJFernandez-BolanosMRobillardREffects of caffeine are more marked on daytime recovery sleep than on nocturnal sleepNeuropsychopharmacology20073296497216936703
- CarrierJPaquetJFernandez-BolanosMEffects of caffeine on daytime recovery sleep: a double challenge to the sleep-wake cycle in agingSleep Med2009101016102419342294
- WalshJKRandazzoACStoneKLSchweitzerPKModafinil improves alertness, vigilance, and executive function during simulated night shiftsSleep20042743443915164895
- HartCLHaneyMVosburgSKComerSDGundersonEFoltinRWModafinil attenuates disruptions in cognitive performance during simulated night-shift workNeuropsychopharmacology2006311526153616395298
- CzeislerCAWalshJKWesnesKAAroraSRothTArmodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled studyMayo Clin Proc20098495897219880686
- CzeislerCAWalshJKRothTModafinil for excessive sleepiness associated with shift-work sleep disorderN Engl J Med200535347648616079371
- Van DongenHPAMaislinGMullingtonJMDingesDFThe cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivationSleep20032611712612683469
- WalshJKSchweitzerPKAnchAMMuehlbachMJJenkinsNADickinsQSSleepiness/alertness on a simulated night shift following sleep at home with triazolamSleep1991141401461866527
- WalshJKSugermanJLMuehlbachMJSchweitzerPKPhysiological sleep tendency on a simulated night shift: adaptation and effects of triazolamSleep1988112512643399778
- WalshJKMuehlbachMJSchweitzerPKHypnotics and caffeine as countermeasures for shiftwork-related sleepiness and sleep disturbanceJ Sleep Res19954808310607218
- SchweitzerPKKoshorekGMuehlbachMJEffects of estazolam and triazolam on transient insomnia associated with phase-shifted sleepHum Psychopharmacol1991699107
- DawsonDCampbellSSTimed exposure to bright light improves sleep and alertness during simulated night shiftsSleep1991145115161798884
- GaudreauHMorettiniJLavoieHBCarrierJEffects of a 25-h sleep deprivation on daytime sleep in the middle-agedNeurobiol Aging20012246146811378253
- DijkDJDuffyJFCzeislerCAAge-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phasesSleep20012456557711480654
- SilvaEJWangWRondaJMWyattJKDuffyJFCircadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adultsSleep20103348149020394317
- AdamMReteyJVKhatamiRLandoltHPAge-related changes in the time course of vigilant attention during 40 hours without sleep in menSleep200629555716453981
- LewyAJAhmedSJacksonJMLSackRLMelatonin shifts human circadian rhythms according to a phase-response curveChronobiol Int199293803921394610
- SackRLHughesRJEdgarDMLewyAJSleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms?Sleep1997209089159415954
- FolkardSArendtJClarkMCan melatonin improve shift workers’ tolerance of the night shift? Some preliminary findingsChronobiol Int1993103153208261530
- SharkeyKMFoggLFEastmanCIEffects of melatonin administration on daytime sleep after simulated night shift workJ Sleep Res20011018119211696071
- SmithMRLeeCCrowleySJFoggLFEastmanCIMorning melatonin has limited benefit as a soporific for daytime sleep after night workChronobiol Int20052287388816298773
- DawsonDEncelNLushingtonKImproving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administrationSleep19951811217761738
- AeschbachDLockyerBJDijkDJUse of transdermal melatonin delivery to improve sleep maintenance during daytimeClin Pharmacol Ther20098637838219606092
- JorgensenKMWittingMDDoes exogenous melatonin improve day sleep or night alertness in emergency physicians working night shifts?Ann Emerg Med1998316997049624308
- JockovichMCosentinoDCosentinoLWearsRLSeabergDCEffect of exogenous melatonin on mood and sleep efficiency in emergency medicine residents working night shiftsAcad Emerg Med2000795595810958143
- JamesMTremeaMOJonesJSKrohmerJRCan melatonin improve adaptation to night shift?Am J Emerg Med1998163673709672452
- Smith-CogginsRHowardSKMacDTImproving alertness and performance in emergency department physicians and nurses: the use of planned napsAnn Emerg Med200648596604604e1604e317052562
- SallinenMHarmaMAkerstedtTRosaRLillqvistOPromoting alertness with a short nap during a night shiftJ Sleep Res199872402479844850
- PurnellMTFeyerAMHerbisonGPThe impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workersJ Sleep Res20021121922712220318
- NicholsonANPascoePARoehrsTSustained performance with short evening and morning sleepsAviat Space Environ Med1985561051143985887
- HarmaMKnauthPIlmarinenJDaytime napping and its effects on alertness and short-term memory performance in shiftworkersInt Arch Occup Environ Health1989613413452707872
- SugermanJLWalshJKPhysiological sleep tendency and ability to maintain alertness at nightSleep1989121061122711086
- SignalTLGanderPHAndersonHBrashSScheduled napping as a countermeasure to sleepiness in air traffc controllersJ Sleep Res200918111919250171
- MatsumotoKHaradaMThe effect of night-time naps on recovery from fatigue following night workErgonomics1994378999078206058
- FallisWMMcMillanDEEdwardsMPNapping during night shift: practices, preferences, and perceptions of critical care and emergency department nursesCrit Care Nurse201131e1e1121459861
- ScheerFASheaTJHiltonMFSheaSAAn endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological nightJ Biol Rhythms20082335336118663242
- SilvaEJDuffyJFSleep inertia varies with circadian phase and sleep stage in older adultsBehav Neurosci200812292893518729646
- BadiaPMyersBBoeckerMCulpepperJHarshJRBright light effects on body temperature, alertness, EEG and behaviorPhysiol Behav1991505835881801013
- CampbellSSDawsonDEnhancement of nighttime alertness and performance with bright ambient lightPhysiol Behav1990483173202255738
- LavoieSPaquetJSelmaouiBRufangeMDumontMVigilance levels during and after bright light exposure in the first half of the nightChronobiol Int2003201019103814680141
- MyersBLBadiaPImmediate effects of different light intensities on body temperature and alertnessPhysiol Behav1993541992028327605
- RugerMGordijnMCMBeersmaDGMde VriesBDaanSAcute and phase-shifting effects of ocular and extraocular light in human circadian physiologyJ Biol Rhythms20031840941914582857
- LowdenAAkerstedtTWibomRSuppression of sleepiness and melatonin by bright light exposure during breaks in night workJ Sleep Res200413374314996033
- RugerMGordijnMCBeersmaDGde VriesBDaanSTime-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposureAm J Physiol Regul Integr Comp Physiol2006290R1413R142016373441
- CostaGGaffuriEGhirlandaGMinorsDSWaterhouseJMPsychophysical conditions and hormonal secretion in nurses on a rapidly rotating shift schedule and exposed to bright light during night workWork Stress19959148157
- CostaGGhirlandaGMinorsDWaterhouseJMEffect of bright light on tolerance to night workScand J Work Environ Health1993194144208153594
- DauratAAguirreAForetJGonnetPKeromesABenoitOBright light affects alertness and performance rhythms during a 24-h constant routinePhysiol Behav1993539299368511209
- CajochenCZeitzerJMCzeislerCADijkDJDose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertnessBehav Brain Res2000115758310996410
- DauratAForetJBenoitOMaucoGBright light during nighttime: effects on the circadian regulation of alertness and performanceBiol Signals Recept2000930931811025337
- CampbellSSDijkDJBoulosZEastmanCILewyAJTermanMLight treatment for sleep disorders: consensus report. III. Alerting and activating effectsJ Biol Rhythms1995101291327632986
- CajochenCMunchMKobialkaSHigh sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength lightJ Clin Endocrinol Metab2005901311131615585546
- LockleySWEvansEEScheerFABrainardGCCzeislerCAAeschbachDShort-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humansSleep20062916116816494083
- RevellVLArendtJFoggLFSkeneDJAlerting effects of light are sensitive to very short wavelengthsNeurosci Lett20063999610016490309
- Phipps-NelsonJRedmanJRDijkDJRajaratnamSMWDaytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performanceSleep20032669570014572122
- RugerMGordijnMCBeersmaDGde VriesBDaanSWeak relationships between suppression of melatonin and suppression of sleepiness/fatigue in response to light exposureJ Sleep Res20051422122716120096
- BonnetMHArandDLThe use of prophylactic naps and caffeine to maintain performance during a continous operationErgonomics199437100910208026448
- EastmanCIMartinSKHow to use light and dark to produce circadian adaptation to night shift workAnn Med199931879810344580
- WeverRAThe Circadian System of Man: Results of Experiments Under Temporal IsolationHeidelbergSpringer-Verlag1979
- AschoffJHoffmannKPohlHWeverRRe-entrainment of circadian rhythms after phase shifts of the zeitgeberChronobiologia1975223781192905
- RevellVLEastmanCIJet lag and its preventionBarkoukisTJMathesonJKFerberRDoghramjiKTherapy in Sleep MedicinePhiladelphiaElsevier2012390401
- RevellVLEastmanCIHow to trick Mother Nature into letting you fy around or stay up all nightJ Biol Rhythms20052035336516077154
- EastmanCIBurgessHJHow to travel the world without jet lagSleep Med Clin2009424125520204161
- EastmanCIBright light in work-sleep schedules for shift workers: application of circadian rhythm principlesRensingLan der HeidenUMackeyMCTemporal Disorder in Human Oscillatory SystemsHeidelbergSpringer-Verlag1987176185
- EastmanCIStewartKTMahoneyMPLiuLFoggLFDark goggles and bright light improve circadian rhythm adaptation to night-shift workSleep1994175355437809567
- MitchellPJHoeseEKLiuLFoggLFEastmanCIConflicting bright light exposure during night shifts impedes circadian adaptationJ Biol Rhythms1997125159104686
- MartinSKEastmanCIMedium-intensity light produces circadian rhythm adaptation to simulated night-shift workSleep1998211541659542799
- BoivinDBJamesFOCircadian adaptation to night-shift work by judicious light and darkness exposureJ Biol Rhythms20021755656712465889
- LewyAJSackRLThe dim light melatonin onset as a marker for circadian phase positionChronobiol Int19896931022706705
- BenloucifSBurgessHJKlermanEBMeasuring melatonin in humansJ Clin Sleep Med20084666918350967
- BurgessHJEastmanCIThe dim light melatonin onset following fixed and firee sleep schedulesJ Sleep Res20051422923716120097
- CagnacciASoldaniRLaughlinGAYenSSCModification of circadian body temperature rhythm during the luteal menstrual phase: role of melatoninJ Appl Physiol19968025298847311
- GoelNLate-night presentation of an auditory stimulus phase delays human circadian rhythmsAm J Physiol Regul Integr Comp Physiol2005289R209R21615790749
- GoelNAn arousing, musically enhanced bird song stimulus mediates circadian rhythm phase advances in dim lightAm J Physiol Regul Integr Comp Physiol2006291R822R82716614052
- EastmanCIMartinSKHebertMFailure of extraocular light to facilitate circadian rhythm reentrainment in humansChronobiol Int20001780782611128297
- MongrainVLavoieSSelmaouiBPaquetJDumontMPhase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningnessJ Biol Rhythms20041924825715155011
- GriefahnBThe validity of the temporal parameters of the daily rhythm of melatonin levels as an indicator of morningnessChronobiol Int20021956157712069038
- BenloucifSGuicoMJReidKJWolfeLFL’Hermite-BaleriauxMZeePCStability of melatonin and temperature as circadian phase markers and their relation to sleep times in humansJ Biol Rhythms20052017818815834114
- GriefahnBKunemundCGolkaKThierRDegenGMelatonin synthesis: a possible indicator of intolerance to shiftworkAm J Ind Med20024242743612382256
- BurgessHJRevellVLMolinaTAEastmanCIHuman phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mgJ Clin Endocrinol Metab2010953325333120410229
- SharkeyKMEastmanCIMelatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work studyAm J Physiol2002282R454R463
- SackRLLewyAJMelatonin as a chronobiotic: treatment of circadian desynchrony in night workers and the blindJ Biol Rhythms1997125956039406035
- CrowleySJLeeCTsengCYFoggLFEastmanCICombinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift workJ Biol Rhythms20031851352314667152
- BaehrEKFoggLFEastmanCIIntermittent bright light and exercise to entrain human circadian rhythms to night workAm J Physiol1999277R1598R160410600904
- RimmerDWBoivinDBShanahanTLKronauerREDuffyJFCzeislerCADynamic resetting of the human circadian pacemaker by intermittent bright lightAm J Physiol2000279R1574R1579
- GronferCWrightKPKronauerREJewettMECzeislerCAEffcacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humansAm J Physiol2004287E174E181
- BurgessHJCrowleySJGazdaCJFoggLFEastmanCIPrefight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright lightJ Biol Rhythms20031831832812932084
- CrowleySJLeeCTsengCYFoggLFEastmanCIComplete or partial circadian re-entrainment improves performance, alertness, and mood during night shift workSleep2004271077108715532201
- LeeCSmithMEastmanCA compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposureChronobiol Int20062385987516887753
- SmithMRCullnanEEEastmanCIShaping the light/dark pattern for circadian adaptation to night shift workPhysiol Behav20089544945618675836
- SmithMREastmanCINight shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend offSleep2008311639164519090319
- SmithMRFoggLFEastmanCIPractical interventions to promote circadian adaptation to permanent night shift work: study 4J Biol Rhythms20092416117219346453
- SmithMRFoggLFEastmanCIA compromise circadian phase position for permanent night work improves mood, fatigue, and performanceSleep2009321481148919928387
- BrainardGCHanifinJPGreesonJMAction spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptorJ Neurosci2001216405641211487664
- ThapanKArendtJSkeneDJAn action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humansJ Physiol200153526126711507175
- LockleySWBrainardGCCzeislerCAHigh sensitivity of the human circadian melatonin rhythm to resetting by short wavelength lightJ Clin Endocrinol Metab2003884502450512970330
- WarmanVLDijkDJWarmanGRArendtJSkeneDJPhase advancing human circadian rhythms with short wavelength lightNeurosci Lett2003342374012727312
- SmithMRevellVLeeCEastmanCBright blue-enriched light did not produce larger phase delays than bright white lightSoc Light Treatment Biol Rhythms Abstr20061830
- DingesDFPowellJWMicrocomputer analyses of performance on a portable, simple visual RT task during sustained operationsBehav Res Methods Instrum Comput198517652655
- DijkDJDuffyJFRielEShanahanTLCzeislerCAAgeing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythmsJ Physiol199951661162710087357
- EastmanCIHow to get a bigger dose of bright lightSleep20113455956021532946
- SmithMRRevellVLEastmanCIPhase advancing the human circadian clock with blue-enriched polychromatic lightSleep Med20091028729418805055
- SmithMREastmanCIPhase delaying the human circadian clock with blue-enriched polychromatic lightChronobiol Int20092670972519444751
- RevellVLBurgessHJGazdaCJSmithMRFoggLFEastmanCIAdvancing human circadian rhythms with afternoon melatonin and morning intermittent bright lightJ Clin Endocrinol Metab200691545916263827
- RevellVLMolinaTAEastmanCIHuman phase response curve to intermittent blue light using a commercially available deviceJ Physiol Epub722012
- PaulMAMillerJCGrayGBuickFBlazeskiSArendtJCircadian phase delay induced by phototherapeutic devicesAviat Space Environ Med20077864565217679560
- GambleKLMotsinger-ReifAAHidaAShift work in nurses: contribution of phenotypes and genotypes to adaptationPLoS One20116e1839521533241
- GumenyukVRothTDrakeCLCircadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorderChronobiol Int20122992893622823876
- BurkeTMarkwaldRMcHillAEvening caffeine phase delays the human circadian clockSleep201235A205
- WittmannMDinichJMerrowMRoennebergTSocial jetlag: misalignment of biological and social timeChronobiol Int20062349750916687322
- CrowleySJAceboCCarskadonMASleep, circadian rhythms, and delayed phase in adolescenceSleep Med2007860261217383934
- National Sleep Foundation2006Sleep in America Poll: Teens and Sleep Available from: http://www.sleepfoundation.orgAccessed August 20, 2012
- EastmanCICircadian rhythms and bright light: recommendations for shift workWork Stress19904245260
