Abstract
Purpose
To systematically review anatomical and functional outcomes subsequent to switching from bevacizumab/ranibizumab to aflibercept monotherapy in patients with treatment-resistant neovascular age-related macular degeneration (nAMD).
Design
Systematic review and meta-analysis.
Methods
Medline, PubMed, Embase, and Cochrane databases were searched up to July 2016 for available scientific literature which met inclusion criteria. Eligible studies reported visual and anatomical outcomes with at least 6 months of follow-up among patients with nAMD and persistent or resistant exudative fluid despite previous anti-vascular endothelial growth factor (VEGF) therapy (bevacizumab and/or ranibizumab) and were switched to aflibercept monotherapy. Mean changes in best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were pooled using random-effects models with 95% confidence intervals (CIs).
Results
Of 82 papers reviewed, 28 studies met inclusion criteria of this review. Pooled results showed a small mean improvement in BCVA at 6 and 12 months following switching (1.11 letters, 95% CI −0.25 to 2.46, P=0.17 and 0.63 letters, 95% CI −0.26 to 1.52, P=0.17, respectively). There was a significant improvement in mean CRT following switching (−61.90 µm, 95% CI −77.10 to −46.80, P<0.001 and −50.00 µm, 95% CI −63.20 to −36.80, P<0.001 at 6 and 12 months, respectively).
Conclusion
Pooled analysis demonstrated significantly improved anatomical outcomes; however, visual function remained stable, having a comparable effect to other anti-VEGF agents in preservation of vision. These patients had poorly responsive chronic disease with limited potential for visual recovery. Switching to aflibercept with frequent monitoring may be a suitable option for patients who have developed treatment resistance.
Introduction
Age-related macular degeneration (AMD) is a progressive, degenerative disease of the retina which is the leading cause of blindness among older people in western countries.Citation1–Citation8 There are 2 main forms of late-stage AMD; “dry” AMD is characterized by the development of either drusen, pigmentary changes or atrophy of the retinal pigment epithelium (RPE) in the macular region (geographic atrophy, GA). Aside from antioxidant therapy, there are no current treatments for dry AMD, although numerous clinical trials are under development.Citation9 Wet AMD or neovascular AMD (nAMD) is characterized by choroidal neovascularization (CNV), an anomalous angiogenic process controlled by growth factors, including vascular endothelial growth factor (VEGF), placental growth factor (PlGF), platelet-derived growth factor, insulin-like growth factor, and angiogenic inhibitors such as pigment epithelium-derived factor.Citation10,Citation11 These choroidal neovascular vessels typically leak and bleed, leading to intra- or subretinal fluid, and lipid exudation which may result in severe vision loss.Citation7 Without treatment, the process usually evolves into fibrous scarring greatly diminishing visual capacity.Citation12
Until the introduction of anti-VEGF agents, treatments most commonly used for nAMD included thermal laser photocoagulation and verteporfin photodynamic therapy (PDT).Citation8 However, neither laser photocoagulation nor PDT offered any significant potential for visual recovery when the central macula is involved. Anti-VEGF therapy has become the standard treatment for nAMD with several anti-VEGF agents available: Avastin® (bevacizumab), Lucentis® (ranibizumab), and more recently Eylea® (aflibercept). Both ranibizumab and aflibercept have been approved by the US Food and Drug Administration (FDA) for use in the eye.Citation13,Citation14 Anti-VEGF therapy has been shown to be effective in the treatment of a number of other ocular diseases, such as diabetic macular edema, and retinal vein occlusions.
Aflibercept, previously known as VEGF Trap (Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA), is the most recent anti-VEGF agent; the molecule serves as a soluble VEGF decoy to impede the growth of new blood vessels by binding to VEGF-A (with a higher affinity than its natural receptors, and both ranibizumab and bevacizumab), VEGF-B, and PlGF. It is therefore thought to be more effective in blocking VEGF and has been effective in naïve eyesCitation15 and was approved for the treatment of nAMD by the FDA in 2011.Citation14
Long-term, repeated use of ranibizumab and bevacizumab has been associated with diminished effectiveness. A significant proportion of patients treated with these therapies, termed “nonresponders”, do not benefit from continued treatment, and vision continues to deteriorate.Citation16–Citation18 Therapeutic options for nonresponders are limited to offering a different therapeutic agent, combining therapy, or decreasing treatment intervals. With up to 25% of patients classed as nonresponders,Citation16 there is a greater need for understanding of treatment resistance to improve treatment strategies.
The purpose of this review is to investigate the functional and anatomical response as well as the effect of differing treatment regimens, and disease chronicity among nAMD patients (resistant or refractory) switched to aflibercept previously treated with other intravitreal anti-VEGF agents (ranibizumab and bevacizumab).
Methods
Search strategy
A search of Medline, PubMed, Embase, and Cochrane databases was undertaken independently by 2 reviewers (KS and TH). The search strategy was based on the combination of medical subject headings and the keywords “neovascular AMD”, “wet AMD treatment”, “aflibercept”, “Eylea”, “age-related macular degeneration”, “ARMD”, “switching”, “refractory”, “anti-vascular endothelial growth factor”, and “anti-VEGF”. Studies published from January 2012 to July 2016 were included in this review. The citations of related articles were examined for additional publications.
When data was missing, unclear, or incomplete, we attempted to contact study authors for clarification and additional information.
Eligibility criteria
This review considered studies that included populations that had undergone previous anti-VEGF therapy with either ranibizumab and/or bevacizumab, before being switched to aflibercept monotherapy due to persistent or resistant exudative fluid secondary to nAMD.
We limited the search to clinical studies published in peer-reviewed, and English language publications. Studies were excluded if participants were not followed for a minimum of 6 months or when only an abstract was published. The key inclusion criteria were the following: (a) patients should be aged 50 years or older with active subfoveal CNV lesions secondary to AMD, who are resistant to previous anti-VEGF therapy; and (b) Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) should be between 75 and 25 letters (20/32 to 20/320 Snellen equivalent).
Study selection
The review authors performed the literature search by screening citations by title and abstract, identified all studies for full review to confirm eligibility, and selected independent studies for their inclusion in the systematic review. Any disagreement between these reviewers was resolved by a third reviewer (AC), and the decision to include and exclude a study was reached by consensus.
Data extraction and quality assessment
The review authors independently screened articles, extracted data, and assessed risks of bias, following the 27-point Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. The review authors then independently assessed the full-text reports and removed articles that were not relevant to the topic.
The authors extracted data and tabulated data such as mean change of visual acuity (VA), central retinal thickness (CRT), and pigment epithelial detachment (PED) height from baseline. When extracting data, the Cochrane handbook was used to obtain standard deviation from range, median, or P-value when present.Citation19 BCVA data was converted to ETDRS letter score.
Data were summarized both qualitatively and quantitatively. This was facilitated by extracting data in regard to the following characteristics from all included studies: study design (retrospective or prospective), study setting (country), patient selection (target population, sample size), patient characteristics (age, gender), and clinical characteristics (BCVA, CRT, PED height, dosing regimens).
Further to the overall meta-analysis, we also stratified the studies by the number of previous anti-VEGF therapies before conversion to aflibercept (<12 injections, 12–23 injections, and ≥24 injections), the number of aflibercept injections received during the course of the study (<4 injections and ≥4 injections for the 6-month studies and <8 injections and ≥8 injections for the 12-month studies), study design type (robustness of prospective vs retrospective studies), and dosing regimen during the course of the study. If studies had missing data regarding the stratification, this was removed from the analysis for the corresponding meta-analysis.
The review authors independently assessed potential sources of bias according to the Downs & Black Checklist.Citation20 All included studies were assessed as fair. Insufficient information was presented in many cases to enable us to assess the quality and strength of studies in 2 domains: measures taken to reduce bias (n=25) and patients lost to follow-up (n=24).
Data synthesis and analysis
A meta-analysis of eligible studies was conducted using Comprehensive Meta-Analysis Version 2.0 (Biostat, Englewood, NJ, USA). All results were subjected to double data entry, and effect sizes were expressed as mean differences (for continuous data) with 95% confidence intervals (CIs). Results were pooled using random-effects models and visualized in forest plots. Meta-regression analysis was used to identify heterogeneity and quality factors that were subsequently used to stratify pooled estimates. Heterogeneity was assessed with the I2 statistic, which is the percentage of between-study heterogeneity that is attributable to variability in the true effect, with 25%, 50%, and >75% indicating low, moderate, and high heterogeneity, respectively.Citation21 In cases where heterogeneity was significant, a random-effects model was used. Publication bias was assessed with funnel plots and Egger’s linear regression.
Results
Description of studies
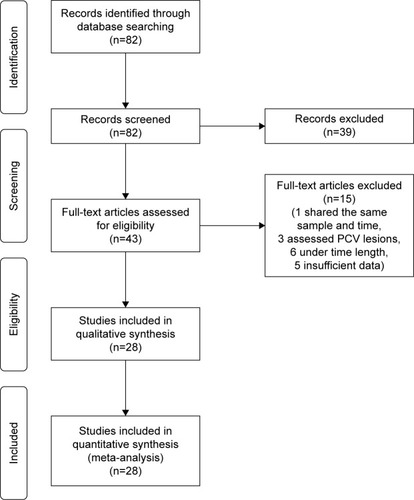
Of the 82 articles identified by the initial search, 43 were found to be potentially relevant for the review. Of these, 15 were excluded – 1 study shared the same sample and time period, 3 assessed polypoidal choroidal vasculopathy rather than CNV lesions, 6 were under the desired time of follow-up, and 5 had insufficient data.
Twenty-eight studies () that met the inclusion criteria for the review were identified. All studies examined the efficacy and safety of aflibercept for patients with nAMD previously treated with bevacizumab and/or ranibizumab. Sample sizes ranged from 11 to 447, with a total of 2,254 eyes included in this meta-analysis. The study duration ranged from 6 to 24 months. The literature search process and reasons for exclusion are summarized in .
Table 1 Characteristics of included studies in the meta-analysis
The majority of included studies reported at least 1 functional and anatomical measure. Reported outcomes included BCVA changes and stability, changes in CRT, macular volume, and PED height. Overall, we included 8 prospectiveCitation22–Citation29 and 20Citation30–Citation49 retrospective studies, with a mean follow-up of at least 6 months after converting to aflibercept. Two articles included the same eyes at different times after conversion.Citation23,Citation24
While we were able to identify more studies that examined the effect of switching to aflibercept, insufficient outcome data prevented them from being included in this meta-analysis.
Baseline characteristics
Baseline characteristics of the patients included within the individual studies were similar across all studies ( and ). The mean age ranged from 70.1 to 83.4 years, and 59% of patients were female. The mean baseline BCVA letter scores ranged from 42.50 to 74.20 ETDRS letters and were similar among the studies. The mean CRT ranged from 228.60 to 449.00 µm. The mean PED height ranged from 122.80 to 313.00 µm.
Table 2 Clinical characteristics of included studies in the meta-analysis
Meta-analysis
Best-corrected visual acuity
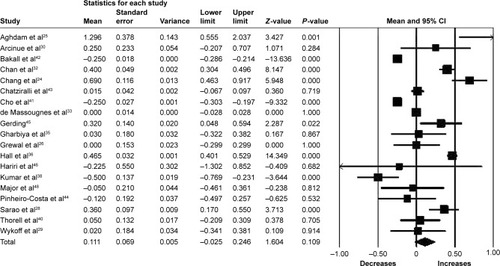
Nineteen studies (n=1,434 eyes) were included in the assessment of BCVA change between baseline and 6 months. Overall, the pooled results did not find a significant increase in BCVA from baseline with a mean increase of 1.11 letters (95% CI −0.25 to 2.46, P=0.11) ().
Figure 2 Forest plot of 19 studies reporting results of best-corrected visual acuity 6 months after the switch to aflibercept.
Abbreviation: CI, confidence interval.
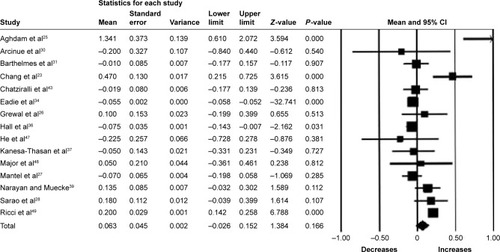
Fifteen studies (n=1,561 eyes) were included in the assessment of BCVA change between baseline and 12 months. There was no significant change in BCVA from baseline with a mean increase of 0.63 letters (95% CI −0.26 to 1.52, P=0.17) (). Significant heterogeneity was found across studies assessing BCVA for 6 and 12 months of follow-up (I2=95.85%, P<0.001 and I2=91.81%, P<0.001, respectively).
Figure 3 Forest plot of 15 studies reporting results of best-corrected visual acuity 12 months after the switch to aflibercept.
Abbreviation: CI, confidence interval.
When the studies were stratified by the number of previous injections prior to switching to aflibercept at 6 months, studies with less mean number of previous injections (<12) showed a greater mean increase of 3.83 letters compared to those which had a greater mean number of previous injections (>24) which showed a lesser mean increase of only 0.05 letter (). Stratification by the number of aflibercept injections showed that studies which had less injections of aflibercept over the course of the study had a greater improvement in BCVA compared to those which had more injections over the course of the study (2.97 vs 0.49 letters at 6 months).
Table 3 Subgroup analysis of pooled BCVA means (95% confidence intervals) in ETDRS letters
Studies with a pro re nata (PRN) regimen without a loading dose had the greatest mean increase in BCVA of 3.47 letters at 6 months. Studies with a regimen of 3 loading doses followed by PRN dosing had the greatest mean increase in BCVA of 2.20 (6 months) and 13.41 letters (12 months). This is compared to studies following a treat-and-extend regimen which had the lowest change in BCVA with a mean decrease of 1.20 and 0.73 letters at 6 and 12 months, respectively. There was significant change in BCVA among prospective studies, exhibiting a greater increase in mean BCVA of 3.96 and 2.79 letters at 6 and 12 months, respectively, compared to those retrospective in nature in which mean BCVA of <1 letter was noted at 6 and 12 months.
Central retinal thickness
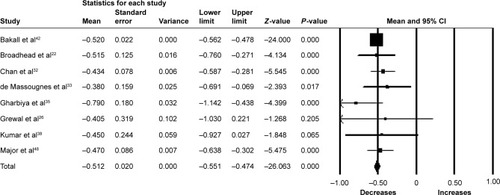
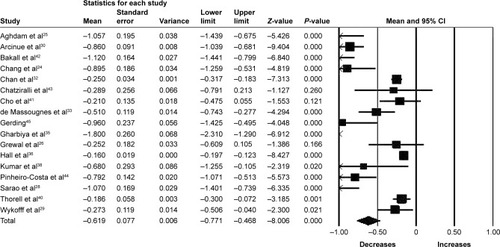
Seventeen studies (1,343 eyes) were included in the meta-analysis of the mean change of CRT between baseline and 6 months. Overall, the pooled results showed a significant reduction in CRT from baseline with a mean reduction of 61.90 µm (95% CI −77.10 to −46.80, P<0.001) ().
Figure 4 Forest plot of 17 studies reporting results of central retinal thickness 6 months after the switch to aflibercept.
Abbreviation: CI, confidence interval.
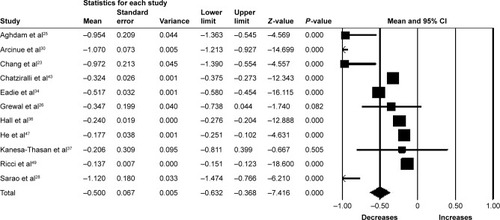
Eleven studies (1,016 eyes) were included in the assessment of CRT change between baseline and 12 months. There was significant reduction in CRT from baseline with a mean reduction of 50.00 µm (95% CI −63.20 to −36.80, P<0.001) (). Significant heterogeneity was found between studies assessing CRT for 6 and 12 months of follow-up (I2=97.01%, P<0.001 and I2=96.95%, P<0.001, respectively).
Figure 5 Forest plot of 11 studies reporting results of central retinal thickness 12 months after the switch to aflibercept.
Abbreviation: CI, confidence interval.
Subgroup analysis of the number of previous anti-VEGF injections and aflibercept injections throughout the study showed similar mean reduction in CRT across the included studies (). Those studies incorporating a dosing regimen of 3 loading doses followed by PRN treatment had a mean reduction of −103.20 (at 6 months) and −95.40 µm (at 12 months) compared to those studies incorporating a dosing regimen of 3 loading doses followed by treat-and -extend treatment which had a mean reduction of −38.40 (6 months) and −24.00 µm (12 months). There was an initial similar difference between study designs, with prospective studies illustrating a greater CRT reduction of −70.00 (6 months) and −85.50 µm (12 months) compared to those retrospective in nature illustrating −58.30 (6 months) and −38.80 µm (12 months).
Table 4 Subgroup analysis of pooled CRT means (95% confidence intervals) in µm
PED height
Eight studies (474 eyes) were included in the analysis of mean change in PED height for 6 months of follow-up. There was a significant reduction in PED height from baseline with a mean reduction of 51.20 µm (95% CI −55.10 to −47.40, P<0.001) (). Heterogeneity (I2=0%, P=0.69) was not detected between studies in this analysis. There was no difference in PED height change across the different dosing regimens and among the study designs (data not shown).
Ocular and systemic adverse events
Reported ocular and systemic adverse events were rare and only reported in 8 of the studies. Another 13 studies indicated that systemic and ocular adverse events were being monitored but with no reported incidence of adverse events. Only a small number of ocular adverse events were reported; among those, endophthalmitis rates were low with 3 reported cases in the cohort of 2,254 eyes (<1%). There were 4 (<1%) reported cases of cataract or lens changes, 3 eyes (<1%) with progression of GA, 3 (<1%) eyes which developed a tear, 2 eyes (<1%) with subretinal hemorrhage, 2 eyes (<1%) with submacular hemorrhage, 1 eye (<1%) with retinal detachment, and 3 eyes with intraocular pressure increase (<1%).
Publication bias
There was evidence of possible publication bias when assessed by the funnel plots and by Egger’s test (P=0.30 BCVA 6 months; P=0.005 BCVA 12 months; P=0.06 CRT 6 months; P=0.02 CRT 12 months; P=0.43 PED 6 months), indicating that the treatment effect was overestimated as majority of studies were having small population sample.
Discussion
In this review, a meta-analysis was used to analyze the efficacy of switching to aflibercept monotherapy in clinical practice among patients with treatment-resistant AMD. The results demonstrated considerable improvement in anatomical outcomes, though functional improvement was limited. Overall, our results show that treatment-resistant patients may benefit from switching to aflibercept in reducing retinal thickness and maintaining visual function.
Although there were significant morphological improvements in terms of CRT and PED height, there were no comparative responses in terms of visual function. The absence of an associated improvement in visual acuity could be due to multiple factors, including better baseline visual acuity, greater foveal thickness, and angiographic lesion type and sizeCitation50–Citation52 limiting the potential for improvement. The chronic nature of nAMD in these patients may progress to atrophic changesCitation12,Citation53,Citation54 and subsequent photoreceptor and RPE damageCitation55 leading to a possible pharmacological ceiling effect. The CATT trials showed an increase in GA with monthly ranibizumab injections;Citation55 thus, it has been suggested aflibercept’s higher binding affinity for VEGF may aggravate preexisting GA in switching patients.Citation56
Furthermore, the chronicity of disease is demonstrated among eyes which received more injections prior to the switch to aflibercept having poorer outcomes than those eyes with fewer previous injections. Additionally, eyes receiving more injections of aflibercept during the study period had minimal improvement in BCVA and CRT reflecting the treatment-resistant nature of this population. Subsequently, a decreased effect over time is suggestive of a drug tolerance as VEGF continues to be expressed as more therapy is administered.Citation57
Prospective studies assessing the switch to aflibercept demonstrated better functional and anatomical improvements than the retrospective studies. Prospective studies typically have a more structured protocol with predefined time points, thus reducing known biases incurred from retrospective study designs.Citation58 The window for visit time-points among retrospective studies may be highly variable, leading to measurement bias.
The pooled results highlight the benefit of a loading dose; the studies which did not include a loading dose did not improve comparatively to those that did.Citation59 This may be a reflection of the chronicity of the disease among these patients requiring more intensive treatment. Few of the included studies reported the frequency of scheduled visits throughout the study period; however, those which did reported monthly follow-up visits supporting the need for more intensive monitoring.
Studies that administered PRN dosing improved more compared to those which administered bimonthly injections over the same period. This is contrary to the VIEW studies among naïve eyes, which did not find any long-term detrimental effects despite the sawtooth pattern of the optical coherence tomography thickness in the bimonthly aflibercept arm.Citation60,Citation61 The number of aflibercept injections administered among the PRN group (range 4.50–5.72) regimens compared to the bimonthly group (range 4.40–5.00) regimens was comparable over the 6 months. Among the studies which had a PRN regimen, only 1 described the number of injections (7.27) over 12 months, and studies which had bimonthly injection had between 5.25 and 10.20 injections. Similarly, PRN dosing regimen displayed the most improvement in contrast to those studies following a loading followed by treat-and -extend regimen (range of injections 3.14–5.30), reflecting the need for closer monitoring in these resistant patients. The slightly higher number of injections within the PRN group may indicate the resistant nature of these eyes, with the majority needing more frequent injections than the bimonthly dosing recommended by the manufacturer. However, it has been reported that aflibercept does not maintain its effect for 2 months in many patients, as 79% of eyes in the subjects with exudative AMD who were incomplete responders to multiple ranibizumab anti-VEGF injections (TURF trials) required treatment on PRN visits,Citation29 indicative of the resistant nature of these patients. The pooled results suggest an individualized PRN treatment regime may yield better results.
The safety profile of aflibercept was similar to bevacizumab and ranibizumab based on information in the included studies.Citation15,Citation62 We reported the outcomes of 28 papers for a total of 2,254 eyes. Although the reporting of adverse events was inconsistent, most of the included studies reported as “no systemic or ocular adverse events occurred”. Among studies which reported adverse events, endophthalmitis and retinal detachment rates were low (<1%).
Several other therapeutic approaches have been suggested to manage treatment resistance in these patients: increasing the frequency between treatment intervals;Citation63,Citation64 increasing the dose of the same anti-VEGF therapy;Citation63,Citation65 and combining anti-VEGF therapy with another treatment modality, such as verteporfin PDT.Citation66 However, switching to a different anti-VEGF agent, such as aflibercept, seems to have the best effect in terms of decreasing CRT, PED height, and the reduction in retinal fluid.
This meta-analysis validates the effectiveness of aflibercept in improving morphology in patients with AMD refractory to ranibizumab and bevacizumab.Citation67,Citation68 This may be because aflibercept binds VEGF-A, VEGF-B, and PlGF, while other anti-VEGFs only bind with VEGF-A. Aflibercept’s higher binding affinity than bevacizumab and ranibizumab may attribute to this better outcome.Citation67 Using aflibercept for those with treatment resistance could potentially inhibit more angiogenic factors improving efficacy.
The results of this meta-analysis should be interpreted with caution for several reasons. Firstly, the available studies generally contained small numbers of participants, which may lead to an overestimation of treatment effect among the individual studies which may accentuate the results of this meta-analysis. Secondly, a majority of the studies were retrospective in nature, and there were no randomized trials to accommodate for confounding variables. Thirdly, there was considerable variability among study designs and dosing regimens.
Conclusion
This analysis shows that patients with nAMD, who have had an inadequate response to ranibizumab and/or bevacizumab, may benefit by switching to aflibercept therapy.
Of importance, the eyes included in this study were poor responders and had limited potential for visual recovery as the disease is both chronic and advanced in nature; nonetheless, they still displayed an improvement. Although vision did not significantly improve, vision stability may have an immense impact on the patient’s quality of life in terms of activities of daily living, independence, and mobility and decrease the risk of comorbidities such as depression.Citation69 As these difficult-to-treat patients are likely to have more chronic disease, recommended bimonthly treatment for naïve eyes may not be adequate for this group. Changing to aflibercept with a loading dose and PRN regimen on an individualized basis may prove to be an effective management strategy.
Acknowledgments
Bayer Corporation Global provided financial support via unrestricted research grant. The sponsor had no role in the design or conduct of this research.
Disclosure
Andrew A Chang is a consultant for Alcon, Bayer, and Novartis. Kimberly Spooner, Thomas Hong, and Wijeyanthy Wijeyakumar report no conflicts of interest in this work.
References
- FriedmanDSO’ColmainBJMuñozBEye Diseases Prevalence Research GroupPrevalence of age-related macular degeneration in the United StatesArch Ophthalmol2004122456457215078675
- KlaverCCWolfsRCVingerlingJRHofmanAde JongPTAge-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam studyArch Ophthalmol199811656536589596502
- WangJJForanSMitchellPAge-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye StudyClin Exp Ophthalmol200028426827311021555
- WongWLSuXLiXGlobal prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysisLancet Glob Health201422e106e11625104651
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ2004821184485115640920
- GehrsKMAndersonDHJohnsonLVHagemanGSAge-related macular degeneration – emerging pathogenetic and therapeutic conceptsAnn Med200638745047117101537
- BresslerNMAge-related macular degeneration is the leading cause of blindnessJAMA2004291151900190115108691
- EmersonMVLauerAKCurrent and emerging therapies for the treatment of age-related macular degenerationClin Ophthalmol20082237738819668729
- U.S. National Institutes of HealthCurrent studies: dry macular degeneration Available from: https://clinicaltrials.gov/ct2/results?term=dry+macular+degeneration&Search=SearchAccessed July 7, 2016
- WangHHartnettMERegulation of signaling events involved in the pathophysiology of neovascular AMDMol Vis20162218920227013848
- WangLLeeAYWiggJPPeshavariyaHLiuPZhangHmiR-126 regulation of angiogenesis in age-related macular degeneration in CNV mouse modelInt J Mol Sci2016176
- JagerRDMielerWFMillerJWAge-related macular degenerationN Engl J Med2008358242606261718550876
- U.S. Food & Drug AdministrationFDA approves new biologic treatment for wet age-related macular degeneration2006 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108685.htmAccessed July 18, 2016
- US Food & Drug AdministrationFDA approves Eylea for eye disorder in older people2011 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280601.htmAccessed July 18, 2016
- HeierJSBrownDMChongVVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- EhlkenCJungmannSBöhringerDAgostiniHTJunkerBPielenASwitch of anti-VEGF agents is an option for nonresponders in the treatment of AMDEye (Lond)201428553854524722504
- BinderSLoss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance?Br J Ophthalmol20129611222157632
- BroadheadGKHongTChangAATreating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degenerationActa Ophthalmol201492871372324925048
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- DownsSHBlackNThe feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventionsJ Epidemiol Community Health19985263773849764259
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- BroadheadGKHongTZhuMResponse of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degenerationRetina201535597598125627086
- ChangAABroadheadGKHongTIntravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration: 12-month safety and efficacy outcomesOphthalmic Res2015552849026637166
- ChangAALiHBroadheadGKIntravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationOphthalmology2014121118819224144450
- AghdamKAPielenAFrammeCJunkerBVisual and anatomic outcomes after conversion to aflibercept in neovascular age-related macular degeneration: 12-month resultsEur J Ophthalmol201626547347826868007
- GrewalDSGillMKSarezkyDLyonATMirzaRGVisual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month resultsEye (Lond)201428789589924833178
- MantelIGianniouCDiraniAConversion to aflibercept therapy versus continuing with ranibizumab therapy for neovascular age-related macular degeneration dependent on monthly ranibizumab treatmentRetina2016361535826166797
- SaraoVParravanoMVerittiDAriasLVaranoMLanzettaPIntra-vitreal aflibercept for chorioidal neovascularization due to age-related macular degeneration unresponsive to ranibizumab therapyRetina201636477077726398691
- WykoffCCBrownDMMaldonadoMECroftDEAflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial)Br J Ophthalmol201498795195524518078
- ArcinueCAMaFBarteselliGSharpstenLGomezMLFreemanWROne-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degenerationAm J Ophthalmol20151593426436.e225461263
- BarthelmesDCampainANguyenPFight Retinal Blindness! Project InvestigatorsEffects of switching from ranibizumab to afliber-cept in eyes with exudative age-related macular degenerationBr J Ophthalmol2016100121640164526994110
- ChanCKJainASaddaSVarshneyNOptical coherence tomographic and visual results at 6 months after transitioning to aflibercept for patients on prior ranibizumab or bevacizumab treatment for exudative age-related macular degeneration (an American Ophthalmological Society thesis)Trans Am Ophthalmol Soc201411216019825646034
- de MassougnesSDiraniAAmbresinADecugisDMarchionnoLMantelIPigment epithelial detachment response to aflibercept in neovascular age-related macular degeneration refractory to ranibizumab: time course and drug effectsRetina201636588188827115852
- EadieJAGottliebJLIpMSResponse to aflibercept in patients with persistent exudation despite prior treatment with bevacizumab or ranibizumab for age-related macular degenerationOphthalmic Surg Lasers Imaging Retina201445539439725230402
- GharbiyaMIannettiLParisiFDe VicoUMungoMLMarencoMVisual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationBioMed Res Int2014201427375424895562
- HallLBZebardastNHuangJJAdelmanRAAflibercept in the treatment of neovascular age-related macular degeneration in previously treated patientsJ Ocul Pharmacol Ther201430434635224552305
- Kanesa-ThasanAGrewalDSGillMKLyonATMirzaRGQuantification of change in pigment epithelial detachment volume and morphology after transition to intravitreal aflibercept in eyes with recalcitrant neovascular AMD: 18-month resultsOphthalmic Surg Lasers Imaging Retina201546663864126114844
- KumarNMarsigliaMMrejenSVisual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degenerationRetina20133381605161223549101
- NarayanDSMueckeJIntravitreal aflibercept treatment in eyes with exudative age-related macular degeneration following prior treatment with intravitreal ranibizumabIndian J Ophthalmol2015631183283626669334
- ThorellMRNunesRPChenGWResponse to aflibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMDOphthalmic Surg Lasers Imaging Retina201445652653325423632
- ChoHShahCPWeberMHeierJSAflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumabBr J Ophthalmol20139781032103523766432
- BakallBFolkJCBoldtHCAflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumabAm J Ophthalmol201315611522.e123706500
- ChatziralliINicholsonLVrizidouEPredictors of outcome in patients with neovascular age-related macular degeneration switched from ranibizumab to 8-weekly afliberceptOphthalmology201612381762177027289179
- Pinheiro-CostaJCostaJMBeatoJNSwitch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practiceOphthalmologica20152333–415516125896317
- GerdingHFunctional and anatomic efficacy of a conversion to aflibercept in eyes with age-related macular degeneration after long-term ranibizumab treatmentKlin Monbl Augenheilkd2015232456056325902122
- HaririADinizBFouLVLamLANittalaMGSaddaSRQuantitative OCT subanalysis of eyes with choroidal neovascularization switched from multiple injections of bevacizumab or ranibizumab to intravitreal afliberceptOphthalmic Surg Lasers Imaging Retina201546219520025707044
- HeLSilvaRAAyoubNMoshfeghiDMLengTExperience with aflibercept for the treatment of neovascular age-related macular degenerationOphthalmic Surg Lasers Imaging Retina201546554254926057757
- MajorJCJrWykoffCCCroftDEAflibercept for pigment epithelial detachment for previously treated neovascular age-related macular degenerationCan J Ophthalmol201550537337726455973
- RicciFParravanoMRegineFAflibercept in persistent neovascular AMD: comparison of different treatment strategies in switching therapyEye (Lond)20163081077108327229701
- FrancisPJThe influence of genetics on response to treatment with ranibizumab (Lucentis) for age-related macular degeneration: the Lucentis Genotype Study (an American Ophthalmological Society thesis)Trans Am Ophthalmol Soc201110911515622253485
- de Oliveira DiasJRRodriguesEBMaiaMMagalhãesOJrPenhaFMFarahMECytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapyBr J Ophthalmol201195121631163721546514
- KrebsIGlittenbergCAnsari-ShahrezaeiSHagenSSteinerIBinderSNon-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degenerationBr J Ophthalmol201397111443144623966368
- MoutrayTAlarbiMMahonGStevensonMChakravarthyURelationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisationBr J Ophthalmol200892336136418303157
- MartinDFMaguireMGFineSLComparison of Age-related Macular Degeneration Treatments Trials (CATT) Research GroupRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- GrunwaldJEDanielEHuangJCATT Research GroupRisk of geographic atrophy in the comparison of age-related macular degeneration treatments trialsOphthalmology2014121115016124084496
- ChengSLengTFactors associated with poor response to aflibercept after switching from ranibizumab or bevacizumab in neovascular age-related macular degenerationOphthalmic Surg Lasers Imaging Retina201647545846527183550
- YangSZhaoJSunXResistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive reviewDrug Des Devel Ther20161018571867
- EuserAMZoccaliCJagerKJDekkerFWCohort studies: prospective versus retrospectiveNephron Clin Pract20091133c214c21719690438
- MenonGChandranMSivaprasadSChavanRNarendranNYangYIs it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc Trial)Eye (Lond)201327895996323743535
- RichardGMonésJWolfSScheduled versus Pro Re Nata Dosing in the VIEW trialsOphthalmology2015122122497250326477840
- FreundKBEngelbertMFineHFIndividualizing the intravitreal anti-VEGF dosing regimen for long-term management of neovascular AMDOphthalmic Surg Lasers Imaging Retina201546550851226057753
- Schmidt-ErfurthUKaiserPKKorobelnikJFIntravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studiesOphthalmology2014121119320124084500
- GasperiniJLFawziAAKhondkaryanABevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisationBr J Ophthalmol2012961142021791509
- ForooghianFCukrasCMeyerleCBChewEYWongWTTachyphylaxis after intravitreal bevacizumab for exudative age-related macular degenerationRetina200929672373119516114
- SchaalSKaplanHJTezelTHIs there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration?Ophthalmology2008115122199220518930553
- WangWHeMZhangXCombined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: a systematic review and meta-analysis of comparative studiesPLoS One2014910e11066725343244
- LazzeriSRipandelliGSartiniMSAflibercept administration in neovascular age-related macular degeneration refractory to previous anti-vascular endothelial growth factor drugs: a critical review and new possible approaches to move forwardAngiogenesis201518439743226346237
- Seguin-GreensteinSLightmanSTomkins-NetzerOA meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with afliberceptJ Ophthalmol20162016409585227042342
- ZhuMWijeyakumarWSyedARVision-related quality of life: 12-month aflibercept treatment in patients with treatment-resistant neovascular age-related macular degenerationGraefes Arch Clin Exp Ophthalmol Epub2016830