Abstract
Purpose
To determine risk factors and clinical signs for severe Acanthamoeba keratitis (AK) by comparing severe cases with mild cases with good prognosis.
Patients and methods
We reviewed medical records of ten cases of AK (five males and five females) referred to our hospital and classified cases into two groups. One eye that required therapeutic keratoplasty and three eyes with a poor visual acuity (<0.2) on last visit were included in the severe group. Six eyes that had good prognosis with a visual acuity of 1.2 on last visit were classified as mild group. We compared patients’ age, the time required for diagnosis, visual acuity on first visit, the history of steroid eye drops use, and other clinical findings.
Results
The average age of the severe group was older than the mild group (P=0.04). The duration between onset and diagnosis of AK and visual acuity on first visit was not statistically different. A history of steroid eye drop use was found in four eyes of the severe group (100%) and four eyes of the mild group (67%). Keratoprecipitates were found in all severe group eyes and one mild group eye during follow-up (P=0.01). One case in the severe group was diagnosed with diabetes mellitus at initial examination. We detected Staphylococcus epidermis by palpebral conjunctival culture in one case of the severe group.
Conclusion
Aging may be a possible risk factor for severe AK. The presence of keratoprecipitates is a possible sign of severe AK. Attention is also required in patients with comorbidities such as diabetes mellitus and bacterial infection.
Introduction
Acanthamoeba keratitis (AK) is a severe and painful corneal infection caused by Acanthamoeba, with a prolonged course featuring remissions and exacerbations.Citation1 AK is an emerging disease with an increasing number of cases presenting each year worldwide, mostly due to the increasing use of contact lens.Citation2 Several papers showed that the incidence of AK was recently increasing in UK, India, and New Zealand.Citation3–Citation5 Additionally, recent AK cases (2013–2015) were reported to be more severe than previous (2009–2012) cases.Citation6 Once established, AK is difficult to treat. Although diagnostic and therapeutic advances have significantly improved overall prognosis, recalcitrant infection and poor outcomes persist in some cases.
Some reports showed that patient-reported duration of symptoms before treatment was reliable in predicting the final visual result and avoiding keratoplasty.Citation7–Citation9 Patients with late diagnosis (>30 days) were reported to have a worse prognosis, poor final visual acuity, as well as prolonged disease period.Citation10 On the contrary, Alfonso-Muñoz et al reported that the infestation depth (within the deep stroma) at the time of diagnosis appeared to be the main risk factor for requiring penetrating keratoplasty, and Tu et al reported that corneal disease staging at presentation with slit-lamp examination was highly predictive.Citation11,Citation12 Carnt et al reported that poor AK outcomes were associated with the presence of severe inflammatory complications, aged >34 years, corticosteroids used before giving antiamoebic therapy, and symptom duration >37 days before antiamoebic therapy.Citation13
The prognosis of AK is important to decide whether aggressive medical or surgical intervention is needed. The purpose of this study was to determine risk factors and clinical signs for severe AK by comparing severe cases with mild cases with good prognosis.
Patients and methods
This was a retrospective comparative case series in a single referral hospital. We reviewed medical records of consecutive ten cases of AK (five males and five females) referred to Saitama Medical Center, Jichi Medical University, between May 2005 and December 2015. All subjects were treated in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of the Jichi Medical University. Written informed consents were obtained from all participants before treatment, as well as additional written informed consents for publication from patients who were described in detail for this study. Patient consent was provided by a parent or legal guardian for any patients under the age of 18 years. According to our IRB approval, patients have the option to opt out of disclosing detailed data including publications. All patients described in the paper have not opted out.
We classified the patients into severe or mild group according to their prognosis. One eye that required therapeutic keratoplasty due to cornea perforation despite intensive conservative therapy and three eyes with slow (over 3 months) response to medical therapy and poor visual acuity (<0.2) on last visit were included in the severe group. Six eyes that showed rapid (within 1 month) response to medical therapy with a best corrected visual acuity (BCVA) of 1.2 on last visit were included in the mild group.
We compared both groups in terms of patient profile, soft contact lens use, systemic disease, the time required for diagnosis, visual acuity on first visit, slit examination on first visit, corneal disease stage on first visit, results of palpebral conjunctiva culture, method of diagnosis, history of steroid eye drops use before diagnosis of AK, existence of keratoprecipitates during follow-up, and cornea scraping frequency. Corneal disease stage was evaluated by slit-lamp biomicroscopy and categorized into one to five levels of progressive anatomic involvement and disease severity (epithelitis, epithelitis with radial neuritis, anterior stromal disease, deep stromal keratitis, or ring infiltrate).Citation12
Statistical analysis
Statistical analysis was performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 2.13.2; The R Foundation for Statistical Computing, Vienna, Austria).Citation14 More precisely, it is a modified version of R commander (version 1.8–4) designed to add statistical functions frequently used in biostatistics. Patient age, gender, contact lens type, systemic disease, slit examination on first visit, the result of palpebral conjunctiva culture, the method of diagnosis, the history of steroid eye drops, and the existence of keratoprecipitates were compared between mild and severe groups using Fisher’s exact test. The time required for presentation, the time required for diagnosis, visual acuity, corneal disease stage at first visit, cornea scraping times, and follow-up period were analyzed using the t-test. Probability (P) values of <0.05 were considered statistically significant.
Results
Patient profile
Patient profiles are shown in . The mean age of patients was 37 (range 25–51) years old in the severe group and 24 (range 15–35) years old in the mild group (P=0.04). One case in the severe group was diagnosed with diabetes mellitus at initial examination (blood sugar level 505 mg/dL, HbA1c value 11.2%), and another case in the severe group had hypertension. All cases were contact lens users, but they had no history of having entered a pool or a bath while wearing contact lens.
Table 1 Patients’ profile of severe Acanthamoeba keratitis cases group and mild Acanthamoeba keratitis cases group
The duration between onset and diagnosis of AK was 240 days in one case of severe group, and in all of the other cases was within 30 days. There was no association between diagnostic delay and severity of AK (P=0.18).
Clinical course
AK was diagnosed by detection of Acanthamoeba cysts in corneal smears in six eyes or characteristic clinical findings of AK in four eyes (). BCVA and slit-lamp examination levels at first visit were not significantly different in both groups. Staphylococcus epidermis was detected by palpebral conjunctiva culture in one case of the severe group, which was resistant to topical antibiotics.
Table 2 Patients’ data at first visit and diagnostic method of severe and mild Acanthamoeba cases
A history of steroid eye drops use was found in all eyes (three with 0.1% betamethasone, one with 0.1% fluorometholone) of the severe group and four of six eyes (all with 0.1% fluorometholone) of the mild group (steroid use P=0.2, type of steroid use P=0.07) (). Mean number of cornea scrapings was higher in the severe group (P=0.07).
Table 3 Steroid eye drop use before diagnosis, the existence of keratoprecipitates during follow-up, cornea scraping times, visual acuity at last visit, and follow-up period
Keratoprecipitates were found in all eyes of the severe group during follow-up, but only in one eye in the mild group (P=0.01). Keratoprecipitates appeared 3–21 days after stopping steroid eye drops in two eyes of the severe group and one eye of the mild group, but they were observed despite steroid eye drop use in two eyes of the severe group (one eye was on betamethasone, the other eye on fluorometholone). Slit-lamp findings associated with keratoprecipitates were one case of keratoneuritis in the mild group, three cases of ring infiltration (two eyes with steroid eye drops use) in the severe group, and one disciform ulcer in the severe group.
Case presentations
S-2
A 51-year-old male was referred to our hospital with keratitis of unknown origin from 11 days prior to visit. He used a monthly type disposable soft contact lens, and his contact lens case was filthy (). He had only been using saline for contact lens care. His BCVA was 0.01. He had a corneal ring ulcer, Descemet’s folds, severe ciliary injection, and severe scleritis (); 0.1% fluorometholone eye drop and 0.5% levofloxacin eye drop were prescribed by the former physician. We found Acanthamoeba cysts by corneal smears and S. epidermis that was resistant to levofloxacin and other some topical antibiotics by palpebral conjunctiva culture. We discontinued 0.1% fluorometholone and levofloxacin and started treatment with corneal epithelial debridement, chlorhexidine gluconate eye drops, antifungal eye drops, 0.3% tobramycin eye drop, 0.5% cefmenoxime eye drop, and systemic antifungal drugs. He presented a disciform infiltration with keratoprecipitates 2 months after referral (). The inflammation eventually tapered off, leaving a corneal scar and mature cataract 12 months after referral (). His visual acuity on last visit was hand movement.
Figure 1 Slit-lamp photographs.
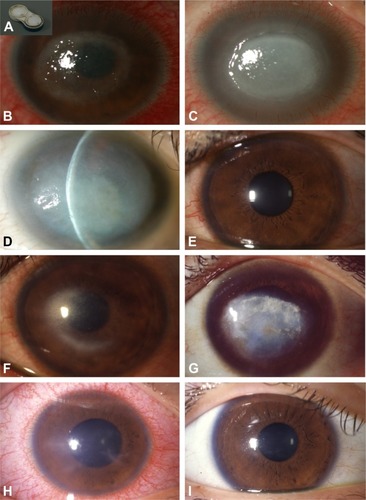
S-4
A 35-year-old male was referred to our hospital diagnosed with herpetic stromal keratitis that repeated exacerbation and remission from 5 months previously. He was a daily disposable soft contact lens user, but he had worn his contact lens consecutively for 3 days just before the onset of keratitis. His BCVA was 0.8, and he had superficial punctate keratopathy and corneal stromal infiltration (). Acyclovir ophthalmic ointment and 0.1% betamethasone eye drop six times a day were prescribed. Corneal smear was negative. Three months after referral, immediately after tapering betamethasone eye drop to three times a day, ring infiltration with keratoprecipitates and severe ciliary injection with severe ocular pain and tearing occurred (). Therefore, we changed the diagnosis to AK based on these clinical findings. After diagnosis, we stopped betamethasone use and started corneal epithelial debridement, chlorhexidine gluconate eye drops, antifungal eye drops, and systemic antifungal drugs. Two years after referral, he had severe corneal scars with no inflammation and his BCVA was 0.07 ().
M-5
A 17-year-old high school girl was referred to our hospital with superficial corneal stromal opacity and ciliary injection with superficial punctate keratopathy due to contact lens use. She had radial neurokeratitis, pseudodendritic keratitis, superficial stromal infiltration, and iritis on her first visit (). Her BCVA was 0.15. She was diagnosed with AK after amoeba cysts were detected by corneal smear. We treated her with corneal epithelial debridement, chlor-hexidine gluconate eye drop, and antifungal eye drops. Three weeks after referral, her BCVA was 1.2 and her cornea was fully recovered ().
Discussion
In this study, the average age of the severe group was older than the mild group. This result is similar to other reports.Citation6,Citation13 This may reflect the fact that aging changes the function of the systemic immune systemCitation15 and may lead to severe keratitis in the elderly. One patient had diabetes in the severe group, which is well-known as a risk factor for infectious keratitis.Citation16 However, systemic diseases were not associated with severity of AK in this study.
We could not find an association between contact lens type and severity of AK in this case series because of too much variation in the type of contact lens used. However, it is worth noting that all our cases were contact lens wearers. The development of AK in contact lens users is strongly related to poor lens hygiene and contaminated water.Citation2,Citation10,Citation13,Citation17–Citation21 In this study, lens care was normal in most patients, but one case in the severe group (S-2) had only been using saline for contact lens care and his contact lens case was filthy. Poor contact lens hygiene may be a risk factor for severe AK. The overnight wear of lenses remains the dominant risk factor for the development of AK,Citation22,Citation23 but only one case in our severe group (S-4) used extended wear lens. The use of contact lens while swimming or bathing is reported to be a risk factor for AK,Citation13 but there were no cases of using contact lens as such in our study.
Some papers report that an early diagnosis and early start of antiamoebic treatment result in a better final visual acuity;Citation4,Citation6–Citation10,Citation13 however, nine out of ten of our patients were diagnosed within 1 month after the onset of AK. There was no association between diagnostic delay and severity of AK in our study. This suggests that there may be other important factors for AK prognosis than early diagnosis.
Visual acuity at presentation was not associated with outcome. Furthermore, corneal disease stage and slit-lamp examination at presentation were not associated with prognosis in our case series, which differed from a previous study, suggesting that corneal disease staging at presentation was highly predictive of outcomes.Citation11,Citation12 However, eight out of ten patients in our series used steroid eye drops before diagnosis, and therefore, visual acuity and slit-lamp examination may have been masked. Furthermore, S-4 only had superficial punctate keratopathy at first visit and was diagnosed as stage 1; however, he had a ring ulcer when AK was diagnosed, after which he was diagnosed as stage 5. Therefore, staging at diagnosis may have a major impact on prognosis.
Severe cases tended to use betamethasone eye drops, and mild cases tended to use fluorometholone eye drops before diagnosis, but there was no significant difference. Betamethasone use may have triggered a bad prognosis. On the contrary, strong inflammation in the severe group may have prompted the physician to prescribe betamethasone eye drops. Because there was no delay in diagnosis of the severe group, we believe that strong inflammation was the reason for betamethasone use.
Keratoprecipitates were found in all severe group eyes and one eye of the mild group (P=0.01). Keratoprecipitates are signs of deep infiltration of Acanthamoeba associated with inflammation in the anterior chamber. Keratoprecipitates may be associated with the use of betamethasone in the severe group because of strong inflammation. The reasons for deep amoeba infiltration may be due to: 1) immune suppression by using steroid eye drops; 2) patients with comorbidities such as age, diabetes, and mixed infection. We examined the relationship of keratoprecipitates presentation and steroid eye drop use. Two eyes in the severe group and one eye in the mild group showed keratoprecipitates 3–21 days after stopping steroid eye drops. This is most likely due to the fact that steroid eye drops masked keratoprecipitates appearance or suppressed immune reaction. Interestingly, another two eyes in severe group had keratoprecipitates regardless of using steroid eye drops (betamethasone: one eye, fluorometholone: one eye). The presence of keratoprecipitates while using steroid eye drops may be a sign of very severe Acanthamoeba infection.
The resistant bacteria in case S-2 may have been a nutrient source for Acanthamoeba. Acanthamoeba may proliferate by consuming bacteria. In this case, coinfection of bacteria may have been one reason of the severity of AK. This particular case in the severe group was interesting because the presence of bacteria in AK was reported to be a requirement for the development of AK.Citation24
Limitation
The limitation of this study is the small number of cases. This may be due to the fact that the number of AK patients has recently been declining in Japan due to increasing public awareness on proper contact lens use publicized by ophthalmic societies.Citation25 If more cases were examined, a more detailed investigation on prognostic factors may be possible.
Conclusion
Aging may be a possible risk factor for severe AK, and the presence of keratoprecipitates especially while using corticosteroids eye drops is a possible sign of severe AK. Attention is also required in patients with comorbidities such as diabetes mellitus and bacterial infection.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Dr Shigeto Shimmura of the Department of Ophthalmology of Keio University School of Medicine for English editing support.
Disclosure
The authors report no conflicts of interest in this work.
References
- MaycockNJJayaswalRUpdate on Acanthamoeba keratitis: diagnosis, treatment, and outcomesCornea201635571372026989955
- JuárezMMTártaraLICidAGAcanthamoeba in the eye, can the parasite hide even more? Latest developments on the diseaseCont Lens Anterior Eye201841324525129273391
- CarntNHoffmanJJVermaSAcanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factorsBr J Ophthalmol Epub2018919
- LalithaPLinCCSrinivasanMAcanthamoeba keratitis in South India: a longitudinal analysis of epidemicsOphthalmic Epidemiol201219211111522364672
- MckelvieJAlshiakhiMZiaeiMPatelDVMcgheeCNThe rising tide of Acanthamoeba keratitis in Auckland, New Zealand: a 7-year review of presentation, diagnosis and outcomes (2009–2016)Clin Exp Ophthalmol201846660060729412494
- RoozbahaniMHammersmithKMRapuanoCJNagraPKZhangQESiuSYAcanthamoeba keratitis: are recent cases more severe?Cornea201837111381138729847494
- ClaerhoutIGoegebuerAvan den BroeckeCKestelynPDelay in diagnosis and outcome of Acanthamoeba keratitisGraefes Arch Clin Exp Ophthalmol2004242864865315221303
- DartJKSawVPKilvingtonSAcanthamoeba keratitis: diagnosis and treatment update 2009Am J Ophthalmol20091484e482487499
- IllingworthCDCookSDKarabatsasCHEastyDLAcanthamoeba keratitis: risk factors and outcomeBr J Ophthalmol19957912107810828562539
- LeeMHAbellRGMitraBFerdinandsMVajpayeeRBRisk factors, demographics and clinical profile of Acanthamoeba keratitis in Melbourne: an 18-year retrospective studyBr J Ophthalmol2018102568769128844988
- Alfonso-MuñozEARoig-RevertMJFernández-LópezEHernández-DíazMAraujo-MirandaRPeris-MartínezCA report of 10 patients with Acanthamoeba keratitisArch Soc Esp Oftalmol2018931049750229885816
- TuEYJoslinCESugarJShoffMEBootonGCPrognostic factors affecting visual outcome in Acanthamoeba keratitisOphthalmology2008115111998200318571729
- CarntNRobaeiDMinassianDCDartJKGAcanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complicationsBr J Ophthalmol2018102101431143529298778
- KandaYInvestigation of the freely available easy-to-use software ‘EZR’ for medical statisticsBone Marrow Transplant201348345245823208313
- DenkingerMDLeinsHSchirmbeckRFlorianMCGeigerHHSC aging and senescent immune remodelingTrends Immunol2015361281582426611154
- BadawiAEMoemenDEl-TantawyNLEpidemiological, clinical and laboratory findings of infectious keratitis at Mansoura Ophthalmic Center, EgyptInt J Ophthalmol2017101616728149778
- JeongHJLeeSJKimJHAcanthamoeba: keratopathogenicity of isolates from domestic tap water in KoreaExp Parasitol2007117435736717574243
- JoslinCETuEYMcmahonTTPassaroDJStaynerLTSugarJEpidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreakAm J Ophthalmol2006142221221716876498
- JoslinCETuEYShoffMEThe association of contact lens solution use and Acanthamoeba keratitisAm J Ophthalmol2007144216918017588524
- KilvingtonSGrayTDartJAcanthamoeba keratitis: the role of domestic tap water contamination in the United KingdomInvest Ophthalmol Vis Sci200445116516914691169
- RadfordCFMinassianDCDartJKAcanthamoeba keratitis in England and Wales: incidence, outcome, and risk factorsBr J Ophthalmol200286553654211973250
- KeayLEdwardsKDartJStapletonFGrading contact lens-related microbial keratitis: relevance to disease burdenOptom Vis Sci200885753153718594345
- StapletonFKeayLEdwardsKThe incidence of contact lens-related microbial keratitis in AustraliaOphthalmology2008115101655166218538404
- NakagawaHHattoriTKoikeNInvestigation of the role of bacteria in the development of acanthamoeba KeratitisCornea201534101308131526203748
- ToriyamaKSuzukiTOhashiYSurvey of the number of Acanthamoeba keratitis cases in JapanNippon Ganka Gakkai Zasshi20141181283224505933

