Abstract
Purpose
To assess the safety and efficacy of transitioning patients whose intraocular pressure (IOP) had been insufficiently controlled on prostaglandin analog (PGA) monotherapy to treatment with travoprost 0.004%/timolol 0.5% fixed combination with benzalkonium chloride (TTFC).
Methods
This prospective, multicenter, open-label, historical controlled, single-arm study transitioned patients who had primary open-angle glaucoma, pigment dispersion glaucoma, or ocular hypertension and who required further IOP reduction from PGA monotherapy to once-daily treatment with TTFC for 12 weeks. IOP and safety (adverse events, corrected distance visual acuity, and slit-lamp biomicroscopy) were assessed at baseline, week 4, and week 12. A solicited ocular symptom survey was administered at baseline and at week 12. Patients and investigators reported their medication preference at week 12.
Results
Of 65 patients enrolled, 43 had received prior travoprost therapy and 22 had received prior nontravoprost therapy (n = 18, bimatoprost; n = 4, latanoprost). In the total population, mean IOP was significantly reduced from baseline (P = 0.000009), showing a 16.8% reduction after 12 weeks of TTFC therapy. In the study subgroups, mean IOP was significantly reduced from baseline to week 12 (P = 0.0001) in the prior travoprost cohort (19.0% reduction) and in the prior nontravoprost cohort (13.1% reduction). Seven mild, ocular, treatment-related adverse events were reported. Of the ten ocular symptom questions, eight had numerically lower percentages with TTFC compared with prior PGA monotherapy and two had numerically higher percentages with TTFC (dry eye symptoms and ocular stinging/burning). At week 12, TTFC was preferred over prior therapy for 84.2% of patients (48 of 57) by the patients themselves, and for 94.7% of patients (54 of 57) by their physicians.
Conclusion
When TTFC replaced PGA monotherapy in patients whose IOP had been uncontrolled, the outcome was a significant reduction in IOP and an acceptable safety and tolerability profile. Most patients and investigators preferred TTFC to prior PGA monotherapy.
Introduction
Travoprost 0.004%/timolol maleate 0.5% fixed combination with benzalkonium chloride (TTFC) gained its first commercial approval as DuoTrav® (Alcon Laboratories, Inc, Fort Worth, TX) eye drops in 2006 for the treatment of patients with open-angle glaucoma or ocular hypertension who need further reduction of intraocular pressure (IOP) from a beta-blocker or a prostaglandin analog (PGA) monotherapy.Citation1 Compared with concomitant therapy, fixed-combination products are advantageous because of increased convenience to the patient (due to dispensing from only one bottle), avoidance of drug washout (as may occur when two individual drugs are administered too quickly in succession), and reduced lifetime exposure to ocular preservatives.
TTFC is composed of a PGA and a beta-blocker, the two drugs that are most often used as first-line therapy for ocular hypertension or glaucoma.Citation2 The combination of these two agents is commonly used to treat patients whose IOP has failed to demonstrate sufficient reduction on monotherapy. Randomized studies have demonstrated that TTFC produces a significant reduction in IOP compared with single-agent timolol or travoprost.Citation3–Citation5
The current study examines replacement therapy for patients receiving insufficient IOP control from PGA monotherapy and incorporates two important elements into its design: evening dosing and transition from monotherapy. The dosing timing is important because the first PGA-based fixed-combination product available, latanoprost 0.005%–timolol maleate 0.5% fixed combination (Xalacom®; Pfizer, Inc, New York, NY), was approved for once-daily morning dosing. Thus, despite the fact that TTFC was approved without a specified timing for its once-daily dosing,Citation1 several studies have used morning dosing to examine its efficacy.Citation5–Citation7 However, outcomes of studies investigating the preferred timing of TTFC dosing have been mixed, with one study showing an efficacy advantage for evening dosingCitation8 and two reporting no difference between morning and evening dosing.Citation9,Citation10 Clearly, additional investigation of the TTFC dosing schedule is warranted. The transition design of the current study, in which medication was changed from PGA monotherapy to TTFC, is desired because it mimics what is often done in routine clinical practice, unlike the randomized studies that have been conducted to compare TTFC to other therapies under artificially controlled conditions.Citation3–Citation5,Citation11–Citation13 The aim of the current study was to assess the safety and efficacy of once-daily TTFC dosed in the evening in patients with open-angle glaucoma or ocular hypertension who had received insufficient IOP reduction with PGA monotherapy.
Methods
Study design
This was a prospective, multicenter, open-label, historical controlled, single-arm, 12-week transition study in patients who had primary open-angle glaucoma, pigment dispersion glaucoma, or ocular hypertension, and who required further IOP reduction despite treatment with PGA monotherapy. At the screening/baseline visit, which was scheduled at 10 am, all patients who enrolled in the study completed the ocular symptom survey, discontinued their PGA monotherapy, and were given travoprost 0.004%/timolol 0.5% fixed combination (DuoTrav® eye drops; Alcon Laboratories, Inc), along with instructions to self-administer the topical ocular medication one drop once daily at 8 pm for 12 weeks. Patients were required to return at week 4 and week 12 (within 1 hour of the time of IOP assessment at the screening/baseline visit) for IOP and safety assessments in both eyes. At week 12, patients completed the ocular symptom survey, and both patients and investigators completed the global preference survey. The protocol was approved by all relevant Institutional Review Boards and the study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. All participating patients provided written informed consent.
Participants
Patients from four sites in Brazil were recruited by investigators to participate in this study. Eligible patients were adults (≥18 years of age) with a clinical diagnosis of primary open-angle glaucoma, pigment dispersion glaucoma, or ocular hypertension in at least one eye [study eye(s)]. IOP, measured at 10 am, had to be between 19 mmHg and 25 mmHg in the study eye(s) and ≤35 mmHg in both eyes after having been treated with PGA monotherapy for at least 2 weeks prior to the screening/baseline visit. If both eyes were eligible for the study, both eyes were treated with study medication, but only the right eye was chosen for analysis. The last dose of PGA must have been instilled correctly the night prior to the screening/baseline visit. IOP had to be considered safe in both eyes, such that clinical stability of vision and the optic nerve was assured throughout the trial. Moreover, in nonstudy eyes, IOP had to be controlled without pharmacologic therapy or on the study medication alone. In addition, patients were required to have a corrected distance visual acuity (CDVA) of 20/200 or better in each eye and those with glaucoma had to have a recent (within 3 months) visual field examination showing a mean deviation better than –15 dB and be without threat to fixation. Finally, eligible patients had to be able to follow instructions, to be willing and able to attend all study visits, and to provide informed consent prior to screening.
Patients were excluded if they met any of the following criteria: any abnormality preventing reliable applanation tonometry in either eye; any opacity or patient uncooperativeness that restricted adequate examination of the anterior chamber of the study eye(s); risk of visual field or visual acuity worsening as a consequence of participation in the trial, in the investigator’s opinion; intraocular conventional surgery or laser surgery in the study eye(s) less than 3 months prior to the screening/baseline visit; progressive retinal or optic nerve disease from any cause; corneal dystrophies in either eye; concurrent infectious/noninfectious conjunctivitis, keratitis, or uveitis in either eye; history of ocular herpes simplex; history or risk of uveitis or cystoid macular edema; severe allergic rhinitis; unwillingness to accept the risk of darkened irides or eyelash changes; known medical history of allergy, hypersensitivity, or poor tolerance to any components of the study medication that was deemed to be clinically significant, in the investigator’s opinion; use of topical or systemic beta-adrenergic blockers or topical or systemic steroids; bronchial asthma or history of bronchial asthma, bronchial hyperreactivity, or severe chronic obstructive pulmonary disease that would preclude the safe administration of a topical beta-blocker; sinus bradycardia, second-degree or third-degree atrioventricular block, sinoatrial block, overt cardiac failure, or cardiogenic shock that would preclude the safe administration of a topical beta-blocker; use of systemic medications known to affect IOP, which had not been on a stable course for 7 days prior to the screening/baseline visit or which had an anticipated dosing change during the course of the study; any clinically significant, serious, or severe medical or psychiatric condition; any condition that, in the investigator’s opinion, would interfere with optimal participation in the study or present a special risk to the patient; participation in any other investigational study within 30 days prior to the screening/baseline visit; women who were pregnant or lactating; and women of childbearing potential who were not using reliable means of birth control.
Outcomes
Efficacy parameters were IOP evaluations, which were performed at each visit using a Goldmann applanation tonometer that had been calibrated within the month prior to the screening/baseline visit. Week 4 and week 12 visits were scheduled within 1 hour of the time of the patient’s screening/baseline visit (ie, 10 am ± 1 hour). The primary efficacy outcome was the change in mean IOP from baseline in patients who transitioned from prior travoprost therapy to TTFC. For secondary efficacy outcomes, the same outcome was measured in the total patient population and in patients who had received prior nontravoprost PGA monotherapy (bimatoprost or latanoprost). Exploratory efficacy parameters assessed were the percentage of patients reaching target IOP (≤18 mmHg) and the percentage of patients achieving ≥2 mmHg reductions in IOP from baseline.
Safety variables included solicited adverse events (AEs, via a solicited ocular symptom survey), unsolicited AEs, CDVA, and slit-lamp biomicroscopy. The solicited ocular symptom survey, which was completed by each patient at the screening/baseline visit and at the week 12 visit, contained eleven questions: ten about the occurrence of individual ocular symptoms (dry eye, photophobia, increased tearing, stinging/burning, crusting, itching, foreign body sensation, irritation, and redness [as judged by the patient and by others]), and one about the ease of instillation of the study medication. This survey is not a validated instrument, but it has been used in a clinical trial published previouslyCitation14 to assess patient experience with other types of IOP-lowering therapies. Adverse events were collected, monitored, and evaluated throughout the study and were recorded at each visit. CDVA was measured in the study eye at each visit using a Snellen visual acuity chart. If patients had more than one error on a line, CDVA values were rounded up to the poorer line. Slit-lamp biomicroscopy was performed at each visit using the investigator’s usual clinical technique. Tolerability was assessed at the week 12 visit via a global preference survey, which involved asking both patients and investigators to choose which therapy they preferred: TTFC or previous PGA monotherapy.
Objectives
The primary objective of this study was to assess the safety and efficacy of TTFC as transition therapy for patients with primary open-angle glaucoma, pigment dispersion glaucoma, or ocular hypertension who had not achieved sufficient reduction in IOP on PGA monotherapy. We hypothesized that, on average, patients switched from any prior PGA monotherapy to TTFC would demonstrate further reductions in IOP while experiencing sufficient tolerability with their new medication.
Statistical methods
Mean change in IOP between prior therapy (at screening/baseline) and TTFC (at week 4 and week 12) was compared using a one-way analysis of variance (ANOVA) test. Assuming a standard deviation (SD) of 2.8 mmHg and an α-level of 0.05, this study provided an 80% power to detect a difference of 1.5 mmHg between travoprost and TTFC if at least 27 patients were analyzed for that cohort (prespecified calculation) or a 90% power to detect a difference of 1.5 mmHg if at least 39 patients were analyzed (post hoc calculation). The percentage of patients who preferred TTFC compared with those who preferred prior therapy or who judged the two treatments to be similar was analyzed using a chi-square test, as was the percentage of investigators who preferred TTFC. Mean age of the two patient cohorts was compared using an unpaired Student’s t-test and both sex and race were compared using a chi-squared test.
An α-level of 0.05 was used to declare statistical significance. All data analyses, which were intent-to-treat (ITT), were two-sided. For patients in the ITT analysis who attended at least one visit but were missing one or more other visits, the last observation was carried forward. If both eyes were eligible for analysis, the right eye was used. Statistical analysis was performed using Statistica 5.1 (StatSoft Inc, São Caetano do Sul, São Paulo, Brazil) by a biostatistician who had been contracted by the study sponsor.
Results
Participants
A total of 65 patients were enrolled and treated with TTFC from June 4, 2009 through November 26, 2010. All 65 patients were included in the ITT population. Two patients in the ITT population who completed the study had an IOP less than 19 mmHg at the screening/baseline visit in the study eye, which violated the inclusion criterion stating that patients must have had an IOP between 19 mmHg and 25 mmHg in the study eye. One patient who had received prior travoprost (for at least 2 weeks) was lost to follow-up before the week 4 visit. Seven additional patients discontinued the study by the week 12 visit (n = 4, lack of response to study medication; n = 1, adverse event; n = 2, lost to follow-up). Therefore, 57 patients completed the study.
Demographics and baseline characteristics
Demographics and baseline characteristics of the ITT population are shown in . The patient population had a mean age of 61.7 years. Forty-three patients had received prior travoprost therapy and 22 had received prior treatment with either bimatoprost (n = 18) or latanoprost (n = 4). No statistical differences were observed in any of the demographics between patients receiving prior travoprost and patients receiving prior nontravoprost therapy.
Table 1 Patient demographics and baseline characteristics
Intraocular pressure
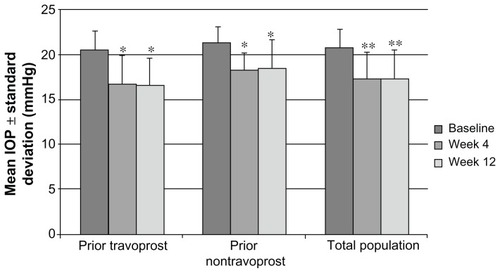
The primary endpoint of the study, mean change in IOP from travoprost to TTFC, demonstrated a significant 18.5% reduction after 4 weeks of TTFC (20.5 ± 2.1 mmHg versus 16.7 ± 3.2 mmHg; P = 0.0001) and a significant 19.0% reduction after 12 weeks of TTFC (20.5 ± 2.1 mmHg versus 16.6 ± 3.0 mmHg; P = 0.0001; ). A similar, signif icant decrease in mean IOP was observed whether examining the total ITT populat ion at week 4 (20.8 ± 2.0 mmHg versus 17.3 ± 2.9 mmHg; P = 0.00002; 16.8%) or week 12 (20.8 ± 2.0 mmHg ver sus 17.3 ± 3.2 mmHg; P = 0.000009; 16.8%) or the cohort of patients receiving prior nontravoprost therapy at week 4 (21.3 ± 1.8 mmHg versus 18.2 ± 2.0 mmHg; P = 0.0001; 14.6%) or week 12 (21.3 ±1.8 mmHg versus 18.5 ±3.1 mmHg; P = 0.0001; 13.1%).
Figure 1 Mean IOP across visits by patient cohort.
**P < 0.0001, baseline versus week 4 or week 12, as measured by ANOVA.
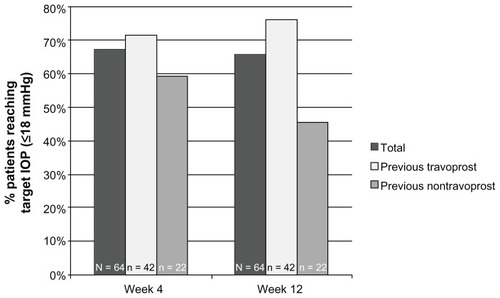
The percentage of patients from the ITT population who reached target IOP (≤18 mmHg) was 67.2% (43 of 64) at week 4 and 65.6% (42 of 64) at week 12 (). Over three-quarters (76.2%; 32 of 42) of patients in the prior travoprost cohort reached target IOP at week 12, and nearly half (45.5%; 10 of 22) did so in the prior nontravoprost cohort.
Figure 2 Percentage of patients reaching target IOP across visits by patient cohort.
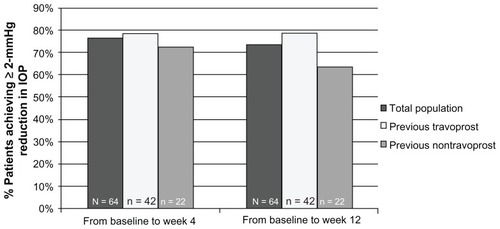
In the total ITT patient population, 76.6% (49 of 64) achieved at least a 2-mmHg reduction in IOP from baseline at the week 4 visit, and 73.4% (47 of 64) did so at the week 12 visit (). Both prior therapy cohorts achieved similar proportions of ≥2 mmHg reductions at the week 4 visit. After 12 weeks of treatment, 78.6% of patients in the prior travoprost cohort (33 of 42) and 63.6% of patients in the prior nontravoprost cohort (14 of 22) reached ≥2 mmHg reductions.
Figure 3 Percentage of patients achieving ≥2 mmHg reductions in IOP across visits by patient cohort.
Adverse events and other safety measures
Patients from the ITT population experienced a total of eight AEs, all of which were mild in severity (). All AEs, except the cold, were related to study medication. One patient who experienced ocular redness and burning discontinued the study as a result of the AE. No serious AEs were reported during the study.
Table 2 Adverse events of all enrolled patients (N = 65)
Among the ITT population, 95.3% of patients at week 12 (61 of 64 with last observation carried forward) had a CDVA in the study eye that was equal to or better than baseline CDVA. One patient had a CDVA in the study eye of 20/30 at the screening/baseline visit and 20/63 at week 4. This reduction was not reported as an AE, and the patient discontinued the study by the week 12 visit due to lack of response to treatment. The distribution of CDVA among patients at week 12 was similar to the distribution at baseline. Slit-lamp biomicroscopy findings at week 12 showed no increases in the number of any ocular abnormality from baseline.
Ocular symptom survey
Of the ten symptoms queried among the ITT population, eight showed a decrease in the incidence scores from the screening/baseline visit to the week 12 visit. The other two demonstrated numerically higher percentages at the week 12 visit compared with baseline (dry eye symptoms [21.1% versus 20.0%] and ocular stinging/burning [40.4% versus 24.6%]; ). More patients considered TTFC easy to administer after 12 weeks of therapy (98.2%; 56 of 57), compared to the ease of administration of their baseline therapy (90.8%; 59 of 65).
Table 3 Survey results, with ocular symptoms at week 12 sorted from lowest to highest incidence
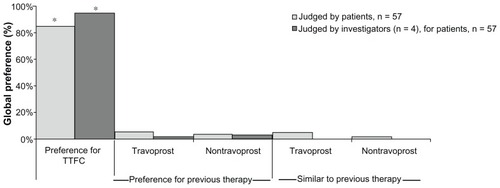
Global preference survey
After transitioning to 12 weeks of treatment with TTFC from prior PGA monotherapy, 84.2% of patients in the ITT population (48 of 57) preferred TTFC compared with 8.8% (5 of 57) who preferred prior therapy and 7.0% (4 of 57) who judged TTFC to be similar to prior therapy (). Significantly more patients preferred TTFC compared with those who preferred previous therapy plus those who judged TTFC to be similar to previous therapy (P < 0.0001). Twelve weeks after patients had transitioned to TTFC from prior therapy, investigators (n = 4) preferred TTFC for 94.7% of their patients (54 of 57) and preferred prior therapy for 5.3% of their patients (3 of 57; P < 0.0001).
Discussion
The current study demonstrated that TTFC dosed in the evening produced a significant, additional reduction in IOP in patients transitioning from PGA monotherapy, whether examining the travoprost or nontravoprost cohort. Nearly two-thirds of enrolled patients reached target IOP and nearly three-quarters achieved at least an additional 2 mmHg reduction in IOP after 12 weeks of TTFC therapy, when compared to prior therapy. These findings indicate that TTFC can further reduce IOP in patients whose IOP failed to achieve sufficient reduction while on PGA monotherapy. Although more patients on prior travoprost therapy appeared to achieve IOP control than those on prior nontravoprost therapy (), the small and unbalanced sizes of the cohorts (n = 22; nontravoprost cohort; n = 42, travoprost cohort) makes it difficult to draw any conclusions from the results of this exploratory outcome measure. A larger, controlled trial would have to be conducted to address this issue.
Previously published studies support the claim that TTFC can provide IOP improvements in patients receiving insufficient benefit from ocular hypotensive therapy. Similar to the current trial design, one study transitioned patients from monotherapy (with a PGA or a beta-blocker) to TTFC.Citation15 After 6 months of TTFC therapy, patients demonstrated a 20% reduction in IOP. In another study, patients were transitioned from monotherapy or combination therapy to TTFC; those on prior monotherapy achieved a 27% reduction in IOP and those on combination therapy demonstrated a 21% reduction after 3 months.Citation14 Finally, additional studies examining the transition of patients from either unfixed or fixed combination therapy with a nontravoprost PGA and a beta-blocker found that even these patients can benefit from transition to TTFC.Citation16,Citation17
As with any clinical study, this trial did have some limitations that need to be considered when interpreting its results. The transition trial design is less controlled than a randomized study, which produces more robust results; however, randomized trial results are often less applicable to the real-world use of a medication. Furthermore, the trial design may have allowed for an imbalance in compliance between the two regimens being evaluated. Oftentimes, patients are more compliant with medications within clinical studies than in the “real world”.Citation18 Because the prior PGA monotherapy was used outside the context of the clinical trial (ie, before the trial began), the possibility exists that patients may have exhibited increased compliance with TTFC during the clinical study, which could have positively impacted the IOP results. The open-label design of the study, and the circumstances of participating in a study and interacting more often with their physician,Citation19 may also have affected the patients’ perception of the study medication. Finally, the limited scope of the solicited symptom survey may have created an incomplete assessment of the tolerability profile.
In conclusion, this study suggests that patients receiving insufficient IOP reduction from PGA monotherapy view transition to travoprost 0.004%/timolol 0.5% fixed combination favorably and can be expected to achieve further IOP improvements.
Acknowledgments
Jennifer Klem, PhD provided medical writing support, which was funded by Alcon Laboratories, Inc. The authors acknowledge Dr Guilherme Guedes, who assisted in study-related operations at Dr Moreira’s site, for critical review of the manuscript. The authors also acknowledge the following coinvestigators for their participation in the trial: Dra Alana Mendonça de Santana and Dra Milena Gavros (for Dr Costa), Dra Sueli Lima Teixeira and Dr. Marcelo Shindy Iwamoto (for Dr Moreira), Dr Diego Tebaldi de Queiróz Barbosa, Dra Sheila Cristina Caniçali and Dra Adriana Chaves de Oliveira (for Dr Paolera), and Dr Mitsuo Hashimoto and Dr Alvio Isao Shiguematsu (for Dra. Silva).
Disclosure
The authors report no conflicts of interest in this work.
References
- Alcon Laboratories IncSummary of product characteristics (SPC) DuoTrav® Eye Drops SolutionHemel HempsteadAlcon Laboratories (UK) Limited2011
- SinghKShrivastavaAMedical management of glaucoma: principles and practiceIndian J Ophthalmol2011Suppl 59S889221150040
- BarnebeyHSOrengo-NaniaSFlowersBEThe safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solutionAm J Ophthalmol200514011715990081
- KonstasAGMikropoulosDHaidichABNtamposKSStewartWCTwenty-four-hour intraocular pressure control with the travoprost/timolol maleate fixed combination compared with travoprost when both are dosed in the evening in primary open-angle glaucomaBr J Ophthalmol200993448148519019932
- SchumanJSKatzGJLewisRAEfficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertensionAm J Ophthalmol2005140224225016086946
- GrossRLSullivanEKWellsDTMallickSLandryTABergaminiMVPooled results of two randomized clinical trials comparing the efficacy and safety of travoprost 0.004%/timolol 0.5% in fixed combination versus concomitant travoprost 0.004% and timolol 0.5%Clin Ophthalmol20071331732219668487
- KitazawaYSmithPSasakiNKotakeSBaeKIwamotoYTravoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubled-masked comparison of safety and efficacyEye (Lond)20112591161116921701528
- KonstasAGTsironiSVakalisANIntraocular pressure control over 24 hours using travoprost and timolol fixed combination administered in the morning or evening in primary open-angle and exfoliative glaucomaActa Ophthalmol2009871717619178390
- DenisPAndrewRWellsDFrirenBA comparison of morning and evening instillation of a combination travoprost 0.004%/timolol 0.5% ophthalmic solutionEur J Ophthalmol200616340741516761242
- SuicSPLausKNDosenVMEkertMMandicZBojicLComparison of evening and morning dosing of travoprost 0.004%/timolol 0.5% fixed combination in 6 month periodColl Antropol201034384785220977071
- CentofantiMOddoneFGandolfiSComparison of travoprost and bimatoprost plus timolol fixed combinations in open-angle glaucoma patients previously treated with latanoprost plus timolol fixed combinationAm J Ophthalmol2010150457558020688314
- KonstasAGMikropoulosDGEmbeslidisTA24-h Intraocular pressure control with evening-dosed travoprost/timolol, compared with latanoprost/timolol, fixed combinations in exfoliative glaucomaEye (Lond)201024101606161320651749
- TeusMAMigliorSLaganovskaGEfficacy and safety of travoprost/timolol vs dorzolamide/timolol in patients with open-angle glaucoma or ocular hypertensionClin Ophthalmol2009362963619997566
- PfeifferNScherzerMLMaierHSafety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from prior mono- or adjunctive therapyClin Ophthalmol2010445946620505839
- MandicZNovak-LausKBojicLSafety and efficacy of monotherapy change to fixed combination (travoprost 0.004%/timolol 0.5%) in 6 months follow up periodActa Clin Croat201049441141921830452
- ScherzerMLLiehneovaINegreteFJSchnoberDTravoprost 0.004%/timolol 0.5% fixed combination in patients transitioning from fixed or unfixed bimatoprost 0.03%/timolol 0.5%Adv Ther201128866167021773673
- RossiGCPasinettiGMBracchinoMSwitching from concomitant latanoprost 0.005% and timolol 0.5% to a fixed combination of travoprost 0.004%/timolol 0.5% in patients with primary open-angle glaucoma and ocular hypertension: a 6-month, multicenter, cohort studyExpert Opin Pharmacother200910111705171119601697
- AndradeSCompliance in the real worldValue Health19981317117316674348
- MargoCEThe placebo effectSurv Ophthalmol1999441314410466586