Abstract
Purpose
To evaluate the efficacy of topical cyclosporine 0.1% in chondroitin sulfate emulsion for the treatment of dry eye.
Methods
This retrospective multicenter study included 100 eyes of 50 dry eye patients aged ≥18 years, with preoperative ocular surface disease index (OSDI) score >12 or corneal staining grade >1 (in either eye) who underwent dry eye treatment with topical cyclosporine 0.1% in chondroitin sulfate emulsion (Klarity-C, ImprimisRx) for 3 months. Postoperative evaluation included comparison of the changes in OSDI score and corneal staining grade after 3 months of treatment from baseline.
Results
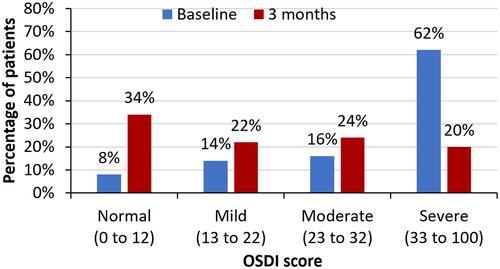
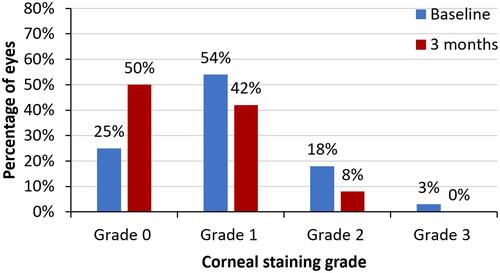
From baseline to 3 months, a statistically significant improvement in mean OSDI scores (38.19 vs 24.18, p <0.001) as well as mean corneal staining grade (3.62 vs 2.20, p <0.001) was observed. The proportion of subjects with severe dry eye decreased from 62% to 20% and more than one-third (34%) of patients were in the normal OSDI range. The percentage of eyes with corneal staining grade of 2 or 3 decreased from 21% (baseline) to 8% at 3 months; 50% of the eyes had corneal staining grade of 0. The treatment was found to be safe with no adverse events observed in the study.
Conclusion
Dry eye treatment with twice daily cyclosporine 0.1% in chondroitin sulfate emulsion was found to be safe and effective in reducing signs and symptoms of dry eye.
Introduction
Dry eye is a chronic multifactorial disease of the tear film and ocular surface that results in symptoms of ocular discomfort, affects vision, and negatively impacts patients’ quality of life.Citation1 Lubricating drops are usually the first line of treatment to restore ocular surface homeostasis.Citation2 While tear supplementation may provide short term symptomatic relief in mild dry eye cases, these methods are generally palliative and inadequate at resolving moderate to severe dry eye symptoms.Citation3,Citation4 Increased osmolarity of the tear film and inflammation of the ocular surface have been recognized as core mechanisms underlying the development of dry eye disease (DED).Citation5 As such, advanced DED cases benefit from topical anti-inflammatory therapies.Citation6
Corticosteroids are potent anti-inflammatory agents that have been found to be quite effective in reducing ocular surface inflammation. However, given the risk of cataract formation, ocular hypertension, and glaucoma with long-term use of steroids, these medications are typically not considered suitable for prolonged treatment of chronic DED.Citation7,Citation8
Cyclosporine is an anti-inflammatory and immunosuppressive agent that has been in use for more than 15 years to reduce ocular surface inflammation and improve tear film dynamics.Citation9–Citation11 The safety profile of cyclosporine is well established.Citation4,Citation10,Citation12 This lipophilic drug functions through the inhibition of calcineurin, preventing the activation of T lymphocytes and associated release of proinflammatory mediators.Citation13 Cyclosporine 0.05% ophthalmic emulsion has been shown to increase tear production and reduce signs and symptoms of DED.Citation4,Citation14–Citation18 Although the recommended dosing regimen of this medication is two times a day, there is some evidence to suggest that the clinical efficacy of the medication increases with more frequent dosing or with higher cyclosporine concentrations.Citation3,Citation7,Citation10,Citation12,Citation19,Citation20
In the present study, the efficacy of a preservative-free, high-concentration cyclosporine (0.1%) in a novel chondroitin sulfate vehicle was evaluated for the reduction of signs and symptoms of dry eye.
Methods
This retrospective multicenter study included case records for 50 patients (100 eyes) who received topical cyclosporine 0.1% in chondroitin sulfate emulsion (Klarity-C, ImprimisRx) for DED treatment at three different sites (Matossian Eye Associates in Hopewell, NJ; Loh Ophthalmology Associates in Miami; and Center for Excellence in Eye Care, Miami) between April and July 2019. The study was conducted in compliance with the study protocol and followed the tenets of Declaration of Helsinki. Salus Independent Review Board (Austin, USA) approved the study with waiver of informed consent as the data were recorded in patient charts as a part of routine clinical practice, and only de-identified patient data was analyzed.
Charts for patients aged ≥18 years who were diagnosed with DED based on either elevated Ocular Surface Disease Index scores (OSDI score >12) or corneal staining grade >1 (in either eye) and underwent dry eye treatment with topical cyclosporine 0.1% (Klarity-C, ImprimisRx) were included in this study.
Subjects with punctal plugs, contact lens wear within 3 months of starting the study drugs, and topical or systemic medications to treat another ocular disease (glaucoma, allergy, infection, etc.) within 4 weeks that may interfere with the interpretation of study results were excluded. Other exclusion criteria included ocular surgery within the last 6 months, ongoing ocular infection/allergy, pterygium, eyelid pathology (eg, trichiasis and entropion), and cyclosporine hypersensitivity. Patients whose 3-month (±15 days) follow-up data was not available were also excluded from the study.
Case records for consecutive patients who received topical cyclosporine 0.1% in chondroitin sulfate emulsion (Klarity-C) for the treatment of dry eye between April and July 2019 were examined and included in the study if they met all inclusion/exclusion criteria. Changes in mean OSDI score (OSDI questionnaireCitation21) and corneal staining (The National Eye Institute/Industry scaleCitation22) after 3 months of cyclosporine 0.1% treatment were compared to baseline. To assess the proportion of eyes with different levels of disease severity, the OSDI score of 0 to 12 was classified as normal, 13 to 22 as mild, 23 to 32 as moderate and 33 to 100 as severe. Likewise, the corneal staining grade was categorized as Grade 0 (score ≤1), Grade 1 (score 2 to 5), Grade 2 (score 6 to 10) and Grade 3 (score 11 to 15).
De-identified patient data was analyzed using SPSS software version 17 for Windows (SPSS Inc., Chicago, IL, USA). Quantitative variables were described using mean ± standard deviation and range where appropriate. Normality of scale data was checked using Shapiro Wilk/Kolmogorov–Smirnov test and quantile-quantile plots. For normally distributed data, paired t-test was used to compare the differences in the means before and after cyclosporine 0.1% treatment. For ordinal or scale data that were not normally distributed, the non-parametric Wilcoxon Signed Rank test was used. A p value of <0.05 was considered statistically significant.
Results
In this multicenter study, charts of 50 patients (100 eyes) who underwent treatment with topical 0.1% cyclosporine in chondroitin sulfate emulsion for dry eye were retrospectively reviewed. The mean age of the patients was 62.4 ± 15.0 years (range 27 to 85 years).
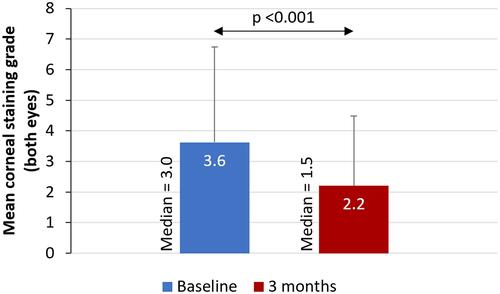
There was a statistically significant improvement in mean OSDI score (38.19 vs 24.18, p <0.001) and a reduction in mean corneal staining grade (3.62 vs 2.20, p <0.001) from baseline to 3 months ( and ). Post-treatment, the proportion of patients with severe dry eye symptoms decreased to 20% from 62% at baseline and more than one third (34%) of patients were in the normal OSDI range after using cyclosporine 0.1% in chondroitin sulfate emulsion for 3 months (). Similarly, the percentage of eyes with a corneal staining grade of 2 or 3 decreased from 21% at baseline to 8% following 3 months of treatment; 50% of the eyes had a corneal staining grade of 0 at 3 months (). Treatment of dry eye with 0.1% cyclosporine in chondroitin sulfate emulsion was found to be safe, with no adverse events observed in the study.
Figure 1 Mean OSDI score at baseline and after 3 months of treatment with topical cyclosporine 0.1% in chondroitin sulfate emulsion.
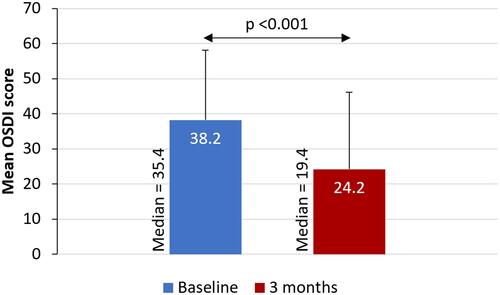
Figure 2 Mean corneal staining grade at baseline and after 3 months of treatment with topical cyclosporine 0.1% in chondroitin sulfate emulsion.
Discussion
In the present study, a higher concentration of cyclosporine (0.1%) in chondroitin sulfate ophthalmic emulsion was used to treat the signs and symptoms of DED. Our results showed that twice-daily dosing of cyclosporine 0.1% in chondroitin sulfate emulsion led to statistically significant improvement in both signs and symptoms after 3 months of treatment.
Cyclosporine is a well-accepted element of the management strategy for moderate to severe DED.Citation23,Citation24 It exhibits both anti-inflammatory and immunomodulatory properties and has long been used to reduce ocular surface inflammation and increase tear production, thereby restoring tear film stability and ocular surface integrity.Citation25
The most commonly available formulation, Restasis, takes 4 to 12 weeks to begin taking effect.Citation9,Citation26 While the recommended daily dose is bid, there is some evidence to suggest that four to five applications per day may provide better relief for patients with advanced DED.Citation27 Numerous studies have shown increased efficacy of cyclosporine at higher concentrations.Citation3,Citation7,Citation10,Citation12,Citation19,Citation27,Citation28 For instance, a comparison of the safety and efficacy of various cyclosporine concentrations (0.05%, 0.1%, 0.2%, 0.4%) showed that all of the concentrations were safe and the highest (0.4%) yielded the greatest improvement in Schirmer test scores.Citation10 In view of the increased efficacy of the drug at higher concentrations, cyclosporine formulations containing twice (0.1%) or nearly twice (0.09%) the concentration of the long-established 0.05% cyclosporine formulation have recently been approved for the treatment of DED.Citation9,Citation12,Citation29 These formulations (Ikervis, Cequa) have been found to be effective and deliver therapeutic concentrations of cyclosporine with minimal discomfort to patients.Citation7,Citation9,Citation12,Citation28,Citation30 A study comparing the safety and efficacy of 0.05% and 0.09% cyclosporine for the management of DED found cyclosporine 0.09% to be more effective and better tolerated.Citation28 In our study, we have similarly found that treatment with high-concentration (0.1%) cyclosporine in chondroitin sulfate emulsion (Klarity-C) is safe and effective in relieving signs and symptoms of dry eye.
The hydrophobic nature and poor aqueous solubility of cyclosporine makes it challenging to formulate a safe and effective ocular delivery system for the drug.Citation20 Traditional formulations of cyclosporine made in oil-based solvents such as castor or corn oil had low ocular tissue bioavailability and were associated with side effects such as blurred vision, burning and stinging, and were poorly tolerated by patients.Citation12 The availability of oil-in-water emulsions (Restasis, Lacrinmune) and micelle-based formulation (TJ Cyporin) of 0.05% cyclosporine have improved the efficacy and bioavailability of the drug and reduced some of the side effects associated with oil-based formulations.Citation29,Citation31
However, poor drug tolerability remains a major reason for noncompliance or treatment discontinuation.Citation7,Citation31,Citation32 Drug tolerability issues associated with 0.05% cyclosporine have been thought to be due to the formulation rather than a reaction to the drug itself. Due to the poor aqueous solubility of cyclosporine, various surfactants are added to the emulsion to improve its solubility and increase drug bioavailability. However, these complex emulsions have free surfactants and free drug in the aqueous phase that may interact with the corneal epithelium and induce pain.Citation33,Citation34 At least 17% of patients reported ocular burning and stinging sensation at the site of instillation.Citation35
In the present study, we evaluated a compounded, non-preserved eye drop that contains 0.1% cyclosporine mixed with several lubricating agents (dextran, glycerol, and hydroxypropylmethylcellulose) in a chondroitin sulfate emulsion. The lubricating and anti-inflammatory effect of chondroitin sulfate is already well established in the literature.Citation16,Citation36,Citation37 The lubricating effect of chondroitin sulfate has been found to prolong tear film break-up time.Citation16 Chondroitin sulfate also acts as a cell membrane stabilizer that enhances healing, reduces cell death and apoptosis.Citation38 It has also been previously postulated that cyclosporine A, which alone may require longer treatment to suppress apoptosis, can be combined with chondroitin sulfate and sodium hyaluronate, owing to their synergistic anti-inflammatory effect speeding up symptomatic improvement in dry eye patients.Citation16 Although we did not specifically study tolerability, the additional lubrication and mild anti-inflammatory effect provided by chondroitin sulfate to the active ingredient (cyclosporine), might contribute to better comfort and patient compliance. While the formulation of cyclosporine 0.1% with chondroitin sulfate showed promising results in relieving dry eye symptoms in the present study, further research is needed to separately quantify the contribution of chondroitin sulfate and cyclosporine 0.1% in patients receiving the compounded formulation.
Treatment of chronic conditions such as DED places a financial burden on patients, and the high cost of cyclosporine is known to be a factor in poor compliance and treatment discontinuation. As such, compounded medications such as Klarity-C may offer patients a cost-effective alternative.
Limitations of the present study include a small sample size, the retrospective nature of the study, and the absence of a control arm. The tolerability of the drug, in terms of burning or stinging upon instillation, could not be evaluated due to the retrospective nature of the study. Future prospective, comparative studies with larger sample sizes could be designed to validate the findings of the present study and determine if the higher dose could shorten the time to efficacy.
Overall, treatment with cyclosporine 0.1% in chondroitin sulfate emulsion was found to be effective in reducing both signs and symptoms of dry eye. Twice-daily dosing of the study drug led to a statistically significant reduction in the mean OSDI score and corneal staining grade score from baseline to 3 months. These preliminary findings are promising and provide direction for future research.
Acknowledgments
Jan Beiting (Wordsmith Consulting, Cary, North Carolina) and Raman Bedi, MD (IrisARC - Analytics, Research & Consulting, Chandigarh, India) provided research, statistical and editorial assistance in the preparation of this manuscript.
Disclosure
The editorial support was funded by ImprimisRx®, San Diego, CA.
Dr William Trattler reports grants from Imprimis, during the conduct of the study; personal fees from Allergan, grants from Sun, Novartis, Kala, outside the submitted work.
Dr Jennifer Loh reports grants from Imprimis, during the conduct of the study; personal fees from Sun, grants from Allergan, grants from Novartis, grants from Kala, outside the submitted work. None of the authors have any other conflicts of interest to disclose.
References
- Singla S, Sarkar L, Joshi M. Comparison of topical cyclosporine alone and topical loteprednol with cyclosporine in moderate dry eye in Indian population: a prospective study. Taiwan J Ophthalmol. 2019;9(3):173–178. doi:10.4103/tjo.tjo_15_18
- Findlay Q, Reid K. Dry eye disease: when to treat and when to refer. Aust Prescr. 2018;41(5):160–163. doi:10.18773/austprescr.2018.048
- Baudouin C, Figueiredo FC, Messmer EM, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol. 2017;27(5):520–530. doi:10.5301/ejo.5000952
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
- Buckley RJ. Assessment and management of dry eye disease. Eye (Lond). 2018;32(2):200–203. doi:10.1038/eye.2017.289
- Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–296. doi:10.5301/ejo.5000779
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. doi:10.2165/00002018-200225010-00004
- Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005;112(10):1790–1794. doi:10.1016/j.ophtha.2005.05.013
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107(5):967–974. doi:10.1016/S0161-6420(00)00035-X
- Wirta DL, Torkildsen GL, Moreira HR, et al. A Clinical Phase II study to assess efficacy, safety, and tolerability of waterfree cyclosporine formulation for treatment of dry eye disease. Ophthalmology. 2019;126(6):792–800. doi:10.1016/j.ophtha.2019.01.024
- Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa(R)) for Dry Eye Disease. Pharm Res. 2019;36(2):36. doi:10.1007/s11095-018-2556-5
- Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. doi:10.1016/j.ajo.2003.10.036
- Kim EC, Choi JS, Joo CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009;147(2):206–213 e203. doi:10.1016/j.ajo.2008.08.015
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi:10.1001/archopht.120.3.330
- Moon JW, Lee HJ, Shin KC, Wee WR, Lee JH, Kim MK. Short term effects of topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol. 2007;21(4):189–194. doi:10.3341/kjo.2007.21.4.189
- Wilson SE, Perry HD. Long-term resolution of chronic dry eye symptoms and signs after topical cyclosporine treatment. Ophthalmology. 2007;114(1):76–79. doi:10.1016/j.ophtha.2006.05.077
- Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea. 2014;33(7):760–767. doi:10.1097/ICO.0000000000000123
- Baiza-Duran L, Medrano-Palafox J, Hernandez-Quintela E, Lozano-Alcazar J, Alaniz-de la OJ. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol. 2010;94(10):1312–1315. doi:10.1136/bjo.2008.150011
- Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi:10.1016/j.ejpb.2017.03.006
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
- Lemp MA. Report of the national eye institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221–232.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628.
- Ridder III WH, Karsolia A. New drugs for the treatment of dry eye disease. Clin Optom (Auckl). 2015;7:91–102. doi:10.2147/OPTO.S68271
- Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005;89(10):1363–1367. doi:10.1136/bjo.2005.070888
- Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005;21(7):1057–1063. doi:10.1185/030079905X50615
- Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28(10):1091–1096. doi:10.1097/ICO.0b013e3181a16472
- Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
- Baudouin C, de la Maza MS, Amrane M, et al. One-year efficacy and safety of 0.1% Cyclosporine a cationic emulsion in the treatment of severe dry eye disease. Eur J Ophthalmol. 2017;27(6):678–685. doi:10.5301/ejo.5001002
- Luchs J. Phase 3 clinical results of cyclosporine 0.09% in a new nanomicellar ophthalmic solution to treatment keratoconjunctivitis sicca. Paper presented at: American society of cataract and refractive surgery (ASCRS) annual meeting; 2018.
- Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond). 2015;5(3):267–285. doi:10.4155/cli.14.135
- Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM, Hollander DA. PERSIST: physician’s Evaluation of Restasis((R)) Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012;6:1971–1976. doi:10.2147/OPTH.S30261
- Coursey TG, Wassel RA, Quiambao AB, Farjo RA. Once-daily cyclosporine-A-MiDROPS for treatment of dry eye disease. Transl Vis Sci Technol. 2018;7(5):24. doi:10.1167/tvst.7.5.24
- Gore A, Attar M, Pujara C, Neervannan S. Ocular emulsions and dry eye: a case study of a non-biological complex drug product delivered to a complex organ to treat a complex disease. Generic Biosimilar Initiative J. 2017;6(1):13–23. doi:10.5639/gabij.2017.0601.004
- Food and Drug Administration. Restasis (cyclosporine) ophthalmic label. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050790s020lbl.pdf. Accessed February 11, 2021.
- Limberg MB, McCaa C, Kissling GE, Kaufman HE. Topical application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. Am J Ophthalmol. 1987;103(2):194–197. doi:10.1016/S0002-9394(14)74226-6
- Llamas-Moreno JF, Baiza-Duran LM, Saucedo-Rodriguez LR, Alaniz-de la OJ. Efficacy and safety of chondroitin sulfate/xanthan gum versus polyethylene glycol/propylene glycol/hydroxypropyl guar in patients with dry eye. Clin Ophthalmol. 2013;7:995–999. doi:10.2147/OPTH.S46337
- Lindstrom RL, Kaufman HE, Skelnik DL, et al. Optisol corneal storage medium. Am J Ophthalmol. 1992;114(3):345–356. doi:10.1016/S0002-9394(14)71803-3