Abstract
Background
Bimatoprost 0.01% was developed for improved tolerability over bimatoprost 0.03%, while maintaining efficacy in lowering intraocular pressure (IOP). This multicenter, prospective, open-label, observational study was designed to investigate the efficacy and tolerability of bimatoprost 0.01% in routine clinical practice.
Methods
Data were collected from 10,337 patients with primary open-angle glaucoma or ocular hypertension attending 1334 centers in Germany. The primary efficacy outcome was mean change in IOP in each eye from baseline to 10–14 weeks after initiation of bimatoprost 0.01%. Target IOP, prior therapies, additional treatments, and adverse events were also assessed. All treatment decisions were at the physicians’ discretion.
Results
Bimatoprost 0.01% significantly lowered mean IOP from baseline by −4.1 mmHg (P < 0.0001) in all patients after a mean of 10.45 weeks. In patients without previous treatment, bimatoprost 0.01% reduced mean IOP from baseline by −6.5 mmHg (P < 0.0001). Bimatoprost 0.01% also significantly reduced IOP in patients previously treated with monotherapy of β-blockers, prostaglandin analogs, carbonic anhydrase inhibitors or bimatoprost 0.03%. No adverse events were reported by 93.9% of patients during treatment with bimatoprost 0.01%; the most commonly reported adverse events were eye irritation (2.0%), ocular hyperemia (1.4%), and conjunctival hyperemia (1.2%). Physicians and patients rated tolerability and adherence as high, and most patients said they would continue with bimatoprost 0.01% treatment.
Conclusion
Bimatoprost 0.01% can produce additional IOP-lowering effects when used in routine clinical practice in patients who have received prior therapy, in addition to lowering IOP in previously untreated patients. A high rate of continuation of therapy with bimatoprost 0.01% was observed in patients who switched from a variety of different medications. The results suggest that bimatoprost 0.01% is a suitable first-choice therapy in patients with primary open-angle glaucoma or ocular hypertension.
Introduction
Glaucoma is a leading cause of visual impairment, with 60 million people worldwide being affected and 8.4 million being bilaterally blind.Citation1,Citation2 Several risk factors for progressive loss of visual field in glaucoma have been identified, including abnormal baseline anticardiolipin antibody levels, older age, raised intraocular pressure (IOP), and female sex.Citation3 However, IOP is currently the only modifiable risk factor for progression. Citation4,Citation5 Every 1 mmHg increase in IOP during follow-up is associated with a 10%–19% increased risk of progression,Citation3,Citation4 and therefore lowering IOP can reduce progression.Citation4,Citation6 Consequently, medical treatments that lower IOP are considered first-line therapy for primary open-angle glaucoma and ocular hypertension.Citation5
Two meta-analyses have shown that the prostaglandin derivative bimatoprost 0.03% (Lumigan® 0.03%) has greater overall ability to lower IOP than the prostaglandin analogs latanoprost (Xalatan®) and travoprost (Travatan®).Citation7,Citation8 A third meta-analysis reported that bimatoprost 0.03% and travoprost were both superior to latanoprost in IOP-lowering efficacy.Citation9 However, this analysis showed that tolerability was better with latanoprost than with bimatoprost 0.03%.Citation9 The adverse event profile of bimatoprost 0.03% is typical of the prostaglandin analogs, with hyperemia, eye irritation, and increased eyelash growth among the commonly reported tolerability issues. These effects are mild in nature and the overall safety profile of bimatoprost 0.03% is good, according to a recent pooled analysis.Citation10 However, any increase in tolerability of glaucoma medications is likely to lead to improved patient adherence.Citation5,Citation11 A new formulation of bimatoprost (0.1 mg/mL; Lumigan 0.01%) has been developed, and shows efficacy equivalent to that of bimatoprost 0.03%, with improved tolerability.Citation12 However, there are currently no published data on the use of bimatoprost 0.01% in routine clinical practice.
The objective of the present study was to investigate the use of bimatoprost 0.01% in clinical practice in Germany in a large number of patients with primary open-angle glaucoma or ocular hypertension. To our knowledge, this is the largest observational study of glaucoma treatment published to date, with over 10,000 patients recruited across several centers.
Methods
Participants
Patients were required to have a diagnosis of primary open-angle glaucoma or ocular hypertension and to be already receiving treatment with bimatoprost 0.01%. Patients were eligible for inclusion regardless of whether they had previously received medical IOP-lowering therapy. Informed consent was obtained from all patients.
Study design
This was a multicenter, prospective, open-label, observational study designed to collect data on the use of bimatoprost 0.01% in routine clinical practice. Patients were treated with bimatoprost 0.01% at a dose determined by their treating physician, and according to the prescribing information, which recommends that bimatoprost 0.01% is applied to the affected eye(s) as one drop once daily in the evening.Citation13 Other IOP-lowering therapies could be used in addition to bimatoprost 0.01% if required. Treatment decisions were at the sole discretion of the treating physician. Because this was a purely observational study in clinical practice, there was no washout period between treatments.
Two assessments were made per patient: a first at baseline and a second at weeks 10–14. The primary efficacy outcome was mean change in IOP (as assessed by tonometry) in each eye from baseline to the end of the study. Efficacy was also assessed in terms of target IOP; targets were set individually for each patient by their treating physician. Other study assessments included previous treatment, additional IOP-lowering medications, early study discontinuation and continuation of bimatoprost 0.01%, and physician-assessed and patient-assessed tolerability of bimatoprost 0.01%. Each investigator also assessed: number and percentage of patients with and without adverse events, frequency of adverse events by Medical Dictionary of Regulatory Activities preferred term, number and percentage of patients with ocular or conjunctival hyperemia, assessment of causal relationship between adverse event and bimatoprost 0.01% (definite, probable, possible, improbable, not assessable, not assessed, or no causal relationship), and occurrence of serious adverse drug reactions. Hyperemia was assessed by the treating physician as present or absent, and no standardized grading system was used. Compliance was recorded by the investigator as better, equal, worse, or not applicable compared with the prior therapy.
Statistical analysis
A target population of 15,000 patients from approximately 3000 centers was planned for this study. This patient number allows the detection of uncommon adverse events with an incidence of >0.02% at least once (α = 0.05, binomial distribution). Data analysis was performed descriptively using SAS software (version 9.1; SAS Institute Inc, Cary, NC) and Medidata software (Medidata GmbH, Konstanz, Germany). Summary statistics included mean, standard deviation, median, minimum and maximum range, interquartile range, and frequency distribution tables, as appropriate for each item. Descriptive statistics were based on the safety population, defined as patients for whom any data were documented. All statistical analyses of efficacy were performed on patients with complete baseline IOP data. Change in IOP from baseline to visit 2 was analyzed using a two-sided paired t-test. Missing data were not replaced, unless the stop date of a medication was missing, in which case the respective medication was counted as ongoing.
Results
Baseline demographics and patient disposition
The first patient was recruited to the study and underwent baseline monitoring in February 2010. Patient monitoring was completed in November 2010. A total of 10,337 patients were enrolled from 1334 participating centers or ophthalmologists in Germany.
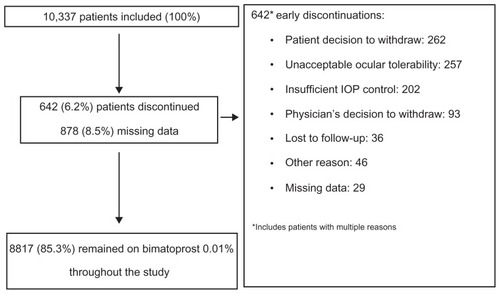
The mean ± SD patient age was 67.3 ± 12.3 years, and 58.1% of the 10,285 patients with information on sex were female. Most patients had a diagnosis of primary open-angle glaucoma (83.6%), and the remainder were diagnosed with ocular hypertension. Participant flow through the study is summarized in . A total of 8817 patients (85.3%) remained on bimatoprost 0.01% throughout the study. There were 642 early discontinuations, of which 257 were due to unacceptable ocular tolerability and 202 were due to insufficient IOP control.
Bimatoprost treatment and additional therapies
Reasons for prescribing bimatoprost 0.01% were insufficient IOP control (52.6% of patients), poor tolerability (28.7%), evidence of glaucomatous disease progression (12.7%), or lack of compliance on prior treatment (7.9%). In the 9664 patients for whom data were available, the mean ± SD duration of bimatoprost 0.01% therapy was 10.45 ± 5.94 weeks. At the end of the study, the dosage of bimatoprost 0.01% was one drop once daily for 96% of patients. Use of one or more additional IOP-lowering medications concomitantly with bimatoprost 0.01% was recorded for 1721 patients (16.6%). The most frequently occurring active ingredient in concomitant IOP-lowering medications was timolol (62.1%), followed by brinzolamide (33.0%) and dorzolamide (28.1%); other active ingredients occurred with lower frequencies.
Prior therapies
Prior to switching to bimatoprost 0.01%, 81.7% of patients (8441/10,337) were recorded as having been previously treated with other IOP-lowering medications (). The remaining 1896 (18.3%) patients either had not been receiving prior IOP-lowering therapy or had no information available regarding previous therapy. Prior monotherapy was used in 54.7% of the total patient population (5654/10,337), while 18.6% of patients used two prior therapies, 6.1% used three prior therapies, and 2.2% used at least four prior therapies. The most frequent active ingredient in prior IOP-lowering therapies was timolol (4146/8441 = 49.1% of patients), followed by brinzolamide (18.6%), latanoprost (16.3%), dorzolamide (14.9%), bimatoprost 0.03% (13.8%), travoprost (9.8%), and brimonidine (9.0%). Other types of prior therapy were used at a frequency of less than 5%, such as tafluprost, which was used in 2.9% of patients.
Table 1 Prior medications taken by >2% of patients, among those whose prior therapy was documented (n = 8441)Table Footnotea
Effect of bimatoprost 0.01% on IOP
In patients with complete data, the mean ± SD baseline IOP was 20.1 ± 4.5 mmHg in each eye. At visit 2, mean IOP in all patients with complete data was 16.0 mmHg in both eyes (reduction from baseline of −4.1 mmHg; P < 0.0001, ), representing a 20.4% reduction in IOP from baseline ().
Figure 2 Percentage reduction from baseline in mean IOP in all patients and in those receiving prior monotherapy (complete data) at 10–14 weeks following initiation of bimatoprost treatment.
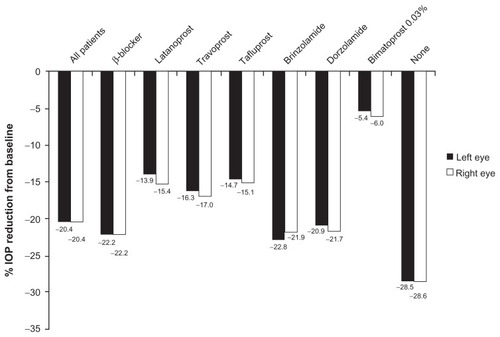
Table 3 Baseline, final, and reduction from baseline in mean IOP (mmHg) in all patients and in those receiving prior monotherapy (complete data) at 10–14 weeks following initiation of bimatoprost treatment
Significant IOP reductions from baseline were observed in patients previously receiving monotherapy with β-blockers (−4.6 mmHg) or with the prostaglandin analogs latanoprost (−2.8 mmHg), travoprost (−3.1 mmHg), tafluprost (−2.8 mmHg), or bimatoprost 0.03% (−1.0 mmHg; ). Significant reductions in mean IOP were also observed in patients with complete data previously receiving the carbonic anhydrase inhibitors brinzolamide (n = 313, −4.4 mmHg; P < 0.0001) or dorzolamide (n = 181, −4.2 mmHg; P < 0.0001) as sole monotherapy. The largest IOP reductions occurred in patients who did not receive prior therapy (−6.5 mmHg; P < 0.0001, ) where the IOP was reduced from baseline by 28.5%.
As stated by the physicians at the end of the monitoring period, target IOP was reached or exceeded in 70.3% of the study population, ranging from 63.9% in patients previously treated with latanoprost monotherapy to 80.0% in patients who previously received β-blocker monotherapy (). A similar percentage of patients reached target or lower IOP when previously treated with brinzolamide monotherapy (75.3%) or dorzolamide monotherapy (79.6%).
Table 2 Physician’s evaluation of achievement of target IOP in all patients, patients on prior β-blocker or prostaglandin analog monotherapy, and in patients on no prior therapy
Safety, tolerability, and compliance
Tolerability and compliance
Physicians rated the tolerability of bimatoprost 0.01% as very good or good in 92.6% of patients. Patients evaluated the tolerability of bimatoprost 0.01% as very good or good in 89.3% of cases. According to the physicians, compliance was improved or unchanged, compared with previous therapy, in 90.9% of previously treated patients. In total, 88.2% of all patients indicated that they wished to continue therapy with bimatoprost 0.01% beyond the end of the study.
Adverse events
The majority of patients (9708/10,337; 93.9%) did not report any adverse events during treatment with bimatoprost 0.01%. The most commonly reported adverse events (>1% of patients) were eye irritation (2.0%), ocular hyperemia (1.4%), and conjunctival hyperemia (1.2%, ). Ocular or conjunctival hyperemia occurred in 2.3% of patients (34/1449) previously treated with β-blocker monotherapy, 2.3% of patients (10/444) previously treated with latanoprost monotherapy, 0.8% of patients (2/238) previously treated with travoprost monotherapy, no patients previously treated with tafluprost monotherapy (0/68), and 2.5% of patients (48/1896) without any prior treatment. Of the total 629 adverse events reported, the investigators assessed 13.5% as being definitely related to bimatoprost 0.01% therapy, 31.6% as probably related, 15.4% as possibly related, and the remainder as unlikely to be related.
Table 4 Adverse events occurring in ≥0.1% of the total population (n = 10,337) classified according to MedDRA 13.1 preferred term
Two patients experienced serious adverse drug reactions. The first was an acute exacerbation of chronic obstructive pulmonary disease with dyspnea in a patient who smoked and was diagnosed with ocular hypertension and chronic obstructive pulmonary disease. This was deemed probably related to study treatment. The episode resolved about 14 days after oral prednisolone treatment was initiated. Bimatoprost 0.01% was discontinued and the patient was switched to brinzolamide 1%. The second serious adverse drug reaction was an asthma attack in a patient with bilateral glaucoma and asthma. This was deemed possibly related to study treatment. The patient recovered about 2 weeks after discontinuation of bimatoprost 0.01%.
Discussion
The aim of this large observational study was to examine the efficacy and tolerability of bimatoprost 0.01% in a routine clinical setting. Significant mean IOP reductions from baseline were observed in 10,337 patients with primary open-angle glaucoma and ocular hypertension treated in Germany with bimatoprost 0.01% over a mean of 10.45 weeks. The great majority (81.7%) had previously received other IOP-lowering therapies prior to using bimatoprost 0.01%, and the most common reason for switching to bimatoprost 0.01% was inadequate IOP lowering with previous therapy. This is in accordance with observations from a previous study, where the most common reason for change in ocular hypotensive medication was lack of IOP lowering, cited in 43% of patients in whom medication was changed.Citation11
In this observational study, treatment with bimatoprost 0.01% for a mean of 10.45 weeks was well tolerated, with 94% of patients experiencing no adverse events. The greatest reductions in IOP (−6.5 mmHg) from baseline were observed in patients without previous IOP-lowering therapy, in whom the baseline IOP was high (22.8 mmHg) compared with the other subgroups, as would be expected in untreated patients. Significant reductions in IOP from baseline were also observed in patients who had previously been receiving monotherapy with β-blockers (−4.6 mmHg), latanoprost (−2.8 mmHg), travoprost (−3.1 mmHg), tafluprost (−2.8 mmHg), brinzolamide (−4.4 mmHg), dorzolamide (−4.2 mmHg), and bimatoprost 0.03% (−1.0 mmHg). The smallest reduction in IOP from baseline was observed in patients switched from bimatoprost 0.03% to bimatoprost 0.01%, the subgroup of patients with the lowest recorded baseline IOP (16.8 mmHg). In this case, the additional IOP lowering achieved was probably due to increased compliance with bimatoprost 0.01% treatment, perhaps because of improved tolerability. Two meta-analyses have shown that bimatoprost 0.03% has greater overall ability to lower IOP than latanoprost or travoprost.Citation7,Citation8 In the meta-analysis by Aptel et al,Citation7 IOP reduction from baseline was significantly greater with bimatoprost 0.03% at all time points measured (8 am, 12 pm, 4 pm, and 8 pm) when compared with latanoprost, and at 8 am and 12 pm when compared with travoprost. In this study, bimatoprost 0.01% treatment provided greater efficacy in terms of IOP lowering in patients previously receiving latanoprost, travoprost, and tafluprost monotherapy.
When tolerability was assessed in previous studies, the incidence of self-reported conjunctival hyperemia was higher with bimatoprost 0.03% than with latanoprost or travoprost.Citation7 The higher incidence of ocular hyperemia reported with bimatoprost 0.03% compared with latanoprost or travoprost prompted the development of bimatoprost 0.01%. In the 12-month study by Katz et al, bimatoprost 0.01% and 0.03% had equivalent efficacy, which was sustained over the entire duration of the study, ie, bimatoprost 0.01% was noninferior to bimatoprost 0.03% in mean IOP change from baseline at all time points analyzed.Citation12 Bimatoprost 0.01% was associated with a significantly lower incidence of treatment-related ocular adverse events compared with bimatoprost 0.03% (38.4 versus 50.8% of patients; P = 0.016).Citation12 There was also an approximate 66% reduction in discontinuations due to treatment-related adverse events with bimatoprost 0.01% versus bimatoprost 0.03%.Citation12 In the current study, eye irritation was the most frequently reported adverse event, occurring in 2.0% of the total population. This is within the expected incidence range, given data from the Katz study, in which eye irritation occurred in 3.8% of patients receiving bimatoprost 0.01% and in 1.6% of patients receiving bimatoprost 0.03%.Citation12 While follow-up was limited to 14 weeks, the number of patients was large, and the incidence of many common adverse events of prostaglandin treatment (eg, eyelash growth and conjunctival hyperemia) decreased during long-term follow-up.Citation14 This would suggest that our approach is unlikely to have missed many adverse events of significance.
Achievement of low levels of IOP slows the progression of glaucomatous optic neuropathy,Citation4,Citation6 and every mmHg increase in IOP during follow-up can equate to an increased risk of progression of 10%–19%.Citation3,Citation15 This observational study demonstrates that bimatoprost 0.01% can produce additional IOP-lowering effects when used in routine clinical practice in patients who have received prior therapy. Bimatoprost 0.01% was also effective in previously untreated patients, suggesting its suitability as a first-choice drug for IOP lowering. European Glaucoma Society guidelines define first-choice treatment as a drug that a physician prefers as an initial IOP-lowering therapy.Citation5 A first-choice therapy should be effective at lowering IOP while maintaining good patient tolerability and promoting patient adherence. Adherence in glaucoma is often overestimated by clinicians, and published measurements range from 5% to 80%.Citation16 In the current study, a high rate of continuation of therapy was observed in patients who switched from a variety of different medications for a number of reasons, including poor tolerability and insufficient IOP lowering.
Although interpretation of our results is limited by the observational, noncontrolled, open-label nature and relatively short duration of the study, it provides a large body of data on the effects of bimatoprost 0.01% in clinical practice. The results suggest that bimatoprost 0.01% is a suitable first-choice therapy in patients with primary open-angle glaucoma or ocular hypertension.
Disclosure
This study was funded by Allergan Pharmaceuticals Ireland. Darwin Healthcare Communications provided medical writing and editorial assistance to the authors for the production of this manuscript, and this assistance was funded by Allergan. Statistical analysis and advice was provided by Medidata GmbH (D-78467 Konstanz, Germany). PE has received consultancy fees from Allergan. MF has received funds from Allergan and Pfizer. SP has received honoraria for giving lectures for Allergan. DS has received honoraria for giving lectures for Allergan and Alcon. HC is an employee of Allergan, Marlow, UK and was involved in the design, conduct, and completion of this study.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ20048284485115640920
- QuigleyHAGlaucomaLancet20113771367137721453963
- ChauhanBCMikelbergFSBalasziAGCanadian Glaucoma Study: risk factors for the progression of open-angle glaucomaArch Ophthalmol20081261030103618695095
- HeijlALeskeMCBengtssonBReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol20021201268127912365904
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edSavona, ItalyEditrice Dogma2008
- Advanced Glaucoma Intervention StudyThe Advanced Glaucoma Intervention Study (AGIS): the relationship between control of intraocular pressure and visual field deterioration. The AGIS InvestigatorsAm J Ophthalmol200013042944011024415
- AptelFCucheratMDenisPEfficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trialsJ Glaucoma20081766767319092464
- van der ValkVWebersCALumleyTA network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressureJ Clin Epidemiol2009621279128319716679
- DenisPLafumaAKhoshnoodBMimaudVBerdeauxGA meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapyCurr Med Res Opin20072360160817355741
- WirtaDVan DenburghAMWengELong-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trialsClin Ophthalmol2011575976521691584
- ZimmermanTJHahnSRGelbLTanHKimEEThe impact of ocular adverse effects in patients treated with topical prostaglandin analogs: changes in prescription patterns and patient persistenceJ Ocul Pharmacol Ther20092514515219284321
- KatzLJCohenJSBatoosinghALTwelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertensionAm J Ophthalmol201014966167120346780
- Allergan Ltd.Lumigan prescribing information Available at: http://www.allergan.com/assets/pdf/lumigan_pi.pdfAccessed March 28, 2012
- WilliamsRDCohenJSGrossRLLong-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4Br J Ophthalmol2008921387139218621791
- GordonMOBeiserJABrandtJDThe Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol200212071472012049575
- OlthoffCMSchoutenJSvan de BorneBWWebersCANoncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based reviewOphthalmology200511295396115885795