 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Metastasectomy represents the standard treatment for improving survival in patients with lung metastases (LMs) from bone (BS) or soft-tissue sarcoma (STS). Recently, radiofrequency ablation (RFA) of the LMs has been proved to be a useful option which can promise the similar effect to metastasectomy. The aim of this study was to determine prognostic factors, including tumor volume doubling time (TVDT), for post-metastatic survival in BS and STS patients treated with metastasectomy and/or RFA of the lung. Forty-eight patients with LMs were retrospectively reviewed. The mean age of the patients at the time of LMs was 56 years. The cohort comprised 27 male and 21 female patients. Eight of the 48 patients had LMs at the point of initial presentation. The mean follow-up period after commencing the treatment for LMs was 37 months. The mean maximum diameter of the initial LMs was 11 mm. The mean number of LMs was 4. The TVDT was calculated using a method originally described by Schwartz. At last follow-up, 5 patients had no evidence of disease, 3 patients were still alive with disease, and 32 patients had died of disease. The 3-year and 5-year post-metastatic survival rates were 32% and 16.8%, respectively. In a Cox univariate analysis, the size (P=0.04) and number of LMs (P<0.001), disease-free interval (P=0.04), curability of the initial LMs (P<0.001), and TVDT (P<0.001) were significantly identified as factors which affect prognosis. In the multivariate analysis, TVDT (P<0.001) and curability of the initial LMs (P<0.001) were confirmed as independent predictors of survival. There was a significant association between the number and curability of the initial LMs (P<0.001). In conclusion, metastasectomy and/or RFA of LMs is recommended for improving survival. However, TVDT and the curability of the LMs should be taken into consideration.
Introduction
Several relevant prognostic factors (PFs) have been defined for bone (BS) or soft-tissue sarcoma (STS) after lung metastasis (LM).Citation1–Citation12 Metastasectomy represents the standard treatment for improving survival in patients with LMs from BS or STS.Citation1–Citation7 Recently, radiofrequency ablation (RFA) of the lung has proved to be a useful option which promise a similar outcome to metastasectomy.Citation13–Citation16 Age, histological tumor grade, disease-free interval (DFI), number of LMs, and tumor volume doubling time (TVDT) are also predictive factors of survival.Citation1–Citation12 The TVDT is an accurate and reproducible method for quantifying the rate and pattern of tumor growth in individual patients.Citation8–Citation12 We previously reported that a shorter TVDT, evaluated using chest computed tomography (CT) scanning, was associated with reduced post-metastatic survival in sarcoma patients.Citation8 However, we included patients who were not treated with metastasectomy and/or RFA of the lung, in addition to those patients who underwent these treatments.
The aim of this study was to determine PFs, including TVDT, for post-metastatic survival in BS or STS patients treated with metastasectomy and/or RFA for the LMs.
Materials and methods
Inclusive patients
Fifty-six patients with LMs from BS or STS were treated with metastasectomy and/or RFA of the lung between 2002 and 2015. Seven patients (12.5%) were excluded from the study because CT scan was performed only 1 time before treatment for LMs, although scanning at 2 points of time is necessary for measuring the TVDT. Another patient (1.8%) with Ewing sarcoma, who had LMs at the time of initial screening, was also excluded from the study because all of the LMs disappeared after systemic neo- and adjuvant chemotherapy without metastasectomy or RFA (the patient underwent metastasectomy after 5 years due to relapsed LMs). In total, 48 patients (85.7%) were retrospectively reviewed. Although 27 (56.2%) of 48 patients were reviewed in a previous study of TVDT, the effect of surgical and interventional procedure was not taken into consideration for analysis of survival.Citation8 This study was approved by the institutional review board of Mie University Hospital. Written informed consent was obtained from all patients for this study including their data to be used in the study.
Method of measuring TVDT
All 48 patients had measurable LMs in at least 2 sequential chest CT images taken ≥14 days apart, before commencing treatment. No patient received chemotherapy during the period of measurement. The TVDT was calculated using a method originally described by Schwartz.Citation9 The tumor volume was calculated as follows, assuming the tumor to have a spheroidal shape:
where a indicates the maximum tumor diameter. The TVDT was calculated using the following equation:
where T2−T1 represents the time interval between 2 measurements and V1 and V2 denote the TVDT at 2 points of measurement. Treatment plans for the LMs were determined by the members of a multidisciplinary team.
Indication for metastasectomy and RFA
Thoracic surgeons assessed the resectability of the LMs after considering the number and location of LMs and the patient’s general condition. The indication for metastasectomy was as follows: (a) no extrapulmonary metastases, (b) well-controlled primary tumor, and (c) possible complete resection of all metastases.
Interventional radiologists assessed the indication for RFA. The indication for RFA was as follows: (a) patients with multiple LMs who were predicted to have respiratory loss after metastasectomy, (b) tumors which were not close to vessels or bronchus, and (c) patients refusing metastasectomy, although metastasectomy was recommended for the initial LMs after the confirmation of diagnosis, unless there were obvious multiple LMs.
We defined curability as complete or incomplete treatment of all initial LMs on CT with metastasectomy or RFA. Complete treatment meant all initial LMs on CT were treated with metastasectomy/RFA. Incomplete treatment meant all initial LMs on CT were not treated with metastasectomy or RFA.
The main aim of this study was to use univariate and multivariate analyses to examine PFs associated with survival in BS or STS patients with LMs.
Statistical analyses
All statistical analyses were conducted using the StatView program for Windows, software version 5.0 (SAS Institute Inc., Cary, NC, USA). Mann–Whitney U tests (quantitative data) and chi-square or Fisher’s exact tests (qualitative data) were performed to assess the statistical association between clinicopathological factors. The DFI was defined as the duration between the time the primary tumor was resected and the date LMs were initially detected (the DFI in patients with LMs at the point of initial presentation was defined as 0 month). Overall survival was defined as the duration between the time of the initial treatment for LMs and the date of death or last follow-up. Survival curves were estimated using the Kaplan–Meier method. Cumulative survival rates were compared using the log-rank test. Univariate and multivariate analyses were conducted using a Cox proportional hazards model to compare overall survival between patients. Probability P-values <0.05 were considered significant in all statistical analyses.
Results
Patients clinicopathological characteristics
In total, 48 patients with LMs from BS or STS were treated with metastasectomy and/or RFA. The mean age of the patients at the time of LMs was 56 years (range, 12–88 years). The cohort comprised 27 male (56.3%) and 21 female (43.7%) patients. Eight of the 48 patients (16.7%) had LMs at the point of initial presentation. The mean follow-up period after commencing the treatment for LMs was 37 months (range, 6–158 months). The mean maximum diameter of the initial LMs was 11 mm (range, 3–35 mm). The mean number of LMs was 4 (range, 1–17). The distribution of LMs, according to histological subtype, was as follows: leiomyosarcoma, n=11; undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma, n=7; osteosarcoma, n=5; synovial sarcoma, n=5; myxofibrosarcoma, n=4; extraskeletal chondrosarcoma, n=3; malignant peripheral nerve sheath tumor, n=3; chondrosarcoma, n=2; myxoid liposarcoma, n=2; malignant granular cell tumor, n=2; and other tumor, n=4. The median and mean DFI for all patients, including those patients with LMs at the point of initial presentation (n=8), was 10.8 months and 22.3 months, respectively. The median and mean DFI for the 40 patients (83.3%) who developed LMs after resection of the primary tumor was 14.3 months and 26.7 months, respectively. The median and mean TVDT for all 48 patients was 35 days and 69 days, respectively (range, 10–506 days). Twenty-seven (56.3%) and 38 (79.2%) of the 48 patients underwent metastasectomy and RFA of the lung for LMs, respectively (metastasectomy alone, n=10; RFA of the lung alone, n=21; both metastasectomy and RFA of the lung, n=17). Twenty-five patients (52.1%) received chemotherapy for LMs ().
Table 1 Patients background
Patient survival and risk factors associated with survival
At last follow-up, 15 patients (31.3%) had no evidence of disease, 3 patients (6.2%) were still alive with disease, and 30 patients (62.5%) had died of disease. Causes of death included LMs (n=25), local tumor progression (n=1), liver metastasis (n=1), brain metastasis (n=1), adrenal metastasis (n=1), and intraabdominal metastasis (n=1). The 3-year and 5-year post-metastatic survival rates were 32% and 16.8%, respectively (). In a Cox univariate analysis, the size (P=0.04) and number of LMs (P<0.001), DFI (P=0.04), curability of the initial LMs (P<0.001), and TVDT (P<0.001) were identified as significant PFs (). In the multivariate analysis, TVDT (P<0.001) and curability of the initial LMs (P<0.001) were confirmed as independent predictors of survival (). There was a significant association between the number and curability of the initial LMs (P<0.001). Conversely, age, gender, the size of the LMs, DFI, and TVDT did not correlate with curability ().
Figure 1 Kaplan–Meier curve of post-metastatic survival in bone or soft-tissue sarcoma patients (n=48) treated with metastasectomy and/or radiofrequency ablation of the lung.
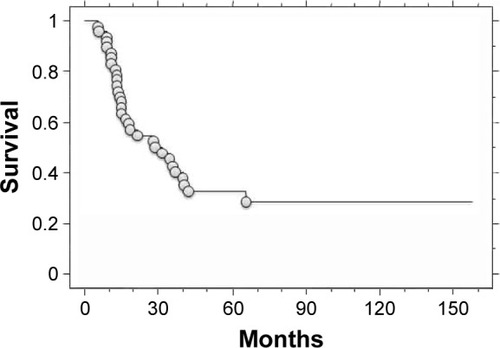
Table 2 Univariate analysis for survival
Table 3 Multivariate analysis for survival
Table 4 Predictive factors which related with curability
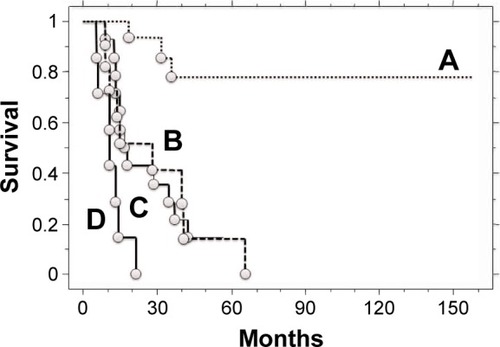
We subsequently analyzed the association between TVDT, curability, and survival. Patients with incomplete treatment and a shorter TVDT had a poorer disease-specific survival (0.0% at 2 years) compared to patients with complete treatment and a longer TVDT (93.3% at 2 years and 85.6% at 3 years; P<0.001). Patients with incomplete treatment and a shorter TVDT also had a poorer disease-specific survival compared to patients with complete treatment and a shorter TVDT (42.9% at 2 years and 28.6% at 3 years; P<0.01) or patients with incomplete treatment and a longer TVDT (51.9% at 2 years and 41.6% at 3 years; P=0.02). There was no significant prognostic difference between patients with incomplete treatment and a longer TVDT and patients with complete treatment and a shorter TVDT. Patients with complete treatment and a longer TVDT had a significantly better survival rate compared to patients with incomplete treatment and a longer TVDT (P<0.001) or patients with complete treatment and a shorter TVDT (P<0.001; ).
Figure 2 Kaplan–Meier curves of post-metastatic survival in bone or soft-tissue sarcoma patients (n=48) with (A) complete treatment and a tumor volume doubling time (TVDT) of >30 days, (B) incomplete treatment and a TVDT of >30 days, (C) complete treatment and a TVDT of ≤30 days, and (D) incomplete treatment and a TVDT of ≤30 days.
Discussion
Of all the patients diagnosed with BS or STS, between 10% and 38% of patients presented with clinically detectable metastases.Citation7,Citation17,Citation18 Regarding the location of these metastases, between 62% and 83% of patients had LMs.Citation17,Citation19,Citation20 Lung metastasectomy is reportedly a pivotal therapeutic option, providing patients with long survival times.Citation3–Citation7 Recently, RFA of the lung has been accepted as a relatively safe and useful therapeutic option for the treatment of unresectable lung cancer and metastatic tumors.Citation13–Citation16 However, even after an apparent complete resection or ablation of the metastases, in 40%–80% of patients, the metastases had recurred.Citation3,Citation20 In our case series, the 3-year and 5-year post-metastatic survival rates were 32% and 16.8%, respectively. The present findings are consistent with those of previously published reports.Citation1–Citation7
From the multivariate analysis, we identified TVDT and curability of the initial LMs as independent predictors of survival. There was a significant association between the number and curability of the initial LMs. Interestingly, patients with incomplete treatment and a shorter TVDT had a poorer disease-specific survival compared to patients with incomplete treatment and a longer TVDT or patients with complete treatment and a shorter TVDT. Furthermore, there was no significant prognostic difference between patients with incomplete treatment and a longer TVDT and patients with complete treatment and a shorter TVDT. Therefore, TVDT and curability of the LMs should be taken into consideration when planning metastasectomy and/or RFA of the lung. Our findings suggest that those patients with LMs who have a longer TVDT should be considered for metastasectomy and/or RFA of the lung even if the lesions are multiple and/or bilateral. Furthermore, if all detectable metastases are controllable, then metastasectomy and/or RFA of the lung should be considered even if the TVDT is short.
Small pulmonary nodules (<5 mm) are easily detectable in STS patients because of highly improved CT technologies.Citation21 In the present study, BS or STS patients were classified into 2 groups according to a TVDT threshold of 30 days. This strategy was selected because the TVDT is simple to calculate, a TVDT of 30 days has important indications for understanding tumor aggressiveness, and the median TVDT was 35 days in our case series. Based on the Schwartz equation, LMs of 3 mm or 5 mm in patients with a TVDT of 30 days would grow to approximately 6 mm or 10 mm in 90 days.Citation9 Similarly, LMs of 3 mm or 5 mm in patients with a TVDT of 10 days would grow to approximately 6 mm or 10 mm in 30 days. Therefore, subsequent CT scans in patients with pulmonary nodules should be taken within 3 months. Metastasectomy and/or RFA of the lung is recommended if the nodules have increased in size at follow-up CT. In that time, TVDT and the curability of the LMs should be taken into consideration. Our results also suggest that patients with poor curability and a shorter TVDT have a significantly higher risk of mortality despite metastasectomy and/or RFA. Therefore, systemic chemotherapy should be considered in those patients. We recommend a TVDT of 30 days as 1 indication of a clinical decision.
There are some limitations to this study: (1) the study population was small for considering associations between different histological tumor types and clinical outcomes, (2) the study had a retrospective design, and (3) treatment was administered at the discretion of the physician, although indications of metastasectomy and/or RFA of the lung were determined after the discussion with the members of a multidisciplinary team. For example, metastasectomy and/or RFA of the lung may be limited in some patients who had multiple metastases by physician’s decision without calculation of TVDT. Further prospective studies will be necessary to validate our findings.
Conclusion
Metastasectomy and/or RFA of the lung is recommended for LMs if the nodules have increased in size. For this procedure, TVDT and the curability of the LMs should be taken into consideration.
Disclosure
The authors report no conflicts of interest in this work.
References
- CassonAGPutnamJBNatarajanGFive-year survival after pulmonary metastasectomy for adult soft tissue sarcomaCancer19926936626681730117
- BillingsleyKGBurtMEJaraEPulmonary metastases from soft tissue sarcoma: analysis of patterns of disease and postmetastasis survivalAnn Surg1999229560261010235518
- WeiserMRDowneyRJLeungDHBrennanMFRepeat resection of pulmonary metastases in patients with soft-tissue sarcomaJ Am Coll Surg2000191218419010945362
- van GeelANHoekstraHJvan CoevordenFMeyerSBrugginkEDBlankensteijnJDRepeated resection of recurrent pulmonary metastatic soft tissue sarcomaEur J Surg Oncol19942044364408076705
- BillingsleyKGLewisJJLeungDHCasperESWoodruffJMBrennanMFMultifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcomaCancer199985238939510023707
- PutnamJBJrRothJAWesleyMNJohnstonMRRosenbergSAAnalysis of prognostic factors in patients undergoing resection of pulmonary metastases from soft tissue sarcomasJ Thorac Cardiovasc Surg19848722602686694417
- HartingMTBlakelyMLJaffeNLong-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcomaJ Pediatr Surg200641119419916410132
- NakamuraTMatsumineAMatsubaraTAsanumaKUchidaASudoAClinical impact of the tumor volume doubling time on sarcoma patients with lung metastasesClin Exp Metastasis201128881982521805254
- SchwartzMA biomathematical approach to clinical tumor growthCancer1961141272129413909709
- RothJAPutnamJBJrWesleyMNRosenbergSADiffering determinations of prognosis following resection of pulmonary metastases from osteogenic and soft tissue sarcoma patientsCancer1985556136113663855684
- JosephWLMortonDLAdkinsPCPrognostic significance of tumor doubling time in evaluating operability in pulmonary metastatic diseaseJ Thorac Cardiovasc Surg197161123324322187
- BlomqvistCWiklundTTarkkanenMElomaaIVirolainenMMeasurement of growth rate of lung metastases in 21 patients with bone or soft-tissue sarcomaBr J Cancer19936824144178347499
- SaumetLDeschampsFMarec-BerardPRadiofrequency ablation of metastases from osteosarcoma in patients under 25 years: the SCFE experiencePediatr Hematol Oncol2015321414925007012
- KoelblingerCStraussSGillamsAOutcome after radiofrequency ablation of sarcoma lung metastasesCardiovasc Intervent Radiol201437114715323670570
- PalussièreJItalianoADescatESarcoma lung metastases treated with percutaneous radiofrequency ablation: results from 29 patientsAnn Surg Oncol201118133771377721638099
- NakamuraTMatsumineAYamakadoKLung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomasCancer2009115163774378119514086
- KaneJMFinleyJWDriscollDKraybillWGGibbsJFThe treatment and outcome of patients with soft tissue sarcomas and synchronous metastasesSarcoma200262697318521331
- PollockREKarnellLHMenckHRWinchesterDPThe national cancer data base report on soft tissue sarcomaCancer19967810224722578918421
- VezeridisMPMooreRKarakousisCPMetastatic patterns in soft tissue sarcomasArch Surg198311889159186307217
- NakamuraTMatsumineAMatsubaraTRetrospective analysis of metastatic sarcoma patientsOncol Lett20112231531822866083
- NakamuraTMatsumineANiimiRManagement of small pulmonary nodules in patients with sarcomaClin Exp Metastasis200926771371819466567

