Abstract
Hepatocellular carcinoma (HCC) is one of the most deadly tumors, and current treatments for the disease are often ineffective. The discovery of the involvement of microRNAs (miRNAs) in hepatocarcinogenesis represents an important area of investigation for the development of their clinical applications. These molecules may act as oncogenes or tumor suppressors by directly or indirectly controlling the expression of key proteins involved in cancer-associated pathways. On the clinical side, because of their tumor-specific expression and stability in tissues and in the circulation, miRNAs have been proposed as novel diagnostic tools for classification and prognostic stratification of HCC. In recent years, the therapeutic potential of miRNAs has been demonstrated in various preclinical studies. Anti-miRNA oligonucleotides and miRNA mimics have been found to have antitumor activity. Moreover, by exploiting tumor-specific expression of miRNA, efforts have been aimed at improving targeting of tumor cells by replicative oncolytic viruses while sparing normal cells. These areas are expected to be explored further in the upcoming years to assess the clinical value of miRNA-based approaches in HCC and cancer in general.
Introduction
Hepatocellular carcinoma (HCC), the most common primary liver cancer, is one of the most prevalent malignant diseases worldwide, and the third most common cause of cancer-related deaths.Citation1 In spite of the development of novel therapeutic strategies, the prognosis of advanced HCC remains poor, with a life expectancy of about six months from the time of diagnosis.Citation2 In most cases, HCC originates on a background of cirrhosis,Citation3 a chronic and diffuse hepatic disease that results from continuous liver injury and regeneration. Increased hepatocyte turnover, inflammation, and oxidative DNA damage are implicated in the pathogenesis of the disease. The prevalent risk factors for HCC are also the cause of liver cirrhosis, and include viral infections (eg, hepatitis B and C) and alcohol consumption; further risk factors include tobacco smoking, exposure to aflatoxin B1 and vinyl chloride, diabetes, and genetic disorders, such as hemochromatosis and alpha-1 antitrypsin deficiency.Citation4–Citation9
HCC is a cancer with a poor prognosis because of the low proportion of cases amenable to curative treatment at diagnosis and the high rate of recurrence following therapeutic intervention. The estimated recurrence rate can be as high as 70%–80% at five years, considering both true recurrences and HCC de novo, and this contributes significantly to the dismal prognosis of HCC. In addition, traditional therapies are not effective for HCC and are too toxic for patients with cirrhosis. Transarterial chemoembolization and radioembolization are the main treatments for intermediate-stage HCC at the present time.Citation10 The only systemic therapy available for advanced HCC is based on the multikinase inhibitor sorafenib,Citation11 which is the most effective therapeutic tool for advanced nonresectable HCC, in which it can slightly improve patient survival.Citation12,Citation13 The survival of patients with advanced HCC treated with sorafenib depends on the absence of liver dysfunction and on the status of the patient.Citation14,Citation15 In the past few years, use of sorafenib in combination with transarterial chemoembolization has significantly improved survival rates in patients with advanced HCC.Citation16,Citation17 New perspectives in cancer treatment have appeared recently with the advent of the microRNAs (miRNAs), a novel class of noncoding small RNAs.
microRNAs
microRNAs are short RNAs (containing 20–24 nucleotides) that play an important role in all biological processes by post-transcriptional regulation of protein-coding genes.Citation18 They constitute a large class of phylogenetically conserved genes, with more than 2000 miRNAs having been discovered in humans.Citation19 miRNAs are transcribed by RNA polymerase II to produce a primary pre-miRNA, cleaved by the Drosha-DGCR8 complex to a shorter pre-miRNA approximately 70 nucleotides long. The pre-miRNA is transported by Exportin-5/RanGTP from the nucleus to the cytoplasm, where it is processed to a short miRNA-miRNA*-duplex by the Dicer-TRPB complex. The *strand is usually degraded, and the other strand becomes the mature miRNA that is incorporated into the RNA-induced silencing complex.
This ribonucleoprotein complex eventually becomes bound to regions of homology present in messenger RNAs (mRNAs), usually within their 3′ untranslated regions. Recruitment of the RNA-induced silencing complex to the target mRNA can promote either degradation or repression of translation. Via these mechanisms, each miRNA can modulate the expression of target protein-coding genes (reviewed recently by Davis-Dusenbery and HataCitation20 and Farazi et alCitation21). Each miRNA can promote the targeting and modulation of expression of tens or even hundreds of mRNAs. On the other hand, each mRNA can be targeted and under the control of several miRNAs. It has also become evident that mRNAs or RNAs highly expressed by pseudogenes or long noncoding RNAs can act as a “sponge” to reduce interaction between miRNAs and other mRNA species. Therefore, the network of interactions between mRNA and miRNA is complex and largely still to be clarified in a comprehensive systemic fashion.
Role of miRNAs in cancer
In view of their role in regulating the expression of protein-coding genes, miRNAs now have a widely recognized role in human carcinogenesis. Initial evidence came from detection of their aberrant expression in all human cancers.Citation22 Some of them are frequently found to be upregulated or downregulated in cancer in comparison with normal tissue. There is now mounting experimental evidence indicating that they may act as oncogenes or tumor suppressors by disrupting regulation of genes encoding for oncoproteins and tumor suppressor proteins.Citation23
In vivo models have provided conclusive proof that miRNAs play a key role in tumorigenesis. Proof of principle of the involvement of miRNA in the development of neoplastic disease was provided by generation of the Eμ-miR155 transgenic mouse that develops a lymphoproliferative B-cell malignancy.Citation24 Further animal models confirmed that deregulated miRNAs could be involved in tumorigenesis in vivo, mostly in hematologic malignancies.Citation25–Citation28 Targeted deletions demonstrated this functional activity in putative tumor suppressor miRNAs; for example, a miR-15a/miR-16-1 knockout mouse model is predisposed to development of an indolent form of leukemia, resembling human chronic lymphocytic leukemia, where deletion of these miRNAs is found in over 60% of cases.Citation29
Role of specific miRNAs in HCC
Involvement of miRNAs in HCC has been demonstrated, as in other cancers. HCC develops via deregulation of various molecular pathways, including p53, RAS/MAPK, PI3K/AKT/mTOR, WNT/β-catenin, MET, MYC, and transforming growth factor beta. Genetic and epigenetic alterations, as well as aberrant miRNA expression, can affect these crucial cancer-associated pathways (for a detailed review, see Negrini et alCitation30). Several studies have shown that expression of miRNA is deregulated in HCC in comparison with normal liver tissue (again, see Negrini et alCitation30 for a comprehensive review). In light of reports from independent studies, consistent deregulation of miR-122, miR-199, miR-221, and miR-21 appears to be particularly important in HCC (). Interestingly, both miR-122 and miR-199a are among the miRNAs most highly expressed in the normal liver.Citation31
Figure 1 Predicted effects of deregulated miRNAs in hepatocarcinogenesis. miR-122, miR-199, miR-221 and miR-21 were shown by various studies to be consistently deregulated in HCC. Via aberrant control of protein-coding gene targets (only the major ones are shown), their deregulation is expected to induce biomolecular effects that promote tumorigenesis
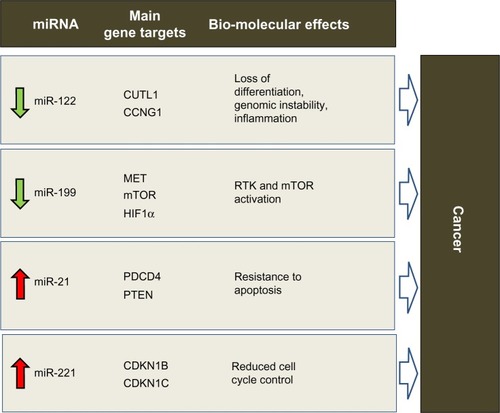
miR-122 is unique among the deregulated miRNAs, in that it is almost exclusively expressed physiologically in the adult liver,Citation32 where it appears to act as a key regulator of the differentiation of adult hepatocytes via repression of genes not specific to the liver.Citation33–Citation35 At the molecular level, this effect is achieved by regulation of CUTL1, a transcriptional repressor of genes specifying terminal differentiation in multiple cell lineages, including hepatocytes. CUTL1 was shown to be the most prominent repressed target of miR-122.Citation33 In HCC, miR-122 is downregulated in approximately 70% of cases, suggesting a tumor suppressor function for this miRNA. Various lines of evidence now support this hypothesis. Enforced expression of miR-122 can induce apoptosis and arrest of the cancer cell cycle,Citation36,Citation37 inhibit tumorigenicity in liver cancer cell lines in vivo,Citation36 and sensitize cells to sorafenib or doxorubicin.Citation36,Citation38 In addition, loss of miR-122 expression in patients with liver cancer is correlated with the presence of metastasisCitation39,Citation40 and a shorter time to recurrence.Citation38 The role of miR-122 in liver cancer has been demonstrated directly by the generation of miR-122 knockout mice.Citation41,Citation42 These mice were characterized by hepatic inflammation, fibrosis, and development of spontaneous tumors similar to HCC, demonstrating the tumor-suppressor function of this miRNA and its important role in liver metabolism and differentiation of hepatocytes. These phenotypic effects could at least in part be understood by identification of miR-122 gene targets. Reduced control of CUTL1, as previously mentioned, may be responsible for the lack of differentiation that characterizes HCC cells. Another known target of miR-122 is cyclin G1,Citation43 which is a negative regulator of p53 and is frequently upregulated in HCC. In a mouse model, the absence of cyclin G1 was associated with less susceptibility to developing liver tumors.Citation44 Reduced levels of miR-122 could lead to inhibition of p53 activity by increasing cyclin G1 levels.Citation38 Loss of miR-122 could also directly affect the intrinsic apoptotic pathway by reduced regulation of the antiapoptotic protein, Bcl-w.Citation45 miR-122 invasive and metastatic properties were instead linked to loss of control on ADAM17 (a disintegrin and member of the metalloproteinase family).Citation39 By targeting ADAM17, miR-122 can reduce in vitro migration and invasion, in vivo tumorigenesis and angiogenesis, and local invasion in the livers of nude mice. A similar effect could also be expected when targeting ADAM10.Citation36
miR-199 has been reported to be consistently downregulated in the majority of HCC,Citation43,Citation46,Citation47 suggesting a tumor suppressive function. All three members of the miR-199 family, ie, miR-199a-1, miR-199a-2, and miR-199b, have emerged as being frequently downregulated in HCC. Phenotypically, enforced expression of miR-199a in HCC cells leads to cell cycle arrest at G1 phase, reduced invasive capability, and enhanced susceptibility to hypoxia. In patients with HCC, downregulation of miR-199a was associated with a higher recurrence rate and shorter time to recurrence after surgery. These effects could be explained by modulation of target genes, such as MET, mTOR, and HIF-1α.Citation48–Citation51 Another important target of miR-199 in HCC is CD44, a transmembrane glycoprotein involved in cell-cell interaction, cell adhesion, and migration.Citation52 Further, absence of control over Discoidin domain receptor-1 tyrosine kinase may promote cell invasion processes in HCC.Citation53
Among the miRNAs that are upregulated in HCC, there is evidence in support of the tumor-promoting activity of miR-221. It is upregulated in 70%–80% of HCC samples.Citation54 From a functional point of view, HCC cells overexpressing miR-221 show increased growth, proliferation, migration, and invasion capability.Citation54–Citation56 miR-221 antagomirs inhibit growth of liver cancer cells, and enforced miR-221 expression was shown to enhance tumorigenesis of cells when implanted in mice. In this setting, overexpression of miR-221 promotes tumor progression and shortens the survival of the animal.Citation57 More recently, a transgenic mouse model characterized by overexpression of miR-221 in the liver was developed. This model demonstrates high susceptibility to HCC in male animals, which can be partly inhibited by challenge with anti-miR-221 oligonucleotides.Citation58 By modulating multiple gene targets, miR-221 has been shown to affect several cancer pathways. The cell cycle could be promoted by modulation of the cyclin-dependent kinase inhibitors, CDKN1B/p27 and CDKN1C/p57.Citation54 Other important targets include the BH3-only protein, Bcl2-modifying factorCitation59 (a proapoptotic protein) and PTEN (a negative regulator in the PI3K-AKT-mTOR pathway).Citation56 DNA damage-inducible transcript 4, another negative regulator of the mTOR pathway, was also identified as a target of miR-221.Citation57 miR-221 was also shown to affect invasion and metastasis by controlling TIMP3, a tissue inhibitor of metalloproteases.Citation56 These examples outline the importance of deregulation of even a single miRNA in cancer. miR-221 is a paradigmatic example of an miRNA regulating multiple pathways at one time.
miR-21 is a potent oncogene when upregulated. It is overexpressed in HCC as well as in several other human malignancies, including breast, colon, lung, pancreas, prostate, and stomach cancers.Citation60 Overexpression of miR-21 in cultured human cells can protect against apoptosisCitation61 and increase tumor cell proliferation and migration.Citation62 In vivo, miR-21 inhibition suppressed cell proliferation and increased apoptosis in a cancer xenograft model.Citation62 In a transgenic mouse model, overexpression of miR-21 led to a pre-B malignant lymphoma that regressed completely when miR-21 was inactivated, partly as a result of apoptosis.Citation28 Another important activity of miR-21 is chemoresistance induced against a variety of anticancer compounds. These multiple effects are linked to several genes targeted by miR-21 (for a recent review describing miR-21 target genes, see Buscaglia and LiCitation63). Among the most important of these is PTEN,Citation64 which promotes cell survival via activation of the PI3K-AKT pathway, and tumor suppressor programmed cell death 4,Citation65,Citation66 a protein believed to have a role in apoptosis induced by transforming growth factor-beta.
Clinical implications
miRNA diagnostics
Profiling of miRNA expression could be a useful tool for classification purposes and for improving prognostic stratification. Previous reviews have summarized the potential applications of miRNAs as diagnostic and prognostic markers in human cancer and in particular liver cancer.Citation30,Citation67,Citation68
Several miRNAs may have potential prognostic significance. summarizes the presently available data. For classification purposes, it is shown that miR-200c, miR-141 and miR-126, alone or in combination, could be used to distinguish primary HCC versus other tumor metastases to the liver with very high accuracy; moreover, the ratio of miR-205 to miR-194 expression could be used to distinguish between gastrointestinal tumors and metastases outside the gastrointestinal system,Citation69 which is important considering that the liver is the main metastatic site for gastrointestinal tumors.Citation70
Table 1 microRNAs with potential prognostic impact in patients with HCC
An emerging area of investigation with regard to miRNAs is their potential use as circulating biomarkers. Because of their different levels in the serum or plasma of patients affected by a range of diseases in comparison with healthy subjects,Citation71 miRNAs could be useful biomarkers for patient follow-up.Citation72 summarizes the studies of miRNAs in serum and plasma, confirming the potential use of miRNAs as sensitive markers for detection of an underlying HCC and for prognostic stratification of the disease. Among the miRNAs, miR-122 and miR-21 levels have been reported by more than one study to be significantly higher in patients with HCC. Because increased levels are also detected in chronic hepatitis, their usefulness as clinical tumor markers needs to be validated further.Citation73–Citation77
Table 2 Circulating microRNAs in liver disease
Overall, these results point to miRNAs being potential biomarkers that could improve our ability to stratify the prognosis and monitor follow-up in patients with HCC. Their stability in formalin-fixed and paraffin-embedded samples as well as in body fluids like serum or plasma is an important property for enabling their detection and quantification in biological samples, which are frequently used in clinical assays. However, at present, prospective studies are still required before miRNAs can be used in the clinic as biomarkers for cancer.
miRNA therapeutics
miRNA inhibition
In the past few years, several lines of evidence have indicated that strategies based on modulation of miRNA activity could be a novel approach to treating cancer (). In 2005, Krutzfeldt et al showed that intravenous administration of specific antagomirs could silence miR-122 in the mouse liver.Citation78 A few years later, Elmen et al demonstrated that inhibition of miR-122 by administration of anti-miRNA oligonucleotides in nonhuman primates was a promising approach for reducing miRNA activity in the adult liver without any evidence of toxicity.Citation79 These proofs of principle established the basis for the various studies that have been performed in cancer models in vivo.
Figure 2 Therapeutic strategies based on modulation of miRNA activity. Summary of preclinical studies (on the left) based on miRNA inhibition, miRNA replacement, and conditionally replicating adenoviruses regulated by miRNA (microRNA) target elements. On the right are clinical trials ongoing using miRNA-based drugs.
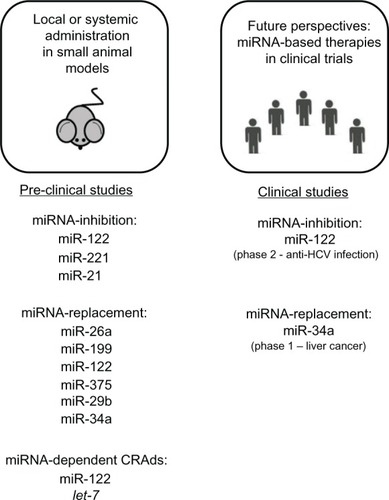
Anti-miR-221 was shown to have antitumor activity, which was demonstrated by intratumoral injections of anti-miRNA oligonucleotides into prostate carcinoma cell-derived tumors,Citation80 in melanoma cell xenotransplants,Citation81 and in multiple myeloma xenografts.Citation82 Park et al showed the ability of anti-miR-221 molecules to reduce proliferation of tumor cells and promote survival in an orthotopic mouse model of HCC.Citation83 Anti-miR-221 was also shown to downregulate miR-221 levels in the liver of the miR-221 transgenic mouse and to achieve a significant reduction in the number and size of tumors in comparison with untreated animals.Citation58
The role of the miR-21 oncomir was investigated using anti-miR-21 molecules in vivo. Use of anti-miR-21 led to complete regression of pre-B lymphoid-like malignancies in mice overexpressing miR-21.Citation28 Anti-miR-21 was also reported to have significant antitumor activity in SCID mice bearing human multiple myeloma xenografts.Citation84 Thus, by confirming the feasibility as well as short-term safety and efficacy of these molecules in large-scale preclinical settings, these studies established the basis for the use of anti-miRNAs in clinical trials.
The first miRNA-targeted drug, a molecule known as miravirsen SPC3649, has been used in various Phase I investigations and is currently in a Phase II clinical trial for the treatment of hepatitis C virus (HCV) infection.Citation85 This trial stems from the discovery of involvement of miR-122 in HCV RNA accumulation, and demonstrated that treatment of chronically infected nonhuman primates with an LNA-modified anti-miR-122 oligonucleotide was well tolerated and led to long-lasting suppression of HCV viremia.Citation86,Citation87
miRNA replacement
In addition to inhibition of oncomirs, another approach to treating cancer is based on restoration of tumor suppressor miRNAs. Several examples of this approach already exist. Enforced expression of miR-26a using an adenoassociated (AAV8) delivery system inhibited tumorigenicity in a myc mouse HCC model.Citation88 Both AAV8 miR-199 and cholesterol conjugated small RNA delivery systems could effectively restore miR-199a/b-3p and reduce tumor size in HCC xenografts.Citation31 The tumor suppressor role of miR-122 in HCC was confirmed by strong inhibition of tumorigenesis using AAV-mediated delivery of miR-122 in a myc mouse HCC model.Citation41 Administration of cholesterol-conjugated 2′-O-methyl-modifed miR-375 mimics significantly suppressed growth of hepatoma xenografts in nude mice.Citation89 miR-29b could sensitize HCC cells to various apoptotic signals and could suppress the ability of HCC cells to form tumors in nude mouse xenograft models.Citation90
Further studies demonstrating the antitumor effectiveness of miRNAs have been reported in other types of tumors and experimental settings.Citation91–Citation93 An important example is restoration of miR-31, the action of which could alter the invasive properties of disseminated tumor cells, raising the possibility of developing miRNA-based strategies for the treatment of metastatic disease.Citation94
The above studies establish miRNAs as promising molecules in cancer therapy. In this context, miR-34a is the first miRNA mimic to reach the clinic (http://clinicaltrials.gov/t2/show/NCT01829971).Citation95 Earlier studies showed that lentivirus expressing miR-34a could prevent tumor formation and progression in mouse models of lung adenocarcinoma induced by K-ras and p53.Citation96 Inhibition of tumor growth and increased survival were also observed in mice bearing multiple myeloma xenografts treated with miR-34a mimics.Citation97 Very recently, expression of miR-34 combined with the cytokine interleukin-24 showed synergistic antitumor activity in a xenograft model of HCC, indicating the possibility of using a multiple-armed miRNA-based viral vector in cancer therapy.Citation98
Oncolytic viruses
Oncolytic viruses are developed to replicate selectively in tumor cells. They are engineered to have cytotoxic effects in tumor cells with minimal toxicity in normal cells. For this reason, they hold promise for the treatment of cancer.Citation99–Citation101 The first conditionally replicating adenovirus (CRAd), known as ONYX-015, carried a deletion in the E1B-55 kDa coding region, which was designed to limit its replication with cells having dysfunctional p53.Citation99 It was used in clinical trials either alone or in combination with chemotherapeutic agents.Citation102–Citation107 Since then, progress has been made in this field, with more selective and potent oncolytic viruses having been entered into clinical trials (see Patel and KratzkeCitation108 for a recent review).
Among the oncolytic viruses in use, the presence of the gene for the immune stimulator granulocyte-macrophage colony-stimulating factor (GM-CSF) is improving significantly antitumor activity. The safety and biological activity of an oncolytic viral vector based on herpes simplex virus type-1, known as OncoVEXGM-CSF (Amgen, Thousand Oaks, CA, USA), has been assessed in several clinical trials.Citation109,Citation110 Oncolytic adenoviruses armed with GM-CSF have also been used successfully in the treatment of patients with advanced metastatic tumors refractory to conventional therapies. Their use resulted in antitumor immunity and increased median overall survival.Citation111–Citation113 Oncolytic poxvirus carrying GM-CSF was also investigated in clinical trials, and demonstrated an oncolytic and immunotherapeutic mechanism of action, tumor responses, and dose-related survival in treated patients with HCC.Citation114–Citation116
To generate safer oncolytic viruses, miRNA-mediated suppression of virus replication has been used successfully to reduce pathogenic effects in normal tissue. The cellular tropism of a picornavirus was modulated by engineering target sequences for muscle-specific miRNAs into the viral genome, thereby avoiding development of lethal myositis in tumor-bearing mice.Citation117 Ylosmaki et al developed a new type of conditionally replicating CRAd regulated by miR-122 target elements within the 3′ untranslated regions of the E1A gene, achieving liver-specific suppression of viral replication and reducing hepatotoxicity.Citation118 At the same time, it was found that viral oncolytic activity was not damaged in targeted tumors in vivo.Citation119 A similar approach has been reported by Cawood et al, who showed that incorporation of miR-122-binding sites to control E1A mRNA significantly reduced adenoviral replication and liver toxicity in mice.Citation120,Citation121 Another approach to E1A regulation combines an miR-122 control with chromogranin-A gene promoter-controlled virus replication, allowing use of high doses of adenovirus for more effective tumor treatment with limited liver toxicity.Citation122 Other miRNAs involved in the control of oncolytic virus replication include a let-7-dependent oncolytic adenovirus, which is able to replicate only in cells lacking miRNA expression, such as HCC cells, and not in normal liver cells.Citation123
These reports have established the potential value of engineered oncolytic viruses in the treatment of human malignancy. In this context, the target sequences of miRNAs could ensure the detargeting from normal tissues of virus replication, which still remains active in tumor cells.
In human cancer, most of the malignant cells are unable to produce tumors when implanted into immunodeficient mice. Still, 1%–2% of these cells maintain cell renewal capabilities and may generate tumors. It has been suggested and demonstrated in several instances that these cells persist in tumors as distinct populations, which are designated as tumor-initiating cells or cancer stem cells.Citation124 Cancer stem cells are essential for the growth of solid tumors and hematologic malignancies, as well as for seeding of metastases. Because they consist largely of nonproliferating cells, they are also intrinsically resistant to traditional therapies, thereby being responsible for relapses following therapy. Hence, it has been suggested that effective therapies against cancer stem cells could potentially lead to complete eradication of tumors.
Several studies have demonstrated the importance of miRNAs in the control of stem cellsCitation125,Citation126 as well as the phenotype of the cancer stem cell.Citation124 In HCC, identification of a cell population called CD133+ which has the characteristics of cancer stem cells, has provided new perspectives for characterization of liver cancer.Citation127 Several lines of evidence indicate that aberrant expression of miRNAs may control and lead to maintenance of liver tumor-initiating cells through aberrant modulation of stem cell-associated genes. summarizes the findings in this area. As indicated earlier, miRNA-based approaches are potentially feasible. Targeting of cancer stem cells using an miRNA-based approach represents a novel area of investigation aimed at eradicating liver tumor-initiating cells, thereby treating HCC.Citation128–Citation130
Table 3 microRNAs and cancer stem cells in HCC
Conclusion
While miRNA-based approaches are not presently used in the clinic, their potential applications are expanding in various areas of interest. Prognostic stratification, follow-up monitoring, and innovative therapeutic approaches are areas that might benefit from use of miRNAs. It should be noted that it has only been during the last 10 years that investigation of miRNAs in cancer has been initiated, and many more studies are needed to move this field forward into the clinical setting. Validation based on prospective studies of the use of miRNAs as cancer biomarkers is needed. Application of miRNA replacement or inhibition approaches need larger preclinical studies to assess their potential efficacy in specific contexts. The present knowledge, as summarized here, should form the basis of studies aimed at development of miRNA-based clinical applications.
Acknowledgments
This work was supported by grants to MN from the Italian Association for Cancer Research, the Italian Ministry of University and Research (project RBAPIIBYNP), and the University of Ferrara.
Disclosure
The authors report no financial conflicts of interests in this work.
References
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- JemalACenterMMDeSantisCWardEMGlobal patterns of cancer incidence and mortality rates and trendsCancer Epidemiol Biomarkers Prev20101981893190720647400
- BoschFXRibesJCleriesRDiazMEpidemiology of hepatocellular carcinomaClin Liver Dis20059219121115831268
- FaraziPADePinhoRAHepatocellular carcinoma pathogenesis: from genes to environmentNat Rev Cancer20066967468716929323
- PurohitVRapakaRKwonOSSongBJRoles of alcohol and tobacco exposure in the development of hepatocellular carcinomaLife Sci20139213923123447
- WuHCSantellaRThe role of aflatoxins in hepatocellular carcinomaHepat Mon20121210 HCCe723823162603
- UccelloMMalaguarneraGCorriereTBiondiABasileFMalaguarneraMRisk of hepatocellular carcinoma in workers exposed to chemicalsHepat Mon20121210 HCCe594323162599
- StarleyBQCalcagnoCJHarrisonSANonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connectionHepatology20105151820183220432259
- DraganiTARisk of HCC: genetic heterogeneity and complex geneticsJ Hepatol201052225225720022654
- ShrimalAPrasanthMKulkarniAVInterventional radiological treatment of hepatocellular carcinoma: an updateIndian J Surg2012741919923372313
- Abou-AlfaGKSchwartzLRicciSPhase II study of sorafenib in patients with advanced hepatocellular carcinomaJ Clin Oncol200624264293430016908937
- LlovetJMRicciSMazzaferroVSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- KaneRCFarrellATMadabushiRSorafenib for the treatment of unresectable hepatocellular carcinomaOncologist20091419510019144678
- KostnerAHSorensenMOlesenRKGronbaekHLassenULadekarlMSorafenib in advanced hepatocellular carcinoma: a nationwide retrospective study of efficacy and tolerabilityScientific World Journal2013201393197223431262
- PadhyaKTMarreroJASingalAGRecent advances in the treatment of hepatocellular carcinomaCurr Opin Gastroenterol201026318919520224395
- BaiWWangYJZhaoYSorafenib in combination with transarterial chemoembolization improves survival of unresectable hepatocellular carcinoma: a propensity-score matching studyJ Dig Dis201314418119023324079
- ZhaoYWangWJGuanSSorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patientsAnn Oncol318201324717869223508822
- BartelDPMicroRNAs: genomics, biogenesis, mechanism, and functionCell2004116228129714744438
- Griffiths-JonesSmiRBase: microRNA sequences and annotationCurr Protoc Bioinformatics2010 Chapter 12: Unit 12.9.1–10
- Davis-DusenberyBNHataAMechanisms of control of microRNA biogenesisJ Biochem2010148438139220833630
- FaraziTAHoellJIMorozovPTuschlTMicroRNAs in human cancerAdv Exp Med Biol201377412023377965
- CalinGACroceCMMicroRNA signatures in human cancersNat Rev Cancer200661185786617060945
- NegriniMFerracinMSabbioniSCroceCMMicroRNAs in human cancer: from research to therapyJ Cell Sci2007120Pt 111833184017515481
- CostineanSZanesiNPekarskyYPre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic miceProc Natl Acad Sci U S A2006103187024702916641092
- SantanamUZanesiNEfanovAChronic lymphocytic leukemia modeled in mouse by targeted miR-29 expressionProc Natl Acad Sci U S A201010727122101221520566844
- EnomotoYKitauraJHatakeyamaKEmu/miR-125b transgenic mice develop lethal B-cell malignanciesLeukemia201125121849185621738213
- ZhaoJLRaoDSBoldinMPTaganovKDO’ConnellRMBaltimoreDNF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignanciesProc Natl Acad Sci U S A2011108229184918921576471
- MedinaPPNoldeMSlackFJOncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphomaNature20104677311869020693987
- KleinULiaMCrespoMThe DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemiaCancer Cell2010171284020060366
- NegriniMGramantieriLSabbioniSCroceCMmicroRNA involvement in hepatocellular carcinomaAnticancer Agents Med Chem201111650052121554203
- HouJLinLZhouWIdentification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinomaCancer Cell201119223224321316602
- Lagos-QuintanaMRauhutRYalcinAMeyerJLendeckelWTuschlTIdentification of tissue-specific microRNAs from mouseCurr Biol200212973573912007417
- XuHHeJHXiaoZDLiver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver developmentHepatology20105241431144220842632
- EsauCDavisSMurraySFmiR-122 regulation of lipid metabolism revealed by in vivo antisense targetingCell Metab200632879816459310
- KrutzfeldtJStoffelMMicroRNAs: a new class of regulatory genes affecting metabolismCell Metab20064191216814728
- BaiSNasserMWWangBMicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenibJ Biol Chem200928446320153202719726678
- MaLLiuJShenJExpression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cellsCancer Biol Ther20109755456120150764
- FornariFGramantieriLGiovanniniCMiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cellsCancer Res200969145761576719584283
- TsaiWCHsuPWLaiTCMicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinomaHepatology20094951571158219296470
- CoulouarnCFactorVMAndersenJBDurkinMEThorgeirssonSSLoss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic propertiesOncogene200928403526353619617899
- HsuSHWangBKotaJEssential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liverJ Clin Invest201212282871288322820288
- TsaiWCHsuSDHsuCSMicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesisJ Clin Invest201212282884289722820290
- GramantieriLFerracinMFornariFCyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinomaCancer Res200767136092609917616664
- JensenMRFactorVMFantozziAHelinKHuhCGThorgeirssonSSReduced hepatic tumor incidence in cyclin G1-deficient miceHepatology200337486287012668979
- LinCJGongHYTsengHCWangWLWuJLmiR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell linesBiochem Biophys Res Commun2008375331532018692484
- MurakamiYYasudaTSaigoKComprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissuesOncogene200625172537254516331254
- JiangJGusevYAdercaIAssociation of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survivalClin Cancer Res200814241942718223217
- JiaXQChengHQQianXLentivirus-mediated overexpression of microRNA-199a inhibits cell proliferation of human hepatocellular carcinomaCell Biochem Biophys201262123724421847633
- FornariFMilazzoMChiecoPMiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cellsCancer Res201070125184519320501828
- KimSLeeUJKimMNMicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2)J Biol Chem200828326181581816618456660
- WangCSongBSongWUnderexpressed microRNA-199b-5p targets hypoxia-inducible factor-1alpha in hepatocellular carcinoma and predicts prognosis of hepatocellular carcinoma patientsJ Gastroenterol Hepatol201126111630163721557766
- HenryJCParkJKJiangJmiR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell linesBiochem Biophys Res Commun2010403112012521055388
- ShenQCicinnatiVRZhangXRole of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasionMol Cancer2010922720799954
- FornariFGramantieriLFerracinMMiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinomaOncogene200827435651566118521080
- MedinaRZaidiSKLiuCGMicroRNAs 221 and 222 bypass quiescence and compromise cell survivalCancer Res20086882773278018413744
- GarofaloMDi LevaGRomanoGmiR-221 and 222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulationCancer Cell200916649850919962668
- PineauPVoliniaSMcJunkinKmiR-221 overexpression contributes to liver tumorigenesisProc Natl Acad Sci U S A2010107126426920018759
- CallegariEElaminBKGiannoneFLiver tumorigenicity promoted by microRNA-221 in a mouse transgenic modelHepatology20125631025103322473819
- GramantieriLFornariFFerracinMMicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocalityClin Cancer Res200915165073508119671867
- VoliniaSCalinGALiuCGA microRNA expression signature of human solid tumors defines cancer gene targetsProc Natl Acad Sci U S A200610372257226116461460
- ChanJAKrichevskyAMKosikKSMicroRNA-21 is an antiapoptotic factor in human glioblastoma cellsCancer Res200565146029603316024602
- SiMLZhuSWuHLuZWuFMoYYmiR-21-mediated tumor growthOncogene200726192799280317072344
- BuscagliaLELiYApoptosis and the target genes of microRNA-21Chin J Cancer201130637138021627859
- MengFHensonRWehbe-JanekHGhoshalKJacobSTPatelTMicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancerGastroenterology2007133264765817681183
- AsanganiIARasheedSANikolovaDAMicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancerOncogene200827152128223617968323
- FrankelLBChristoffersenNRJacobsenALindowMKroghALundAHProgrammed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cellsJ Biol Chem200828321026103317991735
- FerracinMVeroneseANegriniMMicromarkers: miRNAs in cancer diagnosis and prognosisExpert Rev Mol Diagn201010329730820370587
- MinguezBLachenmayerADiagnostic and prognostic molecular markers in hepatocellular carcinomaDis Markers201131318119022045404
- BarshackIMeiriERosenwaldSDifferential diagnosis of hepatocellular carcinoma from metastatic tumors in the liver using microRNA expressionInt J Biochem Cell Biol20104281355136220619223
- HessKRVaradhacharyGRTaylorSHMetastatic patterns in adenocarcinomaCancer200610671624163316518827
- CortezMACalinGAMicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseasesExpert Opin Biol Ther20099670371119426115
- QiJWangJKatayamaHSenSLiuSMCirculating microRNAs (cmiRNAs) as novel potential biomarkers for hepatocellular carcinomaNeoplasma111220136021354223259781
- XuJWuCCheXCirculating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitisMol Carcinog201150213614221229610
- CermelliSRuggieriAMarreroJAIoannouGNBerettaLCirculating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver diseasePLoS One201168e2393721886843
- TomimaruYEguchiHNaganoHCirculating microRNA-21 as a novel biomarker for hepatocellular carcinomaJ Hepatol201256116717521749846
- TrebickaJAnadolEElfimovaNHepatic and serum levels of miR-122 after chronic HCV-induced fibrosisJ Hepatol201358223423923085648
- van der MeerAJFaridWRSonneveldMJSensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte-derived microRNA-122J Viral Hepat201320315816623383654
- KrutzfeldtJRajewskyNBraichRSilencing of microRNAs in vivo with ‘antagomirs’Nature2005438706868568916258535
- ElmenJLindowMSchutzSLNA-mediated microRNA silencing in non-human primatesNature2008452718989689918368051
- MercatelliNCoppolaVBonciDThe inhibition of the highly expressed miR-221 and miR-222 impairs the growth of prostate carcinoma xenografts in micePLoS One2008312e402919107213
- FelicettiFErricoMCBotteroLThe promyelocytic leukemia zinc fnger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanismsCancer Res20086882745275418417445
- Di MartinoMTGullaACantafioMEIn vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myelomaOncotarget20134224225523479461
- ParkJKKogureTNuovoGJmiR-221 silencing blocks hepatocellular carcinoma and promotes survivalCancer Res201171247608761622009537
- LeoneEMorelliEDi MartinoMTTargeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growthClin Cancer Res20131982096210623446999
- LindowMKauppinenSDiscovering the first microRNA-targeted drugJ Cell Biol2012199340741223109665
- LanfordREHildebrandt-EriksenESPetriATherapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infectionScience2010327596219820119965718
- Hildebrandt-EriksenESAarupVPerssonRHansenHFMunkMEOrumHA locked nucleic acid oligonucleotide targeting microRNA 122 is well-tolerated in cynomolgus monkeysNucleic Acid Ther201222315216122545703
- KotaJChivukulaRRO’DonnellKATherapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer modelCell200913761005101719524505
- HeXXChangYMengFYMicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivoOncogene201231283357336922056881
- XiongYFangJHYunJPEffects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinomaHepatology201051383684520041405
- AkaoYNakagawaYHirataIRole of anti-oncomirs miR-143 and -145 in human colorectal tumorsCancer Gene Ther201017639840820094072
- KitadeYAkaoYMicroRNAs and their therapeutic potential for human diseases: microRNAs, miR-143 and -145, function as anti-oncomirs and the application of chemically modified miR-143 as an anti-cancer drugJ Pharmacol Sci2010114327628020953119
- IbrahimAFWeirauchUThomasMGrunwellerAHartmannRKAignerAMicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinomaCancer Res201171155214522421690566
- ValastyanSChangABenaichNReinhardtFWeinbergRAActivation of miR-31 function in already-established metastases elicits metastatic regressionGenes Dev201125664665921406558
- BaderAGmiR-34 – a microRNA replacement therapy is headed to the clinicFront Genet2012312022783274
- KasinskiALSlackFJmiRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinomaCancer Res201272215576558722964582
- Di MartinoMTLeoneEAmodioNSynthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidenceClin Cancer Res201218226260627023035210
- LouWChenQMaLOncolytic adenovirus co-expressing miRNA-34a and IL-24 induces superior antitumor activity in experimental tumor modelJ Mol Med (Berl)201391671572523292172
- BischoffJRKirnDHWilliamsAAn adenovirus mutant that replicates selectively in p53-deficient human tumor cellsScience199627452863733768832876
- MathisJMStoff-KhaliliMACurielDTOncolytic adenoviruses – selective retargeting to tumor cellsOncogene200524527775779116299537
- AlemanyRCancer selective adenovirusesMol Aspects Med2007281425817300834
- GanlyIKirnDEckhardtGA phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancerClin Cancer Res20006379880610741699
- NemunaitisJGanlyIKhuriFSelective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trialCancer Res200060226359636611103798
- GalanisEOkunoSHNascimentoAGPhase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomasGene Ther200512543744515647767
- OpyrchalMAdercaIGalanisEPhase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomasMethods Mol Biol200954270571719565928
- KhuriFRNemunaitisJGanlyIA controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancerNat Med20006887988510932224
- NemunaitisJSwisherSGTimmonsTAdenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancerJ Clin Oncol200018360962210653876
- PatelMRKratzkeRAOncolytic virus therapy for cancer: the first wave of translational clinical trialsTransl Res2013161435536423313629
- HuJCCoffinRSDavisCJA phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factorClin Cancer Res200612226737674717121894
- HarringtonKJHingoraniMTanayMAPhase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neckClin Cancer Res201016154005401520670951
- CerulloVPesonenSDiaconuIOncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patientsCancer Res201070114297430920484030
- PesonenSDiaconuICerulloVIntegrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumorsInt J Cancer201213081937194721630267
- KanervaANokisalmiPDiaconuIAnti-viral and anti-tumor T-cell immunity in patients treated with GMCSF coding oncolytic adenovirusClin Cancer Res201319102734274423493351
- ParkBHHwangTLiuTCUse of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trialLancet Oncol20089653354218495536
- HwangTHMoonABurkeJA mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanomaMol Ther201119101913 –21772252
- HeoJReidTRuoLRandomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancerNat Med201319332933623396206
- KellyEJHadacEMGreinerSRussellSJEngineering microRNA responsiveness to decrease virus pathogenicityNat Med200814111278128318953352
- YlosmakiEHakkarainenTHemminkiAVisakorpiTAndinoRSakselaKGeneration of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNAJ Virol20088222110091101518799589
- YlosmakiELavilla-AlonsoSJaamaaSMicroRNA-mediated suppression of oncolytic adenovirus replication in human liverPLoS One201381e5450623349911
- CawoodRChenHHCarrollFBazan-PeregrinoMvan RooijenNSeymourLWUse of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cellsPLoS Pathog200955e100044019461878
- CawoodRWongSLDiYBabanDFSeymourLWMicroRNA controlled adenovirus mediates anti-cancer efficacy without affecting endogenous microRNA activityPLoS One201161e1615221264344
- LejaJNilssonBYuDDouble-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing abilityPLoS One201051e891620111709
- JinHLvSYangJUse of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cellsPLoS One201167e2130721814544
- NguyenLVVannerRDirksPEavesCJCancer stem cells: an evolving conceptNat Rev Cancer201212213314322237392
- SuhMRLeeYKimJYHuman embryonic stem cells express a unique set of microRNAsDev Biol2004270248849815183728
- HatfieldSDShcherbataHRFischerKANakaharaKCarthewRWRuohola-BakerHStem cell division is regulated by the microRNA pathwayNature2005435704497497815944714
- MaSBiology and clinical implications of CD133(+) liver cancer stem cellsExp Cell Res2013319212613222999864
- OishiNWangXWNovel therapeutic strategies for targeting liver cancer stem cellsInt J Biol Sci20117551753521552419
- LeeTKCheungVCNgIOLiver tumor-initiating cells as a therapeutic target for hepatocellular carcinomaCancer Lett 2012582013 [Epub ahead of print.]
- PangRWPoonRTCancer stem cell as a potential therapeutic target in hepatocellular carcinomaCurr Cancer Drug Targets20121291081109422873219
- BudhuAJiaHLForguesMIdentification of metastasis-related microRNAs in hepatocellular carcinomaHepatology200847389790718176954
- HanZBZhongLTengMJIdentification of recurrence-related microRNAs in hepatocellular carcinoma following liver transplantationMol Oncol20126444545722552153
- JiJShiJBudhuAMicroRNA expression, survival, and response to interferon in liver cancerN Engl J Med2009361151437144719812400
- ViswanathanSRPowersJTEinhornWLin28 promotes transformation and is associated with advanced human malignanciesNat Genet200941784384819483683
- ZhangYGuoXXiongLMicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinomaFEBS Lett2012586244362437023178713
- LiWXieLHeXDiagnostic and prognostic implications of microRNAs in human hepatocellular carcinomaInt J Cancer200812371616162218649363
- GuHGuoXZouLZhuHZhangJUpregulation of microRNA-372 associates with tumor progression and prognosis in hepatocellular carcinomaMol Cell Biochem20133751–2233023291979
- FuXWangQChenJClinical significance of miR-221 and its inverse correlation with p27 Kip(1) in hepatocellular carcinomaMol Biol Rep20113853029303520146005
- RongMChenGDangYIncreased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitroBMC Cancer2013132123320393
- ChenLJiangMYuanWTangHmiR-17-5p as a novel prognostic marker for hepatocellular carcinomaJ Invest Surg201225315616122583011
- HanZBChenHYFanJWWuJYTangHMPengZHUpregulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantationJ Cancer Res Clin Oncol2012138115316122071603
- ChenHYHanZBFanJWmiR-203 expression predicts outcome after liver transplantation for hepatocellular carcinoma in cirrhotic liverMed Oncol20122931859186521786180
- MurakamiYTamoriAItamiSThe expression level of miR-18b in hepatocellular carcinoma is associated with the grade of malignancy and prognosisBMC Cancer2013139923496901
- XuJZhuXWuLMicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/beta-catenin pathwayLiver Int201232575276022276989
- QiPChengSQWangHLiNChenYFGaoCFSerum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infectionPLoS One2011612e2848622174818
- LiJWangYYuWChenJLuoJExpression of serum miR-221 in human hepatocellular carcinoma and its prognostic significanceBiochem Biophys Res Commun20114061707321295551
- GuiJTianYWenXSerum microRNA characterization identifies miR-885-5p as a potential marker for detecting liver pathologiesClin Sci (Lond)2011120518319320815808
- LiuAMYaoTJWangWCirculating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort studyBMJ Open201222e000825
- ShrivastavaSPetroneJSteeleRLauerGMBisceglieAMRayRBUpregulation of circulating miR-20a is correlated with hepatitis C virus mediated liver disease progressionHepatology262013 [Epub ahead of print.]
- JiJYamashitaTBudhuAIdentification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cellsHepatology200950247248019585654
- JiJYamashitaTWangXWWnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinomaCell Biosci201111421711587
- XuCLiuSFuHMicroRNA-193b regulates proliferation, migration and invasion in human hepatocellular carcinoma cellsEur J Cancer201046152828283620655737
- MaSTangKHChanYPmiR-130b promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1Cell Stem Cell20107669470721112564
- JungCJIyengarSBlahnikKREpigenetic modulation of miR-122 facilitates human embryonic stem cell self-renewal and hepatocellular carcinoma proliferationPLoS One2011611e2774022140464
- JiaYLiuHZhuangQTumorigenicity of cancer stem-like cells derived from hepatocarcinoma is regulated by microRNA-145Oncol Rep20122761865187222378186
- ZhangJLuoNLuoYPengZZhangTLiSmicroRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-MybInt J Oncol201240374775622025269
- OishiNKumarMRRoesslerSTranscriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinomaHepatology20125651792180322707408
- WuKDingJChenCHepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer developmentHepatology20125662255226722898879
- XiaHOoiLLHuiKMMiR-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancerHepatology372013 [Epub ahead of print.]
- XiaHOoiLLHuiKMMiR-214 targets beta-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinomaPLoS One201279e4420622962603
