Abstract
Purpose
Pharmaceutical formulation and treatment process attributes, such as dose frequency and route of administration, can have an impact on quality of life, treatment adherence, and disease outcomes. The aim of this literature review was to examine studies on preferences for pharmaceutical treatment process attributes, focusing on research in diabetes, oncology, osteoporosis, and autoimmune disorders.
Methods
The literature search focused on identifying studies reporting preferences for attributes of the pharmaceutical treatment process. Studies were required to use formal quantitative preference assessment methods, such as utility valuation, conjoint analysis, or contingent valuation. Searches were conducted using Medline, EMBASE, Cochrane Library, Health Economic Evaluation Database, and National Health Service Economic Evaluation Database (January 1993–October 2013).
Results
A total of 42 studies met inclusion criteria: 19 diabetes, nine oncology, five osteoporosis, and nine autoimmune. Across these conditions, treatments associated with shorter treatment duration, less frequent administration, greater flexibility, and less invasive routes of administration were preferred over more burdensome or complex treatments. While efficacy and safety often had greater relative importance than treatment process, treatment process also had a quantifiable impact on preference. In some instances, particularly in diabetes and autoimmune disorders, treatment process attributes had greater relative importance than some or all efficacy and safety attributes. Some studies suggested that relative importance of treatment process depends on disease (eg, acute vs chronic) and patient (eg, injection experience) characteristics.
Conclusion
Despite heterogeneity in study methods and design, some general patterns of preference clearly emerged. Overall, the results of this review suggest that treatment process has a quantifiable impact on preference and willingness to pay for treatment, even in many situations where safety and efficacy were the primary concerns. Patient preferences for treatment process attributes can inform drug development decisions to better meet the needs of patients and deliver improved outcomes.
Introduction
The effectiveness of pharmaceutical treatments depends not only on the chemical properties of the medication, but also on how medication is formulated and administered. Differences in treatment regimen and treatment process can have a profound effect on how patients experience pharmaceutical therapy. For example, while some medications are administered orally as tablets or capsules, others require intravenous (IV) administration in a hospital setting. Furthermore, treatment regimens can vary in terms of dose frequency and dose flexibility, including whether medications need to be taken with meals. These pharmaceutical formulation and treatment process attributes (subsequently referred to as “process attributes”) can impact patient adherence, and therefore indirectly affect the efficacy and safety of a medication.Citation1–Citation5 They can also have a direct effect on how patients experience treatment, which can impact health-related quality of life.
One way to examine and quantify the importance that patients place on the treatment process attributes is to use formal preference assessment methods, such as health state utility valuation and discrete choice experiments. These approaches permit quantitative comparison of the relative importance that patients place on a set of treatment attributes. While a substantial amount of research has documented the impact of efficacy and safety on patient preference for various medication options,Citation6–Citation9 less is known about the importance of treatment process attributes. Still, a smaller growing body of research has consistently highlighted the importance of how medications are taken.Citation10–Citation12 In addition, studies that include efficacy and/or safety attributes along with treatment process attributes can also quantify patients’ willingness to accept a risk of adverse events or reduced treatment benefit for the sake of improved comfort or convenience.
The aim of this literature review was to identify and examine published studies presenting preferences for pharmaceutical treatment process attributes. To facilitate synthesis of findings across studies, this review focused only on studies using formal preference assessment methodologies that provide a quantitative estimate of the value of treatment process attributes. Findings from these studies should have direct relevance to researchers working in drug development because results can provide insight into the value that patients place on treatment process attributes. Results may also aid clinicians in selecting treatments with attributes that have the potential to enhance treatment adherence.
Methods
Preference assessment methods
This review focused on studies that have used a range of methodologies to assess and quantify preference for process attributes. Preference assessment methods can be grouped into three broad categories (). Stated preferences are derived from surveys or interviews with an experimental design such as conjoint or contingent valuation studies. Stated preference methods allow researchers to focus on specific attributes, control the way preferences are elicited, and assess preferences for hypothetical products.Citation13–Citation15 Results of these stated preference studies often allow researchers to compare the relative influence of multiple factors on patient preference. A second method commonly used in health care research is the health state utility assessment in which patients or members of the general public perform choice-based tasks to indicate their preferences for their own current health or descriptions of hypothetical health states (often called scenarios or vignettes).Citation16–Citation18 These methods yield utility values on a scale anchored to dead (0) and full health (1) that represent the strength of preferences for various health states, and may be used in cost-utility analyses. Utility studies most frequently focus on quantifying health status, symptoms, and treatment outcomes, but they have also been used to quantify preferences for treatment attributes and treatment processes.Citation10,Citation19 Revealed preferences are derived from actual observed market activities and real-world behavior.Citation14
Figure 1 Preference assessment methods.
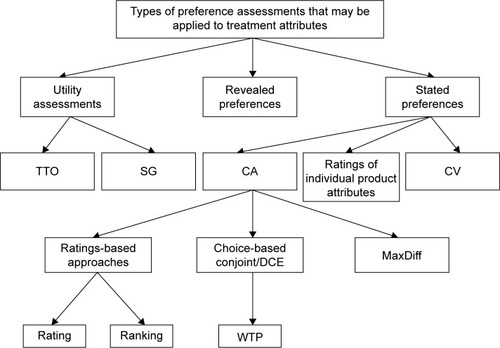
The current literature search was designed to identify stated preference studies and utility studies because these methods can provide a quantitative assessment of specific treatment process attributes. Although revealed preference data can provide an indication of trends across large samples, this methodology is not well suited for identifying preference among specific treatment process attributes. Consequently, the current literature search did not aim to identify revealed preference studies.
Literature search methods
Literature searches were conducted in the following databases: PubMed, EMBASE, Cochrane Library, Health Economic Evaluation Database, and National Health Service Economic Evaluation Database. The list of search terms was developed to identify articles that include the selected methods (ie, stated preference or utility assessment) and attributes related to treatment process. The following search terms (applied to article title and abstract) were intended to identify studies using the relevant preference methods: stated preference(s), time trade-off, TTO, time trade off, standard gamble, conjoint, contingent valuation, discrete choice, discrete-choice, willingness to pay, and willingness-to-pay. Treatment process search terms were intended to identify attributes related to route of administration, dose frequency, dose timing, dose size, convenience, and other process attributes. A full list of treatment process search terms is provided in the Supplementary material.
The search was limited to studies published in English between January 1, 1993 and October 16, 2013. Full-text primary articles were eligible for inclusion. Conference abstracts, editorials, and letters to the editor were excluded. Articles were considered for inclusion if they had both a preference methodology term and a process term. Articles were included if they evaluated preferences for one or more treatment attributes through utility, conjoint, contingent valuation, and/or discrete choice. Articles were excluded if they evaluated preferences for only efficacy and/or safety attributes (without assessment of preferences for treatment attributes, treatment processes, or treatment experience) or if they evaluated preferences through revealed preference rather than stated preference or utility methods. This review included studies examining treatment preferences from the patient perspective (either from patients themselves or nurses as patient proxies) and from general population participants.
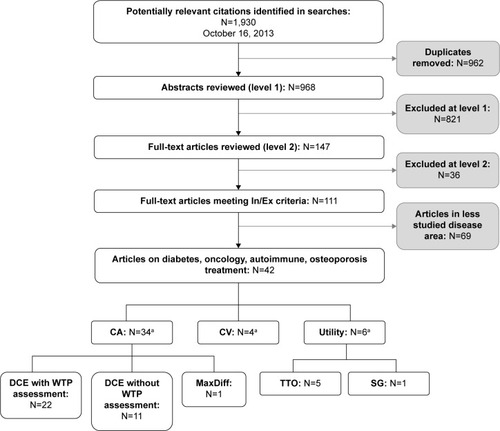
Abstracts of potential studies identified during the literature search (n=968) were screened and examined with regard to the inclusion/exclusion criteria. For any abstract that could not be confidently excluded, full-text articles were obtained and reviewed (n=147). A total of 111 articles met the criteria for inclusion (). Four therapeutic areas were selected for detailed review (ie, diabetes, autoimmune disease, oncology, and osteoporosis) because these were areas with a substantial number of published articles, a range of disease severity, and a variety of treatment process attributes.
Figure 2 Summary of literature search results.
Abbreviations: In, inclusion; Ex, exclusion; CA, conjoint analysis; CV, contingent valuation; DCE, discrete-choice experiment; WTP, willingness to pay; TTO, time trade-off; SG, standard gamble.
Data extraction methods
After articles were selected for inclusion, study characteristics were extracted and organized into table shells so that findings could be examined and summarized across studies. For each article, the following characteristics were captured in the data extraction tables: therapeutic area (diabetes, autoimmune disease, oncology, or osteoporosis), preference assessment method (conjoint, utility, contingent valuation, or multiple methods), respondent samples (patients, proxy, or general population), treatment process attribute results (route of administration, dose frequency, dose timing, dose size, treatment duration, and other), and comparison of treatment process attributes vs efficacy and safety.
As much as possible, an effort was made to present results consistently across studies, including preference for levels within each attribute and relative importance across attributes. However, the level of detail and presentation of results in the source articles varied greatly, and therefore, it was not always possible to extract the same quality or depth of information across studies.
Results
Included articles
A total of 42 studies met inclusion criteria in the following disease areas: 19 diabetes, nine oncology, five osteoporosis, and nine autoimmune. The most commonly used type of preference assessment method was conjoint analysis (n=34), which includes discrete choice experiments (DCEs) with willingness to pay assessment (n=22), DCEs without willingness to pay assessment (n=11), and one MaxDiff study. Other preference methods included utility assessments (n=6) and contingent valuation (n=4). These study methodological categories are not mutually exclusive. For example, there were studies that used both DCE and utility assessment methodology.Citation48 lists three types of stated preference studies, two of which (conjoint analysis and contingent valuation) were identified in the current literature search. No stated preference studies examining ratings of individual product attributes outside the context of a larger treatment profile met the current inclusion criteria.
Most of the studies (n=33) were conducted in patient samples, although some were conducted with general population respondents (n=3) or nurses (n=1) serving as patient proxies. One study included both patient and general population respondents.Citation34 summarizes article categorization, and presents the clinical condition, preference assessment method, respondent sample, and results for each study.
Table 1 Summary of studies included in the review
Treatment process attributes
The most common treatment process attributes examined across the 42 studies were route of administration, dose frequency, dose timing, dose size, and treatment duration. The results for each of these attribute categories are described below and are summarized in . Results in are grouped by therapeutic area rather than treatment process attribute to avoid redundancy, since many studies include more than one treatment process attribute. Results in this section are presented by treatment process attribute to highlight general patterns in preference for treatment process attributes. Statistical results across studies were often not directly comparable. For example, relative preference scores presented in different DCE studies were not necessarily on the same scale, and none of these yield numerical results that are directly comparable to health state utility studies. Therefore, to facilitate interpretation of results across studies, results in are presented in terms of whether preferences for treatment process attributes followed expected or unexpected patterns.
Route of administration
Studies examining preferences among various routes of administration typically yielded findings in the expected direction, with easier or more convenient routes of administration preferred over more difficult routes of administration (). In multiple studies, respondents were found to prefer oral over injectable administration,Citation20–Citation25 inhaled medication over injections,Citation23,Citation26–Citation28 and injections over infusions.Citation19,Citation29 Individual studies also reported a preference for oral over inhaled medicationCitation23 and IV injections over cannula injections.Citation30
Examination of the results across studies highlights several potential factors that could mitigate or influence preference among routes of administration. For example, strength of preference for route of administration may be influenced by both disease status and current treatment.Citation23,Citation27,Citation28,Citation31 One study found that patients with diabetes were willing to pay significantly more for a preferred route of administration (inhaled insulin over injections) than general population respondents.Citation27 Compared with insulin-naïve diabetes patients, insulin-treated patients were found to place less importance on route of administrationCitation31 and were willing to pay significantly less for inhaled insulin instead of insulin injections.Citation28
The strength of preference for route of administration may also be influenced by other characteristics of the treatment itself, including treatment efficacy. Some studies suggest that patients are more willing to accept less convenient routes of administration when compensated by greater clinical benefit. Despite a preference for oral medications, patients with diabetes were willing to accept injectable medication if it was associated with improved glycated hemoglobin (HbA1c)Citation20 or weight reduction.Citation20,Citation24,Citation25 Preference for route of administration was also affected by treatment frequencyCitation21,Citation22 and the location of treatment administration (eg, whether the treatment is administered at home or at a doctor’s office).Citation32
Dose frequency
As expected, most studies examining dose frequency found that less frequent administration was preferred over more frequent administration.Citation20,Citation24,Citation25,Citation33–Citation37 However, there were some instances when patients preferred more frequent dosing.Citation32,Citation38 For example, Augustovski et alCitation38 reported that patients with rheumatoid arthritis preferred weekly treatment over monthly treatment and suggest that this may be to avoid having to remember or plan a less frequent treatment schedule.
Other studies suggest a possible interaction between dose frequency and route of administration. Patients may prefer more frequent dosing via a preferred route of administration over less frequent dosing with a less desirable route of administration.Citation22,Citation39
Finally, one study found that strength of preference for dose frequency could vary by geographic region.Citation40 Patients in Canada and the United Kingdom had a statistically significant preference for fewer doses of oral medication per day, while no significant differences were found in Germany or the United States.
Dose timing
Respondents generally preferred flexible dose timing over dose timing linked to meals or other fixed times.Citation20,Citation34,Citation41 One diabetes study found a potential interaction between dose timing and mode of administration.Citation20 When dosing was less flexible (ie, linked to mealtimes), respondents were willing to pay more for oral over injectable medication than when dosing was more flexible (ie, not linked to mealtimes). Specifically, they were willing to pay €52 per month for tablets instead of injections when dosing was tied to mealtimes, but only €23 per month when doses could be administered at any time of day.
A study by Evans et alCitation34 found that preferences for dose timing were affected by treatment regimen (basal-only vs basal-bolus) and disease status (patients with diabetes vs general population respondents). The preference for flexible basal insulin dosing was less pronounced when administered in a basal-bolus regimen where the timing of the bolus dose was fixed. In a basal-bolus regimen, preference for a once-daily time-flexible injection over a once-daily fixed time injection was significant for general population respondents, but not patients with diabetes.
Number of pills per dose
As expected, patients generally preferred treatment with fewer pills per dose.Citation40,Citation42 However, strength of preference for the number of pills at each dose may be influenced by geographic location. Mohamed et alCitation42 found that in a sample of Swedish patients with Type 2 diabetes, the preference weights did not reveal a significant preference for the number of pills (one or two) for either the once a day or twice a day profiles. However, in the German sample, the preference weights indicate that patients preferred one pill in the morning and one pill in the evening over two pills at each administration.Citation42 Hodgkins et alCitation40 reported that patients in the US and UK were willing to pay significantly more each month for oral medication with a lower pill burden (one pill vs two pills and two pills vs three pills at each dose). However, among patients in Germany and Canada, there was no difference in willingness to pay for the preferred number of pills. In this study, respondents were told to “imagine that you are asked to pay the full cost each month in order to receive these new treatments”, regardless of whether they were typically required to pay for medication in their home country.
Treatment duration
Results of studies evaluating preference for treatment duration suggest that respondents generally prefer shorter treatment durations across disease areas.Citation19,Citation21,Citation22,Citation30 Two studies evaluating preference for duration of psoriasis treatment found that relative preference for treatment duration was influenced by respondent characteristics including comorbid depressionCitation43 and current treatment status.Citation44 Schmieder et alCitation43 found that the duration of treatment is relatively more important to patients with comorbid depression, but other comorbidities such as psoriatic arthritis, diabetes, and cardiovascular disease did not appear to influence the relative importance of treatment duration. Schaarschmidt et alCitation44 found that patients who are currently receiving injectable treatment attached greater importance to treatment duration than patients treated with other treatment modalities.
Relative importance of treatment process compared with efficacy and safety
Results of the preference studies were also examined to compare the importance of treatment process relative to safety and efficacy. In most studies, treatment process attributes were relatively less important than safety and efficacy.Citation23,Citation30,Citation32,Citation40,Citation43,Citation45–Citation51 However, in some instances, treatment process attributes had greater relative importance than some or all efficacy and safety attributes ().Citation20,Citation22,Citation23,Citation25,Citation30–Citation32,Citation36,Citation38–Citation43,Citation45–Citation53
Disease area appears to be the primary factor influencing the importance of treatment process attributes relative to safety and efficacy. This difference in relative importance of treatment process is most obvious when comparing between results of autoimmune and cancer studies. At least one treatment process attribute was found to be relatively more important than safety or efficacy variables in four of the five autoimmune studies but in none of the six cancer studies that included treatment process and safety/efficacy attributes.
Sample characteristics such as disease status and treatment status may also influence the relative importance of treatment process in comparison with safety and efficacy attributes. Casciano et alCitation31 compared subgroups of patients with Type 1 and Type 2 diabetes and found that route of administration was relatively more important than safety (side effects and risk of hypoglycemia) and efficacy attributes (maintenance of blood sugar levels) in the sample of patients with Type 2 diabetes, but not in the sample of patients with Type 1 diabetes. Hauber et alCitation52 found that treatment experience influences the relative importance of daily dosing schedule (an attribute combining dose frequency and dose size) in relation to safety. This study included patients with a low current dosing burden (“light users” – patients taking fewer than five pills per day or taking medication only once a day or as needed) and patients with a high current dosing burden (“heavy users” – patients taking five or more pills per day or taking medications more than once a day). Among heavy users, dosing schedule was less important than safety attributes such as chance of stomach problems, frequency of hypoglycemia, and risk of congestive heart failure. Among light users, preference for daily dosing schedule was more important than stomach problems and risk of congestive heart failure, but less important than frequency of hypoglycemia. Schaarschmidt et alCitation44 found that in a sample of patients with psoriasis, current treatment modality (topical therapy, phototherapy, tablets, injections, and infusions) led to differences in relative importance of magnitude and probability of benefit compared to delivery method, treatment frequency, and treatment duration.
Discussion
This review identified a substantial number of studies that quantitatively assessed preference for treatment process attributes. In many of these studies, it was found that treatment process was less important in determining preference than safety and efficacy. As listed in , this finding was reported across all four disease areas examined in this review, including oncology,Citation45,Citation47,Citation51 diabetes,Citation24,Citation25,Citation36,Citation48 autoimmune disease,Citation38,Citation54 and osteoporosis.Citation32
However, even when safety and efficacy attributes were more important, the treatment process often still had a quantifiable and potentially important impact on preference ().Citation30,Citation32,Citation36,Citation54 In conjoint studies, the impact of various aspects of the treatment process on preference was often quantified in relative importance scores representing the percentage of influence each attribute had on overall preference. In the studies reporting percentages, the impact of treatment process varied widely, accounting for 11.66%Citation31–29.3%Citation39 of treatment preference.
Furthermore, some studies reported that process attributes were equally or more important than safety and efficacy in determining treatment preference. Such results were found in samples of patients with diabetes,Citation24,Citation31 osteoporosis,Citation39 and autoimmune disease,Citation43,Citation44,Citation53 but not in samples of patients with cancer (). Perhaps the importance of treatment process attributes varies by disease condition and severity. For example, patients with cancer, often a terminal disease, were more concerned with safety and efficacy, while treatment process played less of a role in determining preference.
It is also likely that the importance of treatment process relative to treatment efficacy could depend on how outcomes are defined and quantified. Across the studies in this review, the definition of efficacy varied substantially. Given this heterogeneity, it is difficult to draw conclusions regarding the relative importance of treatment process compared to efficacy. When interpreting findings regarding relative preferences, it is important to remember that the way in which the concepts were operationalized (ie, through vignettes, attribute levels, etc) varies across studies, even among those employing the same methodology. The specific context of each study must be considered when interpreting the results, making cross-study comparisons difficult.
A wide range of studies documented preference among levels of treatment process attributes. As expected, these studies typically found that more convenient treatment processes tend to be preferred over more burdensome or more complex treatments (). For example, shorter durations of treatment administration were preferred over longer durations,Citation19,Citation22,Citation30 and less frequent administration was preferred over more frequent administration.Citation20,Citation25,Citation33–Citation37 Fewer tablets at each administration were preferred over a greater number of tablets.Citation40,Citation52 Greater flexibility with regard to dose timing was preferred over less flexibility.Citation20,Citation34,Citation41 Finally, less invasive routes of administration (eg, oral) were preferred over more invasive routes of administration (eg, injection and IV infusion).Citation20,Citation22,Citation23,Citation25
However, it should be noted that there were some exceptions to these patterns of preferences, with some studies failing to find significant differences in the expected direction.Citation46,Citation50,Citation58 In addition, unexpected findings were occasionally reported, such as preferences for more frequent treatment doses ().Citation32,Citation38 Some studies suggest that there may be interactions among multiple treatment process attributes, such as dose frequency and dose timing,Citation20,Citation22,Citation39 and these interactions among multiple treatment process issues could be causing some unexpected findings. Patients may consider each individual treatment process attribute in the larger treatment context of other process characteristics as well as safety and efficacy.
Several limitations of this literature review should be acknowledged. Although a broad literature search was conducted, the decision was eventually made to focus only on four disease areas. Therefore, this review should not be considered a comprehensive review of all published research on the topic. Other limitations stem from the content of the articles that were reviewed. For example, there was substantial variability among articles in terms of preference assessment methods, reported statistics, treatment attribute levels, respondent populations, and disease areas. This variability makes it difficult to compare findings and draw general conclusions. Adding to the difficulty of interpreting findings, levels of treatment process attributes often include multiple characteristics (eg, a blend of dose frequency and mode of administration), which confound the findings. Future studies on patient preference may address these limitations.
Despite inconsistencies in methodology, some general patterns of preference clearly emerged. Overall, the results of this review suggest that treatment process has a quantifiable impact on preference and willingness to pay for treatment, even in many situations where safety and efficacy were the primary concerns. Findings on specific treatment attributes could be used to inform the design of a target product profile for a molecule during early phases of drug development. The target product profile is a summary of drug development described in terms of labeling concepts and is intended to reflect treatment attributes that are believed to provide the greatest benefit and matter most to patients and prescribers.Citation55 This profile is used to shape clinical studies supporting the development of a product and engage regulatory agencies in discussions of registration strategy. Patient preferences for treatment process attributes can serve as valuable input to the design of future studies that target innovative treatment approaches in order to better meet the needs of patients and deliver improved outcomes.
Acknowledgments
Funding for this study was provided by Eli Lilly and Company, Indianapolis, IN, USA. The authors thank Katherine Kim for assistance with data extraction, Clarice P Hayes for assistance with study design, and Amara Tiebout for production assistance.
Supplementary materials
Search terms
The following search terms related to treatment process were applied to article titles and abstracts:
Route of administration: oral, pill, tablet, capsule, chewable, delayed-release, delayed release, sustained-release, sustained release, effervescent, granules, orodispersible, dissolvable, solution, suspension, parenteral, injection, subcutaneous, intramuscular, intravenous, intrathecal, depot, implant, infusion, transmucosal, buccal, nasal, ocular, transdermal, patch, microneedle, microporation, topical, cream, ointment, gel, spray, powder, rectal, vaginal, inhaled, inhaler, pump, intraperitoneal, mode of administration, delivery method, delivery system, drug administration route, drug administration routes, treatment modalities.
Dose frequency: dose, dosing.
Dose timing: food, meal.
Dose size: dosage.
Convenience: convenience, inconvenience.
Other process attributes: onset of action, dietary restriction, laboratory tests, monitoring, taste, sitting upright, treatment attributes, and process attributes.
Disclosure
The study was funded by Eli Lilly and Company (Indianapolis, IN, USA). Four of the authors are employed by the sponsor: Joseph A Johnston, Sarah E Curtis, Henry A Havel, and Stephanie A Sweetana. Three of the authors are employed by Evidera, a company that received support from Eli Lilly and Company for time spent conducting this study: Katie D Stewart, Louis S Matza, and Heather L Gelhorn. A preliminary version of the abstract of this paper was presented at the ISOQOL 22nd Annual Conference, October 21–24, 2015 in Vancouver, BC, Canada, as a poster presentation with interim findings. The actual paper, however, has never been published. The authors report no other conflicts of interest in this work.
References
- Hixson-WallaceJADotsonJBBlakeySAEffect of regimen complexity on patient satisfaction and compliance with warfarin therapyClin Appl Thromb Hemost200171333711190902
- MorrisLSSchulzRMMedication compliance: the patient’s perspectiveClin Ther19931535936068364951
- RauePJSchulbergHCHeoMKlimstraSBruceMLPatients’ depression treatment preferences and initiation, adherence, and outcome: a randomized primary care studyPsychiatr Serv200960333734319252046
- ShikiarRRentzABaroneJDuncansonFKatzEPatient satisfaction with ofloxacin (F) and polymyxin B/neomycin/hydrocortisone (C) in the treatment of otitis externa: results from two randomized clinical trialsJ Manage Care Med2002632427
- ShikiarRRentzAMSatisfaction with medication: an overview of conceptual, methodologic, and regulatory issuesValue Health20047220421515164810
- AllanLHaysHJensenNHRandomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer painBMJ200132272951154115811348910
- GelhornHLStringerSMBrooksAPreferences for medication attributes among patients with type 2 diabetes mellitus in the UKDiabetes Obes Metab201315980280923464623
- JohnsonFROzdemirSMansfieldCCrohn’s disease patients’ risk-benefit preferences: serious adverse event risks versus treatment efficacyGastroenterology2007133376977917628557
- JohnsonFRVan HoutvenGOzdemirSMultiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacyJ Neurol2009256455456219444531
- BrennanVKDixonSIncorporating process utility into quality adjusted life years: a systematic review of empirical studiesPharmacoeconomics201331867769123771494
- DonaldsonCShackleyPDoes “process utility” exist? A case study of willingness to pay for laparoscopic cholecystectomySoc Sci Med19974456997079032837
- SwanJSSainfortFLawrenceWFKuruchitthamVKongnakornTHeiseyDMProcess utility for imaging in cerebrovascular diseaseAcad Radiol200310326627412643553
- BridgesJFStated preference methods in health care evaluation: an emerging methodological paradigm in health economicsAppl Health Econ Health Policy20032421322415119540
- BridgesJFHauberABMarshallDConjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task ForceValue Health201114440341321669364
- RyanMFarrarSUsing conjoint analysis to elicit preferences for health careBMJ200032072481530153310834905
- RowenDBrazierJHealth utility measurementGliedSSmithPThe Oxford Handbook of Health EconomicsNew York, NYOxford University Press2011788813
- TorranceGWMeasurement of health state utilities for economic appraisalJ Health Econ19865113010311607
- TorranceGWFurlongWFeenyDHealth utility estimationExpert Rev Pharmacoecon Outcomes Res2002229910819807322
- MatzaLSCongZChungKUtilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastasesPatient Prefer Adherence2013785586524039408
- BogelundMVilsbollTFaberJHenriksenJEGjesingRPLammertMPatient preferences for diabetes management among people with type 2 diabetes in Denmark – a discrete choice experimentCurr Med Res Opin201127112175218321981417
- de Bekker-GrobEWEssink-BotMLMeerdingWJKoesBWSteyerbergEWPreferences of GPs and patients for preventive osteoporosis drug treatment: a discrete-choice experimentPharmacoeconomics200927321121919354341
- de Bekker-GrobEWEssink-BotMLMeerdingWJPolsHAKoesBWSteyerbergEWPatients’ preferences for osteoporosis drug treatment: a discrete choice experimentOsteoporos Int20081971029103718193329
- GuimaraesCMarraCAColleyLA valuation of patients’ willingness-to-pay for insulin delivery in diabetesInt J Technol Assess Health Care200925335936619619355
- JendleJTorffvitORidderstraleMEricssonANilsenBBogelundMWillingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme resultsJ Med Econ201215Suppl 21522853443
- JendleJTorffvitORidderstraleMLammertMEricssonABogelundMWillingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetesCurr Med Res Opin201026491792320163195
- ChancellorJAballeaSLawrenceAPreferences of patients with diabetes mellitus for inhaled versus injectable insulin regimensPharmacoeconomics200826321723418282016
- SadriHContingent valuation of inhaled insulin: a Canadian perspectiveJ Med Econ2007104475487
- SadriHMacKeiganLDLeiterLAEinarsonTRWillingness to pay for inhaled insulin: a contingent valuation approachPharmacoeconomics200523121215122716336016
- DarbaJRestovicGKaskensLPatient preferences for osteoporosis in Spain: a discrete choice experimentOsteoporos Int20112261947195420838770
- OssaDFBriggsAMcIntoshECowellWLittlewoodTSculpherMRecombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methodsPharmacoeconomics200725322323717335308
- CascianoRMalangoneERamachandranAGagliardinoJJA quantitative assessment of patient barriers to insulinInt J Clin Pract201165440841421401829
- SilvermanSCalderonAKawKPatient weighting of osteoporosis medication attributes across racial and ethnic groups: a study of osteoporosis medication preferences using conjoint analysisOsteoporos Int20132472067207723247328
- BoyeKSMatzaLSWalterKNVan BruntKPalsgroveACTynanAUtilities and disutilities for attributes of injectable treatments for type 2 diabetesEur J Health Econ201112321923020224930
- EvansMJensenHHBogelundMGundgaardJChubbBKhuntiKFlexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off surveyJ Med Econ201316111357136524111563
- HauberABGonzalezJMSchenkelBLoflandJHMartinSThe value to patients of reducing lesion severity in plaque psoriasisJ Dermatolog Treat201122526627521781015
- LloydANafeesBBarnettAHWillingness to pay for improvements in chronic long-acting insulin therapy in individuals with type 1 or type 2 diabetes mellitusClin Ther20113391258126721862132
- OzdemirSJohnsonFRHauberABHypothetical bias, cheap talk, and stated willingness to pay for health careJ Health Econ200928489490119464743
- AugustovskiFBeratarrecheaAIrazolaVPatient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experimentValue Health201316238539323538191
- FraenkelLGulanskiBWittinkDPatient treatment preferences for osteoporosisArthritis Rheum200655572973517013870
- HodgkinsPSwinburnPSolomonDYenLDewildeSLloydAPatient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experimentPatient201251334422077619
- AristidesMWestonARFitzGeraldPLe ReunCManiadakisNPatient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experimentValue Health20047444245415449636
- MohamedAFZhangJJohnsonFRAvoidance of weight gain is important for oral type 2 diabetes treatments in Sweden and Germany: patient preferencesDiabetes Metab201339539740323880594
- SchmiederASchaarschmidtMLUmarNComorbidities significantly impact patients’ preferences for psoriasis treatmentsJ Am Acad Dermatol201267336337222015150
- SchaarschmidtMLUmarNSchmiederAPatient preferences for psoriasis treatments: impact of treatment experienceJ Eur Acad Dermatol Venereol201327218719822225546
- AristidesMChenJSchulzMWilliamsonEClarkeSGrantKConjoint analysis of a new chemotherapy: willingness to pay and preference for the features of raltitrexed versus standard therapy in advanced colorectal cancerPharmacoeconomics2002201177578412201796
- BridgesJFMohamedAFFinnernHWWoehlAHauberABPatients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysisLung Cancer201277122423122369719
- LangerCJFastenauJMForlenzaJBEffectiveness versus convenience: patient preferences for an erythropoietic agent to treat cancer-related anemiaCurr Med Res Opin2007231859217257469
- PolsterMZanuttoEMcDonaldSConnerCHammerMA comparison of preferences for two GLP-1 products – liraglutide and exenatide – for the treatment of type 2 diabetesJ Med Econ201013465566121034377
- PorzsoltFClouthJDeutschmannMHipplerHJPreferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care valuesHealth Qual Life Outcomes2010812521050469
- ShafeyMLupichukSMDoTOwenCStewartDAPreferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experimentBone Marrow Transplant201146796296920935681
- WongMKMohamedAFHauberABPatients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinomaJ Med Econ20121561139114822808923
- HauberABHanSYangJCEffect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetesPatient Prefer Adherence2013793794924086104
- SchaarschmidtMLSchmiederAUmarNPatient preferences for psoriasis treatments: process characteristics can outweigh outcome attributesArch Dermatol2011147111285129422106115
- LichtensteinGRWatersHCKellyJAssessing drug treatment preferences of patients with Crohn’s disease: a conjoint analysisPatient201032113123
- FDA Patient Preference Information – Submission, Review in PMAs, HDE Applications, and De Novo Requests, and Inclusion in Device Labeling Draft Guidance for Industry, Food and Drug Administration Staff, and Other StakeholdersSilver Spring, MDCenter for Biologics Evaluation and Research (CBER)5201526
- GuimaraesCMarraCAColleyLSocioeconomic differences in preferences and willingness-to-pay for insulin delivery systems in type 1 and type 2 diabetesDiabetes Technol Ther200911956757319764835
- GuimaraesCMarraCAGillSA discrete choice experiment evaluation of patients’ preferences for different risk, benefit, and delivery attributes of insulin therapy for diabetes managementPatient Prefer Adherence2010443344021301591
- PintoSLHoliday-GoodmanMBlackCDLeschDIdentifying factors that affect patients’ willingness to pay for inhaled insulinRes Social Adm Pharm20095325326119733826
- SungLAlibhaiSMEthierMCDiscrete choice experiment produced estimates of acceptable risks of therapeutic options in cancer patients with febrile neutropeniaJ Clin Epidemiol201265662763422424607
- TeuffelOChengSEthierMCHealth-related quality of life anticipated with different management strategies for febrile neutropenia in adult cancer patientsSupport Care Cancer201220112755276422350594
- ShinglerSLSwinburnPAliSPerardRLloydAJA discrete choice experiment to determine patient preferences for injection devices in multiple sclerosisJ Med Econ20131681036104223730944

