Abstract
Rheumatoid arthritis (RA) remains a major clinical problem with many patients having continuing systemic inflammatory disease resulting in progressive erosive damage and high levels of disability. A range of pro-inflammatory cytokines including tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6 are involved in RA pathogenesis; these cytokines can be specifically inhibited by biological agents. Tocilizumab (TCZ) is a recombinant humanized anti-IL-6 receptor monoclonal antibody, administered monthly by intravenous infusion that prevents IL-6 signal transduction. There is strong evidence that it is both clinically efficacious and cost-effective. There have been several key clinical trials evaluating the safety and efficacy of TCZ in RA patients. We review five Phase II trials and seven Phase III trials enrolling a total of 626 and 5268 RA patients respectively. The American College of Rheumatology (ACR) response criteria were used as the primary or secondary outcome measure in all trials. Overall these trials demonstrated that TCZ was effective in the treatment of RA in a number of patient groups, including those with an inadequate response to methotrexate (MTX) or TNF inhibition. TCZ use, both as monotherapy and in combination with MTX, improved the signs and symptoms of RA within several weeks of commencing treatment. Additionally, TCZ was shown to reduce radiological disease progression and improve physical function, both as monotherapy and in combination with MTX. A 5-year extension study demonstrated that TCZ sustained good long-term efficacy and safety profiles. TCZ was generally well tolerated. Although its use increased the risk of an adverse event, these were usually mild to moderate in severity and treatment did not increase the risk of a serious adverse event in comparison to controls. Due to moderate increases in serum levels of total cholesterol, triglycerides, high-density lipoproteins and serum transaminases seen in those patients treated with TCZ, as well as severe neutropenia in some, regular blood monitoring of full blood count, liver function and lipids is recommended. Given its clinical efficacy in the treatment of RA, TCZ may be beneficial in the treatment of other autoimmune diseases where IL-6 plays a role in the inflammatory cascade.
Background
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease causing a symmetrical polyarthritis of the large and small joints. Affecting 0.5%–1.0% of the population in the developed world, it typically presents between the ages of 30–50 years, is 2.5 times more common in women than men, and is clinically characterized by joint pain, stiffness and swelling due to synovial inflammation and effusion.Citation1–Citation3 Patients may develop multiple systemic symptoms including fever, fatigue, anemia, anorexia, and osteoporosis, and show an elevation of acute-phase reactants such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).Citation3–Citation6 The disease can lead to a fluctuating progressive course that may result in joint destruction, deformity, disability and premature death.Citation7
Although not fully understood, the etiology of RA is believed to be multifactorial, resulting from interactions between genetic and environmental factors. One theory postulates that a triggering event, possibly autoimmune or infectious in origin, initiates joint inflammation which then precipitates a host of complex immune responses, eventually leading to the joint destruction and systemic complications seen in RA.Citation1,Citation2 Pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1 and IL-6 have all been shown to play an important role in disease pathogenesis in RA.Citation3,Citation5,Citation8
Managing RA
Treatments used in RA include disease modifying antirheumatic drugs (DMARDs) and more recently, biological agents. Traditionally, first-line treatment incorporates conventional DMARDs that relieve inflammatory processes and can slow disease progression.Citation9,Citation10 These include corticosteroids, methotrexate (MTX), leflunomide, sulfasalazine and hydroxychloroquine.Citation11 For patients with an inadequate response to conventional DMARDs, biological agents may be indicated. Biological therapies include the TNF-α inhibitors infliximab, adalimumab, golimumab, certolizumab and etanercept; the IL-1 inhibitor anakinra; the selective modulator of T-cell activation abatacept; and the chimeric auto CD20 B-cell depleting agent rituximab.Citation11,Citation12
Following over a decade of use, anti-TNF-α therapy is now well established as an effective treatment option for RA, especially in patients who experience an inadequate response to DMARDs, including MTX.Citation13,Citation14 Despite this, a significant proportion of patients have a partial but incomplete response (20%–40%) to anti-TNF-α therapy.Citation15 Tocilizumab (TCZ), also known as Actemra® or RoActemra®, was introduced as a new approach for the treatment of RA, targeting the IL-6 pathway.Citation16 It has been approved for use in RA in Europe, the United States, Japan and Canada, amongst others.Citation17
IL-6
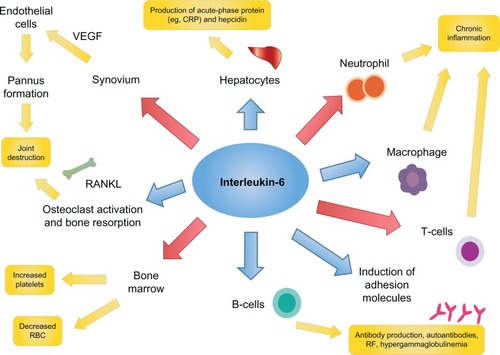
IL-6 is a 26 kDa glycopeptide whose gene is found on chromosome 7.Citation18 It was first cloned in 1986 by Hirano et al.Citation19 Like TNF-α, IL-6 is a pleiotropic pro-inflammatory cytokine with a variety of biologic effects; regulating the immune response, inflammation, bone metabolism, hematopoiesis, and stimulating chemokine production and adhesion molecules in lymphocytes, including acute-phase proteins in hepatocytes ().Citation20–Citation26 IL-6 is produced by lymphocytes, monocytes, fibroblasts, synoviocytes and endothelial cells.Citation20–Citation22,Citation26
IL-6 signal transduction
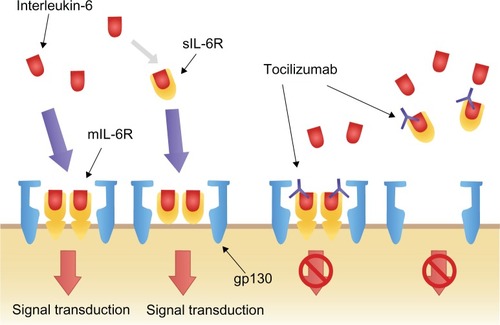
IL-6 exerts its biological activity through two differing IL-6 driven signaling pathways, involving either a membrane bound IL-6 receptor (mIL-6R), or soluble IL-6 receptor (sIL-6R). IL-6 signaling primarily occurs through the mIL-6R via two signal-transducing glycoprotein 130 (gp130) subunits.Citation18,Citation22,Citation27–Citation29 When IL-6 binds to mIL-6R, the homodimerization of gp130 is induced and a high-affinity functional receptor complex of IL-6, IL-6R, and gp130 is formed. In contrast, sIL-6R, lacking the intracytoplasmic portion of mIL-6R, is produced either by the enzymatic cleavage of mIL-6R or by alternative splicing. sIL-6R can bind with IL-6 and then the complex of IL-6 and sIL-6R can form a complex with gp130 ().Citation18,Citation22,Citation27–Citation29
Figure 2 Interleukin-6 signal transduction and blockage by tocilizumab.Reprinted from Biologics, volume 2, Okuda Y, Review of tocilizumab in the treatment of rheumatoid arthritis, pages 75–82, Copyright © 2008, with permission from Dove.Citation26
IL-6 in RA
IL-6 contributes to the pathogenesis of RA by promoting the activation of T-cells and the differentiation of B-cells into immunoglobulin-secreting plasma cells.Citation25–Citation27 It is produced in large quantities by synovial cells and macrophages in patients with RA.Citation25,Citation30 Higher levels of IL-6 and sIL-6R have been found in the serum of RA patients, and levels of IL-6 and sIL-6R have been shown to correlate with both RA disease activity and radiological joint damage.Citation31,Citation32
Enhanced expression of intercellular adhesion molecule-1 allows IL-6 to promote the infiltration of inflammatory cells into synovial tissue, and to act synergistically with TNF-α and IL-1 to promote angiogenesis as a result of increased production of vascular endothelial growth factor (VEGF).Citation33 This increase in VEGF also stimulates pannus formation.Citation18,Citation34 Overproduction of IL-6 induces acute-phase proteins (including CRP and serum amyloid A) and contributes to the systemic manifestations of RA though hepcidin production (anemia), its potent action on the hypothalamic–pituitary–adrenal axis (fatigue), and through its impact on bone metabolism (osteoporosis).Citation18,Citation34 IL-6 may also contribute to the induction and maintenance of the autoimmune process through B-cell modulation and Th17 cell differentiation.Citation18 Taken together, the number of potential mechanisms by which IL-6 may mediate the systemic and articular features of RA, make it an ideal target for the treatment of RA.Citation12
TCZ
TCZ is a recombinant humanized anti-IL-6 receptor monoclonal antibody, administered monthly by intravenous infusion (IV) that prevents IL-6 signal transduction ().Citation16,Citation21 By binding selectively and competitively to both mIL-6R and sIL-6R, TCZ prevents the dimerization of gp130 on the cell membrane and therefore blocks the pro-inflammatory effects of IL-6.Citation16,Citation21 Furthermore, TCZ has the capacity to dissociate IL-6/IL-6R complexes that have already formed.Citation16,Citation21
TCZ is approved in Europe and the United States for the treatment of moderate to severe RA in adult patients who have responded inadequately or have been intolerant to previous therapy with one or more DMARDs or TNF inhibitors.Citation5,Citation35
The objective of this article is to review the key studies of the use of TCZ in RA, addressing efficacy, tolerability and adverse events (AEs).
Key studies of TCZ
Introduction
There have been several key clinical trials evaluating the safety and efficacy of TCZ in RA patients. Numerous primary end-points have been used and include the American College of Rheumatology (ACR) response criteria (ACR20, ACR50, and ACR70 are defined by a 20%, 50%, or 70% clinical improvement from baseline after treatment respectively).Citation36 Other endpoints include the European League Against Rheumatism and the Disease Activity Score of 28 joints (DAS28) improvement criteria;Citation37 the patients’ and physicians’ global assessment of disease activity; the patient’s assessment of pain; the Health Assessment Questionnaire Disease Index (HAQ-DI) score;Citation38 and the acute-phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]).
Phase I/II clinical studies
Monotherapy
A UK Phase I/II study was performed by Choy et al in 2002.Citation23 This randomized, double-blind, placebo-controlled, dose-escalation trial was conducted in 45 patients with active RA as defined by the ACR revised criteria. The study aimed to determine the safety and efficacy of TCZ in single doses. Patients were sequentially allocated to receive one of four single dose cohorts of TCZ (0.1 mg/kg, 1.0 mg/kg, 5.0 mg/kg, or 10.0 mg/kg) or placebo. The primary efficacy endpoint was meeting the ACR20 response criteria 2 weeks after treatment. The ACR20 is considered to be a powerful discriminator between active and placebo treatments.
Choy et al found a significant treatment difference between the TCZ 5.0 mg/kg group and the placebo group, with 55.6% of the TCZ group achieving an ACR20 response compared to 0% in the placebo group. The ESR and CRP values fell significantly in the TCZ 5.0 mg/kg and 10.0 mg/kg groups, normalizing after 2 weeks of treatment. The only AEs noted, were considered to be mild to moderate in severity and were found in approximately equal amounts in both the treatment and placebo groups. No severe AEs were attributed to TCZ. They concluded that inhibition of IL-6 significantly improved the signs and symptoms of RA and normalized acute-phase reactants.Citation23
A Japanese Phase I/II study by Nishimoto et al in 2003 aimed to evaluate the safety and pharmacokinetics of multiple infusions of TCZ in patients with RA. Using an open-label trial, 15 patients with active RA, as defined by the ACR criteria, were administered three doses of TCZ IV at doses of 2 mg/kg, 4 mg/kg, or 8 mg/kg, every 2 weeks for 6 weeks. They evaluated the pharmacokinetics, safety, and clinical efficacy of TCZ. Patients with no safety concerns in regard to TCZ were continued on treatment and reassessed at 6 months.
This study showed that the half-life (T1/2) of TCZ increased with repeated and increased doses. Those given 4 mg/kg and 8 mg/kg TCZ showed a tendency to accumulate TCZ in the blood. In those in whom TCZ levels were detectable in the blood at trough levels, objective inflammatory indicators such as CRP, ESR, and serum amyloid A were completely normalized at 6 weeks, with no statistically significant difference in efficacy amongst the three dose groups. Their findings indicated that IL-6 was a major cytokine responsible for the acute-phase protein production seen in vivo in patients with RA.
Although this was an open-label study, ACR20 and ACR50 response criteria measurements were also assessed. They found that at 6 weeks post-treatment, 60% and 13% of patients achieved ACR20 and ACR50 respectively, and in those that continued on treatment, at 6 months 86.7% and 33.5% achieved ACR20 and ACR50 respectively. TCZ was well-tolerated at all doses with no difference in the frequency of AEs between these groups, and no severe AEs were attributed to TCZ. No new observations of antinuclear antibodies or anti-DNA antibodies, and no anti-TCZ antibodies were detected.
Phase II studies
Monotherapy – previous DMARD failure
A Japanese late Phase II multicenter, double-blind, placebo-controlled trial aimed to evaluate the safety and efficacy of TCZ monotherapy in patients with RA.Citation39 One hundred sixty-four RA patients with an inadequate response to one or more DMARDs or immunosuppressants, were randomized into one of three treatment arms; 3 months of IV infusions every 4 weeks of either TCZ 4 mg/kg, TCZ 8 mg/kg, or placebo infusions. The clinical responses were measured at 3 months using the ACR response criteria and the DAS28 improvement criteria. The mean number of DMARDs tried prior to entry to the trial was between four and five, and the mean duration was 8 years, implying established disease.Citation39
Patients treated with TCZ showed a reduction in disease activity in a dose-dependent manner. At 3 months, an ACR20 response was achieved in 57% and 78% of the 4 mg/kg and 8 mg/kg TCZ groups respectively, compared with only 11% in the placebo group. The 8 mg/kg TCZ dose led to a significantly greater ACR20 response compared to the 4 mg/kg TCZ and placebo groups (P = 0.002 and P < 0.001, respectively). An ACR50 response was seen in 40% of patients treated with 8 mg/kg TCZ compared to only 1.9% in the placebo group (P < 0.001). The ACR70 response was also found to be superior in the 8 mg/kg group compared to the placebo group (16.4% versus 0.0% respectively, P = 0.002). Evaluation using the DAS28 criteria showed a good or moderate response in 90.9% of the 8 mg/kg TCZ group. Complete normalization of the CRP level was observed in 26% and 76% of the TCZ 4 mg/kg and 8 mg/kg groups respectively, compared to only 1.9% of the placebo group. Nishimoto et al concluded that treatment with TCZ was generally well-tolerated and significantly reduced the disease activity of RA.Citation39
Monotherapy – no prior treatment failure
The STREAM extension study (long-term safety and efficacy of TCZ, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with RA)Citation40 was an open-label, long-term extension trial following on from the initial 3-month randomized controlled Phase II trial.Citation39 This trial aimed to evaluate the safety and efficacy of 5-year TCZ monotherapy in patients with refractory RA. Of the 163 patients in the initial late Phase II trial, 143 patients received infusions of 8 mg/kg TCZ monotherapy every 4 weeks. Concomitant therapy with nonsteroidal antiinflammatory drugs and/or oral prednisolone (maximum 10 mg daily) was permitted. Again the clinical responses were measured using the ACR criteria and DAS28 improvement criteria at 5 years.Citation40
At 5 years, 84.0%, 69.1%, and 43.6% of the patients achieved ACR20, ACR50, and ACR70 improvement respectively, and 55.3% achieved DAS28 remission (defined as DAS28 < 2.6). Of the patients receiving corticosteroids at baseline, 88.6% were able to decrease their corticosteroid dose and 31.8% to discontinue them altogether. Nishimoto et al concluded that TCZ demonstrated sustained long-term efficacy.Citation40
Combination therapy – previous DMARD failure
The European CHARISMA study (Chugai humanized anti-human recombinant IL-6 monoclonal antibody study) was a randomized, double-blind, placebo-controlled, multicenter trial investigating the safety and efficacy of multiple infusions of TCZ, both alone and in combination with MTX.Citation14 This 16-week trial included 359 patients with active RA and an inadequate response to 4 weeks of treatment with MTX. Patients received treatment every 4 weeks and were randomized to one of seven treatment arms: TCZ at doses of 2 mg/kg, 4 mg/kg, or 8 mg/kg either as monotherapy with placebo or in combination with MTX, or MTX plus placebo.Citation14
An ACR20 response at week 16 was achieved in 61% and 63% of patients receiving 4 mg/kg and 8 mg/kg TCZ plus placebo (TCZ monotherapy) respectively, compared with only 41% of patients receiving MTX plus placebo (MTX monotherapy) (P < 0.05). In those patients treated with TCZ plus MTX (TCZ combination therapy), an ACR20 response was achieved by 63% and 74% in the TCZ 4 mg/kg and 8 mg/kg groups respectively. TCZ combination therapy demonstrated superior efficacy compared with MTX monotherapy, with a statistically significant ACR50 and ACR70 response at either 4 mg/kg or 8 mg/kg of TCZ plus MTX (P < 0.05).Citation14
In all patients, except those receiving TCZ 2 mg/kg, a dose-related reduction in the DAS28 was observed from week 4 onwards. The majority of patients who received 8 mg/kg of TCZ showed a normalization of the inflammatory markers, CRP and ESR. The CHARISMA study group concluded that TCZ was highly efficacious in decreasing disease activity in RA.Citation14
Phase III trials
Seven Phase III clinical trials were performed to evaluate the efficacy and safety profile of TCZ in patients with RA; these are summarized in –.Citation10,Citation41–Citation46
Table 1 Key trials of tocilizumab
Table 2 Clinical efficacy in Phase III trials
Table 3 Changes in fatigue, pain and disability in key trials
Table 4 Adverse events in Phase III trials
Monotherapy – no prior treatment failure
The AMBITION study (Actemra versus MTX double-blind investigative trial in monotherapy) aimed to investigate the efficacy and safety of TCZ monotherapy versus MTX in patients with moderate to severe active RA.Citation41 This 24-week, double-blind, double-dummy, parallel-group trial randomized 673 patients who had not previously failed MTX or biologics treatment to receive either TCZ 8 mg/kg monotherapy every 4 weeks, oral MTX monotherapy, or placebo for 8 weeks followed by TCZ 8 mg/kg every 4 weeks. Those receiving MTX monotherapy started at a dose of 7.5 mg/week and were titrated to 20 mg/week within 8 weeks. The primary efficacy endpoint was meeting the ACR20 response criteria 24 weeks after treatment.Citation41
Approximately 66% of the patients entering this trial were MTX treatment naïve. At 24 weeks, the intention to treat analysis demonstrated that patients treated with TCZ monotherapy had a statistically superior ACR20 compared to those treated with MTX (69.9% versus 52.5%, respectively, P < 0.001). The proportion of ACR50 (44.1%) and ACR70 (28.0%) responders at week 24 was also statistically superior in those treated with TCZ compared with the MTX group. DAS28 remission was achieved in 33.6% of the TCZ treated group compared to 12.1% in those treated with MTX. By week 24 the TCZ group were five times more likely to achieve DAS28 remission than the MTX group (odds ratio [OR] 5.8; 95% confidence interval [CI] 3.3, 10.4). With respect to laboratory parameters, by week 12 the mean CRP level was within the normal range in the TCZ group, whereas levels remained elevated in the MTX group.Citation41
The AMBITION study group concluded that TCZ monotherapy was superior to MTX monotherapy in RA patients for whom treatment with MTX or biologics had not previously failed.Citation41
Monotherapy – DMARD failure
The SATORI study (study of active-controlled TCZ monotherapy for RA patients with inadequate response to MTX) investigated the clinical efficacy of TCZ monotherapy in patients with moderate to severe active RA with an inadequate response to 8 weeks of low dose MTX.Citation42 This 24-week multicenter, double-blind, randomized controlled trial allocated 125 RA patients to receive either TCZ 8 mg/kg plus MTX placebo (TCZ monotherapy) or TCZ placebo plus MTX 8 mg/week (MTX monotherapy/control) every 4 weeks. The clinical responses were measured using the ACR criteria and the DAS28 response criteria. Serum VEGF levels were also monitored.Citation42
At week 24, the ACR20 response was significantly higher in the TCZ group compared to the MTX treated group (80.3% versus 25.0%, P < 0.001). The ACR50 and ACR70 responses were also significantly higher in the TCZ group at all time points from 4 weeks onwards compared to the MTX group (49.2% versus 10.9% and 29.5% versus 6.3%, respectively, P < 0.001). Similarly, the reduction in DAS28 was greater for the TCZ group (P < 0.001). TCZ also caused a significantly greater reduction in VEGF levels compared with MTX (P < 0.001).Citation42
The SATORI study group concluded that TCZ monotherapy was well- tolerated and provided an excellent clinical benefit in active RA patients with an inadequate response to low dose MTX.Citation42
Combination therapy – DMARD failure
The TOWARD study (the TCZ in combination with traditional DMARD therapy study) was a large multicenter, randomized, double-blind, placebo-controlled trial investigating the efficacy and safety of TCZ combined with conventional DMARDs in patients with moderate to severe active RA.Citation10 This study aimed to represent a diverse clinic population by allowing patients to enroll on the study if they were taking any stable single or multiple agent conventional DMARD, rather than just MTX. Permitted conventional DMARDs included MTX, chloroquine, hydroxychloroquine, parenteral gold, sulfasalazine, azathioprine, and leflunomide. Patients who had an incomplete response to anti-TNF therapy or were previously treated with a cell-depleting agent were excluded.Citation10
A total of 1220 patients were randomized in a 2:1 manner to receive either TCZ 8 mg/kg infusions or placebo infusions every 4 weeks. All patients continued their conventional DMARD therapy at a stable dose. The primary efficacy endpoint was meeting the ACR20 response criteria after 24 weeks.Citation10
At week 24 the proportion of patients achieving an ACR20 response was significantly greater in the TCZ plus DMARD group than in the control group (60.8% and 24.5%, respectively, P < 0.0001). Secondary endpoints of ACR50 and ACR70 responses were also significantly improved in the TCZ group compared to the control group (37.6% versus 9.0% and 20.5% versus 2.9%, respectively, P < 0.0001). DAS28 remission at week 24 was higher in the TCZ group versus the control group (30% versus 3%, respectively, P < 0.0001).Citation10
The levels of inflammatory markers ESR and CRP decreased significantly in the TCZ group versus the control group by week 24 (P < 0.0001 for each), with normalization of CRP seen within 2 weeks in the TCZ group, with maintenance of low levels throughout the study.Citation10
The TOWARD study group concluded that TCZ combined with any of the DMARDs evaluated was safe and effective in reducing articular and systemic symptoms in patients with an inadequate response to these DMARDs alone.Citation10
The OPTION study (TCZ pivotal trial in MTX inadequate responders), a double-blind, randomized, placebo-controlled, parallel group trial, assessed the therapeutic effects of TCZ in patients with moderate to severe active RA who were taking a stable background dose of MTX.Citation43 Six hundred twenty-three patients were randomly assigned into one of three treatment arms: infusions of TCZ 4 mg/kg, TCZ 8 mg/kg, or TCZ-placebo every 4 weeks, all with concurrent MTX at stable pre-study doses (10–25 mg/week). The primary efficacy endpoint was meeting the ACR20 response criteria 24 weeks after treatment.Citation43
At week 24, the proportion of patients achieving ACR20 was significantly higher in the TCZ 4 mg/kg and TCZ 8 mg/kg groups compared to the placebo group (48%, 59%, and 26%, respectively, P < 0.0001). The ACR50 and ACR70 responses were also significantly higher in both the TCZ 4 mg/kg and TCZ 8 mg/kg group compared to the placebo group (ACR50 31%, 44%, and 11%, respectively, P < 0.001; ACR70 12%, 22%, and 2%, respectively, P < 0.001). DAS28 disease remission was reached by 13%, 27%, and 0.8% of the patients in the TCZ 4 mg/kg, TCZ 8 mg/kg, and placebo groups respectively (P < 0.05).Citation43
As seen in other TCZ trials, the mean CRP concentrations normalized by week 2 of treatment with TCZ 8 mg/kg and remained within the normal range until the end of the study (P < 0.0001 versus baseline). ESR also normalized in the 8 mg/kg group in a similar way to CRP (P < 0.0001 versus baseline).Citation43
The OPTION study group concluded that TCZ was an effective therapeutic approach in patients with moderate to severe active RA.Citation43
Combination therapy – anti-TNF failure
The RADIATE study (research on Actemra [TCZ] determining efficacy after anti-TNF failures) examined the efficacy and safety of TCZ in patients with RA refractory to anti-TNF therapy.Citation44 The study design was similar to the OPTION study but the RA patients in RADIATE had previously had an inadequate response to one or more anti-TNF agents. Four hundred ninety-nine patients were randomly assigned to one of three treatment arms: infusions of TCZ 4 mg/kg, TCZ 8 mg/kg, or placebo infusions every 4 weeks, all with stable doses of MTX (10–25 mg/week). The primary efficacy endpoint was meeting the ACR20 response criteria 24 weeks after treatment. Secondary efficacy and safety endpoints were also assessed.Citation44
At week 24, the proportion of patients achieving an ACR20 was significantly higher in the TCZ 4 mg/kg and TCZ 8 mg/kg groups compared to the placebo group (30.4%, 50.0%, and 10.1%, respectively, P < 0.001). The ACR50 and ACR70 responses were also significantly more common in the TCZ 8 mg/kg group (28.8% and 12.4%, respectively) as compared to the placebo group (3.8% and 1.3%, respectively) (P < 0.001 and P = 0.001 respectively, versus control). Patients responded regardless of the most recently failed anti-TNF or the number of failed treatments. DAS28 remission rates at week 24 were dose related, being achieved by 7.6%, 30.1%, and 1.6% of the TCZ 4 mg/kg, 8 mg/kg, and control groups, respectively (P = 0.053 for TCZ 4 mg/kg, P < 0.001 for TCZ 8 mg/kg versus control).Citation44
CRP and ESR levels dropped markedly by week 2 in both the TCZ 4 mg/kg and TCZ 8 mg/kg groups. By week 24 CRP levels had normalized in the TCZ 8 mg/kg group.Citation44
The RADIATE study group concluded that TCZ plus MTX was effective in achieving rapid and sustained improvements in the signs and symptoms of RA in patients with an inadequate response to anti-TNF therapy.Citation44
Radiographic progression with TCZ
Two Phase III studies looked at the effect of TCZ in preventing structural joint damage in RA patients as the primary endpoint; these were the SAMURAICitation45 and LITHECitation46 studies. Both studies had secondary endpoints of clinical efficacy.
Monotherapy – DMARD failure
The SAMURAI study (study of active controlled monotherapy used for RA, an IL-6 inhibitor), an X-ray reader-blind, open-label, randomized controlled trial evaluated the ability of TCZ monotherapy to inhibit progression of structural joint damage in patients with active RA and an inadequate response to at least one DMARD.Citation45
Three hundred six patients with active RA of less than 5 years duration were allocated to receive either infusions of TCZ 8 mg/kg monotherapy or conventional DMARDs (except for leflunomide and anti-TNF therapies), every 4 weeks. The primary endpoint was change in the mean modified Total Shape Score (TSS) at week 52.Citation45
At week 52 the secondary endpoints of ACR20, ACR50, and ACR70 responses were achieved in 78%, 64%, and 44%, respectively, in the TCZ group compared to 34%, 13%, and 6%, respectively, in the conventional DMARDs group (P < 0.001, for each comparison), although clinical efficacy was assessed without blinding. DAS28 remission was achieved in 59% receiving TCZ compared to 3% receiving DMARDs (P < 0.001) at week 52.Citation45
The SAMURAI study group concluded that TCZ monotherapy was generally well-tolerated and provided radiographic benefit in patients with RA.Citation45
Combination therapy – DMARD failure
The LITHE study (TCZ safety and the prevention of structural joint damage) was a randomized, double-blind, placebo-controlled trial aiming to assess the efficacy and safety of TCZ in combination with MTX in preventing structural joint damage and improving physical function and disease activity in patients with RA.Citation46 One thousand one hundred ninety-six patients with active moderate to severe RA, who had an incomplete response to MTX and had not previously failed anti-TNF therapy, were randomized into one of three treatment arms: infusions of either TCZ 4 mg/kg, TCZ 8 mg/kg, or a TCZ placebo every 4 weeks, all in combination with MTX 10–25 mg/week. The length of the controlled phase of the study was 2 years, although only results from the first year were published. The main outcomes measured in the trial were the changes from baseline in the Genant-modified Sharp score and the area under the curve in the HAQ-DI after 52 weeks. Secondary endpoints of disease activity using the ACR response criteria were also assessed.Citation46
At week 52 the ACR20, ACR50, and ACR70 responses in the TCZ 8 mg/kg plus MTX group (56%, 36%, and 20%, respectively) were significantly higher compared to the control group (P < 0.0001 for each response rate). Additionally, a significantly higher proportion of patients treated with TCZ 8 mg/kg plus MTX achieved a DAS28 remission by 52 weeks compared with the control (47.2% versus 7.9%, respectively, P < 0.0001). The LITHE study group concluded that TCZ plus MTX resulted in greater inhibition of joint damage and greater improvement in physical function than MTX alone.Citation46
Effects of TCZ on X-ray damage
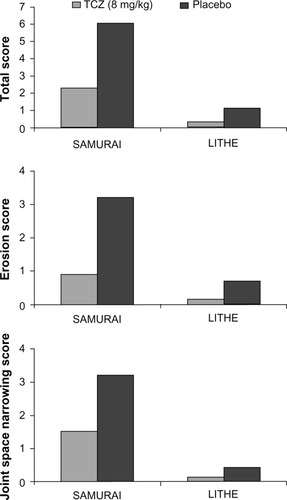
Both the SAMURAI and LITHE studies confirmed that patients treated with TCZ, either as monotherapy or in combination with MTX, had significantly less radiographic progression compared to controls ().Citation45,Citation46
At the end of week 52 of the SAMURAI study, those patients who were treated with TCZ had significantly less radiographic change in TSS than those patients receiving conventional DMARDs, with 56% and 39% having no radiographic progression (change from baseline TSS ≤ 0.5) in the TCZ and DMARD-treated groups respectively (P < 0.01). TCZ was also superior to DMARDs in preventing both erosions and joint space narrowing (P < 0.001 and P < 0.018, respectively).Citation45
In the LITHE study, at the end of 52 weeks, radiographic progression from baseline was reduced by 70% and 74% in the TCZ 4 mg/kg plus MTX and 8 mg/kg plus MTX groups, respectively, compared to the placebo group (MTX alone) (P < 0.0001 for both comparisons), with mean changes in the Genant-modified Sharp score of 0.34, 0.29, and 1.39 for TCZ 4 mg/kg plus MTX, TCZ 8 mg/kg plus MTX, and placebo respectively (P < 0.0001). At 2 years, patients in the TCZ 8 mg/kg plus MTX group had significantly less radiographic progression compared with the placebo group: 85% versus 67% respectively (P < 0.001).Citation46
Effects of TCZ on function
In addition to clinical efficacy, many studies also explored the effect of TCZ on function using various measurements such as the HAQ-DI, functional assessment of chronic illness therapy (FACIT), and the Short Form (36) Health Survey, both mental and physical (SF36-mental and SF36-physical) (summarized in ).Citation10,Citation41–Citation46
All seven of the Phase III clinical studies found that TCZ (at either dose where applicable) significantly improved the modified HAQ-DI scores from baseline, compared to controls.Citation10,Citation41–Citation46 The OPTIONCitation43 and TOWARDCitation10 studies went on to examine the FACIT and SF36-mental and SF36-physical scores and again found that those patients receiving TCZ had a statistically significant improvement from baseline in comparison to controls.Citation10,Citation43
Tolerability and AEs
TCZ was well-tolerated across all seven Phase III trials, both as monotherapy or in combination with MTX or a DMARD.Citation10,Citation41–Citation46 In general, the overall incidence of AEs was similar in both the TCZ and placebo groups ().
The majority of AEs were considered to be mild to moderate in severity.Citation10,Citation41–Citation46 The most common AEs in the TCZ treated groups were infection, often nasopharyngitis, followed by gastrointestinal disturbance, stomatitis, rash, and headache.Citation10,Citation41–Citation46 Common infusion reactions (defined as a reaction that occurred within 24 hours of infusion) included hypertension, rash, nausea, and headache.Citation10,Citation41–Citation46 The incidence of serious infusion reactions and anaphylaxis was rare.Citation10,Citation41–Citation46
In all but one of the trials the incidence of serious AEs (SAEs) was similar in the TCZ and placebo groups.Citation10,Citation41–Citation46 In the RADIATE trialCitation44 more SAEs were seen in the MTX monotherapy (placebo) group than in the TCZ treatment groups.Citation44 Serious infections seen in the TCZ treated groups included septic arthritis, staphylococcal cellulitis, and acute pyelonephritis.Citation10,Citation41–Citation46 No cases of tuberculosis were reported, however one case of asymptomatic Mycobacterium avium-intracellulare was reported in a TCZ treated patient in the TOWARD trial.Citation10
Numerous laboratory abnormalities were reported. These tended to center around deranged liver function tests, lipid abnormalities, and neutropenia.Citation10,Citation41–Citation46 Laboratory abnormalities were often more common in the TCZ groups compared to the controls, with many of the excess TCZ events related to an elevation in total cholesterol, triglycerides, high-density lipoproteins, and low-density lipoproteins, although the majority found no increase in adverse cardiovascular events.Citation10,Citation41–Citation46 Several trials noted neutropenia in the TCZ treated groups, mostly grade 1–2. Both the AMBITION and RADIATE trials reported grade 3 and 4 neutropenia, respectively.Citation41,Citation44 Those in the latter group were withdrawn from the trial.
Systematic reviews
A number of systematic reviews on the use of TCZ in RA have recently been published, with many including meta-analyses of clinical trial data.Citation17,Citation47 These reviews have concentrated on outcomes including clinical efficacy (ACR20, ACR50, ACR70, and DAS28 remission), effect on radiographic progression, function, quality of life, and safety in over 2000 patients receiving TCZ at varying doses, in comparison to either placebo or an active comparator (often MTX).Citation17
Monotherapy
TCZ monotherapy was found to be more effective at improving the ACR50 and ACR70 scores (OR 21.2 ACR50, OR 18.3 ACR70) than placebo or DMARDs alone (OR 2.76 ACR50, OR 2.3 ACR70).Citation17,Citation47 TCZ 8 mg/kg monotherapy was also found to reduce radiographic disease progression compared to MTX alone.Citation17,Citation47
Combination therapy
All studies have consistently found that combination therapy with TCZ plus a DMARD is more effective than placebo (at either the 4 mg/kg or 8 mg/kg dose) at improving the ACR50 and ACR70 (OR 3.79 ACR50, OR 5.94 ACR70 versus DMARD).Citation17 TCZ at a dose of 8 mg/kg was shown to be more likely to lead to DAS28 remission (OR 10.6 versus DMARD) and a greater improvement in HAQ-DI scores, with no significant increase in SAEs.Citation17 Additionally, combination therapy with TCZ plus MTX was found to reduce radiographic disease progression.Citation17
A systematic review by Campbell et al specifically focused on the risk of AEs with TCZ, and found that there was an increased risk of all AEs in patients treated with combination therapy (TCZ 8 mg/kg plus MTX) (OR 1.53) versus MTX alone.Citation47 This included an increased risk of infection (OR 1.3). However, no significant difference in SAEs was seen even in the higher TCZ dose group. Due to differences in reporting of lipid abnormalities and neutropenia across different trials these AEs could not be evaluated.Citation41 In general, the incidence of SAEs were low (1.5 events/100 patient years), but were increased further when TCZ was given in combination with a nonbiologic DMARD (3.6 events/100 patient years).Citation5
Economic aspects
Within the United Kingdom, access to biological therapies conventionally occurs on the basis of approval by the National Institute of Clinical Excellence (NICE). NICE currently recommends TCZ plus MTX in patients with disease refractory to both TNF inhibitors and rituximab, or directly after anti-TNF where rituximab is contraindicated. In addition, a recent update suggests that TCZ may be used as monotherapy in DMARD non-responders as an alternative to TNF inhibitors where MTX cannot be co-prescribed.Citation35
All biological therapies are high cost in comparison to DMARDs, and calculating the cost-effectiveness of such agents is complex. A number of cost-effectiveness models involving biologics have been published, but without details of the cost-effectiveness of TCZ.Citation48,Citation49 However, a recent economic model of the use of TCZ as a first-line biological agent in a sequence of biologics found that TCZ was more cost-effective than standard therapy (etanercept followed by adalimumab, rituximab, and abatacept) due to lower cost and increased benefit.Citation50
Conclusion
Although anti-TNF agents provide an excellent clinical response for most patients, up to 40% of patients fail to respond.Citation15 TCZ is the first biologic targeting IL-6 that is licensed for the treatment of RA.Citation16
The clinical trials of TCZ reviewed showed strong evidence that its use, both as monotherapy and in combination with MTX, improve the signs and symptoms of RA within several weeks of commencing treatment.Citation10,Citation14,Citation23,Citation39–Citation46
Additionally TCZ reduced radiological disease progressionCitation42,Citation46 and improved physical function both as monotherapy and in combination with MTX.Citation10,Citation14,Citation23,Citation39–Citation46 A 5-year extension study by Nishimoto et al demonstrated that TCZ sustained good long-term efficacy and safety profiles.Citation40
More recently, a study by Dougados et al showed that TCZ monotherapy was as effective as combination therapy (TCZ and MTX) in the treatment of RA.Citation51 TCZ monotherapy may be a valuable treatment strategy in suitable RA patients who are intolerant of MTX.
TCZ was generally well tolerated. Although its use increased the risk of an AE, these were usually mild to moderate in severity and treatment did not increase the risk of SAEs in comparison to controls. Due to moderate increases in serum levels of total cholesterol, triglycerides, high-density lipoproteins, and serum transaminases seen in those treated with TCZ, as well as severe neutropenia in some, regular blood monitoring of full blood count, liver function and lipids is recommended.Citation10,Citation41–Citation46 Whilst these short-term efficacy and safety profiles are encouraging, further long-term safety data are required to better characterize the risk–benefit profile of TCZ.
With advances in pharmacogenomics and the field of stratified medicine expanding, biologics targeting differing pro-inflammatory cytokines may offer new hope for patients who have failed treatment targeting other inflammatory cytokines. Given its clinical efficacy in the treatment of RA, TCZ may be beneficial in the treatment of other autoimmune diseases where IL-6 plays a role in the inflammatory cascade.
Disclosure
Israa Al-Shakarchi and Nicola Gullick have received no external grants or personal funding from any industrial sponsor. David L Scott receives grant funding support from Arthritis Research UK and The National Institute for Health Research. In the past 3 years Professor Scott has received no personal funding from any industrial sponsor. The authors report no other conflicts of interest in this work.
References
- MajithiaVGeraciSARheumatoid arthritis: diagnosis and managementAm J Med20071201193693917976416
- Al-ShakarchiIFragility Fractures in Rheumatoid Arthritis – A systematic review of the literatureProceedings of the British Society of Rheumatology ConferenceApril 21–23, 2010Birmingham, UK
- SmolenJSAletahaDKoellerMWeismanMHEmeryPNew therapies of rheumatoid arthritisLancet200737096021861187417570481
- NishimotoNYoshiazakiKMaedaKToxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical studyJ Rheumatol20033071426143512858437
- Navarro-MillánISinghJCurtisJRSystematic review of Tocilizumab for rheumatoid arthritis: A new biologic agent targeting the Interleukin-6 receptorClin Ther2012344788802 e322444783
- HashizumeMMiharaMThe roles of Interleukin-6 in the Pathogenesis of Rheumatoid ArthritisArthritis20112011
- OldfieldVDhillonSPloskerGLTocilizumab: a review of its use in the management of rheumatoid arthritisDrugs200969560963219368420
- FiresteinGSEvolving concepts of rheumatoid arthritisNature2003423693735636112748655
- GaffoASaagKGCurtisJRTreatment of rheumatoid arthritisAm J Health Syst Pharm200663242451246517158693
- GenoveseMCMcKayJDNasonovELInterleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs. The Tocilizumab in combination with traditional disease modifying anti-rheumatic drug therapy studyArthritis Rheum200858102968298018821691
- OlsenNJSteinCMNew drugs for rheumatoid arthritisN Engl J Med2004350212167217915152062
- HushawLLSawaquedRSweisGCritical appraisal of tocilizumab in the treatment of moderate to severe rheumatoid arthritisTher Clin Risk Manag2010614315220421913
- FeldmannMMainiRNAnti-TNF α therapy of rheumatoid arthritis; what have we learnt?Ann Rev Immunol20011916319611244034
- MainiRNTaylorPCSzechinsjiJCHARISMA Study GroupDouble-blind randomized Controlled clinical trial of the interleukin-6 receptor antagonist, Tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexateArthritis Rheum20065492817282916947782
- HetlandMLChristensenIJTarpUAll Departments of Rheumatology in DenmarkDirect comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide DANIBO registryArthritis Rheum2010621223220039405
- NishimotoNKishimotoTHumanized antihuman IL-6 receptor antibody, tocilizumabHandb Exp Pharmacol200818115116018071945
- SinghJABegSLopez-OlivoMAtocilizumab for rheumatoid arthritis: a cochrane systematic reviewJ Rheumatol2011381102020952462
- DayerJChoyETherapeutic targets in rheumatoid arthritis: the interleukin-6 receptorRheumatology2010491152419854855
- HiranoTYasukawaKHaradaHComplementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulinNature1986324609273763491322
- KishimotoTInterleukin-6: from basic science to medicine-40 years in immunologyAnn Rev Immunol20052312115771564
- MimaTNishimotoNClinical value of blocking Il-6 receptorCurr Opin Rheumatol200921322423019365268
- KishimotoTInterleukin-6 and its receptor in autoimmunityJ Autoimmun19925Suppl A1231321380241
- ChoyEHIsenbergDAGarrodTTherapeutic benefit of blocking Interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis. A randomized, double-blind, placebo-controlled, dose-escalation trialArthritis Rheum200246123143315012483717
- FonsecaJESantosMJCanhãoHChoyEInterleukin-6 as a key player in systemic inflammation and joint destructionAutoimmun Rev20098753854219189867
- HiranoTMatsudaTTurnerMExcessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritisEur J Immunol19881811179718012462501
- OkudaYReview of tocilizumab in the treatment of rheumatoid arthritisBiologics200821758219707430
- Rose-JohnSSchellerJElsonGJonesSAInterleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancerJ Leukoc Biol200680222723616707558
- HiranoTInterleukin 6 and its receptor: ten years laterInt Rev Immunol1998163–42492849505191
- WardLDHowlettGJDiscoloGHigh affinity interleukin-6 receptor is a hexameric complex consisting of two molecules each of interleukin-6 receptor and gp-130J Biol Chem19942693723286232898083235
- LallyFSmithEFilerAA novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synoviumArthritis Rheum200552113460340916255036
- DasguptaBCorkillMKirkhamBGibsonTPanayiGSerial estimation of interleukin-6 as a measure of systemic disease in rheumatoid arthritisJ Rheumatol199219122251556695
- KotakeSSatoKKimKJInterleukin-6 and soluble interleukin-6 receptors in the synovial fluid from rheumatoid arthritis patients are responsible for osteoclast-like cell formationJ Bone Miner Res199611188958770701
- NakaharaHSongJSugimotoMAnti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor (VEGF) production in rheumatoid arthritisArthritis Rheum20034861521152912794819
- NishimotoNInterleukin-6 as a therapeutic target in candidate inflammatory diseasesClin Pharmacol Ther201087448348720182422
- Tocilizumab for the treatment of rheumatoid arthritis (rapid review of technology appraisal guidance 198)NICE technology appraisal guidance 247 [webpage on the Internet]LondonNational Institute for Health and Clinical Excellence2012 Available from: http://guidance.nice.org.uk/TA198Accessed Sep 12, 2012
- FelsonDTAndersonJJBoersMAmerican College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritisArthritis Rheum19953867277357779114
- van GestelAMHaagsmaCJvan RielPLValidation of rheumatoid arthritis improvement criteria that include simplified joint countsArthritis Rheum19984110184518509778226
- van der HeijdeDMvan’t HofMAvan RielPLJudging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity scoreAnn Rheum Dis199049119169202256738
- NishimotoNYoshizakiKMiyasakaNTreatment of rheumatoid arthritis with humanized anti–interleukin-6 receptor antibody – a multicenter, double-blind, placebo-controlled trialArthritis Rheum20045061761176915188351
- NishimotoNMiyasakaNYamamotoKKawaiSTakeuchiTAzumaJLong-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension studyAnn Rheum Dis200968101580158419019888
- JonesGSebbaAGuJComparison of tocilizumab monotherapy versus monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION studyAnn Rheum Dis2010691889619297346
- NishimotoNMiyasakaNYamamotoKStudy of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapyMod Rheumatol2009191121918979150
- SmolenJSBeaulieuARubbert-RothAOPTION InvestigatorsEffect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trialLancet2008371961798799718358926
- EmeryPKeystoneETonyHPIL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumor necrosis factor biological: results from a 24-week multicenter randomized placebo-controlled trialAnn Rheum Dis200867111516152318625622
- NishimotoNHashimotoJMiyasakaNStudy of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x-ray reader-blinded randomized controlled trial of tocilizumabAnn Rheum Dis20076691162116717485422
- KremerJMBlancoRBrzoskoMTocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to Methotrexate – results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one yearArthritis Rheum201163360962121360490
- CampbellLChenCBhagatSSParkerRAÖstörAJRisk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trialsRheumatology (Oxford)201150355256221078627
- PuolakkaKBlåfieldHKauppiMCost-effectiveness modeling of sequential biologic strategies for the treatment of moderate to severe rheumatoid arthritis in FinlandOpen Rheumatol J20126384322582103
- SchoelsMWongJScottDLEconomic aspects of treatment options in rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritisAnn Rheum Dis2010696995100320447950
- DiamantopoulosABenucciMCapriSEconomic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in ItalyJ Med Econ201215357658522313326
- DougadosMKisselKSheeranTAdding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomized controlled strategy trial in rheumatoid arthritis (ACT-RAY)Ann Rheum Dis2013721435022562983