Abstract
Purpose
Multiple sclerosis (MS) is a chronic demyelinating, degenerative disease requiring long-term treatment. Patient adherence to treatment may be challenging in such scenarios, especially since treatment often involves self-injection, for example, with interferon beta-1b during therapy. BETACONNECT™ is a novel electronic autoinjector for patient support in interferon beta-1b administration. The purpose of this survey was to assess patient satisfaction with the BETACONNECT™ device and its features.
Patients and methods
A total of 2,299 MS patients using the BETACONNECT™ device were asked to participate in a survey in October 2014. All of these candidates participated in the BETAPLUS® program and had provided written informed consent. The participants were asked to answer 13 device-related questions.
Results
Of these candidates, 1,365 replied to the questionnaire, with more than 60% of the participants being 40–59 years of age. Among them, 69% were women and 21% were men (10% not specified). Approximately half of the participants received treatment with interferon beta-1b for more than 5 years. Most participants (85%) had used self-injection devices before, with 59% previously using BETACOMFORT®, 23% using BETAJECT® Comfort, and 3% using BETAJECT® Lite, while less than 4% manually injected interferon beta-1b. The majority of the participants had received the BETACONNECT™ device from a BETAPLUS® nurse (87%) and 48% had already used the device for more than 2 months (49% for 2 months or less). Among the participants, more than 90% evaluated the BETACONNECT™ device as “very helpful” or “helpful” in supporting their interferon beta-1b therapy with only marginal sex differences. Features that were rated “very important” by more than half of the participants included adjustability of injection speed and depth, contact sensor for avoidance of unintentional release, optical and acoustic signals, and rechargeable battery.
Conclusion
The vast majority of patients rated the BETACONNECT™ device as very helpful or helpful for their treatment with interferon beta-1b, and many considered most features as “very important”. In conclusion, usage of the BETACONNECT™ autoinjector may facilitate interferon beta-1b therapy and support adherence to long-term therapeutic regimen.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, commonly manifesting between 20 and 40 years of age. Among neurological disorders, MS is the most common reason for permanent disability in young adults and for premature retirement.Citation1 Strategies for early intervention in MS commonly comprise first-line disease-modifying drugs (DMDs), including injectable interferon beta preparations for long-term administration.Citation2
Although common first-line DMDs have been shown to be effective, safe, and tolerable for decades, therapeutic success is particularly dependent on patients’ adherence to long-term application of their prescribed medication.Citation1,Citation3 Besides forgetfulness, which in some cases might be caused by cognitive impairment, reduced fine motor skills due to MS progression may complicate self-injections and promote nonadherence.Citation3,Citation4 In addition, injection anxiety, being tired of injections, insufficient efficacy, depression, injection site reactions, and flu-like symptoms are common reasons for nonadherence to therapeutic regimens.Citation3,Citation4
In order to overcome injection-related factors interfering with patient comfort and treatment adherence, a variety of injection devices for most DMDs have been developed and evaluated with respect to patient satisfaction.Citation5–Citation11 Besides simplicity, safety, and reliability,Citation7 patients favor specific features of injection devices such as adjustable injection settings,Citation10 hidden needle, multidose cartridge, and acoustic signaling,Citation11 as well as on-screen instructions, skin sensor, and confirmation of end of injection.Citation6
Here, we report the results of a survey conducted among MS patients using the new electronic autoinjector BETACONNECT™ for subcutaneous interferon beta-1b therapy (BETAFERON®). The aim of this survey was to investigate patient satisfaction with the features of the device in daily routine and its support in interferon beta-1b treatment.
Materials and methods
Patients
In October 2014, 2,299 patients were contacted for this survey. Prerequisites were participation in the BETAPLUS® program,Citation12–Citation14 usage of the BETACONNECT™ device, and the patients’ previous consent to be contacted in written form. Of these patients, 1,730 (75%) were women and 569 (25%) were men. Most MS patients who received the questionnaire were between 40 and 59 years of age (56%). The remainder of the patients were distributed as follows: 2.3% (<20 years), 9.3% (20–29 years), 19.6% (30–39 years), 11.9% (>59 years), and 0.9% (not specified).
BETACONNECT™ device
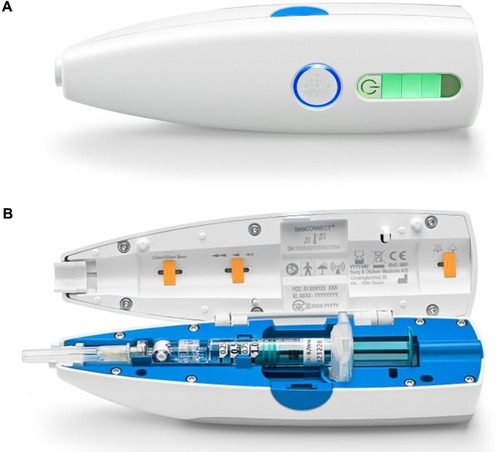
The BETACONNECT™ device is a novel autoinjector for self-administration of interferon beta-1b, which was introduced into the German market in May 2014 (Bayer Vital GmbH, Leverkusen, Germany). In comparison with preceding models (BETACOMFORT®, BETAJECT® Comfort, and BETAJECT® Lite), BETACONNECT™ has several unique features: BETACONNECT™ is an electronic device (previous models were mechanical), which is equipped with a reminder function for the next injection, a contact sensor for avoidance of unintentional release, and the possibility of transferring the injection history in a calendar view into myBETAapp® (myBETAapp® is currently being adjusted for connectivity to BETACONNECT™ – until finalization of the app, accruing data are collected and stored in the device). In addition, the injection process is adjustable for speed in three different settings. The newly developed four-phase injection technology of BETACONNECT™ includes an additional step compared with the preceding model (BETACOMFORT® with three-phase injection technology): After injection, the needle remains in the skin for a short while in order to minimize injection site reactions. Furthermore, the novel device is equipped with a rechargeable battery.
Additional features of this device are silent electronic injection with a hidden needle, adjustable injection depth (according to personal preferences, patients can choose from 8, 10, and 12 mm injection depths), and optical and acoustic signals at the end of the injection ().
Figure 1 The BETACONNECT™ device.
Questionnaire
The questionnaire and a cover letter were sent to the patients by mail. The questionnaire comprised 13 questions, covering receipt of the BETACONNECT™ device (eg, by a BETAPLUS® nurse) and support with respect to handling instructions, the duration of the interferon beta-1b therapy, previous utilization of autoinjectors, and duration of the BETACONNECT™ usage. In addition, several questions addressed patient satisfaction with the BETACONNECT™ device and its features regarding support in the interferon beta-1b therapy. Particularly, patients were asked to evaluate specific functions of the device such as hidden needle during the entire injection process, adjustability of injection speed and depth, contact sensor for avoidance of unintentional release, optical and acoustic supervision of the injection process, reminder function, and the rechargeable battery. Furthermore, participants were asked to indicate the probability of recommending the BETACONNECT™ device.
The results of the survey are represented as mean values. Statistical tests were not performed.
Results
The questionnaire was answered by 1,365 participants, corresponding to 59% of the contacted MS patients. gives an overview over the demographic characteristics of the patients. Among the participants, 69% were women and 21% were men (10% not specified). Most participants were ≥40 years old (30% of the total population between 40 and 49 years and 40% ≥50 years of age), with similar age distribution between sexes (). Half of the patients received interferon beta-1b-treatment for more than 5 years at the time of this survey, while 21% received interferon beta-1b therapy for 2–5 years, 11% for 1–2 years, and 16% for less than 1 year. Consequently, most of the patients had received their MS diagnosis before 2010 (64%), while the remainder of the patients were diagnosed between 2010 and 2014. Again, no relevant differences were observed between men and women ().
Table 1 Demographic characteristics of participants
At the time of the survey, nearly half of the participants (48%) had already used the BETACONNECT™ device for more than 2 months, 38% between 1 and 2 months, and 11% for less than 1 month (). The majority of patients had received their device by a BETAPLUS® nurse (87%), followed by medical personnel in clinical practice (9%) (). Most patients were experienced in using injection devices (BETACOMFORT®: 59%, BETAJECT® Comfort: 23%, BETAJECT® Lite: 3%), while only 4% of the patients had injected interferon beta-1b manually before receiving BETACONNECT™ (11% not specified) ().
Table 2 BETACONNECT™ – specific characteristics of participants
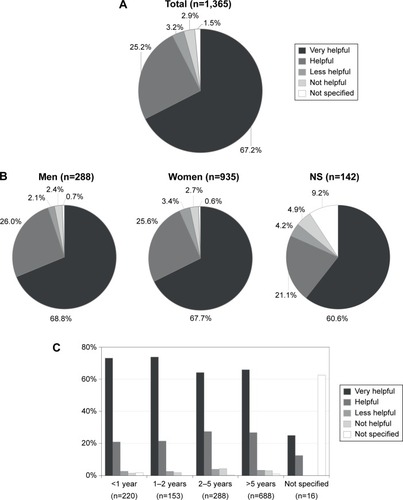
The BETACONNECT™ device was rated as “very helpful” in supporting interferon beta-1b therapy by 67% of the total population (n=1,365) and as “helpful” by 25%. Only 6% of the patients regarded the BETACONNECT™ as “less helpful” or “not helpful” (1.5% not specified) (). Again, no major differences were observed between men and women (). Utilization of the BETACONNECT™ seems to be more helpful early in therapy, as 73% of the patients voted “very helpful” within the first year of therapy and 74% within the second year. The proportion of patients regarding the device as “very helpful” declined to 64% of patients who were treated with interferon beta-1b for 2–5 years and to 66% of patients treated for ≥5 years (). This effect was even more pronounced within the first year of therapy: 81% of the patients who had started interferon beta-1b treatment within the previous 6 months regarded BETACONNECT™ as “very helpful” (data not shown). Among participants not specifying the duration of their therapy, the proportion who also did not specify their rating of BETACONNECT™ nearly reached 63% ().
Figure 2 Rating of the support provided by the BETACONNECT™ device in interferon beta-1b therapy.
Abbreviation: NS, not specified.
Further, the questionnaire addressed specific functions and features of the BETACONNECT™ device. At first, participants were asked to evaluate which functions of the BETACONNECT™ device were most supportive in their therapy. Most patients appreciated easy handling of the device (23%), followed by more comfortable injection (14%), no need of manual injections (9%), less skin irritations (6%), availability of a reminder function (6%), and further features (27% not specified) (n=1,365, ). The vast majority of patients in the rating had used injection devices before (between 81% and 92%; data not shown). Of those patients opting “less skin irritations”, 97% rated the device as “very helpful/helpful” and 90% used the device at least for 1 month already. Within the category “other” (3%) (), patients most frequently appreciated that BETACONNECT™ is a good device and a modern advancement. Further responses in this section included less side effects, individual settings, and no noise from a mechanic spring anymore (data not shown). Interestingly, more women rated the substitution of manual injections to be especially supportive than men (11% vs 6%, respectively; data not shown).
Figure 3 Rating of the BETACONNECT™ function and features in patient support (n=1,365).
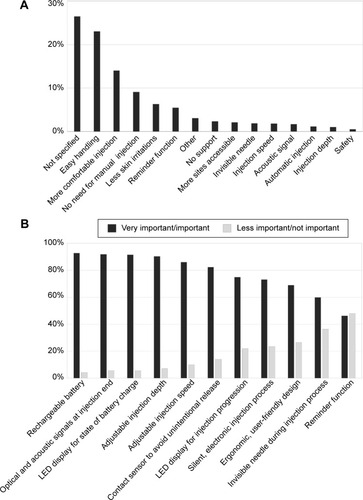
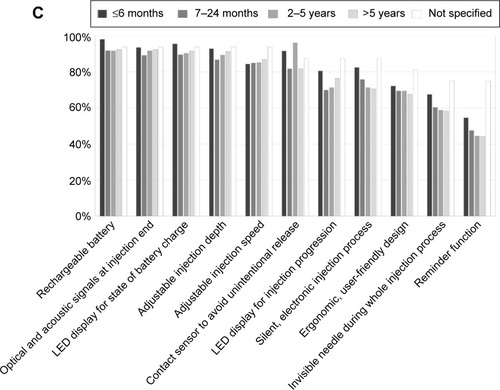
Subsequently, patients evaluated the importance of individual BETACONNECT™ features (n=1,365; multiple selections possible). Most important features were the rechargeable battery (93%), optical and acoustic signals at injection end (92%), LED display for state of battery charge (91%), adjustable injection depth (90%) and speed (86%), and contact sensor (82%) (), with no relevant differences between men and women (data not shown). The ratings were similar after stratifying for the different BETACONNECT™ features for duration of interferon beta-1b treatment (). With regard to the rating categories “less important/not important”, the reminder function (48%), invisible needle (36%), ergonomic design (27%), silent injection process (24%), and LED display for injection progress (22%) were most often chosen (), regardless of therapy duration (), age, or time since diagnosis (data not shown).
Also, participants were asked to score BETACONNECT™ with grades from “very good” to “unsatisfactory”. In this rating, nearly 90% of the patients evaluated the device as “very good/good”, while only 2.5% rated the autoinjector as “inadequate/unsatisfactory” ().
Figure 4 Evaluation of the BETACONNECT™.
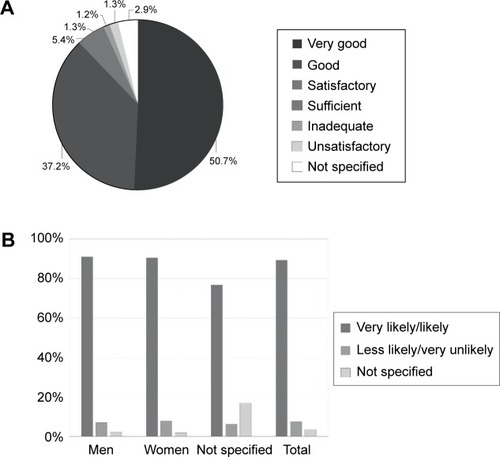
Finally, patients were asked if they would recommend the BETACONNECT™ device for interferon beta-1b therapy. While 89% of the total population (n=1,365) would recommend the device (men: 91%; women: 90%, patients without sex specification: 77%), only 7.4% of the patients rated this question with “less likely/very unlikely” (3.5% not specified) (). Among the latter patients, 42% (n=14/33) criticized the contact sensor (eg, minimal changes in pressure or low pressure leading to interruption of the injection process). In addition, 12% of all the participants (n=163/1,365) suggested improvements of the contact sensor, which may prevent injections if the application pressure is too low or if pressure changes during the injection process (eg, abdominal tissue).
Discussion
The application of first-line DMDs over decades is common in MS therapy, and has been shown to be effective and safe also in elderly patients.Citation2,Citation15 However, following strictly defined self-injection schedules is challenging for many patients. The development of autoinjection devices supports improving treatment-related challenges including injection anxiety, injection fatigue, and injection site reactions.Citation4,Citation16,Citation17 Further, the use of autoinjectors has been shown to positively correlate with adherence.Citation14 Additional features of the devices, eg, suggesting suitable injection sites for injections or a reminder function,Citation18 may simplify application of medication and increase adherence rates, which are known to be low in chronic diseases.Citation19,Citation20
The aim of this survey was to evaluate patient satisfaction with the newly developed autoinjection device BETACONNECT™ and its features in the daily routine of MS patients.
The higher ratio of female-to-male participants in this survey reflects known sex differences in MS prevalence.Citation2 Most of the participants were older than 40 years, which was not unexpected as 64% of the participants were already diagnosed with MS before 2010 and 50% received interferon beta-1b treatment in the long term (>5 years), corresponding to an earlier clinical onset of MS. In the literature, the median clinical onset is approximately 29 years of age.Citation2 The majority of patients participating in our survey had experiences with injection devices, but only 4% had injected interferon beta-1b manually before switching to BETACONNECT™. This might impact the perceived support of the device on interferon beta-1b therapy. Hence, the rating “very helpful/helpful” (>92%) may not simply be attributable to the fact that an autoinjector is used, but rather it reflects the high acceptance of the specific features of the device.
The BETACONNECT™ device might especially facilitate the initiation of interferon beta-1b treatment, as the highest proportion of the participants regarded the device as “very helpful” within the first 6 months of therapy. This rating declined over time. However, as the majority of participants had already received interferon beta-1b treatment for more than 5 years, the number of patients with shorter treatment was rather small, yielding unreliable estimates for the other treatment duration categories. In a comparable survey among previously treatment-naïve patients using interferon beta-1a, the offered device for interferon beta-1a administration was appreciated by more than 90% of the participants.Citation11 Therefore, electronic autoinjection devices may especially offer support early in therapy.
The most important aspect in supporting interferon beta-1b therapy in this survey was the easy handling of the device, which has also been rated with high priority in a previous survey.Citation21 This was followed by injection comfort and the cease of manual injections as well as a reduction in skin irritations. Interestingly, the switch from manual injections was rated especially supportive by women compared to men (11% of women vs 6% of men). However, absolute numbers, especially in men, were small.
Among the participants, a rechargeable battery and the LED display for state of battery charge were among the most appreciated features, suggesting the preference of a reusable device over a disposable device. In addition, the optical and acoustic signals at injection end and the adjustable settings regarding injection depth and speed were highly rated, which is in line with previous findings.Citation6,Citation10 The contact sensor was among features receiving major criticism, as 163 of all participants (12%) suggested improvements in the contact sensor, especially with respect to potential interruption of the injection process due to low pressure or minimal changes in pressure. In general, the contact sensor initiates the injection process reliably without the need of high pressure. Albeit the prerequisite for proper injection is a minimal resistance of the tissue, in order for the sensor to be activated. This might be impaired in obese or elderly patients with reduced connective tissue as well as in the abdominal tissue of women shortly after labor.
“Less/not important” features included the reminder function and the invisible needle. In another survey, the latter was rated most useful.Citation11 The difference in our study may partially be explained by the small number of patients (n=56) in this study compared with our study (n=1,365). Given that forgetfulness is a common reason for nonadherence,Citation3,Citation4 the reminder function was integrated into the device. The fact that most participants in the survey had already been injected with interferon beta-1b for more than 5 years might have influenced the rating of the reminder function. These participants are experienced in therapy and familiar with the injection schedule (every other day). Further research might help to understand whether a reminder function might positively influence adherence early in MS therapy.
Finally, the high proportion of patients rating BETACON-NECT™ as “very good/good” (nearly 90%) and the similar proportion who would recommend the device reflect the overall satisfaction of the patients with BETACONNECT™.
Conclusion
The electronic autoinjector BETACONNECT™ attained high degrees of patient satisfaction with respect to support of interferon beta-1b treatment. Many patients would recommend the device and considered most features as “very important”, especially optical and acoustic signaling and individually adjustable injection settings. In conclusion, the BETACONNECT™ autoinjector may facilitate interferon beta-1b treatment, especially at the onset of therapy, and foster adherence to long-term therapeutic regimen.
Acknowledgments
The study was funded by Bayer Vital GmbH, the manufacturer of BETAFERON®. Medical writing services from Dr Carmen Koch, employee of KW medipoint, were also funded by Bayer Vital GmbH.
Disclosure
This study was sponsored by Bayer Vital GmbH, Leverkusen, Germany. IW, AS, TS, and JV are salaried employees of Bayer Vital GmbH. NP is a salaried employee of Vitartis Medizin-Service GmbH. The authors report no other conflicts of interest in this work.
References
- BayasAImproving adherence to injectable disease-modifying drugs in multiple sclerosisExpert Opin Drug Deliv201310328528723339371
- WingerchukDMCarterJLMultiple sclerosis: current and emerging disease-modifying therapies and treatment strategiesMayo Clin Proc201489222524024485135
- LugaresiARottoliMRPattiFFostering adherence to injectable disease-modifying therapies in multiple sclerosisExpert Review Neu-rother201414910291042
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol2009256456857619444532
- PhillipsJTFoxEGraingerWTuccilloDLiuSDeykinAAn open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex(R) prefilled syringe in multiple sclerosis subjectsBMC Neurol20111112621999176
- DevonshireVArbizuTBorreBPatient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb studyBMC Neurol2010102820433746
- BayasAJappGFuldaUKallmannBAInjection devices in MS therapy: survey on neurologists, MS-nurses and patientsNervenheilkunde2010295762
- BrochetBLemaireGBeddiafAEpicure Study GReduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devicesRev Neurol20061626–773574016840982
- de SaJUrbanoGReisLAssessment of new application system in Portuguese patients with relapsing-remitting multiple sclerosisCurr Med Res Opin20102692237224220687777
- Verdun di CantognoERussellSSnowTUnderstanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practicesPatient Prefer Adherence2011517318021573048
- D‘ArcyCThomasDStonemanDParkesLPatient assessment of an electronic device for subcutaneous self-injection of interferon beta-1a for multiple sclerosis: an observational study in the UK and IrelandPatient Prefer Adherence20126556122298944
- KohlmannTWangCLipinskiJThe impact of a patient support program for multiple sclerosis on patient satisfaction and subjective health statusJ Neurosci Nurs2013453E3E1423636073
- PozzilliCSchweikertBEcariUOentrichWBuggeJPQuality of life and depression in multiple sclerosis patients: longitudinal results of the BetaPlus studyJ Neurol2012259112319232822527232
- PozzilliCSchweikertBEcariUOentrichWBetaPlus Study groupSupportive strategies to improve adherence to IFN beta-1b in multiple sclerosis – results of the betaPlus observational cohort studyJ Neurol Sci20113071–212012621636099
- LamplCNaglSArnasonBEfficacy and safety of interferon beta-1b sc in older RRMS patients – a posthoc analysis of the BEYOND studyJ Neurol201326071838184523504050
- HuppertsRBeckerVFriedrichJMultiple sclerosis patients treated with intramuscular IFN-beta-1a autoinjector in a real-world setting: prospective evaluation of treatment persistence, adherence, quality of life and satisfactionExpert Opin Drug Deliv2015121152525430947
- LugaresiADurastantiVGasperiniCSafety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa studyClin Neuropharmacol200831316717218520983
- ZettlUKBauer-SteinhusenUGlaserTHechenbichlerKLimmrothVStudyGEvaluation of an electronic diary for improvement of adherence to interferon beta-1b in patients with multiple sclerosis: design and baseline results of an observational cohort studyBMC Neurol20131311724011220
- WHOAdherence to Long-Term Therapies – Evidence for ActionGenevaWorld Health Organization200392 4 154599 2
- OsterbergLBlaschkeTAdherence to medicationNew Engl J Med2005353548749716079372
- LugaresiAFlorioCBrescia-MorraVPatient adherence to and tolerability of self-administered interferon beta-1a using an electronic autoinjection device: a multicentre, open-label, phase IV studyBMC Neurol201212722390218

