Abstract
Background
Low birth weight (LBW) neonates face a significantly higher risk of complications and mortality compared to those with normal birth weight. This risk is particularly pronounced in low-income countries, where access to quality health care and adequate nutrition is limited. However, there is insufficient data on mortality for LBW neonates while receiving parenteral feedings. Therefore, this study was aimed to investigate the incidence and determinants of mortality among admitted LBW neonates receiving parenteral feeding at Neonatal Intensive Care Units (NICUs) in selected hospitals in Addis Ababa.
Methods
A prospective follow-up study was conducted in selected hospitals’ NICU from March to June 2022. Data were collected using a structured questionnaire and checklist, and analyzed using STATA software. The assumption for survival analysis was assessed using Kaplan–Meier survival curves and a global test. Bi-variable and multivariable Cox regression analyses were performed to identify determinants of mortality. A p-value of less than 0.05 was used to declare as a significant predictor.
Results
Two hundred eighty-nine neonates with their indexed mothers were enrolled for a total of 2242 days. During this follow-up time sixty-six neonates were died, making the incidence rate of mortality among LBW neonates 29.438 per 1000 person-day. Birth weight less than 1000 grams (AHR: 9.539; 95% CI: 2.272, 40.038), admission Apgar score of three or less (AHR: 5.894 95% CI: 1.320, 26.315), edematous malnutrition (AHR: 3.389; 95% CI: 1.355, 8.474) and not initiated trophic feeding (AHR: 7.324; 95% CI: 3.453, 15.532) were identified as a significant predictors for neonatal mortality.
Conclusion
This study revealed a high incidence of mortality among low birth weight (LBW) neonates who received parenteral feeding. Furthermore, factors such as birth weight, trophic feeding initiation, low Apgar score, and edematous malnutrition were identified as predictors for mortality among LBW neonates who received enteral feeding. Therefore, future studies should prioritize optimizing enteral feeding practices and enhancing the quality of care provided to LBW neonates.
Introduction
Globally, an estimated 14.7% of all babies born were low birth weight (LBW) in 2020,Citation1,Citation2 with one-third of these LBW neonates born in Africa, majority in Eastern and Western Africa. To address this, World Health Assembly has set a nutrition target to reduce low birth weight by 30% between 2012 and 2030.Citation3 However, progress in reducing low birth weight prevalence has been slow or lacking across all regions, with the most significant decrease occurring in South Asia (only 4.5% over 20 years, from 29.4% to 24.9%).Citation1–4
Neonatal mortality is a significant concern worldwide.Citation5,Citation6 Estimates from the World Health Organization (WHO) indicated that neonatal mortality rate in Sub-Saharan African countries is approximately 28 deaths per 1000 live births, nearly 1 in 36 newborns dies within the first 28 days of life.Citation3,Citation4 Various factors contribute to the high neonatal mortality rates in the region, including limited access to quality healthcare services, poverty, inadequate nutrition, infectious diseases, and social determinants of health.Citation7–9
Low birth weight neonates specifically have an incomparably high risk of mortality than normal weight counterparts.Citation5,Citation6 Low birth weight accounts for approximately 60–80% of neonatal deaths, highlighting its substantial impact on neonatal mortality rates. It is not death alone, survivors of LBW also face lifelong consequences, including a higher risk of stunted growth, lower intelligence quotient (IQ), and adult-onset chronic conditions, such as obesity and diabetes.Citation2–4
Limited access to quality prenatal care, healthcare facilities, and adequate nutrition in low- and middle-income countries contributes to the high burden of LBW-related mortality, which is multifactorial among LBW neonates.Citation4 LBW have immature digestive system, feeding intolerance, a risk of aspiration while feeding, and they need specialized and individualized nutrition. These challenges makes the healthcare providers to encounter several difficulties in managing the transition of LBW neonates to the extra uterine life.Citation10
Improving the health of low birth weight neonates and reducing their mortality rates in Sub-Saharan African countries requires a comprehensive approach. This includes strengthening healthcare systems, increasing access to quality prenatal care, promoting skilled birth attendance, enhancing neonatal resuscitation skills, ensuring access to essential newborn care, and addressing social determinants of health such as poverty and education.Citation2–4,Citation11 Additionally, mothers need to prioritize balanced nutrition and rest, receive adequate antenatal care, avoid smoking and limit alcohol consumption, refrain from taking un-prescribed medications, avoid exposure to toxins, prevent infections, ensure appropriate inter-pregnancy spacing, and seek genetic counselling as needed.Citation9,Citation12–15
There has been no previous study on mortality among low birth weight neonates receiving parenteral feeding. However, in Ethiopia, the magnitude of neonatal mortality among admitted neonates in general has been reported to range from 13% to 18.6%.Citation16–19 Additionally, the incidence of neonatal mortality among preterm neonates in Black-Lion Hospital was found to be 39.1 per 1000 neonate-days.Citation20 Various maternal factors such as antepartum hemorrhage,Citation16 pregnancy-induced hypertension,Citation16,Citation21,Citation22 multiple pregnancy,Citation16,Citation23 place of delivery,Citation16,Citation21 premature rupture of membranes,Citation20,Citation22 antenatal care,Citation17,Citation23 maternal diabetes mellitusCitation20 were identified. Factors related to the neonates include LBW,Citation16,Citation17,Citation22 perinatal asphyxia,Citation16,Citation17,Citation22,Citation23 neonatal sepsis,Citation16,Citation17,Citation20,Citation22 respiratory distress,Citation20,Citation22 prematurity,Citation17,Citation18,Citation20,Citation21,Citation23 hypothermia,Citation18,Citation21 low Apgar score,Citation20,Citation23 meconium aspiration syndrome,Citation22 and admission blood sugar level.Citation23
Given the scarcity of data on neonatal mortality rates among low birth weight neonates, particularly those receiving parenteral feeding in the NICU, both globally and locally, and majority of the available studies on neonates in general have been retrospective in nature. Therefore, the main objective of this prospective follow-up study was to investigate the incidence and determinants of neonatal mortality among LBW neonates receiving parenteral feeding in selected referral hospitals’ NICUs. The prospective nature of the study and its focus on parenteral feeding, makes this study crucial for understanding the factors contributing to their mortality, guiding healthcare professionals in making informed decisions, improve survival rate, and reduce mortality among these neonates. Additionally, the results can contribute to the development of targeted interventions aimed at reducing mortality rates in this vulnerable population when they are on parenteral feeding.
Method and Materials
Study Area and Period
Institution-based prospective follow up study was conducted from March to June 2022 in six randomly selected public hospitals with NICU in Addis Ababa City, Ethiopia. The city has eleven public hospitals equipped with NICU. Of these hospitals, Gandhi Memorial Hospital, Zewditu Memorial Hospital, Menelik II Referral Hospital, St Peter Specialized Hospital, St’ Paul’s Hospital Millennium Medical College, and Tikur Anbessa Specialized Hospital were selected by lottery method.
Source Population
The source population were all LBW neonates admitted to NICU in public hospitals of Addis Ababa.
Study Population
All selected LBW neonates receiving parenteral feeding and their respective caretakers in the selected six public hospitals during the study period.
Eligibility Criteria
All admitted LBW neonates receiving parenteral feeding in selected hospital’s to NICU during the study period were included. Neonates with major congenital cardiac disease, GI defect and those neonates diagnosed with necrotizing enterocolitis by the most senior physician were excluded.
Sample Size Determination
The sample size was calculated using Epi-Info software version 7.2.5.0. For the first objective (incidence), a calculation was performed using 25.07Citation24 proportion (p), 80% power, and 95% confidence level, resulting in a sample size of 289. For the second objective (determinant factors), the sample size was calculated using the double population proportion formula, considering the following assumptions: p1 = 35.2, p2 = 14.4 and a risk ratio of 2.144. This consideration yielded a sample size of 224. Therefore, the larger sample size (289) was taken.
Sampling Procedure
Initially, all the eleven public hospitals with NICU in Addis Ababa were identified, and six of them (50%) were selected using a lottery method. Subsequently, the total study population was estimated based on the previous three months’ data retrieved from the admission logbook (340 low birth weight neonates admission per month), and proportional allocation was carried out for each hospital based on the case flow. All eligible LBW neonates admitted to NICU and their indexed mothers in the selected hospital within the study period were included consecutively until we reached the predetermined quota. From the total participants, 59 (20.4%) were from Tikur Anbesa Specialized Hospital, 43 (14.9%) from Zewditu Hospital, 57 (19.7%) from Gandhi Memorial Hospital, 38 (13.1%) from Menelik II referral Hospital, 38 (13.1%) from St. Peter Hospital, and 54 (18.7%) from St Paul Millennium medical College. During the follow-up three month there were 1128 low birth weight neonates admitted.
Operational Definitions
Neonate
A newborn infant, or neonate, is a child under 28 days of age.Citation1
Parenteral Feeding
A newborn who received all of his/her need of fluid, and/or nutrition or any supplementation parenterally.
Full Enteral Feeding
Newborn infants receive all of their nutrition as milk feeds (either human milk or formula) enteral through tube feeding or orally and do not receive any supplemental parenteral fluids or nutrition.Citation1,Citation4
Time to Event
Time from birth of an LBW infant to death.
Censored
LBW neonates who were no longer under follow-up due to events such as being transferred to another institution or leaving against medical advice, as well as those who did not achieve full feeding before the study concluded (at 28 days).
Event
Refers to the occurrence of the outcome of interest (death in LBW neonates).
Follow Up Time
Time from recruiting up to either the study subjects died or censored.Citation25
Trophic Feeding
A small volume (12 cc/Kg/d or less) of enteral nutrition insufficient for the neonate’s nutritional needs but producing some positive gastrointestinal or systemic benefit.Citation4,Citation26
Survival Time
The length of time in days followed starting from birth to death or full enteral feeding.
Data Collection Tools and Procedure
Interviewers administered structured questionnaire and data extraction checklist was used to collect the data. The extraction tool has four components (Socio-demographic variables, prenatal variables, neonatal variables, and management and health service-related variables). It was adapted from previous related studiesCitation1,Citation14,Citation18,Citation19,Citation23,Citation24,Citation27 and modified based on the available data and the care given at the hospital. Six data collectors, one for each hospital, and one supervisor, collect the data and provide supportive supervision throughout the data collection process, respectively.
Data Quality Control
A structured pre-tested questionnaire was developed and used to collect the data. The pre-test was done on 5% of randomly selected LBW neonates in Ras-Desta Damtew Memorial Hospital. Data like infection prevention practice guideline compliance, incomplete vital sign documentation, specific infectious agents, and family satisfaction with the care were difficult to get. Therefore, necessary correction and modification were made accordingly.
The data collectors had a minimum of BSc in neonatal nursing and a daylong training was given for them. Throughout the data collection period and before data entry we maintained strict and continuous supervision to ensure the validity and consistency of the data, and each questionnaires were examined for consistency and completeness. As a result, we did not encounter any missing data. In cases of inconsistent data, we promptly communicated with the data collectors and cross-checked the information with the medical charts for accuracy. Moreover, double data entry was done.
Data Processing and Analysis
Data were cleaned, coded, and entered to Epi-Data version 4.6 and exported to STATA version 16 for analysis. Descriptive statistics were done to show the distribution of socio-demographic, prenatal, and neonatal characteristics among participants and indexed mother. The time to death during the follow-up was estimated by Kaplan Meier failure curve. The Log rank test was also employed to compare statistical differences between groups of independent variables for the survival function. The Schoenfeld residual global test for proportionality assumption was checked, and its result (P-value = 0.5964) suggested the satisfaction for the assumption. Variables with P-value < 0.25 in the bi-variable cox-regression analysis were entered into the multivariable analysis. Variance inflation factor was also used to assess multi-collinearity. In multivariable analysis variables having a p-value < 0.05 were considered as predictors of neonatal mortality. Crude and adjusted hazard ratios with 95% CI, were used to show the strength of association with the outcome variable.
In order to determine the most appropriate model, the log-likelihood and Akaike Information Criteria (AIC) were utilized. The model with the lowest AIC value was deemed the best-fitted model. In this study, the Weibull regression model achieved the minimum AIC value of 725.649, thus making it the chosen fitted model. To further assess the goodness of fit, the Cox-Snell residual test was employed. The results of this test indicated that the model closely aligned with the bisector, implying a good fit between the predicted and observed values.
Result
Two-hundred eighty-nine LBW neonates receiving parenteral feeding were included in the follow-up. During the follow-up, two neonates were discharged from the hospital for reasons of discharge against medical advice and referral to other institution (one for each).
Sociodemographic, Medical, and Obstetric Characteristic of Indexed Mothers
One hundred seventy-three (59.9%) of the indexed mothers’ age was between 25 and 35. Sixteen women had no ANC follow-up at all, 127 (43.9%) were primiparous, and 150 (51.9%) mothers gave birth by spontaneous vaginal delivery ().
Table 1 Sociodemographic, Medical, and Obstetric Characteristic of Indexed Mothers, Whose Neonates Were Admitted in Selected Hospitals of Addis Ababa, Ethiopia (n = 289)
Characteristics of Participant Neonates Receiving Parenteral Feeding at NICU in Hospitals of Addis Ababa
One hundred fifty-four neonates were male, 168 (58.1%) neonates were delivered at a gestation between 32 and 36 weeks, and 101 (34.9%) weighed 1500 to 1999 grams ().
Table 2 Characteristics of Participant Neonates Admitted at NICU in Selected Hospitals of Addis Ababa, Ethiopia, 2023 (n = 289)
Participant Neonates’ Diagnosis and Findings During the Follow-Up
During the follow-up period, certain conditions were identified in a percentage of neonates receiving parenteral feeding at NICU in Addis Ababa hospitals. Hypothermia was detected in 55% of all neonates, while early onset sepsis was diagnosed in 33.6% of the participants. Additionally, hyper-bilirubinemia affected 6.2% of the neonates, and hospital-acquired infections were occurred in 22.8% of the cases ().
Table 3 Participant Neonates’ Diagnosis and Findings During the Follow-Up at NICU in Selected Hospitals of Addis Ababa, Ethiopia, 2023 (n = 289)
Incidence of Mortality Among LBW Neonates Receiving Parenteral Feeding in NICU
Two hundred eighty nine neonates who received parenteral feeding were enrolled for a total of 2242 days (15,694 hours), and during these follow-up sixty-six neonates were died. Which makes the incidence rate of mortality among LBW neonates receiving parenteral feeding 0.029438 (95% CI: 0.0231277, 0.03747) per person-day/29.438 per 1000 person-day. The median follow-up time were 22 days, and the mean and median survival time among died parenteral feeding neonates were 7.818 (CI: 6.492, 9.143) and 5 days (95% CI: 5, 7), respectively.
Determinants of Mortality Among LBW Neonates Receiving Parenteral Feeding in NICU
To assess the fulfilment of assumptions for survival analysis with Cox regression, a global test was conducted. The test yielded a chi-square value of 29.45 and a corresponding p-value of 0.5964. Additionally, a Kaplan–Meier survival curve was generated and is depicted in . The results of the global test indicate that there is no significant violation of assumptions for survival analysis with Cox regression.
Figure 1 Kaplan-Meier Survival curve for mortality among LBW neonates admitted to NICU in selected Hospitals 15.
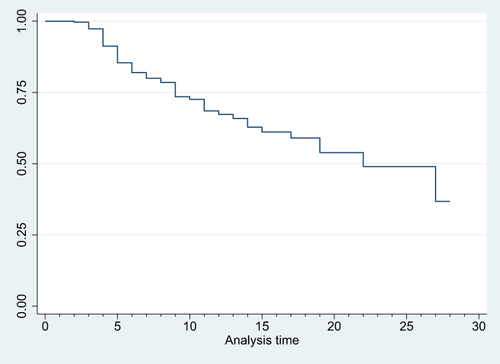
Figure 2 Kaplan-Meier Survival curve for mortality among LBW neonates admitted to NICU based on their APGAR score on admission 16.
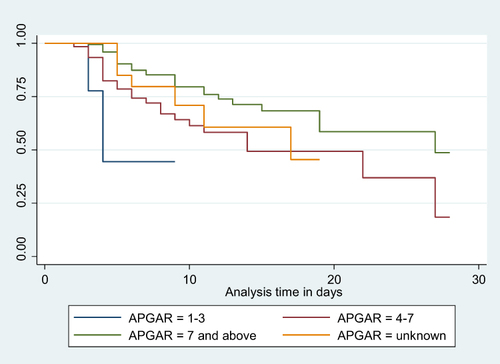
Figure 3 Kaplan-Meier Survival curve for mortality among LBW neonates admitted to NICU based on their birth weight 17.
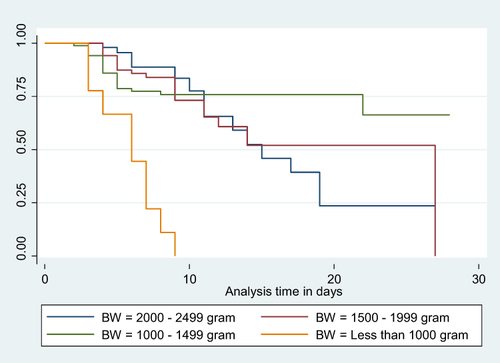
Figure 4 Kaplan-Meier Survival curve for mortality among LBW neonates admitted to NICU based on oedematous malnutrition status 17.
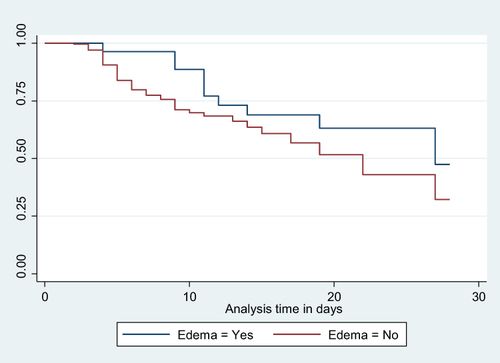
Figure 5 Kaplan-Meier Survival curve for mortality among LBW neonates admitted to NICU based on their trophic feeding initiation status 18.
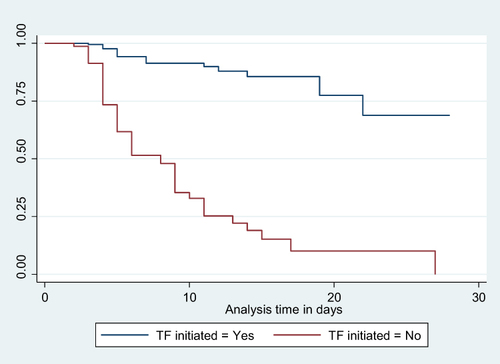
In the bivariate Cox regression analysis, maternal age, residence, educational level, ANC follow-up, gestational age, labor onset, pregnancy-induced hypertension, and multiple pregnancies were found to be associated with mortality among neonates receiving parenteral feeding at a p-value less than 0.25. Similarly, neonatal characteristics such as birth weight, weight for age, Apgar score at admission, respiratory distress, perinatal asphyxia, jaundice, apnea, dehydration, edematous malnutrition, need for respiratory support, receiving Kangaroo Mother Care, phototherapy, and initiation of trophic feeding were associated at a p-value less than 0.25.
All of these variables were then entered into a multivariable Cox regression analysis. Birth weight, Apgar score at admission, edematous malnutrition, and initiation of trophic feeding were found to have a significant association with mortality among neonates receiving parenteral feeding at a p-value of less than 0.05.
Neonates born with a birth weight of less than 1000 grams faced a significantly increased risk of mortality, with a hazard ratio (AHR) of 9.539 (95% CI: 2.272, 40.038), compared to those weighing between 2000 and 2499 grams. Similarly, neonates admitted with an Apgar score of three or less had a substantially higher risk of mortality, with an AHR of 5.894 (95% CI: 1.320, 26.315), when compared to neonates with a score of seven or higher. Notably, neonates who experienced edematous malnutrition exhibited a significantly higher likelihood of mortality, with an AHR of 3.389 (95% CI: 1.355, 8.474), compared to their counterparts. Furthermore, neonates who did not initiate trophic feeding were at a significantly higher risk of mortality, with an AHR of 7.324 (95% CI: 3.453, 15.532), compared to those who did ().
Table 4 Bi-Variable and Multivariable Cox Regression Analysis for Predictors of Neonatal Mortality Among LBW Neonates Admitted to NICU in Selected Hospitals
Discussion
In this study, the observed incidence rate of neonatal mortality among admitted low birth weight (LBW) neonates receiving parenteral feeding was 29.438 per 1000 person-days (95% CI: 0.023, 0.037). These findings align with previous studies conducted in southern Ethiopia (27 per 1000 neonate-days)Citation28 and Felege Hiwot Comprehensive Specialized Hospital (35.3 per 1000 person-days).Citation29 However, the incidence rate in this study was lower than that reported in studies conducted in North West Ethiopia (75.63 per 1000 neonate-days).Citation30 This discrepancy may be attributed to the fact that the current study took place in hospitals equipped with well-staffed NICUs, which can decrease the mortality as they can give better care and proper diagnosis, in contrast to the other studies. Additionally, the relatively short follow-up duration and the exclusion of neonates with severe congenital abnormalities may have influenced the results.
Conversely, the incidence rate in this study was higher than that reported in a study conducted in Burkina Faso (1.93 per 1000 persons-days).Citation27 This disparity may be explained by the higher mean birth weight in the Burkina Faso study, and the inclusion of both normal and abnormal birth weight neonates.Citation27
Parenteral feeding LBW neonates with a birth weight less than 1000 grams faced a significantly higher risk of mortality, with an adjusted hazard ratio (AHR) of 9.539 (95% CI: 2.272, 40.038), compared to those weighing between 2000 and 2499 grams at birth. This finding is consistent with studies conducted in various countries, including North West Ethiopia,Citation30 Eritrea,Citation31 South Africa,Citation32 India,Citation33 Burkina Faso,Citation27 Nigeria,Citation34 and Indonesia.Citation35 The increased risk might be attributed to physiological immaturity, such as underdeveloped lungs, heart, liver, digestive system, and immune system, as well as a higher prevalence of congenital malformations. These factors contribute to a higher susceptibility to health complications, including respiratory distress syndrome, jaundice,Citation36 infections such as sepsis, pneumonia, and meningitis,Citation24,Citation36 necrotizing enterocolitis,Citation37,Citation38 intraventricular hemorrhage,Citation39–41 difficulties in maintaining body temperature, challenges in feeding, apnea, anemia, and other complications.Citation34,Citation42–44
Parenteral feeding LBW neonates with an Apgar score of three or less upon admission had a significantly higher risk of mortality, with an adjusted hazard ratio (AHR) of 5.89, compared to neonates with a score of seven or more. This finding is supported by studies conducted in various countries, including China,Citation45 United States,Citation46,Citation47 Sweden,Citation48,Citation49 Eritrea,Citation31 Cameroon,Citation50 Nigeria,Citation34 and Southern and Northern Ethiopia.Citation51–53 Since Apgar score is a tool for quickly assessing the overall health and well-being of new-born, serving as an indicator of potential health issues or complications that can contribute to an increased risk of mortality.Citation52 Neonates with low Apgar scores may experience perinatal asphyxia, respiratory distress from various causes (such as respiratory distress syndrome, meconium aspiration syndrome, or other respiratory conditions leading to poor oxygenation and respiratory failure),Citation52 birth trauma, infections, and congenital abnormalities,Citation51,Citation54 all of which can contribute to their higher mortality risk. This is helpful for health care providers to make informed decisions and better manage parenteral feeding neonates with lower Apgar score to prevent the risk of adverse outcomes.
Similarly, parenteral feeding LBW neonates who experienced edematous malnutrition were 3.389 times more likely (AHR: 3.389 95% CI: 1.355, 8.474) to die compared to their counterparts. This finding is supported by studies conducted in Zimbabwe and Zambia.Citation55 The reason for this could be that malnourished neonates may have poor immune status, water and electrolyte imbalances, low energy reserves, and difficulties in regulating body temperature.
Furthermore, parenteral feeding LBW neonates who did not start trophic feeding were 7.324 times (AHR: 7.324 95% CI: 3.453, 15.532) more likely to die compared to their counterparts. This finding is supported by a systematic review of trials that demonstrated a lower risk of mortality, shorter length of hospital stay, and reduced infection rates among low birth weight (LBW) and preterm neonates who received early enteral feeding.Citation56 Additional studies have also shown similar results.Citation57 The reason for this might be that early enteral feeding promotes physiologic gut maturity,Citation58 neurological development,Citation58,Citation59 and better cognitive outcomes.Citation59,Citation60 In contrast, its absence results in the decreased growth of a healthy gut microbiome, which is essential for immune system development, nutrient metabolism, and protection against pathogens.
When interpreting the findings of this study, it is important to consider the following limitations. Firstly, the study was conducted on admitted LBW neonates who received parenteral feeding at NICU, so the results may not be applicable to all admitted neonates or the entire neonatal period. Additionally, as this study was done in hospitals found in Addis Ababa, it does not represent the situation in Ethiopia. However, as a strength, it provides results that are more specific for LBW neonates during parenteral feeding.
Conclusion
This study identified a high incidence of mortality among LBW neonates receiving parenteral feeding in the study area. Predictors for mortality among LBW neonates receiving enteral feeding included birth weight less than 1000 grams, initiating trophic feeding, having a low Apgar score, and experiencing edematous malnutrition. Therefore, additional study should focus on optimizing enteral feeding practices such as use of human milk over cow’s milk formula, timing of enteral feeding initiation, duration of minimal enteral/trophic feeds and optimizing human milk fortification. Additionally, it is crucial to examine quality of care practices for LBW neonates with low Apgar scores at birth in order to reduce neonatal mortality.
Abbreviations
AHR, adjusted hazard ratio; CHR, crude hazard ratio; CI, confidence interval; WHO, World Health Organization; LBW, Low Birth Weight; SSA, Sub-Sahara African Countries; KMC, kangaroo mother care; ANC, Antenatal care; NICU, neonatal intensive care unit.
Ethics Approval and Consent to Participate
Approval to carry out this study was received from Ethical review board of Addis Ababa University. Written informed consent was also obtained from all mothers of the included neonates, and confidentiality of the collected data were assured throughout the study. Moreover, this study complies with the declaration of Helsinki and related documents.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request [email protected].
Disclosure
The authors declared that there is no conflict of interest related to the conducting or publication of this research.
Acknowledgment
We express our gratitude to all participants and data collectors who took part in this study.
Additional information
Funding
References
- World Health Organization. UNICEF-WHO Low Birthweight Estimates: Levels and Trends 2000-2015. World Health Organization; 2019.
- World Health Organization. UNICEF/WHO Low Birthweight Estimates: Levels and Trends 2000-2020. World Health Organization; 2023.
- World Health Organization. Global Nutrition Targets 2025: Low Birth Weight Policy Brief. World Health Organization; 2014.
- World Health Organization. WHO Recommendations for Care of the Preterm or Low-Birth-Weight Infant. World Health Organization; 2022.
- Veloso FCS, Kassar L, Oliveira MJC, et al. Analysis of neonatal mortality risk factors in Brazil: a systematic review and meta-analysis of observational studies. J de ped. 2019;95(5):519–530. doi:10.1016/j.jped.2018.12.014
- Daemi A, Ravaghi H, Jafari M. Risk factors of neonatal mortality in Iran: a systematic review. Med J Islamic Republic Iran. 2019;33:87. doi:10.34171/mjiri.33.87
- Akombi BJ, Renzaho AM. Perinatal mortality in sub-saharan Africa: a meta-analysis of demographic and health surveys. Anna Global Health. 2019;85(1). doi:10.5334/aogh.2348
- Usman F, Imam A, Farouk ZL, Dayyabu AL. Newborn mortality in sub-saharan Africa: why is perinatal asphyxia still a major cause? Ann Glob Health. 2019;85(1):112. doi:10.5334/aogh.2541
- Tekelab T, Chojenta C, Smith R, Loxton D. The impact of antenatal care on neonatal mortality in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2019;14(9):e0222566.
- Banait N, Basu S, Desai P, et al. Feeding of low birth weight neonates. Journal of Neonatology. 2020;34(1–2):28–51. doi:10.1177/0973217920938522
- Tura G, Fantahun M, Worku A. The effect of health facility delivery on neonatal mortality: systematic review and meta-analysis. BMC Preg Child. 2013;13(1):18. doi:10.1186/1471-2393-13-18
- Kenyanya JO, Kikuvi G, Wanzala P, Nyagero J. Maternal factors associated with low birth weight among neonates born at Thika Level 5 Hospital in Kiambu County, Kenya. Interna J Hea Sci. 2023;6(5):58–97. doi:10.47941/ijhs.1464
- Debele EY, Dheresa M, Tamiru D, et al. Household food insecurity and physically demanding work during pregnancy are risk factors for low birth weight in north Shewa zone public hospitals, Central Ethiopia, 2021: a multicenter cross-sectional study. BMC Pediatrics. 2022;22(1):419. doi:10.1186/s12887-022-03480-2
- Khan JR, Islam MM, Awan N, Muurlink O. Analysis of low birth weight and its co-variants in Bangladesh based on a sub-sample from nationally representative survey. BMC Pediatrics. 2018;18(1):100. doi:10.1186/s12887-018-1068-0
- Kouser W, Bala K, Sahni B, Akhtar N. Epidemiological determinants of low birth weight: a prospective study. J Family Med Pri Care. 2020;9(7):3438–3443. doi:10.4103/jfmpc.jfmpc_414_20
- Eyeberu A, Shore H, Getachew T, Atnafe G, Dheresa M. Neonatal mortality among neonates admitted to NICU of Hiwot Fana Specialized University Hospital, eastern Ethiopia, 2020: a cross-sectional study design. BMC Pediatrics. 2021;21(1):125. doi:10.1186/s12887-021-02598-z
- Mohamed HA, Shiferaw Z, Roble AK, Kure MA. Neonatal mortality and associated factors among neonates admitted to neonatal intensive care unit at public hospitals of somali regional state, Eastern Ethiopia: a multicenter retrospective analysis. PLoS One. 2022;17(5):e0268648. doi:10.1371/journal.pone.0268648
- Ahmed AT, Farah AE, Ali HN, Ibrahim MO. Determinants of early neonatal mortality (hospital based retrospective cohort study in somali region of Ethiopia). Sci Rep. 2023;13(1):1114. doi:10.1038/s41598-023-28357-x
- Abera T, Bayisa L, Bekele T, Dessalegn M, Mulisa D, Gamtessa LC. Neonatal mortality and its associated factors among neonates admitted to wollega university referral hospital neonatal intensive care unit, East Wollega, Ethiopia. Glob Pediatr Health. 2021;8:2333794X2110301. doi:10.1177/2333794X211030157
- Yared Asmare A, Hussien M, Kenean G, et al. Determinants of neonatal mortality among preterm births in Black Lion Specialized Hospital, Addis Ababa, Ethiopia: a case–cohort study. BMJ Open. 2022;12(2):e043509.
- Wake GE, Chernet K, Aklilu A, et al. Determinants of neonatal mortality among neonates admitted to neonatal intensive care unit of Dessie Comprehensive and Specialized Hospital, Northeast Ethiopia; an unmatched case-control study. Front Public Health. 2022;1:10.
- Alemu AY, Belay GM, Berhanu M, Minuye B. Determinants of neonatal mortality at neonatal intensive care unit in Northeast Ethiopia: unmatched case-control study. Trop Med Health. 2020;48(1):40. doi:10.1186/s41182-020-00232-9
- Jemal B, Abebe T, Zemedkun A, et al. Determinants of neonatal mortality in the neonatal intensive care unit of dilla University Referral Hospital, Southern Ethiopia; 2019-2020; A matched, case-control study. Heliyon. 2022;8(9):e10389. doi:10.1016/j.heliyon.2022.e10389
- Pabbati J, Subramanian P, Renikuntla M. Morbidity and mortality of low birth weight babies in early neonatal period in a rural area teaching hospital, Telangana, India. Int J Contemp Pediatr. 2019;6(4):1582. doi:10.18203/2349-3291.ijcp20192759
- Pagano M, Gauvreau K, Mattie H. Prin Biostat. CRC Press; 2022.
- Finkel YMD, Baker, Robert D, Rosenthal PMD, Sherman PMMD, Sondheimer JMMD. A critical perspective on trophic feeding. J Pediatr Gastroenterol Nutri. 2004;38(3):237–238. doi:10.1002/j.1536-4801.2004.tb12154.x
- Coulibaly A, Baguiya A, Millogo T, Meda IB, Koueta F, Kouanda S. Predictors of mortality of low birth weight newborns during the neonatal period: a cohort study in two health districts of Burkina Faso. Int J Gynecol Obstet. 2016;135(S89–S92). doi:10.1016/j.ijgo.2016.08.006
- Orsido TT, Asseffa NA, Berheto TM. Predictors of Neonatal mortality in Neonatal intensive care unit at referral Hospital in Southern Ethiopia: a retrospective cohort study. BMC Preg Childbirth. 2019;19(1):1–9. doi:10.1186/s12884-019-2227-5
- Woelile TA, Kibret GT, Workie HM, et al. Survival status and predictors of mortality among low-birth-weight neonates admitted to the neonatal intensive care unit at Felege Hiwot Comprehensive Specialized Hospital. In: Pediatr Health Med Therap. Ethiopia: Bahir Dar; 2020:451–466.
- Wondie WT, Zeleke KA, Wubneh CA. Incidence and predictors of mortality among low birth weight neonates in the first week of life admitted to the neonatal intensive care unit in Northwestern Ethiopia comprehensive specialized hospitals, 2022. multi-center institution-based retrospective follow-up study. BMC Pediatr. 2023;23(1):489. doi:10.1186/s12887-023-04319-0
- Andegiorgish AK, Andemariam M, Temesghen S, Ogbai L, Ogbe Z, Zeng L. Neonatal mortality and associated factors in the specialized neonatal care unit asmara, Eritrea. BMC Public Health. 2020;20(1):1–9. doi:10.1186/s12889-019-8118-x
- Michaelis IA, Krägeloh-Mann I, Manyisane N, Mazinu MC, Jordaan ER. Prospective cohort study of mortality in very low birthweight infants in a single centre in the eastern cape province, South Africa. BMJ Paediatr Open. 2021;5(1):e000918. doi:10.1136/bmjpo-2020-000918
- Basu S, Rathore P, Bhatia B. Predictors of mortality in very low birth weight neonates in India. Singapore Med J. 2008;49(7):556.
- Uleanya ND, Aniwada EC, Ekwochi U, Uleanya ND. Short term outcome and predictors of survival among birth asphyxiated babies at a tertiary academic hospital in Enugu, South East, Nigeria. African Health Sci. 2019;19(1):1554–1562. doi:10.4314/ahs.v19i1.29
- Iskandar W, Andayani Y, Marlia L, Burhan B, Primadi A. The influence of gestational age and birth weight on neonatal mortality. Glob Med Health Commun. 2020;8(3):239–244.
- Ayukarningsih Y, Pratiwi ST, Lailani N. The overview of low birth weight infants with incidence of neonatal jaundice in perinatology ward at Dustira Hospital. 12th Annual Scientific Meeting, Medical Faculty, Universitas Jenderal Achmad Yani, International Symposium On” Emergency Preparedness and Disaster Response During COVID. Vol. 19. 2021. Atlantis Press.
- Cho H, Lee EH, Lee K-S, Heo JS. Machine learning-based risk factor analysis of necrotizing enterocolitis in very low birth weight infants. Sci Rep. 2022;12(1):21407. doi:10.1038/s41598-022-25746-6
- Riskin A, Riskin-Mashiah S, Itzchaki O, et al. Mode of delivery and necrotizing enterocolitis in very preterm very-low-birth-weight infants. J Mater Fetal Neon Med. 2021;34(23):3933–3939. doi:10.1080/14767058.2019.1702947
- Özek E, Kersin SG. Intraventricular hemorrhage in preterm babies. Turkish Arch Pediatr/Türk Pediatri Arşivi. 2020;55(3):215.
- MacLeod R, Paulson JN, Okalany N, et al. Intraventricular haemorrhage in a Ugandan cohort of low birth weight neonates: the IVHU study. BMC Pediatr. 2021;21(1):12. doi:10.1186/s12887-020-02464-4
- Sancak S, Topcuoğlu S, Karatekin G. Evaluation of intracranial hemorrhage incidence and risk factors in very low birth weight preterm newborns. Haydarpaşa Numune Med J. 2020;60(4):426.
- Murthy S, Godinho MA, Guddattu V, Lewis LES, Nair NS. Risk factors of neonatal sepsis in India: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0215683. doi:10.1371/journal.pone.0215683
- Glaser MA, Hughes LM, Jnah A, Newberry D. Neonatal sepsis: a review of pathophysiology and current management strategies. Adv Neon Care. 2021;21(1):49–60. doi:10.1097/ANC.0000000000000769
- Shinde R, Haridas K, Nagar P, Parakh H. A study of survival of very low birth weight neonates in a tertiary care hospital. Int J Contemp Pediatr. 2019;6(2):857–862. doi:10.18203/2349-3291.ijcp20190743
- Mu Y, Li M, Zhu J, et al. Apgar score and neonatal mortality in China: an observational study from a national surveillance system. BMC Preg Childbirth. 2021;21(1):47. doi:10.1186/s12884-020-03533-3
- Gillette E, Boardman JP, Calvert C, John J, Stock SJ. Associations between low Apgar scores and mortality by race in the United States: a cohort study of 6,809,653 infants. PLOS Med. 2022;19(7):e1004040. doi:10.1371/journal.pmed.1004040
- Chen H-Y, Blackwell SC, Chauhan SP. Association between apgar score at 5 minutes and adverse outcomes among Low-Risk pregnancies. J Mater Fetal Neon Med. 2022;35(7):1344–1351. doi:10.1080/14767058.2020.1754789
- Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. New England J Med. 2020;383(1):49–57. doi:10.1056/NEJMoa1915075
- Razaz N, Cnattingius S, Joseph K. Association between Apgar scores of 7 to 9 and neonatal mortality and morbidity: population based cohort study of term infants in Sweden. BMJ. 2019;365. doi:10.1136/bmj.l1656
- Ndombo PK, Ekei QM, Tochie JN, et al. A cohort analysis of neonatal hospital mortality rate and predictors of neonatal mortality in a sub-urban hospital of Cameroon. Italian J Pediatr. 2017;43(1):1–8. doi:10.1186/s13052-017-0369-5
- Desta M, Tadese M, Kassie B, Gedefaw M. Determinants and adverse perinatal outcomes of low birth weight newborns delivered in Hawassa University Comprehensive Specialized Hospital, Ethiopia: a cohort study. BMC Res Notes. 2019;12(1):1–7. doi:10.1186/s13104-019-4155-x
- Genie YD, Kebede BF, Zerihun MS, Beyene DT. Morbidity and mortality patterns of preterm low birthweight neonates admitted to referral hospitals in the Amhara region of Ethiopia: retrospective follow-up study. BMJ Open. 2022;12(7):e054574. doi:10.1136/bmjopen-2021-054574
- Gebreheat G, Teame H. Survival and mortality of preterm neonates in a neonatal intensive care unit in Northern Ethiopia: a retrospective cohort study. Sci Rep. 2022;12(1):600. doi:10.1038/s41598-021-04521-z
- Gebreheat G, Tadesse B, Teame H. Predictors of respiratory distress syndrome, sepsis and mortality among preterm neonates admitted to neonatal intensive care unit in northern Ethiopia. J Pediatr Nurs. 2022;63:e113–e20. doi:10.1016/j.pedn.2021.09.029
- Sturgeon JP, Mufukari W, Tome J, et al. Risk factors for inpatient mortality among children with severe acute malnutrition in Zimbabwe and Zambia. Europ J Clin Nutri. 2023;77(9):895–904. doi:10.1038/s41430-023-01320-9
- Chitale R, Ferguson K, Talej M, et al. Early enteral feeding for preterm or low birth weight infants: a systematic review and meta-analysis. Pediatrics. 2022;150(Supplement 1). doi:10.1542/peds.2022-057092E.
- Jeziorczak PM, Frenette RS, Aprahamian CJ. Lack of enteral feeding associated with mortality in prematurity and necrotizing enterocolitis. J Surg Res. 2022;270:266–270. doi:10.1016/j.jss.2021.09.028
- Shanahan KH, Yu X, Miller LG, Freedman SD, Martin CR. Early serum gut hormone concentrations associated with time to full enteral feedings in preterm infants. J Pediatr Gastroenterol Nutri. 2018;67(1):97–102. doi:10.1097/MPG.0000000000001987
- Tottman AC, Bloomfield FH, Cormack BE, Harding JE, Taylor J, Alsweiler JM. Sex-specific relationships between early nutrition and neurodevelopment in preterm infants. Pediatr Res. 2020;87(5):872–878. doi:10.1038/s41390-019-0695-y
- Kwok T, Dorling J, Gale C. Early enteral feeding in preterm infants. Seminars in Perinatology. 2019;43(7):151159. doi:10.1053/j.semperi.2019.06.007

