Abstract
Overactive bladder (OAB) syndrome is a common, complex, and challenging condition. To assist the management of these patients, the European Association of Urology (EAU) updates its guidelines annually. This review reports the presentations from the symposium titled “Dealing with complex OAB patient profiles: in or out of the EAU guidelines?” held at the 32nd EAU Annual Congress in March 2017 in London. The symposium focused on three groups of OAB patients: women who may also suffer pelvic organ prolapse, stress urinary incontinence, the genitourinary syndrome of menopause (GSM); patients at risk of cognitive impairment; and elderly patients. The aim of the symposium was to determine how the 2017 EAU guidelines can best assist physicians, as well as to assess the benefits of fesoterodine in these patients. The EAU guidelines recommend antimuscarinic agents (grade A) for the medical treatment of OAB. In women, OAB is correlated with GSM, both of which are underdiagnosed and undertreated. Fesoterodine decreases OAB symptoms and the associated limitation of physical activity. A combination of fesoterodine and vaginal estrogens is appropriate for OAB associated with GSM. In patients at risk of cognitive impairment, prescribers should pay particular attention to the choice of medication. Fesoterodine is a Pgp substrate with limited ability to cross the blood–brain barrier, which may explain the lack of negative effects on the central nervous system observed in clinical trials of this agent. OAB should not be regarded as a normal consequence of aging. Fesoterodine has been extensively investigated in the elderly, and is the only anticholinergic drug licensed for OAB in this population, rated B (beneficial) according to the Fit for the Aged classification for lower-urinary-tract symptoms. The EAU guidelines are a valuable resource for physicians managing patients with OAB, and the pharmacological properties of fesoterodine offer credible clinical advantages in these three patient groups.
Introduction
Overactive bladder (OAB) syndrome is defined as “urinary urgency, with or without urgency urinary incontinence [UUI], usually with increased daytime frequency and nocturia, in the absence of infection or other obvious pathology”.Citation1,Citation2 OAB can be a complex and challenging syndrome in certain patient groups. The prevalence of OAB is increasing worldwide in association with the growth of the aging population, with 11% of the global population affected in 2008 and predicted to increase to 20%, or 546 million individuals, by 2018.Citation3 OAB has a greater impact on the social and functional domains of quality of life (QL) than that of diabetes.Citation4
The diagnosis and management of OAB merits particular attention, given that this syndrome can affect many patient groups: both men and women, those with other comorbidities, and those taking a mix of medications.Citation5 As a result, the onus is on the physician to collect an accurate history, examine the patient, and investigate if other causes can better explain the symptoms, and then to treat the patient accordingly. The European Association of Urology (EAU) guidelinesCitation6 have been developed to assist health-care professionals in dealing with complex cases in a structured way based on the available evidence. These are updated every year, and form a framework for clinicians to assist in the diagnosis and management of OAB.
This review reports on lectures given at the symposium “Dealing with complex OAB patient profiles: in or out of the EAU guidelines?” held at the 32nd Annual Congress of the EAU in March 2017 in London. The symposium, sponsored by Pierre Fabre Laboratories, aimed to answer the question: Do these guidelines help the physician in dealing with challenging patient groups, or should we vary from them? It focused on three challenging patient groups: women who may also suffer from pelvic organ prolapse (POP), stress UI (SUI) or the effects of menopause; OAB patients at risk of cognitive impairment; and elderly patients, in whom the prevalence of OAB is high and whose specificities are important to consider. The EAU recommendations for these three patient groups, as well as the published evidence on fesoterodine,Citation7 an antimuscarinic agent, are presented and discussed.
Management of women with OAB
OAB is frequently unrecognized in women
Data collected in Belgium from women aged 40 years or over visiting their general practitioner for any reason who answered the Bladder Control Self-Assessment Questionnaire (B-SAQ) revealed that 18% of them were affected by moderate–severe SUI and a third had mild bladder-control symptoms.Citation8 In this study, urgency and nocturia were found to be the most bothersome (higher score of bother on the B-SAQ) symptoms. In another study,Citation9 women from the Finnish population register were contacted to assess the bother associated with 12 different lower-urinary-tract symptoms (LUTS). From an individual perspective, UUI was found to be the symptom most likely to be rated as bothersome by patients and SUI was second. Women and men with OAB symptoms report that OAB affects their health-related QL (HRQL): they have significantly poorer work productivity, sexual satisfaction, higher rates of major depressive symptoms, and less physical activity than controls.Citation10,Citation11 Despite this, many women do not seek medical assistance,Citation9,Citation12 leaving the disorder unrecognized.
OAB is a dynamic disease, particularly in women
In Austria, women with OAB (21–81 years) were followed for a mean of 6.5 years. The prevalence of OAB was 19% at baseline and 27% at the end of the study. With time, remission occurred in 40% of women, improvement occurred in 12%, disease was stable in 41%, and it progressed in 7%. Therefore, natural history shows that OAB is a dynamic disease that can have a long-lasting and stable course, remit, or progress with age.Citation13
Sex differences in OAB show that women are more often bothered by symptoms
The OAB prevalence found in the EPIC study was 12%, and it reached 16% in the National Overactive Bladder Evaluation (NOBLE) program in the US, with no significant differences between women and men. However, the type of symptoms may differ. For instance, UI seems to be more frequent in women (13% versus 5% in men in the EPIC study). Women (66%) were more likely to be bothered by frequency of urination than men (46%).Citation14
Specific risk factors for women
In Sweden, OAB prevalence, measured in female twins from the Swedish twin registry, was 19% in those who had a history of gestational diabetes mellitus (GDM) and 11% in women without GDM. After adjusting for age, parity, body-mass index (BMI), DM, and smoking, GDM was associated with almost-doubled odds of OAB (OR 1.88, 95% CI 1.26–2.8).Citation15
A systematic review of observational studies on OAB and metabolic syndrome, a cluster of risk factors that includes central obesity, dyslipidemia, hypertension, insulin resistance, and glucose intolerance, concluded that there was a link between OAB and metabolic syndrome.Citation16 Women with a BMI >30 are twice as likely to have OAB with urge incontinence than women with a BMI <24.Citation14 Weight loss should be encouraged, in addition to therapy for OAB symptoms.
In a cross-sectional Dutch study,Citation17 POP symptoms were present in 11% of women. The prevalence of urgency, frequency, UI, or any OAB symptoms was significantly increased in those with POP. Treatment of this independent risk factor was able to improve OAB symptoms. The other risk factors found in this study were incontinence surgery in the past, age >75 years, overweight, and postmenopausal status.
Genitourinary syndrome of menopause (GSM), formerly called vulvovaginal atrophy (VVA), has been designated to facilitate the identification of VV and urinary estrogen deprivation-associated signs and symptoms. GSM includes genital symptoms of dryness, burning, and irritation; impaired sexual function with lack of lubrication, discomfort, or pain; and urinary symptoms with frequency, nocturia, urgency, SUI, and urinary-tract infections when other vulvar and urinary pathologies possibly responsible for similar symptomatology have been excluded.Citation18
The female lower urinary tract is a target organ for estrogens, and estrogen deprivation leads to changes in periurethral tissues (decrease in collagen) and urethral mucosa (atrophy). This could be the rationale for the use of estrogen supplementation in the treatment of the LUTS disorders of postmenopausal women.Citation19,Citation20 In the AGATA study,Citation21 among postmenopausal women asking for a routine gynecological examination, 79% were diagnosed with GSM based on the patient’s sensation of vaginal dryness, any objective sign of VVA and a pH >5. Patients also reported dyspareunia (78%), burning (57%), itching (57%), and dysuria (36%). Only 30% of participants had been previously diagnosed with VVA. GSM appeared to be highly prevalent, underdiagnosed, and inadequately treated. Conserved sexual function may prevent or delay the loss of vaginal elasticity.Citation21 In another studyCitation22 in 45- to 60-year-old women, vaginal dryness was associated with a greater OAB prevalence (ratio 1.75, P=0.012). Health professionals should adopt a proactive response to women with genital atrophy to identify and treat OAB.
Robinson et alCitation23 reported that 70% of women relate the onset of UI to their final menstrual period, corresponding to estrogen decrease. First-line therapies include vaginal moisturizers and lubricants;Citation24 however, estrogen therapy remains the standard therapy for symptomatic women who suffer from moderate–severe GSM. Local treatment is preferred. There are few absolute contraindications for estrogen use, especially if used topically.Citation25 Even in breast cancer patients, there are now data suggesting that ultralow-dose topical estriol can be used safely, with excellent results in improving vaginal microflora, quality of sex life, and urinary function.Citation26 The estrogen–antimuscarinic combination could be a good option to treat OAB in women with GSM.
Fesoterodine in women with OAB
The primary goals of pharmacological therapy for women with OAB are to relieve symptoms and improve HRQL. In a pilot study,Citation27 menopausal patients with OAB symptoms were treated either with fesoterodine 4 mg/day for a week and then with 8 mg/day if tolerance was good, or with the same dos-age of fesoterodine combined with topical vaginal estrogens once daily. After 12 weeks, the fesoterodine-alone group showed a significant improvement in OAB symptoms, as measured with the Overactive Bladder Questionnaire–short form (P<0.0001), HRQL (P=0.0002), and Sexual Quality of Life – female (SQOL-F; P=0.02). Compared to fesoterodine alone, the combination group showed a reduction in OAB-symptom severity (10 versus 23.3, P=0.35), higher HRQL score (96.9 versus 84.6, P=0.75), and higher SQOL-F score (99 versus 81, P=0.098).
In a prospective study,Citation28 women with OAB were treated with flexible doses of fesoterodine combined with behavioral counseling over 8 weeks. Physical activity was assessed using two questions of the Short Form 12 and categorized into three levels of limitation: none, moderate, or severe limitations. Women treated with fesoterodine reported less bother from urinary symptoms and fewer restrictions in physical activity, showing that fesoterodine reduces the impact of OAB on physical activity.
The SAFINACitation29 trial defined the goals of OAB patients, mainly women (80%), using the Self-Assessment Goal Achievement questionnaire. Patients were treated with flexible doses of fesoterodine for 12 weeks. Patients’ treatment aims were more focused on the completion of tasks, rather than on reducing particular symptoms. With regard to symptoms, the most frequently expressed goals were to reduce nocturia, urgency, and frequency. At the end of the study, 81% of patients treated with fesoterodine declared that their goals were “somewhat achieved”, “achieved”, or “greatly exceeded/exceeded their expectations”.
EAU guidelines for OAB
The 2017 EAU guidelinesCitation6 for women presenting with UI recommend (after initial assessment and discussion of OAB management) that patients are offered individual behavioral and physical therapies. When medical treatment is advised, antimuscarinic agents are recommended (grade A). The EAU recommendations dedicated to women meet the conclusions of the studies presented, notably:
on risk factors: “Encourage obese women with UI to lose weight and maintain weight loss (grade A)”
on activity: “Counsel female athletes experiencing UI with intense physical activity that it will not predispose them to UI in later life (grade C)”
on estrogen therapy: “Offer postmenopausal women with UI vaginal estrogen therapy, particularly if other symptoms of VVA are present (grade A)”; “Vaginal estrogen therapy for VVA should be prescribed long-term. In women with a history of breast cancer, the treating oncologist needs to be consulted (grade C)”.
Dealing with OAB in patients at risk of cognitive impairment
Specific factors in patients at risk of cognitive impairment
Being prefrail or frail, having a functional comorbidity index (number of comorbidities 0–18) ≥2, and being aged ≥85 years have been found to be associated with anticholinergic medication use.Citation30 However, one of the major issues of treating these patients is the difficulty in assessing their exposure to and impact of drugs with anticholinergic effects. The serum anticholinergic assay has been proposed as a measure of anticholinergic burden, but assay levels do not necessarily reflect the treatment used by the patient.Citation30 Few patients report central nervous system (CNS) symptoms, because they are not able to recognize the cognitive impairment or changes in memory or may think these are related to aging and not to their medications.Citation31 The effects of anticholinergics on cognition vary substantially among studies. This may be due to several factors, including age, clinical condition, and the medicines used.Citation30,Citation32 Recognition of how many drugs the patient is taking and the variety of different therapeutic areas they involve, including antihistamines, antidepressants, anti-emetics, antipsychotics, muscle relaxants, and antivertigo, cardiovascular, gastrointestinal, or antiparkinsonian drugs, which have anticholinergic propertiesCitation33 is important, and they should be identified as such. Patients can be considered at risk of cognitive impairment where there is preexisting CNS impairment or when they take multiple medications or some with anticholinergic activity, particularly when oxybutynin is used in vulnerable elderly patients.Citation31,Citation34
The role of the blood–brain barrier
The blood–brain barrier (BBB) is a complex, heterogeneous, and dynamic membrane at the level of the cerebral capillaries that limits the entry of unwanted molecules into the brain. Antimuscarinic agents cross the BBB via passive diffusion according to their lipophilicity and molecular weight.Citation34–Citation38 Some antimuscarinics are removed from the brain via an active transport mechanism facilitated by Pgp, which limits the levels of drugs in the brain. The BBB can be impaired by different causes, including aging, medication, or systemic diseases (inflammatory; neurodegenerative diseases, such as Alzheimer’s, Parkinson’s disease, and multiple sclerosis; vascular conditions; neoplasia; brain edema; meningitis; and trauma).Citation34,Citation39
Fesoterodine in OAB patients at risk of cognitive impairment
As a result of its pharmacological properties, fesoterodine crosses the BBB poorly, which in fact is an advantage in terms of any potential negative effect on cognitive function. It has the highest molecular weight of the antimuscarinics and low lipophilicity (); moreover, the active moiety of fesoterodine, 5-HMT, is pumped out of the brain by Pgp, an efflux transporter.Citation7,Citation33,Citation38,Citation40–Citation42 Trospium is a quaternary amine and a hydrophilic molecule that does not cross the BBB.
Table 1 Pharmacological and pharmacokinetic properties of antimuscarinic agents
The effect of fesoterodine on cognitive function has been evaluated in a crossover studyCitation43 where healthy older individuals (mean age 72.2 years) received over four different periods fesoterodine 4 mg × 6 days, fesoterodine 4 mg × 3 days + fesoterodine 8 mg × 3 days, placebo × 6 days, or placebo × 6 days with alprazolam 1 mg on day 6. A battery of cognitive tests (detection task, identification task, one-card learning task, continuous paired-associate learning task, Groton maze learning task, and the Rey auditory verbal learning test) were performed on days 1 and 6 of each period. Unlike alprazolam, which induced sedation and a large and statistically significant deterioration, fesoterodine at doses of 4 mg and 8 mg showed no statistically significant effects compared with placebo on any of the cognitive functions, including memory.
The effects of flexible-dose fesoterodine were tested in a double-blind, placebo-controlled Vulnerable Elderly Study.Citation44 Vulnerable elderly subjects were defined as having a risk of deteriorating health with a score ≥3 on the Vulnerable Elders Survey (VES) 13 and a score ≥20 on the Mini-Mental State Examination (MMSE), indicating possible mild cognitive impairment (MMSE maximum score 30, scores ≥27 correspond to normal cognition, 20–26 indicate some cognitive impairment). In both the placebo and fesoterodine groups, patients had a high number of comorbidities (mean number per patient slightly over eight, range one to 27) and comedications (mean number per patient slightly over eight, range one to 40). Treatment with a flexible dose of fesoterodine over 12 weeks showed significantly greater efficacy versus placebo in reducing the mean number of UUI episodes and micturitions per 24 hours. There was no decrease in mean MMSE scores for subjects receiving fesoterodine or placebo, showing that fesoterodine did not worsen cognitive impairment. Fesoterodine was well tolerated, with a safety profile comparable to that seen in other studies in younger populations. The most frequent adverse events were a dry mouth and constipation, and no new safety signals were identified.
2017 EAU guidelines and cognitive function
The 2017 EAU guidelines contain a specific chapter dedicated to antimuscarinic agents, the elderly, and cognition, and recommend caution notably in the long-term use of antimuscarinic treatment in elderly patients, especially those who are at risk of, or have, cognitive dysfunction. Moreover the total antimuscarinic load has to be evaluated and if additional antimuscarinic load is to be avoided the use of mirabegron should be considered.Citation6
Concerning antimuscarinic agents, the EAU guidelines state that “solifenacin, darifenacin, fesoterodine, and trospium have been shown not to cause cognitive dysfunction in elderly people”.Citation6
Elderly with OAB
Specific factors in elderly patients with OAB
Although the prevalence of OAB increases with age, it should not be considered a normal consequence of aging.Citation45 Treatment should be chosen on an individual basis, taking into account tolerability, absence of drug interactions, and cognitive safety.Citation46 OAB and UUI in the elderly may have serious consequences, notably in cases of nocturia. Elderly women with UI have a 26% higher risk of falls and 34% greater risk of fractures. Urgency and UUI significantly predict 10-year mortality in men, and UUI significantly predicts mortality in women.Citation47–Citation49 The OAB Re-Contact Study, a self-reported Internet survey in patients aged 65 years or over, demonstrated that diagnosed versus undiagnosed and treated versus untreated respondents had better mental scores, better QL, and less impairment of their activity.Citation50
Fesoterodine in elderly patients
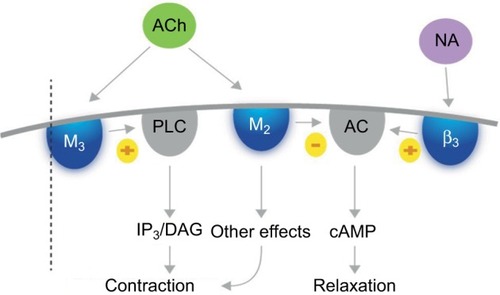
Fesoterodine possesses four interesting pharmacological properties that give it a therapeutic profile adapted to many OAB patients, including the elderly.Citation51–Citation53 Firstly, fesoterodine’s active metabolite, 5-HMT, has limited ability to cross the BBB, and as a Pgp substrate, it is actively pumped out of the brain. As a result, fesoterodine presents a safe profile with respect to cognitive impairment. Secondly, the molecule does not undergo first-pass hepatic metabolism, but is metabolized by ubiquitous peripheral esterases that are not dependent on the individual’s enzymatic equipment and not affected by age. This property might explain the consistency of response across patients and the consistent results in clinical trials. Thirdly, fesoterodine is a prolonged-release medication, permitting once-daily dosing. Finally, fesoterodine has a balanced affinity for M2 and M3 receptors. All muscarinic-receptor subtypes have been detected in the human bladder; however, the M2 and M3 receptors seem to be the most important in the regulation of the complex process of bladder function. The M2 subtype outnumbers the M3 subtype, and is the most highly expressed in the urothelium of the human bladder. However, M3 receptors are thought to be most important for detrusor contraction, which is decreased in cases of blockade by anti-M3 agents.Citation54,Citation55 Both subtypes are coupled to G proteins, but their signal-transduction pathways differCitation54 (). Activation of M2 receptors reverses the sympathetic β-adrenoreceptor-mediated relaxation of the detrusor. Therefore, by blocking M2 receptors with M2 antimuscarinic agents, fesoterodine inhibits this effect, resulting in bladder relaxation and an increase in bladder capacity.Citation56 The M2 receptor might also play an indirect role in mediating bladder contraction by enhancing the contractile response to M3-receptor activation, and minor M2-receptor-mediated contractions might also occur. Moreover, in some disease states, the contribution of M2 receptors to detrusor contraction might increase.Citation54 The result of fesoterodine’s balanced M2–M3 affinity is a double effect on contraction control and relaxation.
Figure 1 Actions of acetylcholine on M2 and M3 receptors.
Abbreviations: ACh, acetylcholine; cAMP, cyclic adenosine monophosphate; NA, noradrenaline; β3, beta-3 adrenergic receptor; M2, M3, muscarinic receptors.
Several studies have evaluated the efficacy and safety of fesoterodine in elderly OAB patients. In the SOFIA study in an aging population,Citation57 patients aged 65 years or older were randomized to fesoterodine or placebo and treated for 12 weeks. Approximately a third of participants were older than 75 years. At week 4, 52% of patients treated with fesoterodine chose to increase their dose from 4 mg to 8 mg, with a further 16% choosing to increase their dose similarly at week 8, and only 4% reduced their dose during the study, supporting the use of flexible dosing in an elderly population. In comparison with placebo, fesoterodine induced a statistically significant decrease in urgency episodes per 24 hours, in nocturnal micturitions, and incontinence-pad use, with an improvement in QL and a reduction in symptom-bother scores. The rate of severe dry mouth was low (3%).
Most of the patients who completed the SOFIA study continued in an open study for a further 12 weeks.Citation58 The subjects who had received fesoterodine during the double-blind phase maintained the improvement in urgency episodes over the open-label phase, and had a lower percentage of dry mouth and constipation (7% and 2%, respectively, versus 28% and 6% in the group that had received placebo in the double-blind phase), as well as a lower discontinuation rate due to adverse events (4% versus 10%). Constipation is usually considered an adverse event; however, in this age-group, which frequently shows irritable bowel disease and loose stools, constipation could have a positive impact and can even improve QL.Citation59
A pooled post hoc analysis of trialsCitation60 exploring the efficacy and safety of fesoterodine in elderly patients demonstrated a significant decrease in UUI episodes per 24 hours in three groups (<65, 65–74, or ≥75-years of age) of patients treated with fesoterodine (8 mg) and a higher positive treatment response (improved or greatly improved) in patients treated with fesoterodine (4 and 8 mg) than with placebo in the three age-groups, with a dose-dependent effect.
The Fit for the Aged (FORTA) classification ranks medical treatments according to the level of evidence provided by prospective control studies performed in elderly patients. This results from a systematic independent literature review of studies aimed at evaluating the elderly population through an international consensus-validation process and an independent quality assessment of variation within the studies. The FORTA classification was first established for cardiology medications, and is now used for different kinds of treatments. It guides physicians as to the suitability of medicines for older patients in an everyday clinical setting, and includes four categories (). In urology, FORTA-LUTS classifies the appropriateness of oral drugs for long-term treatment of LUTS in older persons. Fesoterodine is classified B (beneficial), and has the highest level of evidence of the available OAB treatments. Most of the other antimuscarinic agents are classified as C (careful), because the few studies that have been performed in aging patients were not prospective, but involved post hoc or subgroup analyses of data.Citation61
Table 2 Rating of OAB medical drugs on the LUTS-FORTA classification
A cost-analysis studyCitation62 evaluating the treatment costs of vulnerable (≥ 65 years with a VES score ≥3 and risk of deteriorating health) OAB patients and including fesoterodine-drug costs, health-care resources, and OAB-related comorbidities (falls/fractures, urinary tract infections, depression, and nursing home) found that fesoterodine was cost-saving compared to no OAB pharmacotherapy, and could save US$1,616 per patient per year. Fesoterodine accounted for only 4% of the total medical costs in the vulnerable elderly.
EAU guidelines and the elderly OAB patient
The word “elderly”, although not defined, usually refers to individuals 65 years old or over. In addition to guidelines related to cognitive function that also frequently concern elderly patients, the 2017 EAU guidelines recommend that older people being treated for UI should be prescribed nonpharmacological treatments first (grade C). They also consider that antimuscarinic drugs are effective in elderly patients (grade 1B). With natural aging, UI becomes more common and elderly people may require special consideration and specific interventions, taking into account their preferences, but OAB should not be considered a normal consequence of aging.
Discussion
Some interesting points have been raised during the discussion of the specificities of the three patient groups in light of the existing fesoterodine clinical data and the EAU recommendations. In women, OAB is correlated with GSM, and both are underdiagnosed and undertreated. Antimuscarinics are classed as grade A in the recommendations for treatment of women with OAB. Fesoterodine is associated with a reduction in OAB symptoms, as well as in perceived limitations in physical activity. Fesoterodine combined with vaginal estrogens is appropriate for women with OAB associated with GSM.
In all patients, and particularly patients at risk of cognitive impairment, prescribers should be careful with medications. Fesoterodine has a limited ability to cross the BBB and is also a Pgp substrate, which could explain its lack of negative effects on the CNS, as confirmed by clinical evidence. Evaluation of cognitive function in healthy older subjects has confirmed that fesoterodine does not impair it.
Elderly people need careful consideration, and OAB should not be considered a normal consequence of aging. Fesoterodine offers an interesting combination of pharmacological properties with clinical benefits, and is generally well tolerated in older people. Fesoterodine has been studied extensively in elderly patients, and is the only OAB drug rated FORTA B (beneficial).Citation61
In neurological diseases, such as multiple sclerosis, cognitive impairment appears to be linked to neurological lesions and to the degree of disability measured with the Expanded Disability Status Scale.Citation63 Cognitive impairment is impacted by the deterioration in neurological status that occurs in this disease. For Parkinson’s disease, the impact seems more linked to anticholinergic exposure, as these patients are very sensitive to the side effects of antimuscarinic drugs.Citation64 Parkinson’s, multiple sclerosis, and also brain-trauma patients, who are sometimes young, are known to be at high risk of cognitive impairment. Tests evaluating cognitive impairment are not routinely performed, and remain within the competence of specialists or appropriately trained physicians.
Another point raised was to determine if drug-induced side effects persist over the duration of treatment or if they recede after a certain time. In a studyCitation65 investigating the rate of dry mouth in female patients treated with fesoterodine, a greater proportion of women with dry mouth reported improvement in their urinary symptoms, and the authors suggested that dry mouth may be a marker of pharmacological potency. In clinical practice, increasing the fesoterodine dose from 4 mg to 8 mg is generally associated with an increase in the rate of dry mouth; however, women who are satisfied with the improved control of their symptoms may be less troubled by a dry mouth. Usually, there is a balance between efficacy and side effects, which are better tolerated when the patient shows a better response, although important individual variations are observed. The response to treatment is rapid, with the full effect reached at 2–4 weeks. In this respect, in most countries, and especially in the UK, nurses have the skills and can take the time to explore their patients’ needs and expectations and explain the possible side effects. Specialist nurse intervention in the management of incontinence has been evaluated in several studies, some showing more active detection of patients, gain in QL, or reduction of symptoms, with high patient satisfaction and cost-effectiveness.Citation66 Overall, clinical experience has shown that side effects seem to decrease with time, possibly because of the natural course of the disease, and because the patient adapts their treatment accordingly. Prospective studies evaluating the evolution of side effects after 5 or 10 years have not yet been performed; however, long-term treatment (24 and 36 months) with fesoterodine has been shown to be well tolerated, with no new safety concerns identified.Citation67
Our objective here was to discuss the EAU guidelines, though guidelines on UI are available from other societies, such as the National Institute for Health and Care Excellence (NICE, UK) or the American Urologic Association (AUA). These guidelines, which may vary in their recommendations, were compared by Syan and BruckerCitation68 in 2016.
Conclusion
OAB can be complex in its presentation, and occurs as part of a broader patient profile. The updated EAU guidelines are valuable in assisting physicians with the management of these complex patients. An appropriate assessment of the patient is a prerequisite to applying the EAU guidelines in complex groups of patients, including women who have specific risk factors and conditions; patients at risk of cognitive impairment who may have comorbidities, take multiple medications, or show BBB deterioration; and elderly patients who are frequently frail and vulnerable and should be treated with caution. Fesoterodine at doses of 4 mg and 8 mg have been studied extensively in these three patient groups, and has been shown to have pharmacological properties that confer clear clinical advantages: fesoterodine is efficient, irrespective of sex or age, is well tolerated in older and vulnerable patients, and does not cause impairment in cognitive function.
Acknowledgments
Participation in the satellite symposium “Dealing with complex OAB patient profiles: in or out of the EAU guidelines?” at the 32nd Annual Congress of the EAU in March 2017 in London and writing assistance were sponsored by Pierre Fabre (Castres, France).
Disclosure
JH has received support as a consultant/lecturer from Astellas, Allergan, BlueWind, Urogyn BV, and Pierre Fabre, and for scientific studies and trials from Astellas, Boston Scientific, Ipsen, BlueWind, and Urogyn BV. MEP has received support as a consultant/lecturer from Astellas, Boston Scientific, and Pierre Fabre. ECK has received support as consultant and/or lecturer and/or investigator from Astellas, Allergan, Boston Scientific, Coloplast, Ipsen, Medtronic, Pfizer, Lilly, Pierre Fabre, and Uromedica. PTH has received support as lecturer from Astellas, Boston, and Pierre Fabre, and as a consultant from Allergan and Speciality European Pharma. The authors report no other conflicts of interest in this work.
References
- AbramsPCardozoLFallMThe standardisation of terminology of lower urinary tract function: report from the Standardisation sub-Committee of the International Continence SocietyNeurourol Urodyn200221216717811857671
- DrakeMJDo we need a new definition of the overactive bladder syndrome? ICI-RS 2013Neurourol Urodyn201433562262424838519
- IrwinDEKoppZSAgatepBMilsomIAbramsPWorldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstructionBJU Int201110871132113821231991
- AbramsPKelleherCJKerrLARogersRGOveractive bladder significantly affects quality of lifeAm J Manag Care2000611 SupplS580S59011183901
- BrownJSMcGhanWFChokrovertySComorbidities associated with overactive bladderAm J Manag Care2000611 SupplS574S57911183900
- BurkhardFCBoschJLCruzFUrinary incontinence [guidelines]2017 Available from: https://uroweb.org/guideline/urinary-incontinenceAccessed July 4, 2017
- Toviaz (fesoterodine fumarate) [prescribing information]2016 Available from: http://www.medicines.org.uk/emc/medicine/20928Accessed July 4, 2017
- de RidderDRoumeguèreTKaufmanLOveractive bladder symptoms, stress urinary incontinence and associated bother in women aged 40 and above: a Belgian epidemiological surveyInt J Clin Pract201367319820423409688
- AgarwalAEryuzluLNCartwrightRWhat is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and womenEur Urol20146561211121724486308
- CoyneKSSextonCCIrwinDEKoppZSKelleherCJMilsomIThe impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC studyBJU Int2008101111388139518454794
- CoyneKSSextonCCClemensJQThe impact of OAB on physical activity in the United States: results from OAB-POLLUrology201382479980623953610
- TaylorJMcGrotherCWHarrisonSCAssassaPRLower urinary tract symptoms and related help-seeking behaviour in South Asian men living in the UKBJU Int200698360560916925760
- HeidlerSMertCTemmlCMadersbacherSThe natural history of the overactive bladder syndrome in females: a long-term analysis of a health screening projectNeurourol Urodyn20113081437144121661037
- EapenRSRadomskiSBGender differences in overactive bladderCan J Urol201623Suppl 12926924589
- TettamantiGIliadouANPedersenNLBelloccoRAltmanDAssociation between gestational diabetes mellitus and subsequent overactive bladder among premenopausal female twinsBJOG2013120101289129523647812
- BunnFKirbyMPinkneyEIs there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studiesInt J Clin Pract201569219921725495905
- de BoerTASlieker-ten HoveMCBurgerCWVierhoutMEThe prevalence and risk factors of overactive bladder symptoms and its relation to pelvic organ prolapse symptoms in a general female populationInt Urogynecol J201122556957521104400
- PortmanDJGassMLGenitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause SocietyMaturitas201479334935425179577
- IosifCSBekassyZPrevalence of genito-urinary symptoms in the late menopauseActa Obstet Gynecol Scand19846332572606730943
- BlakemanPJHiltonPBulmerJNOestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen statusBJU Int2000861323810886079
- PalmaFVolpeAVillaPCagnacciAVaginal atrophy of women in postmenopause: results from a multicentric observational study – the AGATA studyMaturitas201683404426421474
- JuliatoCRBaccaroLFPedroAOCosta-PaivaLLui-FilhoJPinto-NetoAMSubjective urinary urgency in middle age women: a population-based studyMaturitas201685828726857885
- RobinsonDToozs-HobsonPCardozoLThe effect of hormones on the lower urinary tractMenopause Int201319415516224336244
- Calleja-AgiusJBrincatMPThe urogenital system and the menopauseClimacteric201518Suppl 1182226366796
- LethabyAAyelekeRORobertsHLocal oestrogen for vaginal atrophy in postmenopausal womenCochrane Database Syst Rev20168CD00150027577677
- BuchholzSMögeleMLintermansAVaginal estriol-lactobacilli combination and quality of life in endocrine-treated breast cancerClimacteric201518225225925427450
- ChughtaiBFordeJCBuckJThe concomitant use of fesoterodine and topical vaginal estrogen in the management of overactive bladder and sexual dysfunction in postmenopausal womenPost Reprod Health2016221344026883688
- ChuCMHarvieHSSmithALAryaLAAndyUUThe impact of treatment of overactive bladder on physical activity limitationsJ Womens Health (Larchmt)201625880180527135856
- RantellACardozoLKhullarVPersonal goals and expectations of OAB patients in the UKNeurourol Urodyn20173641194120027564470
- LampelaPPaajanenTHartikainenSHuupponenRCentral anticholinergic adverse effects and their measurementDrugs Aging2015321296397426518014
- ChancellorMBooneTAnticholinergics for overactive bladder therapy: central nervous system effectsCNS Neurosci Ther201218216717422070184
- LampelaPTaipaleHHartikainenSAssociation between anticholinergic load and frailty in community-dwelling older peopleJ Am Geriatr Soc201664367167227000355
- CetinelBOnalBRationale for the use of anticholinergic agents in overactive bladder with regard to central nervous system and cardiovascular system side effectsKorean J Urol2013541280681524363860
- ChancellorMBStaskinDRKayGGSandageBWOefeleinMGTsaoJWBlood-brain barrier permeation and efflux exclusion of anticholinergics used in the treatment of overactive bladderDrugs Aging201229425927322390261
- HawkinsRAO’KaneRLSimpsonIAViñaJRStructure of the blood-brain barrier and its role in the transport of amino acidsJ Nutr20061361 Suppl218S226S16365086
- GellerEJCraneAKWellsECEffect of anticholinergic use for the treatment of overactive bladder on cognitive function in postmenopausal womenClin Drug Investig20123210697705
- ScheifeRTakedaMCentral nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderlyClin Ther200527214415315811477
- KerdraonJRobainGJeandelCImpact on cognitive function of anticholinergic drugs used for the treatment of overactive bladder in the elderlyProg Urol2014241167268125214448
- ZeeviNPachterJMcCulloughLDWolfsonLKuchelGAThe blood-brain barrier: geriatric relevance of a critical brain-body interfaceJ Am Geriatr Soc20105891749175720863334
- Medicines and Healthcare Products Regulatory AgencyPublic assessment report – mutual recognition procedure: Mictonorm XL 45 mg modified release capsules (propiverine hydrochloride)2011 Available from: http://www.mhra.gov.uk/home/groups/par/documents/websitere-sources/con143796.pdfAccessed July 4, 2017
- WaggAVerdejoCMolanderUReview of cognitive impairment with antimuscarinic agents in elderly patients with overactive bladderInt J Clin Pract20106491279128620529135
- MalhotraBGandelmanKSachseRWoodNMichelMCThe design and development of fesoterodine as a prodrug of 5-hydroxymethyl tolterodine (5-HMT), the active metabolite of tolterodineCurr Med Chem200916334481448919835561
- KayGGMaruffPScholfieldDEvaluation of cognitive function in healthy older subjects treated with fesoterodinePostgrad Med20121243715
- DubeauCEKrausSRGrieblingTLEffect of fesoterodine in vulnerable elderly subjects with urgency incontinence: a double-blind, placebo controlled trialJ Urol2014191239540423973522
- ErdemNChuFMManagement of overactive bladder and urge urinary incontinence in the elderly patientAm J Med20061193 Suppl 1293616483866
- Lechevallier-MichelNMolimardMDartiguesJFFabrigouleCFourrier-RéglatADrugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID studyBr J Clin Pharmacol200559214315115676035
- NakagawaHNiuKHozawaAImpact of nocturia on bone fracture and mortality in older individuals: a Japanese longitudinal cohort studyJ Urol201018441413141820727545
- BrownJSVittinghoffEWymanJFUrinary incontinence: does it increase risk for falls and fractures?Am Geriatr Soc2000487721725
- NuotioMTammelaTLLuukkaalaTJylhäMUrgency and urge incontinence in an older population: ten-year changes and their association with mortalityAging Clin Exp Res200214541241912602577
- LeeLKGorenAZouKHPotential benefits of diagnosis and treatment on health outcomes among elderly people with symptoms of overactive bladderInt J Clin Pract2016701668126662296
- CardozoLKhullarVWangJTGuanZSandPKFesoterodine in patients with overactive bladder syndrome: can the severity of baseline urgency urinary incontinence predict dosing requirement?BJU Int2010106681682120151972
- García-BaqueroRMadurgaBGarcíaMVFernándezMARosetyJMÁlvarez-OssorioJLNew perspectives of treatment with fesoterodine fumarate in patients with overactive bladderActas Urol Esp20133728391 Spanish23374672
- KhullarVRovnerESDmochowskiRNittiVWangJGuanZFesoterodine dose response in subjects with overactive bladder syndromeUrology200871583984318342923
- AbramsPAnderssonKEMuscarinic receptor antagonists for overactive bladderBJU Int20071005987100617922784
- SellersDJChess-WilliamsRMuscarinic agonists and antagonists: effects on the urinary bladderHandb Exp Pharmacol201220837540022222707
- ChappleCRMuscarinic receptor antagonists in the treatment of overactive bladderUrology2000555A Suppl334610767450
- WaggAKhullarVMarschall-KehrelDFlexible-dose fesoterodine in elderly adults with overactive bladder: results of the randomized, double-blind, placebo-controlled study of fesoterodine in an aging population trialJ Am Geriatr Soc201361218519323350833
- WaggAKhullarVMichelMCOelkeMDarekarABitounCELong-term safety, tolerability and efficacy of flexible-dose fesoterodine in elderly patients with overactive bladder: open-label extension of the SOFIA trialNeurourol Urodyn201433110611423460503
- BulchandaniSToozs-HobsonPParsonsMMcCootySPerkinsKLatthePEffect of anticholinergics on the overactive bladder and bowel domain of the electronic personal assessment questionnaire (ePAQ)Int Urogynecol J2015264533725323310
- KrausSRRuiz-CerdáJLMartireDWangJTWaggASEfficacy and tolerability of fesoterodine in older and younger subjects with overactive bladderUrology20107661350135720974482
- OelkeMBecherKCastro-DiazDAppropriateness of oral drugs for long-term treatment of lower urinary tract symptoms in older persons: results of a systematic literature review and international consensus validation process (LUTS-FORTA 2014)Age Ageing201544574575526104505
- QinLLuoXZouKHSnedecorSJEconomic impact of using fesoterodine for the treatment of overactive bladder with urge urinary incontinence in a vulnerable elderly population in the United StatesJ Med Econ201619322923526488196
- RoccaMAAmatoMPDe StefanoNClinical and imaging assessment of cognitive dysfunction in multiple sclerosisLancet Neurol201514330231725662900
- Perez-LloretSPeraltaMCBarrantesFJPharmacotherapies for Parkinson’s disease symptoms related to cholinergic degenerationExpert Opin Pharmacother201617182405241527785919
- WeissbartSJLewisRSmithALHarvieHSMillerJMAryaLAImpact of dry mouth on fluid intake and overactive bladder symptoms in women taking fesoterodineJ Urol201619551517152226682757
- Holtzer-GoorKMGaultneyJGvan HoutenPCost-effectiveness of including a nurse specialist in the treatment of urinary incontinence in primary care in the NetherlandsPLoS One20151010e013822526426124
- ChappleCOelkeMKaplanSAScholfieldDArumiDWaggASFesoterodine clinical efficacy and safety for the treatment of overactive bladder in relation to patient profiles: a systematic reviewCurr Med Res Opin20153161201124325798911
- SyanRBruckerBMGuideline of guidelines: urinary incontinenceBJU Int20161171203326033093

