Abstract
Substituted cathinones are synthetic analogs of cathinone that can be considered as derivatives of phenethylamines with a beta-keto group on the side chain. They appeared in the recreational drug market in the mid-2000s and now represent a large class of new popular drugs of abuse. Initially considered as legal highs, their legal status is variable by country and is rapidly changing, with government institutions encouraging their control. Some cathinones (such as diethylpropion or pyrovalerone) have been used in a medical setting and bupropion is actually indicated for smoking cessation. Substituted cathinones are widely available from internet websites, retail shops, and street dealers. They can be sold under chemical, evocative or generic names, making their identification difficult. Fortunately, analytical methods have been developed in recent years to solve this problem. Available as powders, substituted cathinones are self-administered by snorting, oral injestion, or intravenous injection. They act as central nervous system stimulants by causing the release of catecholamines (dopamine, noradrenaline, and serotonin) and blocking their reuptake in the central and peripheral nervous system. They may also decrease dopamine and serotonin transporter function as nonselective substrates or potent blockers and may inhibit monoamine oxidase effects. Nevertheless, considerable differences have been found in the potencies of the different substituted cathinones in vitro. Desired effects reported by users include increased energy, empathy, and improved libido. Cardiovascular (tachycardia, hypertension) and psychiatric/neurological signs/symptoms (agitation, seizures, paranoia, and hallucinations) are the most common adverse effects reported. Severe toxicity signs compatible with excessive serotonin activity, such as hyperthermia, metabolic acidosis, and prolonged rhabdomyolysis, have also been observed. Reinforcing potential observed in animals predicts a high potential for addiction and abuse in users. In case of overdose, no specific antidote exists and no curative treatment has been approved by health authorities. Therefore, management of acute toxic effects is mainly extrapolated from experience with cocaine/amphetamines.
Introduction
Many recreational drugs have been synthesized chemically in recent decades as legal alternatives to scheduled cocaine and amphetamine/methamphetamine stimulants. Among them, synthetic derivatives of cathinone, also called substituted cathinones, have become increasingly popular among recreational drug users. The growing problem of the abuse of these psychoactive drugs, especially affecting youths, has prompted concerns from both health care providers and legal authorities. Moreover, in response to market trends and legislative controls, synthesis of new cathinone derivatives by introducing chemical modifications has dramatically increased the diversity of these substances. At the same time, since the appearance of substituted cathinones, ever-increasing data on the identification or analysis methods, pharmacokinetics or pharmacodynamics, animal or human toxicity, and addictive potential are becoming available. The aim of the current contribution is to present updated information on substituted cathinones, useful for poisons centers, clinical toxicologists, and emergency physicians.
Chemical structure and designation
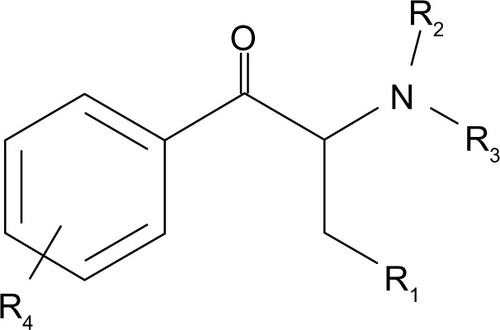
Substituted cathinones are a large family of synthetic beta-keto phenethylamine (2-amino-1-phenyl-1-propanone) derivatives chemically related to the parent compound cathinone, a monoamine alkaloid naturally present in the khat plant, differing only in the functionality present at the beta-carbon; ie, ketone oxygen. As with amphetamines, both systematic (International Union of Pure and Applied Chemistry) and non-standard nomenclature is common in cathinone chemistry. The use of acronyms is also widespread. As a consequence of the beta-keto substituent, it is also common practice for widely accepted amphetamine acronyms to be augmented with the prefix “bk”. The most common cathinones and their related trade names are listed in . The general structure of a cathinone derivative () shows substitution patterns at four locations of the cathinone molecule; eg, on the carbon atom linked to the carbon in alpha position (R1), on the nitrogen atom (R2 and R3), and on the phenyl group (R4). Substituents in R1, R2, R3, and R4 may vary, and include: alkyl, alkoxy, alkylenedioxy, haloalkyl, halide, and phenyl. However, differences between substituted cathinones may be limited to the simple addition of a methyl group (). For some substituted cathinones, nitrogen may be incorporated in a cyclic pyridinyl structure leading to the series of compounds presented in . Some others are methylenedioxy-substituted analogs differing from their corresponding methylenedioxy amphetamines by the addition of only a beta-ketone group (). Moreover, besides substituted 2-amino-1-phenyl-1-propanones, some 1-amino-1-phenyl-2-propanone derivatives are now emerging, including isoethcathinone and isopentedrone. All these compounds are chiral, and can exist in two stereoisomer forms. Aside from their usual trade names, substituted cathinones may be intentionally mislabeled and retailed under slang names as bath salts, plant food, or research chemicals and be labeled not for human consumption. They may be referred to by specific street names, such as meow-meow, MCAT, meph, subcoca-1, and so on, for mephedrone or by generic brand names as Energy 1, 2, or 3 (NRG1, 2, or 3), Blizzard, Blue silk, and Ivory snow among others. Qualitative analyses have been carried out on so called legal highs or NRG that have been obtained from different websites. They showed that: 1) the real composition of many of the products were found to differ markedly from information given to the purchaser, and 2) the majority of these products contained a mixture of substituted cathinones,Citation1 which increased poisoning risk.
Table 1 List of the most common substituted cathinones
Table 2 Related structure of several alkylcathinones
Table 3 Structure of pyrrolidinyl substituted cathinones
Table 4 Chemical structure of methylenedioxy amphetamines and cathinones
Current and developing analytical methods for detection
Immunochemistry
As a general rule, immunoassay screening methodologies used to detect methamphetamines fail to detect cathinone derivatives but can give false positive reactions for some.Citation2 We tested the cross-reactivity of some cathinones using the semi-quantitative AxSYM amphetamine/methamphetamine II assay that utilizes Fluorescence Polarization Immunoassay (FPIA) (Abbott Laboratories, Chicago, IL, USA). We evaluated the responses obtained from aqueous solutions of 14 substituted cathinones at three concentrations: 1 mg/L, 10 mg/L, and 100 mg/L. Even at 100 mg/L, pentedrone, pentylone, α-pyrrolidinovalerophenone (PVP), and methylenedioxypyrovalerone (MDPV) did not react with the test; the most sensitive cathinones that reacted in the assay at 10 mg/L had the least substitutions on the ethyl amine chain: ethylone, mephedrone, methylone, methedrone, and 4-methylethcathinone (MEC). A cross-reaction study with the nal von minden methamphetamine Drug-Screen® test (nal von minden GmbH, Regensburg, Germany), confirmed the poor response of cathinones with these screening tests. Looking at these data obtained with marketed amphetamine/methamphetamine assays, even if cross-reactivity is chemical structure-dependent, a positive result is not expected in a user’s urine, except perhaps in the context of massive intoxication. By contrast, it was recently shown that MDPV cross-reacts with the phencyclidine (PCP) immunoassay used in hospitals.Citation3 Recently, in commercial enzyme-linked immunosorbent assays, eight cathinone derivatives among 30 designer drugs that were evaluated demonstrated cross-reactivity at concentrations at low as 0.15 mg/L when tested against the Randox Mephedrone/Methcathinone ELISA kit (RANDOX Toxicology, Crumlin, UK) recently developed for forensic specific cathinones screening in urine and blood specimens.Citation4
Gas chromatography-mass spectrometry
The method of choice to rapidly identify substituted cathinones in powders remains gas liquid chromatography connected to electron-impact mass spectrometry (GC-MS).Citation1,Citation5,Citation6 In our own experience, when we used a 5% phenyl methylpolysiloxane fused-silica capillary column (30 m × 0.25 mm × 0.25 μm), oven temperature ramped from 100°C (hold time: 1 minute) at 8°C/minute to 280°C (1.5 minutes), helium flow at 1 mL/minute, the total runtime was 25 minutes and the rank order (retention time) of the 14 analyzed cathinones was as follows: flephedrone < metamfepramone < ethcathinone < mephedrone < pentedrone < amfepramone < 4-MEC < methedrone < methylone < PVP < ethylone < butylone < pentylone < MDPV. The selected mass spectrometry (MS) data that we collected in the literature and in our own data bank are summarized in and permit the identification of the most common substituted cathinones. As a general rule, mass spectra show base peaks in the low mass and small or absent intensities for the molecular ions (M+). The mass-spectral fragmentation pattern of cathinone derivatives under electron impact conditions includes the formation of different iminium ions at m/z 44, 58, 72, 86, and 100. For cathinones with a pyrrolidine ring in the side chain, fragmentation leads to the formation of characteristic ions at m/z 70, 55, 42, and 41, results of the pyrrolidine ring degradation. The alternative cleavage reaction that is typical for aromatic ketones results in the formation of the product of decarboxylation, the phenyl cation (m/z 77).Citation7 All isomers of fluoromethcathinone show significant fragments at m/z 95 and m/z 123; these correspond to the fluorophenyl cation and fluorobenzoyloxy cation, respectively.Citation8 The limit of quantification in biologic fluids for the GC-MS method developed by Torrance and Cooper in 2010 was 100 μg/L.Citation9 This value is acceptable in fatal cases but appears inadequate to measure concentrations in user’s blood.
Table 5 Gas-chromatography mass spectrometry identification of substituted cathinones
Liquid chromatography-mass spectrometry
Different liquid chromatography-tandem-mass spectrometry (LC-MS-MS) screening or semi-quantitative/quantitative methods have been published to identify and quantify substituted cathinones in urine, oral fluid, live and post-mortem whole blood, and post mortem tissues,Citation10–Citation14 or using dried blood spots.Citation15 More recently, rapid screening using direct analysis in real time mass spectrometry (DART-MS) or using portable mass-spectrometer equipped with desorption electrospray ionization (DESI-MS) shortened the time of the sensitive and selective examination of substituted cathinones from various substrates.Citation16,Citation17 A classic reverse phase C18 or a phenyl column is typically used for the chromatographic separation prior to analysis by LC-tandem MS using electrospray positive ionization multiple reaction monitoring (MRM) mode.Citation18 MRM transitions for cathinones are presented in . For all substances, the dominant Q1 (first quadripole) ions were the protonated molecular ions ([M+H]+). The product ion ([M+H-H2O]+) is generally the most abundant in Q3, except for amfepramone. Cathinones with unsubstituted ring structures produced m/z 105 ions ([Ph-C≡O]+) in significant abundance. For all substances, at least two product ions of acceptable abundance can be obtained.Citation19 In order to complete targeted LS-MS-MS screening, LC coupled to high resolution quadripole time-of-flight MS (LC-Q-TOF-MS) methods have been developed to identify new structures of cathinones or fragmented ions in “legal highs”Citation20,Citation21 or biological samples.Citation22,Citation23 Zuba described that under electrospray ionization (ESI)-Q-TOF-MS conditions, the loss of water and α-cleavage of the amine end are the most characteristic features.Citation20 For the 3,4-methylenedioxy cathinone derivatives, the loss of the methylenedioxy group CH4O2 (48.02) and the formation of the cation [M+H-CH4O2]+ are observed.Citation21 The limits of quantification for the assay of substituted cathinones in serum using an electrospray ionization source in positive polarity are of the order of 10 μg/L with a linear dynamic range of 10 to 500 μg/L. Accordingly, LC-MS-MS is the only method that presents sufficient sensibility to quantify substituted cathinones in biological fluids.
Table 6 Liquid chromatography–tandem mass spectrometry identification of substituted cathinones
Extraction procedures for quantification in biological samples
Different sample preparations are proposed including basic liquid-liquid extractionCitation24 and solid-phase extraction methods.Citation25 Consistent with our personal data, commercially available liquid-liquid extraction tubes, such as Toxi.tubes A® (Agilent Technologies, Santa Clara, CA, USA), can be considered as an alternate. For LC-MS-MS, a single precipitation step of methanol denatured protein by centrifugation was recommended by Sørensen before injection in the liquid chromatograph system.Citation19
Experimental pharmacology and neuropharmacology
In vitro
As with amphetamines or cocaine, substituted cathinones act as central nervous system stimulants, via increasing synaptic concentration of catecholamines, such as dopamine, serotonin, and norepinephrine. These monoamines are released into the synapse where their concentrations are regulated, at least in part, by reuptake proteins (dopamine [DAT], serotonin [SERT], norepinephrine [NET] transporters).Citation26 In uptake and release assays performed in rat synaptosomes or in HEK293 cells that stably expressed human SERT, NET, and DAT, cathinone derivatives are able to strongly inhibit these uptake transporters, but may also stimulate release of the three monoamines from intracellular stores. Nevertheless, considerable differences have been found in the pharmacology of the different substituted cathinones in vitro. Mephedrone, methylone, ethylone, butylone, and 4-fluoromethcathinone (FMC) are relatively non-selective transporter substrates that are fully efficacious in the release assays.Citation27,Citation28 Ethylone acts as non-selective DAT (half maximal inhibitory concentration [IC50] =2.5 μM), SERT (4.5 μM), and NET (2.5 μM) inhibitors similar to cocaine. Methylone inhibits the activities of DAT (4.8 μM), SERT (15.5 μM), and NET (0.5 μM) but not γ-αminobutyric acid (GABA) transporter-1.Citation29,Citation30 Eshleman et al found that 4-FMC was an efficient releaser of dopamine, effective from 10 nM, as potent as mephedrone, but more potent than methylone.Citation28 These substituted cathinones are also serotonin releasers. Comparing the substituted methcathinone efficacies to those of methamphetamine on SERT, mephedrone, and methylone had similar potencies to those of methamphetamine, while butylone and 4-FMC were less efficacious. With respect to NET, methamphetamine, 4-FMC, mephedrone, and methylone elicited a lower release of norepinephrine.Citation28
In contrast, pyridinyl cathinones produce no dopamine or serotonin efflux but act as specific potent blockers of DAT and NET.Citation27,Citation29,Citation30 Pyrovalerone and MDPV exhibited very high affinity for the DAT in the low nanomolar range (30 nM±5 nM and 10 nM±2 nM, respectively) and NET (60 nM±5 nM and 80 nM±2 nM, respectively) and poor affinity for SERT in the micromolar range (4.97±0.03 μM and 2.86±0.1 μM respectively).Citation29 In 2013, Eshleman et al determined that naphyrone had high affinities for DAT (11 nM), SERT (15 nM), and NET (59 nM), high potency for uptake inhibition, and no drug-induced substrate release.Citation28 Furthermore, pyrovalerone and MDPV were the most potent DAT inhibitors (IC50 =35 nM and 31 nM, respectively) and NET (IC50 =43 nM and 44 nM, respectively) among all of the drugs studied by Simmler et al,Citation29 including other cathinones, amphetamines, and cocaine. In the short term, whereas some cathinones, such as mephedrone, behave as dopamine-releasing agents (depolarizing current) similar to methamphetamine and may be categorized in methamphetamine-like releasers, some others, such as MDPV, act as dopamine-reuptake inhibitors (hyperpolarizing current) similar to cocaine (cocaine-like monoamine reuptake inhibitors).Citation31,Citation32 Additionally, substituted cathinones have no affinity for dopamine receptors and act as low potency serotonin (5HT)1A receptor partial agonists or 5HT2A/5HT2C antagonists.Citation28
In vivo
After acute administration, cathinone derivatives share actions on central dopamine systems involved in the regulation of behavioral reinforcement, motor coordination, and thermoregulation.Citation33 In 2012, Marusich et al concluded that in vivo all cathinones caused increased locomotor activity, with different cathinones showing different potencies, magnitudes of stimulation, and duration of action.Citation32 MDPV, mephedrone, and 3-FMC produced the most pronounced initial stimulation similar to cocaine or methamphetamine, whereas methedrone produced the weakest initial stimulation. Oral administration of methylone (15 mg/kg and 30 mg/kg) induced a dose-dependent increase in locomotor activity in rats.Citation34 Cathinone 1 mg/kg or 4 mg/kg, mephedrone 10 mg/kg, and 3,4-methylenedioxy-N-methylamphetamine MDMA 10 mg/kg caused similar hyperactivity in rats with evidence of locomotor sensitization following intermittent dosing with cathinone and mephedrone.Citation35 MDPV and naphyrone produced locomotor stimulant effects that lasted much longer than those of cocaine or methamphetamine.Citation36 Moreover, stimulation of motor activity following administration of a wide range of MDPV doses (1 mg/kg to 30 mg/kg) was affected by ambient temperature; ie, was potentiated in warm ambient temperature.Citation37 By contrast, MDPV was interpreted to be a less potent disruptor of thermoregulation compared to methamphetamine.Citation38 Consistent with locomotor activation, substituted cathinones may produce hyperactivity, ataxia, convulsions, and stereotyped movements.Citation32 They may also inhibit food intake in a less potent way than amphetamine.Citation39
After a binge-like regimen of mephedrone and methylone, long-term changes in either neurochemistry or cognitive function have been observed in rodents with some variances depending on the product used. Mephedrone reduced working memory performance but did not affect neurotransmitter levels while methylone had little effect on behavior but produced a widespread depletion of serotonin levels in rats. Nevertheless, some discrepancies have been noticed among species since methylone did not cause significant change in serotonin level in mice. Accordingly, animal models have to be considered with caution when screening these psychoactive drugs of abuse.Citation33
All cathinone derivatives are chiral and can exist in two stereoisomer forms that may differ in their pharmacological potencies. For instance, the S-isomer of methcathinone exhibits stronger central nervous system stimulating effects than its R-enantiomer.Citation40
In animal models of addiction, cathinone demonstrated reinforcing potential. In rats trained to discriminate amphetamine from saline, cathinone and methcathinone resulted in stimulus generalization with methcathinone being the most potent;Citation40 rhesus monkeys that were previously trained to self-administrate cocaine, did not discriminate between cathinone and cocaine;Citation41 in a taste aversion test procedure used by Goudie in 1987, cathinone was less potent than amphetamine;Citation42 and cathinone was equipotent to d-amphetamine in rats trained to discriminate the stimulus induced by the administered drug in a two-lever, food-motivated operant task.Citation43 In rats, MDPV, methylone, mephedrone, naphyrone, flephedrone, and butylone fully substituted for the discriminative stimulus effects of cocaine and methamphetamine.Citation36 In rats trained to self-administer MDPV or methamphetamine, dose-substitution studies demonstrated that behavior was sensitive to dose for both drugs, but MDPV showed greater potency and efficacy than methamphetamine.Citation38 In mice trained to discriminate 0.3 mg/kg MDPV from saline, cumulative doses of MDPV, MDMA and methamphetamine fully substituted for the MDPV training stimulus. In a study evaluating the abuse potential of MDPV by assessing its ability to support intravenous self-administration (0.05 mg/kg to 0.2 mg/kg) and to lower thresholds for intracranial self-stimulation, MDPV had reinforcing properties.Citation44 These rodent studies suggest that, like amphetamines, cathinone derivatives may be able to induce tolerance and dependence.
Human use of substituted cathinones
Modalities of administration
Substituted cathinones are most commonly nasally insufflated or orally ingested. Rectal administration, intramuscular or intravenous injection, and smoking or inhalation, have also been reported. Among 362 patients admitted to Texas poison centers during 2010–2011, the route of exposure was 47.8% by inhalation alone and 28.7% by ingestion alone.Citation45 The typical dose range varies between the different cathinone derivatives. According to ErowidCitation46 or Drugs-ForumCitation47 websites, where users are invited to begin with a small dose then to gradually increase the dose, a typical first time oral dose of mephedrone or methylone ranges between 50 mg and 100 mg; a dose of 150–250 mg seems to be the usual dose in experienced users. Sumnall and WoodingCitation48 reported that a light effect is obtained with an oral 25–100 mg, while a strong effect requires a higher dose of 125–250 mg. Insufflating doses are lower, ranging between 15–25 mg for a light effect to 75–125 mg for a strong effect. In addition, 80–120 mg of butylone and 20–100 mg of buphedrone are described as common oral doses. By contrast, users reported lower oral doses of MDPV or naphyrone between 5–30 mg and 10–50 mg, respectively.
Recreational effects
In humans, substituted cathinones have stimulant and entactogenic properties. Desired or “pleasant” effects most often described by users of synthetic cathinones include euphoria, intensification of sensory senses, increased sociability, increased energy, mental stimulation, empathy connection, openness, increased sensory perception, decreased inhibition, and sexual arousal.Citation49
Acute and chronic physical and psychological adverse/toxic effects
For the 51 adolescent exposures reported to Texas poison centers, the medical outcome was known or suspected to be serious in 74.5%.Citation45 Physical signs of synthetic cathinone intoxication are characteristically associated with a sympathomimetic toxidrome with the most common feature being agitation in more than 50% of users. Other common features include tachycardia, heart palpitation, arrhythmia, increased blood pressure, capillary dilatation, and hemorrhage. Patients can also display headache, pupil dilatation, nausea/vomiting, suppressed appetite, bruxism, hyperthermia, dehydration, hot flushes, sweating, blue/cold extremities, and seizures.Citation50 In 15 patients admitted to an emergency department following mephedrone use, the pattern of clinical toxicity included agitation in 53%, tachycardia in 40%, systolic hypertension in 20%, and seizures in 20%.Citation51 In overdose patients, physical manifestations range from severe hyperthermia, rhabdomyolysis, hyponatremia, acidosis,Citation52 and seizures to those as severe as stroke, cerebral edema, cardiorespiratory collapse, myocardial infarction, multiple organ failure, and death. A case of severe neurologic toxicity and hyponatremia, followed by rhabdomyolysis, and consistent serotonin toxicity related to ethcathinone and methylone poisoning was reported in 2012.Citation53 Several cases of myocardial infarction and mephedrone related myocarditis have been reported.Citation54,Citation55 Levine et al reported three cases of acute compartment syndromes (decreased tissue perfusion) attributed to synthetic cathinones (PVP, MDPV) with complete recovery in two patients; one patient remained in renal failure and continued to receive hemodialysis 5 months later.Citation56 In a fatal case related to the consumption of methylone, steatosis of the heart muscle, congenital heart disease, and bronchial asthma could have been the predisposing factors for sudden cardiac death that occurred in the presence of methylone.Citation57 Among 62 mephedrone young victims collected in the UK National Programme on Substance Abuse Deaths database, mephedrone alone was identified at postmortem on eight occasions.Citation58
Behavioral effects include insomnia, confusion, agitation, panic attack, anxiety, severe paranoia, hallucinations, psychosis, suicidal ideation, self-mutilation, and aggressive, violent, and self-destructive behavior.Citation59 White matter abnormalities with greatest severity of damage underlying executive motor areas have been observed in patients with a distinctive extrapyramidal syndrome due to intravenous ephedrone abuse.Citation60 Moreover, ephedrone is synthesized from ephedrine or pseudoephedrine by potassium permanganate oxidation and there is a risk for manganese-induced extrapyramidal system damage including rigidity, hypokinesia, gait disorders, and hallucinations.Citation61 In addition, the binge use of cathinone drugs may result in the development of the syndrome of excited delirium, including extreme agitation, violent behavior, confused speech, paranoia, and hallucinations.Citation62
As a remark, nasal pain or bleeding is an associated local effect related to the irritant properties of mephedrone administered by the nasal route.Citation50
Addictive potential
The potency of abused drugs to activate the brain reward circuitry (dopamine system) increases the risk of potential for abuse and addiction in humans. In contrast, a relative activation of the serotonin system would be linked to a reduction in abuse potential.Citation29,Citation63–Citation65 Thus, DAT/SERT inhibition ratio and dopamine/serotonin release potency have been proposed to predict the psychostimulant effect in humans. Mephedrone induced strong feelings of craving in most usersCitation66 and multiple cases of mephedrone dependence have been described in the medical literature.Citation67 In a Scottish survey carried out prior to the classification of mephedrone, 4.4% of mephedrone users reported a daily use and 17.5% reported “addiction/dependence” symptoms.Citation50 Likewise, Prosser and Nelson stated strong cravings to repeat or increase doses after taking mephedrone.Citation49 As a result of a paucity of data, long-term effects of substituted cathinone use including the potential for addiction/dependence remain largely unknown.
Pharmacokinetics
Mephedrone, methylone, ethylone, MDPV, and naphyrone showed high blood–brain barrier permeability in an in vitro model with mephedrone and MDPV exhibiting particularly high permeability.Citation29 Pyrrolidine ring and tertiary amino groups improve liphophilicity.Citation68 In rats, the plasma concentrations versus time curve after intravenous administration of 10 mg/kg methylone were described by a two-compartment model with distribution and terminal phases of α=1.95 hour 1 and β=0.72 hour 1. The steady state apparent volume of distribution and total plasma clearance was 2.39 L/kg and 0.53 L/hour, respectively. Maximal concentration values were achieved within 0.5 hour and 1 hour. Absolute bioavailability was about 80% after oral administration.Citation34 In a sympathomimetic toxicity case report, naphyrone half-live was estimated at around 34 hours and was consistent with the known long-lasting effect of naphyrone.Citation69 Insufflation provides a quicker onset of action and shorter duration of effect compared to ingestion, with the total experience lasting a few hours. The central action of buphedrone, which is stronger than methylcathinone, persists after nasal application for about 6 hours.Citation70
Distribution
In postmortem cases analyzed for methylone, mephedrone, and MDPV, analysis of several tissue samples (liver, kidney, and spleen) show that methylone does not sequester in a particular tissue type after death.Citation71 Following lethal intoxication, MDPV was fairly uniformly distributed among tissues at a value of approximately 0.4 to 0.6 mg/L (blood, brain, muscle, spleen, lung, and kidneys). Levels in different brain regions reflected a higher concentration in parietal, cerebellum, medulla, and occipital regions than in the frontal and lentiform nucleus.Citation10
Metabolism
The designer drug PPP (α-pyrrolidinopropiophenone) is extensively metabolized by rats. The main pathways were hydroxylation of the pyrrolidine ring with subsequent dehydrogenation to the corresponding lactam, hydroxylation of the aromatic ring in position 4′ or double dealkylation of the pyrrolidine ring to the corresponding primary amine partly followed by reduction of the keto group to the corresponding secondary alcohol.Citation72,Citation73 All other pyrrolidinophenone-derived designer drugs were mainly metabolized at their aromatic substituents, by oxidation of the methyl group (MPPP, MPHP [methyl-α-pyrrolidinohexanophe none]), by O-demethylation of the methoxy moiety (MOPPP), or by demethylation of the methylenedioxy moiety (MDPPP). Hydroxylation of the side chain could be observed for the derivative with long side chain (MPHP). Metabolic phase 2 pathways were methylation, glucuronidation, or sulfation.Citation74 Initial hydroxylation of the 4′-methyl moiety of MPPP and MPHP, O-demethylation of MOPPP, and demethylenation of MDPPP were catalyzed by cytochrome P450 (CYP) 2D6 (major) and CYP2C19; CYP1A2, CYP2B6, and CYP2C9 were additionally involved in MPPP and MPHP hydroxylation to a minor extent.Citation74 Furthermore, MPPP is a substrate of CYP2D6 and may represent a kinetic drug interaction risk.Citation75
Concentrations in biological samples
Concentrations of substituted cathinone in blood reported after recreational use appear to be below 0.1 mg/L. By contrast, in cases of acute toxicity, blood levels often exceed this value. Meanwhile, blood concentration does not appear to accurately predict outcome regarding fatalities.
In a study that surveyed mephedrone concentrations in impaired driving cases (n=32), blood concentrations ranged up to 0.74 mg/L (mean: 0.21 mg/L); in cases in which mephedrone was the only drug detected (n=9) concentrations ranged up to 0.66 mg/L (mean: 0.27 mg/L).Citation76 In a case of recreational use of naphyrone that produced acute sympathomimetic toxicity, naphyrone concentrations were 0.03 mg/L and 0.02 mg/L, 40 hours and 60 hours after drug intake, respectively.Citation69 In a patient with signs of toxic liver damage and rhabdomyolysis followed by renal insufficiency repeatedly treated by hemodialysis, MPHP was found in serum in a concentration of approximately 0.1 mg/L.Citation77 In a 23-year old man with a prior psychiatric history arriving via emergency medical service for bizarre behavior, suicidality, and hallucinations, serum and urine were found to contain MDPV at concentrations of 0.186 mg/L and 0.136 mg/L, respectively, and flephedrone at concentrations of 0.346 mg/L and 0.257 mg/L, respectively.Citation78 The concentrations measured in several fatal cases in which death was attributed directly to cathinone toxicity alone are outlined in . In a series of 13 positive hair cases for mephedrone, concentrations ranged from 0.2 ng/mg to 313.2 ng/mg.Citation79 Postmortem MDPV was detected in hair at 11.7 μg/gCitation10 or 22 ng/10 mm.Citation80
Table 7 Blood concentrations measured in several fatal cases
Clinical management of acute and chronic adverse effects and addiction
There are limited reliable data to guide clinicians in managing patients with toxicity due to substituted cathinones. Management of users with acute toxic effects is mainly extrapolated from experience with longer established stimulants or hallucinogenic drugs.
Diagnostic tests
Routine laboratory tests in the work-up of a potentially synthetic cathinone toxic patient include: 1) a basic metabolic panel with blood glucose levels, serum electrolyte concentrations (sodium and potassium), and serum osmolarity if hyponatremia is present, liver and kidney function tests (transaminases, alanine transaminase/aspartate transaminase [ALT/AST], gamma-glutamyl transpeptidase [GGT], alkaline phosphatase, total and unbound bilirubin, creatinine, and urea); 2) a complete blood cell count with coagulation studies (activated partial thromboplastin time, prothrombin time, platelet count, and p-dimer); 3) cardiac markers (troponin and creatine kinase MB); and 4) total creatinine kinase to exclude potential rhabdomyolysis and myoglobin in urine if there are signs of rhabdomyolysis.Citation81 The typically available urine toxicology screens will not detect the vast majority of synthetic cathinones (see section on Current and developing analytical methods for detection), but could be useful to detect other possibly ingested substances. More specific and sensitive chromatographic methods associated with MS could successfully identify substituted cathinone. Electrocardiograms might also be useful especially if the patient is profoundly tachycardic and electroencephalogram might be indicated if seizure is suspected or observed.
Clinical exam
In addition to the aforementioned laboratory tests, detailed physical and neurological exams are imperative. Common physical signs of use and intoxication include: tachycardia, hypertension, hyperthermia, diaphoresis, mydriasis, hyperreflexia, myoclonus, seizures, tremors, irritability, anxiety, and paranoia. A proper history taking of the patient is also required, but is sometimes difficult to obtain as patients frequently present highly intoxicated and are uncooperative due to various psychiatric symptoms and agitation. Moreover, the level of agitation frequently requires physical restraints and safety protocol/monitoring implementation.
The combination of various bath-salt compounds present in available products, and other illegal substances or alcohol involved, can lead to significant differences in presentation, with signs of catecholamine and serotonergic toxicities ranging from mild neurological and neurovegetative symptoms to more severe symptoms. Concurrent use of serotonergic drugs may also increase the risk of serotonergic syndrome leading to multiple system failures, most notably cardiac, renal, and neurologic.
Treatment
Appropriate supportive care and addressing any complications is the primary treatment for the acutely intoxicated patient. No specific antidote exists for synthetic cathinone exposure and no curative treatment is approved by health authorities. Hydration with intravenous fluids should be initiated along with measures to actively cool patients if they are hyperthermic. Hypertonic saline or water restriction should be prescribed if the patient becomes hyponatremic.Citation82 Agitation and seizures associated with acute toxidrome should be managed with benzodiazepines as a first step. Antipsychotics (intramuscular haloperidol or oral risperidone) could be an effective therapeutic alternative to treat excitement, violent behavior, or psychosis.Citation83 However, haloperidol may contribute to hyperthermia and dysrhythmias.Citation84 As a general rule, the use of dopamine blocking (antipsychotic) agents that may potentially exacerbate thermoregulatory disturbances should be carefully managed in cathinone-intoxicated patients. A case of treating cathinone dependence and comorbid depression using bupropion was reported in 2012, but research regarding the potential effectiveness of bupropion in these cases is needed.Citation85 Electroconvulsive therapy improved persistent psychotic symptoms observed in a patient who discontinues repeated use of MDPV.Citation86 In 15 mephedrone users admitted to a London emergency department, 20% required treatment with benzodiazepines predominantly for management of agitation.Citation87 The clinical experience of two regional poison centers in the US reported the use of benzodiazepines (n=125), antipsychotics (n=47), and propofol (n=10) to treat severe medical outcomes (one death, eight major, and 130 moderate) in 236 synthetic cathinone users.Citation88
After recovery, substituted cathinone abusers should be referred for psychiatric consultation. Spiller et al reported the primary disposition of these patients: 49% were treated and released from the emergency department, 21% were admitted to critical care, 12% were admitted to psychiatry, and 12% were lost to follow up.Citation88 Unfortunately, to date, no pharmacological therapy has been approved by the US Food and Drug Administration (FDA) to treat psychostimulant addiction.Citation89
Legal status
Cathinone derivatives have different legal status around the world. Cathinone and methcathinone are listed in Schedule I of the United Nations 1971 Convention on Psychotropic Substances. Amfepramone and pyrovalerone are in Schedule IV of that Convention, but a large number of synthetic cathinone derivatives are not under international control. Recently, the World Health Organization (WHO) expert committee did not consider that the abuse liability of methylbenzodioxolylbutanamine (MBDB) would constitute a significant risk to public health that could necessitate its placement under international control.
In the US, pyrovalerone is a Schedule V substance.Citation90 In 2011, the Department of Justice issued a final order in the Federal Register, temporarily placing the three synthetic stimulants, mephedrone, MDPV, and methylone, under Schedule I of the Controlled Substances Act. On July 2012, MDPV and mephedrone were permanently banned. Recently, the Synthetic Cathinones Control Act of 2013 planned to provide for the specified placement of methylone and 14 other substituted cathinones on Schedule I of the Controlled Substances Act.Citation91
The European Council adopted a decision on submitting mephedrone to control measures across the European Union in 2010.Citation92 A few other cathinone derivatives, such as methylone, butylone, MDPV, and flephedrone, are controlled in some Member States of the EU under drug control or equivalent legislation. In addition, generic control in the UK under the UK’s Misuse of Drugs Act in April 2010 and France in August 2012 covers a wide group of cathinone derivatives.
Availability and usage demographics
The misuse of synthetic cathinones is not new. Methcathinone, originally used as an antidepressant in the former Soviet Union in the 1930s, went on to be used recreationally during the 1970s and 1980s. The emergence of the popularity of methcathinone in countries such as the US rang some alarm bells in the 1990s. Although mephedrone was first synthesized in 1929, it really became an attractive cocaine and MDMA replacement during the early 2000s.Citation93 The first seizure of substituted cathinone (capsules containing mephedrone) was reported in 2007 in Finland. In 2008 mephedrone was also detected in the UK and other scandinavian countries. By the end of 2010 it had been detected in 31 European and neighboring countries, suggesting widespread availability throughout Europe.Citation92 In the UK, mephedrone first appeared widespread among clubbers before enjoying popularity among young adults and teenagers.Citation67
Nowadays, substituted cathinones are largely available online and in retail stores for recreational public or private use. Cathinones are mostly encountered as a white, off-white, or slightly yellow-colored fine powder, as racemic mixtures and hydrochloride salts, and mainly sold in small packages of 200 mg to 500 mg, sometimes in the form of capsule or tablet. The synthesis of these cathinone derivatives is relatively easy and Internet sites are also used for the purchase of the starting materials, the most common being the appropriate propiophenone or alpha-bromopropiophenone.Citation5
Prior to being banned under the UK’s Misuse of Drugs Act in April 2010, mephedrone, which is reported as easy to obtain, was widely used among school and college/university students: 205 of 1,006 surveyed individuals had used mephedrone on at least one occasion.Citation50 Interviewed mephedrone users tended to obtain mephedrone mainly through friends/acquaintances, dealers, and less commonly from Internet-based head shops to avoid “drug user” identities. Following the ban, they reported a greater reliance on dealers, a change in packaging, and a rise in prices.Citation94 By contrast, despite media attention focusing on synthetic cathinone use as a growing epidemic, the prevalence of use among a population of 2,349 students in a large university in the Southeastern US was extremely rare (1.07%).Citation95
Aside from this problematic misuse, it is important to remember that several cathinones, such as diethylpropion, bupropion, or pyrovalerone have been used medically as antidepressants and that bupropion is still currently used as a smoking cessation aid.
Conclusion
Substituted cathinones, initially considered as “legal highs”, have been used in the recreational drug markets not only for their own hedonic and euphoric effects but as a replacement for other tightly regulated stimulants, such as cocaine or amphetamine. Experimental and clinical reports clearly demonstrate the acute cardiovascular and central nervous system toxicity due to the intensive abuse and binge intake patterns of these substances, in combination with the high risk of death related to the powerful stimulation of the catecholaminergic system. Furthermore, the dopaminergic stimulation of the reward system could explain the development of dependence after frequent consumption of cathinone derivatives. In addition, these “highs” are often taken in conjunction with other recreational drugs or alcohol, which increases the potential for health-related complications. Despite their now illegal status in many countries, substituted cathinones continue to be prevalent drugs of abuse with MDPV and mephedrone being the chief substances detected in blood and urine from patients hospitalized for cathinone derivative overdose. Accordingly, clinicians should be aware of this developing trend as an explanation for patients presenting with unexplained delirious toxidromes or secondary psychoses. Routine toxicology screens may not detect the presence of these compounds and more specific methods may be required for identification and quantification in biological samples. This analytical step is crucial in clinics and forensics to clarify the extent to which peripheral and central monoamine systems might be affected. Indeed, concentrations of substituted cathinones are measured in a rather narrow window between use and fatality cases which may further differ from one compound to another. All information collected will be useful for professional continuing education of the clinician pertaining to new trends of substituted cathinone abuse. Meanwhile, substituted cathinones represent only part of synthetic derivatives available on the online market. An ever-increasing diversity of new products not under control are manufactured to supply an ever-increasing and diversified demand for psychoactive substances. In conclusion, it seems that: 1) emergency departments will continue to encounter patients suffering from complications caused by synthetic stimulants; 2) clinicians should remain vigilant of the risk of increasing morbidity and mortality associated with these products; and 3) as key health care practitioners, physicians and pharmacists must continue to feed databases of case reports and help promote awareness of this problem in their communities.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrandtSDFreemanSSumnallHRMeashamFColeJAnalysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinonesDrug Test Anal20113956957521960541
- OjanperäLAHeikmanPKRasanenIJUrine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependant patients by gas chromatography-mass spectrometryTher Drug Monitoring201133257263
- MacherAMPendersTMFalse-positive phencyclidine immunoassay results caused by 3,4-methylenedioxypyrovalerone (MDPV)Drug Test Anal20135213013222611039
- SwortwoodMJHearnWLDecaprioAPCross-reactivity of designer drugs, including cathinone derivatives, in commercial enzyme-linked immunosorbent assaysDrug Test Anal2013
- McDermottSDPowerJDKavanaghPO’BrienJThe analysis of substituted cathinones. Part 2: an investigation into the phenylacetone based isomers of 4-methylmethcathinone and N-ethylcathinoneForensic Sci Int20112121–3132121775082
- KavanaghPO’BrienJFoxJThe analysis of substituted cathinones. Part 3. Synthesis and characterisation of 2,3-methylenedioxy substituted cathinonesForensic Sci Int20122161–3192821907509
- ZubaDAdamowiczPByrskaBDetection of buphedrone in biological and non-biological material – two case reportsForensic Sci Int20132271–3152022981959
- ArcherRPFluoromethcathinone, a new substance of abuseForensic Sci Int20091851–3102019195800
- Tor ranceHCooperGThe detection of mephedrone (4-methylmethcathinone) in 4 fatalities in ScotlandForensic Sci Int20102021–3e62e6320685050
- WymanJFLavinsESEngelhartDPostmortem tissue distribution of MDPV following lethal intoxication by “bath salts”J Anal Toxicol201337318218523408250
- O’ByrnePMKavanaghPVMcNamaraSMStokesSMScreening of stimulants including designer drugs in urine using a liquid chromatography tandem mass spectrometry systemJ Anal Toxicol2013372647323316030
- BellCGeorgeCKicmanATTraynorADevelopment of a rapid LC-MS/MS method for direct urinalysis of designer drugsDrug Test Anal201137–849650421744513
- Strano-RossiSCadwalladerABde la TorreXBotrèFToxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometryRapid Commun Mass Spectrom201024182706271420814976
- MarinettiLJAntonidesHMAnalysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of resultsJ Anal Toxicol201337313514623361867
- AmbachLHernández RedondoAKönigSWeinmannWRapid and simple LC-MS/MS screening of 64 novel psychoactive substances using dried blood spotsDrug Test Anal Epub2013719
- LesiakADMusahRACodyRBDominMADaneAJShepardJRDirect analysis in real time mass spectrometry (DART-MS) of “bath salt” cathinone drug mixturesAnalyst2013138123424343223636110
- VircksKEMulliganCCRapid screening of synthetic cathinones as trace residues and in authentic seizures using a portable mass spectrometer equipped with desorption electrospray ionizationRapid Commun Mass Spectrom201226232665267223124656
- WangXVernikovskayaDIAbdelrahmanDRHankinsGDAhmedMSNanovskayaTNSimultaneous quantitative determination of bupropion and its three major metabolites in human umbilical cord plasma and placental tissue using high-performance liquid chromatography-tandem mass spectrometryJ Pharm Biomed Anal20127032032922682512
- SørensenLKDetermination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography-electrospray tandem mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci201187911–12727736
- ZubaDIdentification of cathinones and other active components of “legal highs” by mass spectrometric methodsTrends Anal Chem20123215
- FornalEIdentification of substituted cathinones: 3,4-Methylenedioxy derivatives by high performance liquid chromatography-quadrupole time of flight mass spectrometryJ Pharm Biomed Anal201381821319
- SundströmMPelanderAAngererVHutterMKneiselSOjanperäIA high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urineAnal Bioanal Chem2013405268463847423954996
- GottardoRSorioDMusileGScreening for synthetic cannabinoids in hair by using LC-QTOF MS: A new and powerful approach to study the penetration of these new psychoactive substances in the populationMed Sci Law2014541222723842479
- AmmannDMcLarenJMGerostamoulosDBeyerJDetection and quantification of new designer drugs in human blood: Part 2 – Designer cathinonesJ Anal Toxicol201236638138922593565
- MayerMBenkoAHuszárASimultaneous determination of 4-substituted cathinones (4-MMC, 4-MEC and 4-FMC) in human urine by HPLC-DADJ Chromatogr Sci201351986186623192736
- MeltzerPCButlerDDeschampsJRMadrasBK1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitorsJ Med Chem20064941420143216480278
- BaumannMHPartillaJSLehnerKRPsychoactive “bath salts”: not so soothingEur J Pharmacol20136981–31523178799
- EshlemanAJWolfrumKMHatfieldMGJohnsonRAMurphyKVJanowskyASubstituted methcathinones differ in transporter and receptor interactionsBiochem Pharmacol201385121803181523583454
- SimmlerLDBuserTADonzelliMPharmacological characterization of designer cathinones in vitroBr J Pharmacol2013168245847022897747
- SogawaCSogawaNOhyamaKMethylone and monoamine transporters: correlation with toxicityCurr Neuropharmacol201191586221886563
- CameronKKolanosRVekariyaRVerkariyaRDe FeliceLGlennonRAMephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporterPsychopharmacology (Berl)2013227349349923371489
- MarusichJAGrantKRBloughBEWileyJLEffects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in miceNeurotoxicology20123351305131322922498
- den HollanderBRozovSLindenAMUusi-OukariMOjanperäIKorpiERLong-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedronePharmacol Biochem Behav2013103350150923099177
- López-ArnauRMartínez-ClementeJCarbóMlPubillDEscubedoECamarasaJAn integrated pharmacokinetic and pharmacodynamic study of a new drug of abuse, methylone, a synthetic cathinone sold as “bath salts”Prog Neuropsychopharmacol Biol Psychiatry201345647223603357
- ShortallSEMacerolaAESwabyRTBehavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the ratEur Neuropsychopharmacol20132391085109523051939
- GatchMBTaylorCMForsterMJLocomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinonesBehav Pharmacol2013245–643744723839026
- FantegrossiWEGannonBMZimmermanSMRiceKCIn vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activityNeuropsychopharmacology201338456357323212455
- AardeSMHuangPKCreehanKMDickersonTJTaffeMAThe novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in ratsNeuropharmacology20137113014023597511
- ZelgerJLSchornoHXCarliniEABehavioural effects of cathinone, an amine obtained from Catha edulis Forsk.: comparisons with amphetamine, norpseudoephedrine, apomorphine and nomifensineBull Narc198032367816911034
- GlennonRAYousifMNaimanNKalixPMethcathinone: a new and potent amphetamine-like agentPharmacol Biochem Behav19872635475513575369
- YanagitaTIntravenous self-administration of (-)-cathinone and 2-amino-1-(2,5-dimethoxy-4-methyl)phenylpropane in rhesus monkeysDrug Alcohol Depend1986172–31351413743404
- GoudieAJImporting khat, legal but dangerousLancet198728571134013412890946
- SchechterMDRosecransJAGlennonRAComparison of behavioral effects of cathinone, amphetamine and apomorphinePharmacol Biochem Behav19842021811846718445
- WattersonLRKufahlPRNemirovskyNEPotent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV)Addict Biol2012
- ForresterMBSynthetic cathinone exposures reported to Texas poison centersAm J Drug Alcohol Abuse201238660961522541001
- EROWID [homepage on the Internet] Available from: http://www.erowid.orgAccessed on: January 5, 2014
- Drugs-Forum [homepage on the Internet]SIN Foundation2012 Available from: http://www.drugs-forum.comAccessed on January 5, 2014
- SumnallHWoodingOMephedrone: an update on current knowledgeLiverpoolCentre for public health, Liverpool John Moores University2010
- ProsserJMNelsonLSThe toxicology of bath salts: a review of synthetic cathinonesJ Med Toxicol201281334222108839
- DarganPIAlbertSWoodDMMephedrone use and associated adverse effects in school and college/university students before the UK legislation changeQJM20101031187587920675396
- WoodDMDarganPIMephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicityProg Neuropsychopharmacol Biol Psychiatry201239222723322564711
- SammlerEMFoleyPLLauderGDWilsonSJGoudieARO’RiordanJIA harmless high?Lancet2010376974274220801405
- Boulanger-GobeilCSt-OngeMLalibertéMAugerPLSeizures and hyponatremia related to ethcathinone and methylone poisoningJ Med Toxicol201281596121755421
- NicholsonPJQuinnMJDoddJDHeadshop heartache: acute mephedrone ‘meow’ myocarditisHeart201096242051205221062771
- JamesDAdamsRDSpearsRNational Poisons Information ServiceClinical characteristics of mephedrone toxicity reported to the UK National Poisons Information ServiceEmerg Med J201128868668920798084
- LevineMLevitanRSkolnikACompartment syndrome after “bath salts” use: a case seriesAnn Emerg Med201361448048323318022
- KovácsKTóthARKeresztyEM[A new designer drug: methylone related death]Orv Hetil20121537271276 Hungarian22318528
- SchifanoFCorkeryJGhodseAHSuspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, “meow meow”) in the United KingdomJ Clin Psychopharmacol201232571071422926609
- RossEAReisfieldGMWatsonMCChronisterCWGoldbergerBAPsychoactive “bath salts” intoxication with methylenedioxypyrovaleroneAm J Med2012125985485822682791
- StepensAStaggCJPlatkajisABoudriasMHJohansen-BergHDonaghyMWhite matter abnormalities in methcathinone abusers with an extrapyramidal syndromeBrain2010133Pt 123676368421036949
- SikkKHaldreSAquiloniusSMManganese-induced parkinsonism in methcathinone abusers: bio-markers of exposure and follow-upEur J Neurol201320691592023347399
- PendersTMLangMCPaganoJJGoodingZSElectroconvulsive therapy improves persistent psychosis after repeated use of methylenedioxypyrovalerone (“bath salts”)J ECT2013294e59e6023609518
- RothmanRBBaumannMHDerschCMAmphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotoninSynapse2001391324111071707
- WeeSAndersonKGBaumannMHRothmanRBBloughBEWoolvertonWLRelationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogsJ Pharmacol Exp Ther2005313284885415677348
- BaumannMHClarkRDWoolvertonWLWeeSBloughBERothmanRBIn vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the ratJ Pharmacol Exp Ther2011337121822521228061
- BruntTMPoortmanANiesinkRJvan den BrinkWInstability of the ecstasy market and a new kid on the block: mephedroneJ Psychopharmacol201125111543154720826554
- JohnsonLAJohnsonRLPortierRBCurrent “legal highs”J Emerg Med20134461108111523528960
- CoppolaMMondolaR3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed onlineToxicol Lett20122081121522008731
- DerungsASchietzelSMeyerMRMaurerHHKrähenbühlSLiechtiMESympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone)Clin Toxicol (Phila)201149769169321740148
- BilińskiPHołowniaPKapka-SkrzypczakLWojtyłaADesigner Drug (DD) abuse in Poland; a review of the psychoactive and toxic properties of substances found from seizures of illegal drug products and the legal consequences thereof. Part II--piperazines/piperidines, phenylethylamines, tryptamines and miscellaneous ‘others’Ann Agric Environ Med201219487188223311821
- CawrseBMLevineBJuferRADistribution of methylone in four postmortem casesJ Anal Toxicol201236643443922582221
- SpringerDFritschiGMaurerHHMetabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4′-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci20037962253266
- StaackRFMaurerHHMetabolism of designer drugs of abuseCurr Drug Metab20056325927415975043
- MaurerHHKraemerTSpringerDStaackRFChemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsisTher Drug Monit200426212713115228152
- PritzkerDKanungoAKilicarslanTTyndaleRFSellersEMDesigner drugs that are potent inhibitors of CYP2D6J Clin Psychopharmacol200222333033212006905
- CosbeySHPetersKLQuinnABentleyAMephedrone (methylmethcathinone) in toxicology casework: a Northern Ireland perspectiveJ Anal Toxicol2013372748223354334
- SauerCHoffmannKSchimmelUPetersFTAcute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP)Forensic Sci Int20112081–3e20e2521444164
- ThorntonSLGeronaRRTomaszewskiCAPsychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantificationJ Med Toxicol20128331031322528592
- MartinMMullerJFTurnerKDuezMCirimeleVEvidence of mephedrone chronic abuse through hair analysis using GC/MSForensic Sci Int20122181–3444822041623
- NameraAUrabeSSaitoTA fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of α-pyrrolidinobutiophenone and α- pyrrolidinovalerophenone on blood and/or hairForensic Toxicol2013312338343
- Mas-MoreyPVisserMHWinkelmolenLTouwDJClinical toxicology and management of intoxications with synthetic cathinones (“bath salts”)J Pharm Pract201326435335723178412
- ProsserJMNelsonLSThe toxicology of bath salts: a review of synthetic cathinonesJ Med Toxicol201281334222108839
- KasickDPMcKnightCAKlisovicE“Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone useAm J Drug Alcohol Abuse201238217618022221190
- BlomMTBardaiAvan MunsterBCDifferential changes in QTc duration during in-hospital haloperidol usePLoS One201169e2372821961030
- Lev-RanSA case of treating cathinone dependence and comorbid depression using bupropionJ Psychoactive Drugs201244543443623457895
- PendersTMLangMCPaganoJJGoodingZSElectroconvulsive therapy improves persistent psychosis after repeated use of methylenedioxypyrovalerone (“bath salts”)J ECT2013294e59e6023609518
- WoodDMGreeneSLDarganPIClinical pattern of toxicity associated with the novel synthetic cathinone mephedroneEmerg Med J201128428028220581379
- SpillerHARyanMLWestonRGJansenJClinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United StatesClin Toxicol (Phila)201149649950521824061
- TaylorSBLewisCROliveMFThe neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humansSubst Abuse Rehabil20134294324648786
- JohnsonLAJohnsonRLPortierRBCurrent “legal highs”J Emerg Med20134461108111523528960
- GermanCLFleckensteinAEHansonGRBath salts and synthetic cathinones: An emerging designer drug phenomenonLife Sci2013In press
- European Monitoring Centre for Drugs and Drug Addiction [webpage on the Internet]EMCDDA: Drug-related topics – Drug profilesLisbonEMCDDA2013 Available from: http://www.emcdda.europa.eu/publications/drug-profilesAccessed January 5, 2014
- BruntTMPoortmanANiesinkRJvan den BrinkWInstability of the ecstasy market and a new kid on the block: mephedroneJ Psychopharmacol201125111543154720826554
- McElrathKO’NeillCExperiences with mephedrone pre- and post-legislative controls: perceptions of safety and sources of supplyInt J Drug Policy201122212012721242082
- StognerJMMillerBLInvestigating the ‘bath salt’ panic: the rarity of synthetic cathinone use among students in the United StatesDrug Alcohol Rev201332554554923718639
- ZaitsuKKatagiMKamataHTMikiATsuchihashiHDiscrimination and identification of regioisomeric β-keto analogues of 3,4-methylenedioxyamphetamines by gas chromatography-mass spectrometryForensic Toxicol2008264551
- WestphalFJungeTRing positional differentiation of isomeric N-alkylated fluorocathinones by gas chromatography/tandem mass spectrometryForensic Sci Int20122231–39710522940190
- AbiedallaYFAbdel-HayKDeRuiterJClarkCRSynthesis and GC-MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV)Forensic Sci Int20122231–318919722995045
- SpringerDFritschiGMaurerHHMetabolism and toxicological detection of the new designer drug 4′-methoxy-alpha-pyrrolidinopropiophenone studied in rat urine using gas chromatography-mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci20037932331342
- GilDAdamowiczPSkulskaATokarczykBStanaszekRAnalysis of 4-MEC in biological and non-biological material – three case reportsForensic Sci Int20132281–3e11e1523562144
- WikströmMThelanderGNyströmIKronstrandRTwo fatal intoxications with the new designer drug methedrone (4-methoxymethcathinone)J Anal Toxicol201034959459821073814
- WestphalFJungeTRösnerPFritschiGKleinBGirreserUMass spectral and NMR spectral data of two new designer drugs with an alpha-aminophenone structure: 4′-methyl-alpha-pyrrolidinohexanophenone and 4′-methyl-alpha-pyrrolidinobutyrophenoneForensic Sci Int20071691324216962275
- BrandtSDWoottonRCDe PaoliGFreemanSThe naphyrone story: The alpha or beta-naphthyl isomer?Drug Test Anal201021049650220886463
- WestphalFJungeTGirreserUGreiblWDoeringCMass, NMR and IR spectroscopic characterization of pentedrone and pentylone and identification of their isocathinone by-productsForensic Sci Int20122171–315716722115724
- RojekSKłysMStronaMMaciówMKulaK“Legal highs” – toxicity in the clinical and medico-legal aspect as exemplified by suicide with bk-MBDB administrationForensic Sci Int20122221–3e1e622648055
- KeshaKBoggsCLRippleMGMethylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literatureJ Forensic Sci20135861654165923822613
- MaskellPDDe PaoliGSeneviratneCPounderDJMephedrone (4-methylmethcathinone)-related deathsJ Anal Toxicol201135318819121439157
- PearsonJMHargravesTLHairLSThree fatal intoxications due to methyloneJ Anal Toxicol201236644445122589523
- CarbonePNCarboneDLCarstairsSDLuziSASudden cardiac death associated with methylone useAm J Forensic Med Pathol2013341262823403480