Abstract
Schizophrenia is a complex neuropsychiatric disease with documented clinical and genetic heterogeneity, and evidence for neurodevelopmental origins. Driven by new genetic technologies and advances in molecular medicine, there has recently been concrete progress in understanding some of the specific genetic causes of this serious psychiatric illness. In particular, several large rare structural variants have been convincingly associated with schizophrenia, in targeted studies over two decades with respect to 22q11.2 microdeletions, and more recently in large-scale, genome-wide case-control studies. These advances promise to help many families afflicted with this disease. In this review, we critically appraise recent developments in the field of schizophrenia genetics through the lens of immediate clinical applicability. Much work remains in translating the recent surge of genetic research discoveries into the clinic. The epidemiology and basic genetic parameters (such as penetrance and expression) of most genomic disorders associated with schizophrenia are not yet well characterized. To date, 22q11.2 deletion syndrome is the only established genetic subtype of schizophrenia of proven clinical relevance. We use this well-established association as a model to chart the pathway for translating emerging genetic discoveries into clinical practice. We also propose new directions for research involving general genetic risk prediction and counseling in schizophrenia.
Introduction
Schizophrenia is arguably one of humankind’s most severe diseases.Citation1,Citation2 Driven by new genetic technologies and advances in molecular medicine, recently there has been concrete progress in understanding some of the specific genetic origins of this complex psychiatric illness, summarized in several recent reviews.Citation2–Citation8 There has, however, been little focus on how we might practically apply the findings.Citation9–Citation11 In this review, we critically appraise recent developments in terms of immediate clinical utility. We use the well-established association of schizophrenia with microdeletion 22q11.2 as a model to chart the pathway for translating emerging genetic discoveries into clinical practice. We also propose new directions for research involving general genetic risk prediction and counseling in schizophrenia.
Clinical features of schizophreniaCitation9,Citation12
Schizophrenia is a common psychiatric illness that typically involves lifelong but treatable changes in thinking, behavior, and emotions. It has a lifetime morbid risk of approximately 1%. The principal symptoms are psychotic in nature: delusions (false beliefs), hallucinations (false perceptions), and thought disorder (disorganization of thought processes). In addition to these “positive” symptoms, there are also “negative” symptoms of blunted affect (reduced emotional expression), poverty of speech, anhedonia (reduced ability to feel pleasure), and amotivation, as well as disorganization of behavior and emotions. Depression, anxiety, irritability, agitation, sleep disturbance, and cognitive impairments, including changes in attention, memory, insight, and judgment, are also common. Onset of schizophrenia occurs most commonly in early adulthood from 17 to 30 years of age, but can arise in childhood (in <1% of cases) through to the elderly age range. The diagnosis of schizophrenia is a clinical diagnosis, based on course of illness as well as cross-sectional symptoms. Diagnostic reliability is high when standard diagnostic criteria are combined with a direct examination and thorough history, including information from the patient, relatives, and others, to differentiate schizophrenia from other psychotic disorders. As with most neuropsychiatric disorders, there are no characteristic neuropathological findings (), which, coupled with the absence of any diagnostic tests and substantial clinical heterogeneity (variable signs and symptoms), emphasizes the importance of detailed expert phenotyping (cf “shallow phenotyping”).Citation13
Table 1 Schizophrenia within the context of other common complex neuropsychiatric diseases (as of 2011)
Genetic epidemiology of schizophrenia
Psychiatric genetics has historically focused in large part on the study of schizophrenia and its epidemiology.Citation14 It is well-established that the heritability of schizophrenia is >80%,Citation15–Citation17 amongst the highest known for complex genetic disorders.Citation18 Consistent evidence from family, twin, and adoption studies over the past century strongly indicates that predisposition is largely genetically determined (MIM #181500).Citation19–Citation24 Nongenetic factors, for example, marijuana use and hypoxiamediated factors like birth complications or childhood head injury, increase risk for schizophrenia only modestly.Citation9,Citation25–Citation32 Studies of schizophrenia in twinsCitation33,Citation34 support reduced penetrance (genetic variants that do not express in every carrier as disease) and variable expression (variants that express as different diseases in different carriers),Citation35,Citation36 which is common to most human genetic diseases. As for most diseases, there is also substantial evidence for genetic heterogeneity and probably allelic heterogeneity.Citation18,Citation36 Gene– gene interaction (epistasis) is likely in schizophreniaCitation37,Citation38 and indeed is ubiquitous in nature.Citation39 Molecular evidence for early predictions of spontaneous (de novo) mutations in schizophrenia,Citation40,Citation41 recently reframed as the “common disease – rare alleles” model,Citation42 adds to the complex genetic picture. Thus, it is not surprising, in hindsight, that classic Mendelian inheritance patterns are very rarely observed in schizophrenia, and that elucidation of causal genetic factors has been so challenging. Researchers have remained undeterred, because of the myriad possible benefits for patients, families, and clinicians that could result from an improved understanding of the genetic etiology of schizophrenia ().
Table 2 Potential future roles for molecular genetics in the clinical management of schizophrenia
22q11.2 deletion syndrome
To date, 22q11.2 deletion syndrome (22q11.2DS; previously DiGeorge syndrome and velocardiofacial syndrome) is the only established genetic subtype of schizophrenia of proven clinical relevance.Citation3,Citation43 The association of 22q11.2DS with schizophrenia followed soon after the discovery in the early 1990s that the 22q11.2 deletion was the underlying molecular anomaly unifying several, seemingly distinct, clinical syndromes first described in the 1960s and 1970s.Citation44,Citation45 Many of the features suggested above with respect to the genetic epidemiology of schizophrenia, including spontaneous mutations, reduced penetrance, and variable expressivity, are found in 22q11.2DS.
Molecular origins and epidemiology
22q11.2DS is associated with a hemizygous microdeletion on chromosome 22q11.2 of variable length (typically 3 Mb) and, in some cases, variable position within this region.Citation9,Citation46 There is no apparent critical region at this locus for any major phenotype, including schizophrenia.Citation47–Citation50 Most deletions are flanked by segmental duplicationsCitation51 and occur as de novo mutations mediated by nonallelic homologous recombination.Citation46,Citation52 Only 5%–10% of cases have been found to be inherited from transmitting parents,Citation53–Citation56 most frequently mothers with mild neuropsychiatric phenotypes.Citation57 Nevertheless, 22q11.2DS is the most common genomic disorderCitation58 in humans, with an oft-cited estimated prevalence in the general population of 1 in 3000–4000 live births that is likely to be an underestimate.Citation59,Citation60 Fluorescence in situ hybridization using a standard 22q11.2 probe (D22S75 or TUPLE1) has been used since 1992 to detect most deletions in this region,Citation9 but is now being superseded by clinical microarrays that should increase diagnostic yield.Citation46,Citation61,Citation62 Several lines of evidence support the generally pathogenic nature of 22q11.2 deletions and the high penetrance of observable phenotypes,Citation3,Citation63,Citation64 so that no distinction is typically made between individuals with 22q11.2 deletions and individuals with 22q11.2DS.
Association with schizophrenia
Several studies have confirmed that 22q11.2DS accounts for approximately 1% of all cases of schizophrenia (see Bassett et alCitation65 and references therein). Conversely, an estimated 22.5% of adults with 22q11.2DS develop schizophreniaCitation66 or a related psychotic disorder.Citation67 In other words, a 22q11.2 deletion is a variant of large effect, associated with a greater than 20-fold increase in risk for schizophrenia.Citation9 The clinical expression of the schizophrenic illness is essentially indistinguishable from that found in the general population with respect to prodrome, age at onset, presentation, cognitive profile (except for lower mean IQ), and, according to limited data available, response to treatment.Citation43,Citation66–Citation71 Thus 22q11.2DS represents the best available specific genetic model of schizophrenia, with minimized genetic heterogeneity and substantial evidence this is a representative form of this illness.Citation2 Empirical evidence for a strong negative selective pressureCitation57 and high rate of recombination at the 22q11.2 locusCitation72 is consistent with the common disease – rare variant model for schizophrenia.Citation42
Clinical relevance
For patients with schizophrenia, a clinician today should be armed with a high index of suspicion for 22q11.2DS and/or consistently use established clinical screening criteria to detect features suggesting this genetic diagnosis, such as dysmorphic facies, a nasal voice, congenital anomalies, and/or learning difficulties.Citation65,Citation73 This would prompt a comprehensive diagnostic assessment, including developmental, medical, and family history, and a physical examination by a clinician experienced in genetic syndromes and dysmorphology.Citation9 Genetic testing would follow if sufficient features were present to support a clinical diagnosis of 22q11.2DSCitation73 and should proceed in all patients with comorbid mental retardation and/or multiple congenital anomalies.Citation3,Citation61
In a patient with schizophrenia, detection of a 22q11.2 deletion is clinically relevant.Citation9 Consensus clinical practice guidelinesCitation46 now exist that detail opportunities for anticipatory care and optimizing medical management of associated features, and for genetic counseling that can be informed by extensive (and rapidly expanding) knowledge of pathogenesis, recurrence risk, and lifelong expression, natural history, and clinical outcomes.Citation54,Citation56,Citation57,Citation63,Citation66,Citation67,Citation70,Citation74–Citation88 Careful attention to the commonly accompanying endocrine and neurological features in particular may be helpful in the psychiatric management of patients with 22q11.2DS.Citation9,Citation63 As is common in patients with schizophrenia, the psychiatrist may be the only physician the patient sees regularly and may therefore be expected to provide primary care for accompanying medical conditions, and/or have the responsibility for arranging appropriate investigations and follow-up.
For patients already diagnosed with 22q11.2DS, what are the potential implications of knowing about the risk for schizophrenia prior to first onset of psychosis? As for schizophrenia in the general population,Citation29 there is little evidence for any additional environmental factor(s) affecting risk of psychosis in 22q11.2DS.Citation9,Citation89 Avoiding substance use, particularly early marijuana use, and lifelong general health measures such as good nutrition, and physical and mental exercise, may decrease risk to some extent.Citation9 Common genetic modifiers of some effect may exist,Citation90,Citation91 as for the congenital cardiac phenotype,Citation92,Citation93 but have not been convincingly demonstratedCitation67,Citation94–Citation96 (see Philip and BassettCitation48 for more details), and additional copy number variation/variants (CNV) do not seem to play a major role in expression of schizophrenia.Citation53 ProspectiveCitation97–Citation99 and retrospectiveCitation68,Citation70,Citation100 research is ongoing to identify specific neurocognitive and neuroimaging predictors of future psychotic symptoms, as none are yet known. The greatest potential benefit of early diagnosis of a 22q11.2 deletion would likely be to facilitate the recognition of the early stages of schizophrenia or another psychiatric illness, and promptly seeking expert help in diagnosis and effective treatment.Citation9,Citation78 Limiting the duration of untreated psychiatric illness is associated with better prognosis.Citation101 Psychosis in an adolescent or young adult with 22q11.2DS should also be easier to diagnostically classify as schizophrenia in its early stages because of the significant association between these two elements,Citation102 despite the potential additional diagnostic complexities that could be posed by comorbid mental retardation.Citation103
Schizophrenia in the molecular age
Foreshadowed by the association with 22q11.2 deletions, there is further emerging evidence that multiple rare variants contribute significantly to the genetic vulnerability for schizophrenia. Other select genomic disorders caused by rare, recurring CNV represent emerging genetic subtypes of schizophrenia of growing clinical importance (). This encouraging progress comes after several decades of genome-wide and targeted molecular studies, representing essential, though largely unfruitful, tests of standard genetic hypotheses usually involving common variants at the level of nucleotide sequence (eg, single nucleotide polymorphisms [SNPs]) and DNA structure (eg, copy number polymorphisms [CNPs]). In retrospect, these studies were driven by genetically naïve expectations for schizophrenia. These included that there would exist (1) a single major locus, (2) common genetic variants of large effect, and (3) variants specific to schizophrenia (ie, not frequently associated with other conditions).
Table 3 The current established and emerging genetic subtypes of schizophrenia are characterized by large, rare, recurring copy number variation and variable expressivity
Genome-wide linkage and association studies
Associations with schizophrenia
Initially, several genome-wide linkage studies of multiply affected families identified regions where candidate genes for schizophrenia are likely to be. Some of these loci have been replicated and/or supported by meta-analyses,Citation9,Citation18,Citation104–Citation108 including 1q21-q23, 6p22, 8p21, and 13q32-q34.Citation109–Citation118 As expected,Citation119 however, there are also negative studies of all loci.Citation18,Citation120–Citation122 Despite a few highly significant findings that have led, for example, to identification of functional SNPs in candidate genes,Citation123 one of the most important collective contributions of these studies has been the confirmation that there is no unifying single gene mutation for familial forms of schizophrenia.
In addition, there have now been several large-scale, case-control genome-wide association studies (GWAS) of schizophrenia using SNP-based microarray technology. Overall, this strategy, based on studying common SNPs, has proven to be relatively ineffective for the study of complex neuropsychiatric diseases, compared with the relative successes for auto immune diseases like diabetes and inflammatory bowel disease.Citation124 Some of these GWAS failed to find significant evidence of association and/or have not replicated weak associations.Citation125–Citation129 Others have reported common sequence variants of very small effect, including several in the human leukocyte antigen (HLA) region (6p).Citation130–Citation137 The latter findings recall early studies of protein-based HLA polymorphisms.Citation138,Citation139 Meta-analysis using GWAS data from thousands of individuals with schizophrenia has revealed a few weak-effect associations, in the ZNF804A gene.Citation140–Citation143 GWAS of common structural variants such as CNPs, while fewer in number, have yielded comparable findings of nominal effect.Citation127,Citation144
GWAS using common SNPs and data on drug dosages and treatment response in schizophrenia (ie, pharmacogenomics studies) are reviewed elsewhere.Citation145,Citation146 These show modest results similar to those for diagnosis of schizophrenia, and broad clinical applications still represent more a dream than a reality; the family history with respect to treatment response remains arguably the best predictor of treatment efficacy and side effects.Citation147 Findings from thousands of targeted candidate gene association studies of schizophrenia using individual SNPs (and, occasionally, specific rare sequence variants), catalogued online in the SzGene databaseCitation148 (www.szgene.org) and the database of Genotypes and Phenotypes (www.ncbi.nlm.nih.gov/gap), are either negative altogether or sporadically positive without replication and/or positive but with modest effect size. None are associated with a relative risk for schizophrenia anything approaching that of the ɛ4 variant of the APOE gene relative risk for Alzheimer disease ().Citation9 Taken together, the GWAS and targeted association study results conclusively indicate there are no common genetic variants of large effect for schizophrenia.
Clinical relevance
Although linkage approaches and GWAS of common variants have yielded a collection of several dozen candidate genes, the individual effect sizes of associated variants are modest. Mutation testing in these genes has no role in the clinic at this time. Larger and larger sample sizes have ensured that variants of smaller and smaller effect may be detected. Importantly, a GWAS design lacks the power to detect rare sequence variants of large effect, and it is rare variants that may collectively play a major role in the etiology of schizophrenia.Citation124 While preliminary findings from the first whole exome sequencing studies in schizophrenia are solely in the realm of scientific discovery at this time, they provide evidence in support of a de novo mutational paradigm at the sequence level.Citation149,Citation150 In time, these studies may provide clinically relevant findings. However, the most notable evidence today for clinically important rare genetic variants in schizophrenia comes from studies of CNV – that is, structural genomics.
Rare copy number variation and emerging genetic subtypes
The discovery that there is substantial structural genomic variation in the human genome that contributes to both normal variation and to susceptibility for disease is one of the major scientific advances in recent years.Citation5,Citation64,Citation151–Citation155 Consistent with early reports of the association of schizophrenia with rare microscopically visible chromosomal abnormalitiesCitation156 and the established association with 22q11.2 deletions,Citation43,Citation66,Citation67,Citation69 there is now substantial evidence for the importance of diverse rare structural variants in causing schizophrenia.Citation3,Citation5 These structural variants are mainly comprised of specific examples of large (eg, >500 kb) CNV that are consistently enriched in schizophrenia samples, and absent or extremely uncommon (ie, “rare”) in control populations.
Associations with schizophrenia
Following the discovery of 22q11.2 deletions in schizophrenia, the next tier of large, rare CNV findings includes a set of emerging genetic subtypes of schizophrenia ().Citation3 In contrast to 22q11.2DS, the epidemiology and basic genetic parameters such as penetrance and expression remain uncertain for these genomic disorders, and there has been little to no study of the dosage-sensitive genes that may cause the associated phenotypes.Citation64 Collectively, however, this second tier of CNV may be as or more common in schizophrenia than 22q11.2 deletions.Citation3,Citation5 Those variants identified to date include large (>500 kb), rare, recurring (flanked by segmental duplications), hemizygous losses (deletions) and gains (duplications) at several loci (). All involve numerous genes and are consistently identified in large-scale case-control CNV studies of schizophrenia, albeit each individually at apparently low prevalence. A recent review has suggested pooled odds ratios for schizophrenia of 8 or higher for four of these five genomic disorders,Citation5 and for 15q11-q13 duplications of maternal origin, an initial report based on four cases suggested an odds ratio for schizophrenia of 7.3.Citation157 As with 22q11.2 deletions,Citation3 these rare structural variants may be expressed as other psychiatric illnesses and/or developmental conditions, such as autism spectrum disorders (ASDs) and epilepsy (). On the other hand, the few studies of rare CNV in bipolar disorder indicate little overlap of these forms of schizophrenia with this major mood disorder.Citation5,Citation158 This would be consistent with the historical clinical separation of schizophrenia and bipolar disorder based on presentation, course, and outcome, and the perhaps less “neurodevelopmental” nature of bipolar disorder. The variability of expression of 22q11.2 deletions and these other rare structural variants is shining new light on schizophrenia and on the genetically related spectrum of neuropsychiatric disorders.Citation3 There are also other potential susceptibility factors for schizophrenia of likely smaller effect (),Citation5 such as hemizygous deletions at 15q11.2Citation159–Citation164 and 17q12,Citation162,Citation165 and duplications at 1q21.1Citation159,Citation164,Citation166 and 16p13.11.Citation160,Citation167 These appear to be similarly or more variable in their expression and less penetrant with respect to schizophrenia, or indeed any major phenotype, than 22q11.2 deletions and the five emerging genetic subtypes.Citation64,Citation168–Citation179 All await more detailed study, especially of expression in adults.
Table 4 Pathway to clinical utility for copy number variation and genomic disorders associated with schizophrenia (as of 2011)
outlines some of the many remaining issues with respect to clinical translation for possible genetic subtypes of schizophrenia. For instance, there are limited or inconsistent data on CNV inheritance status or comorbidity in most of the existing studies, and numerous systematic methodological issues that complicate the assessment of prevalence and penetrance.Citation3,Citation180 Truly inclusive population-based prevalence samples of schizophrenia (eg, community catchments) are difficult to obtain, and many of the initial large case-control studies may have implicitly or explicitly excluded subjects with dysmorphic features, birth defects, learning difficulties, and/or known syndromes.Citation3 Such systematic ascertainment biases in sample collection for large-scale case-control studies suggest that the prevalence of genomic disorders in schizophrenia may be underestimated. As an example, the expected prevalence of 22q11.2 deletions in schizophrenia is about 1%,Citation65,Citation181 but a pooled estimated prevalence based on large consortium-based case-control studies is approximately 0.3%.Citation5 There are also few data concerning fundamental issues such as the possible effects of sex, ethnicity (most studies to date have involved Caucasians), or sampling from genetic isolates. Nonetheless, this initial wave of genome-wide studies of CNV provides replicated associations of schizophrenia with specific rare variants. This supports a more general mutational mechanism involving large rare CNV that substantially elevate risk for schizophrenia, especially more developmental forms of the disease.Citation3,Citation182
Neurodevelopmental implications
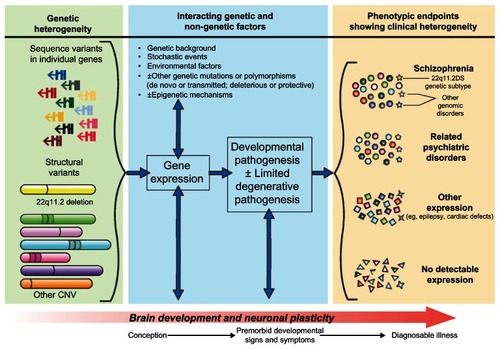
Notably, many of the structural variants associated with schizophrenia implicate a dosage effect of neurodevelopmental genes involved with neuronal proliferation, migration, or synapse formation.Citation2,Citation182 Although there are few studies that have examined age at onset of schizophrenia,Citation166 it may be that rare CNV have a greater impact on such genes in individuals with younger onset (eg, childhood, age <12 years) compared with onset at older ages.Citation182 This would be consistent with a previously reported greater prevalence of chromosomal abnormalities and 22q11.2 deletions in childhood-onset schizophrenia.Citation183 Several other lines of evidence, including brain imaging, premorbid clinical signs, and associations with minor dysmorphic features, have previously indicated that early changes in neurodevelopment may be involved in the pathogenesis of schizophrenia.Citation2,Citation35,Citation184 The current genetic neurodevelopmental model of the etiopathogenesis of schizophrenia () has important consequences with respect to the potential for pre-symptomatic prediction and, ultimately, attenuation, delay, or prevention of psychosis, as well as present-day genetic counseling (outlined below).
Figure 1 Neurodevelopmental model of schizophrenia, informed by new molecular genetic discoveries. One or more transmitted or de novo sequence or structural mutations, involving one or more genes, and acting individually or interactively, is proposed as the initial causal event. The pathway from genotype to phenotype is formulated as a dynamic process beginning at or before conception, and involving gene expression (including, but not limited to, protein activity) and interaction with normal brain development and neuronal plasticity mechanisms, and likely multiple other genetic and non-genetic factors. Different phenotypic endpoints are possible, and specific factors that dictate variable expression of ostensibly the same genetic loading are largely unknown and may be variant-specific. These resulting phenotypes could include clinically diagnosable schizophrenia, other psychiatric illnesses, other conditions including disorders of development, or no detectable expression. For example, a 22q11.2 deletion (yellow structural variant) may be expressed as schizophrenia and/or a related psychiatric disorder and/or another developmental disorder (yellow stars).
Abbreviation: CNV, copy number variation.
Clinical relevance
Use of 22q11.2DS as the benchmark for clinical applicability of molecular genetics in schizophrenia highlights the gaps that currently exist at the level of translating recent genetic results involving other large rare CNV to inform clinical management of patients (). Optimism about eventual direct benefits for patients and their families stems from the observations that effect sizes are generally large and that the spectrum of disorders involved in variable expression may tend to be multisystem and/or developmental in nature. This may be related to the multiple genes usually involved in large CNV, and in turn suggests that penetrance for any observable phenotype, as opposed to schizophrenia per se, will be fairly high. Meaningful prediction of some associated conditions would then be possible, creating opportunities for anticipatory care and improved medical management, as already exist for 22q11.2DS.Citation3 Eventually, such genetic variants may also assist in diagnostic subtyping of schizophrenia.
Psychiatric disorders in general appear to have a poorly understood, nuanced connection to the various associated structural variants.Citation153 Determinants of disease specificity may be other, perhaps more common, genetic, epigenetic, stochastic, and/or environmental modifiers. There has been little research as yet on such additional factors ().Citation185 Likely the research that will have the most clinical impact will involve unbiased sampling, family studies, and detailed study of the variable expression and natural history of individual variants. This will in turn facilitate specific care recommendations and prediction of comorbidities, and eventually prognosis and drug response ().Citation3 To date, in order to gain sample sizes sufficient to detect signals in genetically heterogeneous populations, many researchers have sacrificed: (1) detailed phenotyping of the probands, (2) the ability to return to individual participants after analyses, and (3) the familial context necessary to assess de novo status and segregation patterns. These features represent, from a clinician’s point of view, unfortunate consequences of the study design of much of the large-scale genetics research conducted thus far in the twenty-first century. The longstanding practice of DNA sample anonymity, and other formal barriers between research participation and clinical care, is more and more at odds with a conflicting “duty to warn” in this new era of molecular medicine, where actionable and clinically relevant information is increasingly likely to be obtained.Citation186,Citation187 Researchers, clinicians, and genetic counselors must begin to consider new strategies for when and how to routinely return to genotypic information and subsequently inform research participants and their clinicians of medically pertinent findings, keeping in mind that the interpretation of any particular variant is subject to change as new data accrues.
Lack of data on potential utility at this time, especially given limited resources, suggests that clinical genome-wide microarray testing is not yet justified for individuals with schizophrenia, except for the minority with syndromic and/or neurodevelopmental features such as mental retardation or multiple congenital anomalies.Citation3,Citation61,Citation65 Similarly, calls for routine targeted clinical testing on the basis of a single study where penetrance and information about expression remain unknown (eg, 7q36.3 duplications of various sizes implicating the VIPR2 geneCitation188) appear dangerously premature. The effectiveness of personal genomic information in tailoring interventions and improving health outcomes has not yet been convincingly demonstrated for emerging genetic subtypes of schizophrenia, nor for susceptibility factors that may be relevant for this complex disease. There are also limited to no data with respect to the ethical, legal, social, and economic implications of widespread personal genomic testing.Citation189 As for other diseases like ASDs,Citation61,Citation190 careful consideration and professional consensus are needed to decide how to apply such genomic knowledge in clinical practice. Use of well-recognized standards and guidelines for clinical genetic testing, such as the ACCE Model Process for Evaluating Genetic Tests (available from: www.cdc.gov/genomics/gtesting/ACCE/), may be helpful in this regard. Their application quickly exposes the many gaps in our fundamental knowledge base with respect to the clinical and analytic validity, and clinical utility, of genetic testing for most CNV in schizophrenia, particularly compared with other diseases and genetic variants such as breast/ovarian cancer and BRCA1 and BRCA2 mutations.Citation191
Genetic risk and counseling issues
Genetic counseling for schizophrenia largely continues to focus on recurrence risk based on family history, but recent molecular genetic discoveries are now having a significant impact in specific cases. Post-onset (ie, phenotype first), the identification and disclosure of a well-established genetic variant (eg, a 22q11.2 deletion) that is strongly associated with a stigmatized illness like schizophrenia may be highly valued by the patient and family for its explanatory value.Citation186,Citation192 Such variants also provide the potential to inform reproductive decision making, including the possible availability of prenatal detection.Citation3 Even in the absence of such genetic variants, genetic counseling for schizophrenia may still represent an informative and therapeutic intervention. However, there are limited empiric data about potential benefits at present.
Genomic disorders and genetic subtypes
Identifying genetic subtypes of schizophrenia offers new possibilities with respect to recurrence risk prediction. In the case of an individual with 22q11.2DS-schizophrenia and no other affected relatives (including the spouse or partner), for example, knowledge of the proband’s 22q11.2 deletion bifurcates the risk scenario. Transmission of the 22q11.2 deletion would imply a recurrence risk for schizophrenia of approximately 20%–25% in each offspring, whereas a failure to transmit the deletion would theoretically decrease that risk to the standard population rate (~1%). With no knowledge about the 22q11.2 deletion status, offspring would have an a priori averaged (though individually far less informative) recurrence risk for schizophrenia that is comparable to the standard empiric recurrence risk of 13%.Citation20,Citation147 Also, identification of a de novo 22q11.2 deletion, or other putatively causal de novo CNV, in an individual with schizophrenia would be expected to lower the recurrence risk for siblings and nieces/nephews to nearly the population rate.Citation10 We note from experience that for schizophrenia the risk of recurrence in siblings and nieces/nephews is the dominant concern expressed in most genetic counseling sessions, given the significantly decreased reproductive fitness associated with schizophrenia and concomitantly few direct offspring.Citation57,Citation193,Citation194
As new diagnoses of specific genomic disorders and increasing evidence for their involvement in schizophrenia flood clinical practice, the need for more psychiatric genetic counseling research and training will be increasingly apparent. First, the association of specific large rare CNV with schizophrenia, as outlined above ( and ), effectively means that incidental predictive “genetic testing” for schizophrenia is now a reality of clinical practice, because of the use of clinical microarrays as a first-tier diagnostic test for developmental delay/mental retardation, multiple congenital anomalies (even in utero), or ASD.Citation10,Citation61 Proposed benefits and disadvantages of predictive testing are discussed elsewhere.Citation147,Citation195,Citation196 Genetic counselors, however, may be reluctant to disclose the possibility of psychotic illness to parents even in the context of well-established risk factors such as 22q11.2 deletions. Stigma,Citation197 lack of knowledge about the illness and its treatment, and concerns about generating anxietyCitation79 may play a role in deferral or non-disclosure,Citation198 despite some evidence that opportunities to anticipate and prepare for such an illness would have been valued by the parents. Citation186 Second, genetic counseling of the adolescent and adult patients themselves may be complicated by cognitive impairments and/or psychiatric symptoms.Citation147,Citation199 There are surprisingly few empirical studies of the optimal content and process, and of the effectiveness and ensuing outcomes, of genetic counseling in these instances,Citation199,Citation200 even for well-recognized genomic disorders like 22q11.2DSCitation57 or Williams syndrome.Citation201
On the other hand, clinical microarrays also give results unrelated to current genomic disorders and about which there is much uncertainty with respect to interpretation. Today, proposed workflow algorithms for determining pathogenicity are likely to label most individual CNV identified, other than the relatively established like those underlying 22q11.2DS and other genomic disorders, as “variants of unknown significance” or “VOUS.”Citation153,Citation202 This is of particular concern for smaller (atypical) rare CNV at loci that may be associated with emerging genetic subtypes of, or possible susceptibility factors for, schizophrenia. The nature of schizophrenia poses additional challenges. For example, parents of adult patients are less likely to be available for testing to determine de novo/inherited status, and patients may be poor historians with respect to medical and/or family history. Clinical interpretation of many CNV findings will thus remain a major challenge for the foreseeable future.
Familial schizophrenia
Individuals from multiplex families arguably have the greatest need for risk prediction and genetic counseling. However, empiric recurrence risk figures cannot be quantitatively modified to account for multiple affected relatives or a bilineal (ie, maternal and paternal) family history.Citation147,Citation203 The sole exceptions relate to the situations where both parents, or one parent and one sibling, are affected, for which some recurrence risk data are available.Citation9,Citation147,Citation204 There is also no ability to adjust risk to take into account relatives with schizophrenia spectrum conditions and/or other neuropsychiatric diseases.Citation147,Citation203 Also, unlike in Huntington disease before mutation identification, Citation9,Citation205,Citation206 knowledge of linkage or association information where it exists for individual families cannot be meaningfully incorporated into illness prognostication, given the modest effect size of alleles identified.
As for any individual with schizophrenia, clinicians should be aware of features consistent with testable conditions like 22q11.2DS. However, 22q11.2 deletions are less likely to be co-segregating with schizophrenia in multiply affected families, as they are associated with a strong negative selective pressure.Citation57 Other large rare CNV associated with schizophrenia, for example, microduplications not associated with multisystem/syndromic features,Citation157,Citation180 may have less effect on reproductive fitness and thus a higher likelihood of contributing to the burden of illness in multiply affected families, though this remains to be shown. There is growing empiricalCitation207 and theoreticalCitation208,Citation209 evidence that, as for Parkinson disease,Citation210 familial and “sporadic” schizophrenia may not be molecularly distinct entities. This underscores the need for a greater focus in future studies on both the inheritance status of genetic variants shown to be associated with schizophrenia, and on the extent of co-segregation of these variants with other neuropsychiatric illness and developmental conditions within families.
Idiopathic schizophrenia
Often overlooked in our collective enthusiasm for the promise of new genetic discoveries are the sobering realizations that the vast majority of cases of schizophrenia are “idiopathic” and that, as for most conditions,Citation211 family history remains the cornerstone for individualized disease prediction. When the schizophrenia is not a syndromic form of the illness,Citation9 of genetically testable origin,Citation61 or originating in the context of a multiplex family, patients and their relatives are unlikely to be seen by a genetics professional unless presented with an unrelated concern.Citation147 Much has been written and repeated over the past several decades about the optimal content and process of “multifactorial” genetic counseling,Citation9,Citation147,Citation196,Citation204,Citation212–Citation214 despite low rates of genetic counseling referralsCitation215 and a continued scarcity of evidence in support of the desirabilityCitation216 or effectivenessCitation217 of the genetic counseling intervention. To assess these key issues, and also prepare for the growing role of personalized molecular genetic information, there is an urgent need for data-driven reports of the genetic counseling of patients with idiopathic schizophrenia and their relatives.
Recent advances in schizophrenia genetics may still be germane to contemporary genetic counseling however, even in the absence of personalized application. For example, presenting schizophrenia to consultands as a neurodevelopmental disorder (),Citation35 with psychosis as a later stage manifestation often preceded by a prodromal period,Citation2 has the potential to further modify false beliefs that upbringing, lifestyle decisions, or other “triggers”Citation204 are either necessary or sufficient to cause an illness that otherwise would not have developed. Proof that de novo mutations play an important causal role in some cases may help in dispelling a popular misconceptionCitation218 that “genetic” and “inherited” are synonymous terms. Especially in the absence of an affected first- or second-degree ancestor, de novo mutation is a highly plausible theory of causation that may decrease a sense of family blame or shame. Finally, presenting evidence for genetic and epigenetic differences between monozygotic twins can help to explain discordant twin pairs without needing to resort to the unsupported assumption of powerful environmental factors in these rare cases.Citation219–Citation222 The role of independent environmental factorsCitation29 may be less, and that of gene-environment interactionsCitation25,Citation223,Citation224 and stochastic effectsCitation225,Citation226 may be greater, than initially supposed.
As yet, the full “risk architecture” of schizophrenia, and the extent to which risk factors may be modifiable, is unknown.Citation2 With respect to idiopathic schizophrenia, there is a forced reliance on family history and associated crude empiric recurrence risks, unmodified quantitatively by any other clinical or demographic variables.Citation203 Few attempts have been made to update or validate these recurrence risks, despite the potential increase in the proportion of individuals with schizophrenia who partner with someone else with schizophrenia (ie, assortative mating),Citation227 new conceptualizations of the genetically relevant schizophrenia spectrum,Citation3 and increasing opportunities for molecular characterization. Initial attempts to generate a “risk score” from multiple variants with weak association with schizophrenia may be promising avenues for future research,Citation131,Citation228,Citation229 but are not yet meaningful in a clinical genetic counseling context. In addition, the factors of primary interest in genetic counseling (ie, those that increase individual recurrence risk substantially) do not necessarily have much effect on average risk, the primary focus of most retrospective studies of schizophrenia, and vice versa.Citation230 Partial risk prediction as afforded by proven moderate to high penetrance variants such as 22q11.2 deletions may be the best case scenario. There are likely to be fundamental limits on precise individualized genetic risk prediction due to the complex architecture of common traits, including common variants of very small effect, rare variants that cannot be fully enumerated, and complex epistatic interactions, as well as stochastic and possible environmental factors.Citation231 The potential “added value” of genome-wide data (eg, derived from next-generation sequencing) in tailoring risk estimates would also need to be weighed against many other factors. These include the cost of, and expertise needed for, the molecular analysis (which is still prohibitive for widespread use, particularly in publicly funded health care systems) and the interpretation of results (as great or greater than molecular analytic costs, and less likely to decrease over time).Citation231 Such barriers will impede widespread application of new genetic technologies in clinical practice more generally for the foreseeable future. Personal genome sequencing as a single universal genetic test that is cost-effective and of broadly applicable clinical utility remains a distant, though much wished for, prospect.Citation232
Conclusion
Schizophrenia is a complex neuropsychiatric disease with documented clinical and genetic heterogeneity, and little is known about the associated pathophysiology apart from strong evidence for neurodevelopmental origins. The elucidation of specific causes and mechanisms for schizophrenia that is beginning to be derived from advances in molecular genetics and related research promises to help many families afflicted with this illness. However, much work needs to be done to move the recent surge of genetic research discoveries into the clinic. In particular, specific large rare structural variants (CNV) have been convincingly implicated in targeted studies over two decades (with respect to 22q11.2 deletions) and more recently in several large-scale genome-wide case-control studies of schizophrenia. Clinical interpretation of most individual loci remains unclear as yet because the associated epidemiology and basic genetic parameters (such as penetrance and expression) are not yet well characterized. For now, 22q11.2 deletions represent the cutting-edge of clinically applicable molecular genetics in schizophrenia. New opportunities in risk prediction and genetic counseling are exciting avenues for future research.
Acknowledgments
The authors thank Sean Bekeschus for his assistance with aesthetic design of the figure. This work was supported by Canadian Institutes of Health Research grants (MOP-97800, MOP-111238, MOP-53216), a Vanier Canada Graduate Scholarship (GC), and a Canada Research Chair in Schizophrenia Genetics and Genomic Disorders (ASB).
Disclosure
The authors have no actual or potential conflicts of interest to disclose in this work.
References
- Where next with psychiatric illness?Nature1988336619595963185737
- InselTRRethinking schizophreniaNature2010468732118719321068826
- BassettASSchererSWBrzustowiczLMCopy number variations in schizophrenia: critical review and new perspectives on concepts of genetics and diseaseAm J Psychiatry2010167889991420439386
- GejmanPVSandersARKendlerKSGenetics of schizophrenia: new findings and challengesAnnu Rev Genomics Hum Genet20111212114421639796
- HochstenbachRBuizer-VoskampJEVorstmanJAOphoffRAGenome arrays for the detection of copy number variations in idiopathic mental retardation, idiopathic generalized epilepsy and neuropsychiatric disorders: lessons for diagnostic workflow and researchCytogenet Genome Res20111353–417420222056632
- KimYZerwasSTraceSESullivanPFSchizophrenia genetics: where next?Schizophr Bull201137345646321505112
- KirovGThe role of copy number variation in schizophreniaExpert Rev Neurother2010101253220021318
- Rodriguez-MurilloLGogosJAKarayiorgouMThe genetic architecture of schizophrenia: new mutations and emerging paradigmsAnnu Rev Med201263638022034867
- BassettASChowEWCHodgkinsonKAGenetics of schizophrenia and psychotic disordersSmollerJWRosen SheidleyBTsuangMTPsychiatric Genetics: Applications in Clinical PracticeArlingtonAmerican Psychiatric Publishing, Inc200899130
- CollierDAVassosEHoldenSPatchCMcGuirePLewisCAdvances in the genetics of schizophrenia: will high-risk copy number variants be useful in clinical genetics or diagnostics?F1000 Med Rep200916120948719
- Moreno-De-LucaDCubellsJFCopy number variants: a new molecular frontier in clinical psychiatryCurr Psychiatry Rep201113212913721253883
- MueserKTJesteDVClinical Handbook of SchizophreniaNew YorkGuilford Press2008
- BrzustowiczLMBassettASPhenotype matters: the case for careful characterization of relevant traitsAm J Psychiatry200816591096109818765489
- HarperPSA Short History of Medical GeneticsNew YorkOxford University Press2008
- CannonTDKaprioJLonnqvistJHuttunenMKoskenvuoMThe genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling studyArch Gen Psychiatry199855167749435762
- CardnoAGGottesmanIITwin studies of schizophrenia: from bow-and- arrow concordances to star wars Mx and functional genomicsAm J Med Genet2000971121710813800
- KendlerKSDiehlSRThe genetics of schizophrenia: a current, genetic-epidemiologic perspectiveSchizophr Bull19931922612858322035
- KirovGO’DonovanMCOwenMJFinding schizophrenia genesJ Clin Invest200511561440144815931379
- GottesmanIIShieldsJHansonDRSchizophrenia, the Epigenetic PuzzleNew YorkCambridge University Press1982
- GottesmanIIWolfgramDLSchizophrenia Genesis: The Origins of MadnessNew YorkFreeman1991
- KendlerKSGruenbergAMTsuangMTPsychiatric illness in first-degree relatives of schizophrenic and surgical control patients. A family study using DSM-III criteriaArch Gen Psychiatry19854287707794015321
- LowingPAMirskyAFPereiraRThe inheritance of schizophrenia spectrum disorders: a reanalysis of the Danish adoptee study dataAm J Psychiatry19831409116711716614222
- MoldinSOGottesmanIIAt issue: genes, experience, and chance in schizophrenia – positioning for the 21st centurySchizophr Bull19972345475619365994
- TienariPJWynneLCAdoption studies of schizophreniaAnn Med19942642332377946240
- AbdelMalikPHustedJChowEWBassettASChildhood head injury and expression of schizophrenia in multiply affected familiesArch Gen Psychiatry200360323123612622655
- ByrneMBrowneRMulryanNLabour and delivery complications and schizophrenia. Case-control study using contemporaneous labour ward recordsBr J Psychiatry200017653153610974958
- HenquetCDi FortiMMorrisonPKuepperRMurrayRMGene-environment interplay between cannabis and psychosisSchizophr Bull20083461111112118723841
- McGuffinPAshersonPOwenMFarmerAThe strength of the genetic effect. Is there room for an environmental influence in the aetiology of schizophrenia?Br J Psychiatry199416455935997921708
- MathesonSLShepherdAMLaurensKRCarrVJA systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophreniaSchizophr Res20111331–313314221999904
- MooreTHZammitSLingford-HughesACannabis use and risk of psychotic or affective mental health outcomes: a systematic reviewLancet2007370958431932817662880
- TienariPImplications of adoption studies on schizophreniaBr J Psychiatry Suppl19921852581389042
- WahlbergKEWynneLCOjaHGene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of SchizophreniaAm J Psychiatry199715433553629054783
- GottesmanIIBertelsenAConfirming unexpressed genotypes for schizophrenia. Risks in the offspring of Fischer’s Danish identical and fraternal discordant twinsArch Gen Psychiatry198946108678722802925
- KringlenECramerGOffspring of monozygotic twins discordant for schizophreniaArch Gen Psychiatry198946108738772802926
- BassettASChowEWO’NeillSBrzustowiczLMGenetic insights into the neurodevelopmental hypothesis of schizophreniaSchizophr Bull200127341743011596844
- BassettASChowEWWaterworthDMBrzustowiczLGenetic insights into schizophreniaCan J Psychiatry200146213113711280081
- PrasadKMTalkowskiMEChowdariKVMcClainLYolkenRHNimgaonkarVLCandidate genes and their interactions with other genetic/environmental risk factors in the etiology of schizophreniaBrain Res Bull2010833–4869219729054
- RischNLinkage strategies for genetically complex traits. I. Multilocus modelsAm J Hum Genet19904622222282301392
- PhillipsPCEpistasis – the essential role of gene interactions in the structure and evolution of genetic systemsNat Rev Genet200891185586718852697
- BookJASchizophrenia as a gene mutationActa Genet Stat Med195342–313313913137881
- PenroseLSMutation in manActa Genet Stat Med19566216918213410479
- McClellanJMSusserEKingMCSchizophrenia: a common disease caused by multiple rare allelesBr J Psychiatry200719019419917329737
- BassettASChowEWSchizophrenia and 22q11.2 deletion syndromeCurr Psychiatry Rep200810214815718474208
- McDonald-McGinnDMZackaiEHThe history of the 22q11.2 deletion [webpage on the Internet]Philadelphia, PAClinical Genetics Center – The Children’s Hospital of Philadelphiand Available from: http://www.cbil.upenn.edu/VCFS/history.htmlAccessed January 4, 2012.
- ShprintzenRJGoldbergRGolding-KushnerKJMarionRWLate- onset psychosis in the velo-cardio-facial syndromeAm J Med Genet19924211411421308357
- BassettASMcDonald-McGinnDMDevriendtKPractical guidelines for managing patients with 22q11.2 deletion syndromeJ Pediatr20111592332339e121570089
- KarayiorgouMSimonTJGogosJA22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophreniaNat Rev Neurosci201011640241620485365
- PhilipNBassettACognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndromeBehav Genet201141340341221573985
- Sandrin-GarciaPAbramidesDVMartelliLRRamosESRichieri- CostaAPassosGATypical phenotypic spectrum of velocardiofacial syndrome occurs independently of deletion size in chromosome 22q11.2Mol Cell Biochem20073031–291717426930
- WeksbergRStachonACSquireJAMolecular characterization of deletion breakpoints in adults with 22q11 deletion syndromeHum Genet2007120683784517028864
- EdelmannLPanditaRKMorrowBELow-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndromeAm J Hum Genet19996441076108610090893
- StankiewiczPLupskiJRGenome architecture, rearrangements and genomic disordersTrends Genet2002182748211818139
- BassettASMarshallCRLionelACChowEWSchererSWCopy number variations and risk for schizophrenia in 22q11.2 deletion syndromeHum Mol Genet200817244045405318806272
- CohenEChowEWWeksbergRBassettASPhenotype of adults with the 22q11 deletion syndrome: A reviewAm J Med Genet199986435936510494092
- DigilioMCAngioniADe SantisMSpectrum of clinical variability in familial deletion 22q11.2: from full manifestation to extremely mild clinical anomaliesClin Genet200363430831312702165
- McDonald-McGinnDMTonnesenMKLaufer-CahanaAPhenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net!Genet Med200131232911339373
- CostainGChowEWSilversidesCKBassettASSex differences in reproductive fitness contribute to preferential maternal transmission of 22q11.2 deletionsJ Med Genet2011481281982422051516
- LupskiJRGenomic disorders ten years onGenome Med2009144219439022
- KobrynskiLJSullivanKEVelocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromesLancet200737095961443145217950858
- McDonald-McGinnDMSullivanKEChromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome)Medicine (Baltimore)201190111821200182
- MillerDTAdamMPAradhyaSConsensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomaliesAm J Hum Genet201086574976420466091
- TokuyasuTACotterPDSegravesRDetection of single clone deletions using array CGH: identification of submicroscopic deletions in the 22q11.2 deletion syndrome as a model systemAm J Med Genet A2007143A992593217394204
- BassettASChowEWHustedJClinical features of 78 adults with 22q11 Deletion SyndromeAm J Med Genet A2005138430731316208694
- CooperGMCoeBPGirirajanSA copy number variation morbidity map of developmental delayNat Genet201143983884621841781
- BassettASCostainGFungWLClinically detectable copy number variations in a Canadian catchment population of schizophreniaJ Psychiatr Res201044151005100920643418
- FungWLMcEvillyRFongJSilversidesCKChowEWCBassettASElevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndromeAm J Psychiatry2010167899820693476
- MurphyKCJonesLAOwenMJHigh rates of schizophrenia in adults with velo-cardio-facial syndromeArch Gen Psychiatry1999561094094510530637
- van AmelsvoortTHenryJMorrisRCognitive deficits associated with schizophrenia in velo-cardio-facial syndromeSchizophr Res2004702–322323215329299
- BassettASChowEWAbdelMalikPGheorghiuMHustedJWeksbergRThe schizophrenia phenotype in 22q11 deletion syndromeAm J Psychiatry200316091580158612944331
- ChowEWWatsonMYoungDABassettASNeurocognitive profile in 22q11 deletion syndrome and schizophreniaSchizophr Res2006871–327027816753283
- StoddardJNiendamTHendrenRCarterCSimonTJAttenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndromeSchizophr Res20101181–311812120056393
- Torres-JuanLRosellJSanchez-de-la-TorreMFiblaJHeine-SunerDAnalysis of meiotic recombination in 22q11.2, a region that frequently undergoes deletions and duplicationsBMC Med Genet200781417397557
- BassettASChowEW22q11 deletion syndrome: a genetic subtype of schizophreniaBiol Psychiatry199946788289110509171
- AntshelKMFremontWRoizenNJADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndromeJ Am Acad Child Adolesc Psychiatry200645559660316670654
- BassettASChowEWHustedJPremature death in adults with 22q11.2 deletion syndromeJ Med Genet200946532433019246480
- BooijJvan AmelsvoortTBootECo-occurrence of early-onset Parkinson disease and 22q11.2 deletion syndrome: Potential role for dopamine transporter imagingAm J Med Genet A2010152A112937293820949509
- De SmedtBSwillenAVerschaffelLGhesquierePMathematical learning disabilities in children with 22q11.2 deletion syndrome: a reviewDev Disabil Res Rev200915141019213009
- GreenTGothelfDGlaserBPsychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndromeJ Am Acad Child Adolesc Psychiatry200948111060106819797984
- HercherLBruennerGLiving with a child at risk for psychotic illness: the experience of parents coping with 22q11 deletion syndrome: an exploratory studyAm J Med Genet A2008146A182355236018698620
- JolinEMWellerRAJessaniNRZackaiEHMcDonald-McGinnDMWellerEBAffective disorders and other psychiatric diagnoses in children and adolescents with 22q11.2 Deletion SyndromeJ Affect Disord20091191–317718019269692
- KapadiaRKBassettASRecognizing a common genetic syndrome: 22q11.2 deletion syndromeCMAJ2008178439139318268261
- LawrenceSMcDonald-McGinnDMZackaiESullivanKEThrombocytopenia in patients with chromosome 22q11.2 deletion syndromeJ Pediatr2003143227727812970648
- McDonald-McGinnDMKirschnerRGoldmuntzEThe Philadelphia story: the 22q11.2 deletion: report on 250 patientsGenet Couns1999101112410191425
- McDonald-McGinnDMZackaiEHGenetic counseling for the 22q11.2 deletionDev Disabil Res Rev2008141697418636638
- OskarsdottirSPerssonCErikssonBOFasthAPresenting phenotype in 100 children with the 22q11 deletion syndromeEur J Pediatr2005164314615315565286
- RyanAKGoodshipJAWilsonDISpectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative studyJ Med Genet199734107988049350810
- VantrappenGDevriendtKSwillenAPresenting symptoms and clinical features in 130 patients with the velo-cardio-facial syndrome. The Leuven experienceGenet Couns19991013910191424
- ZaleskiCBassettASTamKShugarALChowEWMcPhersonEThe co-occurrence of early onset Parkinson disease and 22q11.2 deletion syndromeAm J Med Genet A2009149A352552819208384
- ChowEWHustedJWeksbergRBassettASPostmaturity in a genetic subtype of schizophreniaActa Psychiatr Scand2003108426026812956826
- GothelfDEliezSThompsonTCOMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndromeNat Neurosci20058111500150216234808
- VorstmanJAChowEWOphoffRAAssociation of the PIK4CA schizophrenia-susceptibility gene in adults with the 22q11.2 deletion syndromeAm J Med Genet B Neuropsychiatr Genet2009150B343043318646052
- GuoTMcDonald-McGinnDBlonskaAGenotype and cardiovascular phenotype correlations with TBX1 in 1,022 velo-cardiofacial/digeorge/22q11.2 deletion syndrome patientsHum Mutat201132111278128921796729
- SwabyJASilversidesCKBekeschusSCComplex congenital heart disease in unaffected relatives of adults with 22q11.2 deletion syndromeAm J Cardiol2011107346647121257016
- BassettASCaluseriuOWeksbergRYoungDAChowEWCatechol- O-methyl transferase and expression of schizophrenia in 73 adults with 22q11 deletion syndromeBiol Psychiatry200761101135114017217925
- BootEBooijJAbelingNDopamine metabolism in adults with 22q11 deletion syndrome, with and without schizophrenia – relationship with COMT Val(1)/(1)Met polymorphism, gender and symptomatologyJ Psychopharmacol201125788889521447540
- IkedaMWilliamsNWilliamsHJFailure to confirm association between PIK4CA and psychosis in 22q11.2 deletion syndromeAm J Med Genet B Neuropsychiatr Genet2010153B498098220052689
- AntshelKMShprintzenRFremontWHigginsAMFaraoneSVKatesWRCognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up studyJ Am Acad Child Adolesc Psychiatry201049433334420410726
- GothelfDFeinsteinCThompsonTRisk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndromeAm J Psychiatry2007164466366917403981
- GothelfDHoeftFUenoTDevelopmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndromeJ Psychiatr Res201145332233120817203
- ChowEWHoAWeiCVoormolenEHCrawleyAPBassettASAssociation of schizophrenia in 22q11.2 deletion syndrome and gray matter volumetric deficits in the superior temporal gyrusAm J Psychiatry2011168552252921362743
- EmsleyRChilizaBSchoemanRPredictors of long-term outcome in schizophreniaCurr Opin Psychiatry200821217317718332666
- BrometEJKotovRFochtmannLJDiagnostic shifts during the decade following first admission for psychosisAm J Psychiatry2011168111186119421676994
- WernerSStawskiMKnowledge, attitudes and training of professionals on dual diagnosis of intellectual disability and psychiatric disorderJ Intellect Disabil Res2011
- BadnerJAGershonESMeta-analysis of whole-genome linkage scans of bipolar disorder and schizophreniaMol Psychiatry20027440541111986984
- LewisCMLevinsonDFWiseLHGenome scan meta-analysis of schizophrenia and bipolar disorder, part II: SchizophreniaAm J Hum Genet2003731344812802786
- NgMYLevinsonDFFaraoneSVMeta-analysis of 32 genome-wide linkage studies of schizophreniaMol Psychiatry200914877478519349958
- OwenMJWilliamsNMO’DonovanMCThe molecular genetics of schizophrenia: new findings promise new insightsMol Psychiatry200491142714581932
- WaterwortDMBassettASBrzustowiczLMRecent advances in the genetics of schizophreniaCell Mol Life Sci200259233134811915947
- BlouinJLDombroskiBANathSKSchizophrenia susceptibility loci on chromosomes 13q32 and 8p21Nat Genet199820170739731535
- BrzustowiczLMHonerWGChowEWLinkage of familial schizophrenia to chromosome 13q32Am J Hum Genet19996541096110310486329
- BrzustowiczLMHodgkinsonKAChowEWHonerWGBassettASLocation of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22Science2000288546667868210784452
- EkelundJHovattaIParkerAChromosome 1 loci in Finnish schizophrenia familiesHum Mol Genet200110151611161711468279
- GurlingHMKalsiGBrynjolfsonJGenomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1- 11.23Am J Hum Genet200168366167311179014
- HolmansPARileyBPulverAEGenomewide linkage scan of schizophrenia in a large multicenter pedigree sample using single nucleotide polymorphismsMol Psychiatry200914878679519223858
- LinMWShamPHwuHGCollierDMurrayRPowellJFSuggestive evidence for linkage of schizophrenia to markers on chromosome 13 in Caucasian but not Oriental populationsHum Genet19979934174209050933
- ShawSHKellyMSmithABA genome-wide search for schizophrenia susceptibility genesAm J Med Genet19988153643769754621
- StefanssonHSigurdssonESteinthorsdottirVNeuregulin 1 and susceptibility to schizophreniaAm J Hum Genet200271487789212145742
- StraubREMacLeanCJO’NeillFAWalshDKendlerKSSupport for a possible schizophrenia vulnerability locus in region 5q22-31 in Irish familiesMol Psychiatry1997221481559106240
- VielandVJThermometers: something for statistical geneticists to think aboutHum Hered200661314415616770079
- FaraoneSVHwuHGLiuCMGenome scan of Han Chinese schizophrenia families from Taiwan: confirmation of linkage to 10q22.3Am J Psychiatry2006163101760176617012687
- GoesFSZandiPPMiaoKMood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33Am J Psychiatry2007164223624717267786
- SuarezBKDuanJSandersARGenomewide linkage scan of 409 European-ancestry and African American families with schizophrenia: suggestive evidence of linkage at 8p23.3-p21.2 and 11p13.1-q14.1 in the combined sampleAm J Hum Genet200678231533316400611
- WrattenNSMemoliHHuangYIdentification of a schizophrenia- associated functional noncoding variant in NOS1APAm J Psychiatry2009166443444119255043
- GershonESAlliey-RodriguezNLiuCAfter GWAS: searching for genetic risk for schizophrenia and bipolar disorderAm J Psychiatry2011168325325621285144
- AthanasiuLMattingsdalMKahlerAKGene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohortJ Psychiatr Res2010441274875320185149
- LenczTMorganTVAthanasiouMConverging evidence for a pseudoautosomal cytokine receptor gene locus in schizophreniaMol Psychiatry200712657258017522711
- NeedACGeDWealeMEA genome-wide investigation of SNPs and CNVs in schizophreniaPLoS Genet200952e100037319197363
- SchanzeDEkiciABGawlikMPfuhlmannBReisAStoberGEvaluation of risk loci for schizophrenia derived from genome-wide association studies in a German populationAm J Med Genet B Neuropsychiatr Genet2011156219820321302348
- SullivanPFLinDTzengJYGenomewide association for schizophrenia in the CATIE study: results of stage 1Mol Psychiatry200813657058418347602
- O’DonovanMCCraddockNNortonNIdentification of loci associated with schizophrenia by genome-wide association and followupNat Genet20084091053105518677311
- PurcellSMWrayNRStoneJLCommon polygenic variation contributes to risk of schizophrenia and bipolar disorderNature2009460725674875219571811
- RietschelMMattheisenMDegenhardtFAssociation between genetic variation in a region on chromosome 11 and schizophrenia in large samples from EuropeMol Psychiatry2011
- RipkeSSandersARKendlerKSGenome-wide association study identifies five new schizophrenia lociNat Genet2011431096997621926974
- ShiJLevinsonDFDuanJCommon variants on chromosome 6p22.1 are associated with schizophreniaNature2009460725675375719571809
- StefanssonHOphoffRASteinbergSCommon variants conferring risk of schizophreniaNature2009460725674474719571808
- YamadaKIwayamaYHattoriEGenome-wide association study of schizophrenia in Japanese populationPLoS One201166e2046821674006
- YueWHWangHFSunLDGenome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2Nat Genet201143121228123122037552
- CroweRRThompsonJSFlinkRWeinbergerBHLA antigens and schizophreniaArch Gen Psychiatry1979362231233420545
- EberhardGFranzenGLowBSchizophrenia susceptibility and HL-A antigenNeuropsychobiology1975142112171226230
- ShiYLiZXuQCommon variants on 8p12 and 1q24.2 confer risk of schizophreniaNat Genet201143121224122722037555
- SteinbergSde JongSAndreassenOACommon variants at VRK2 and TCF4 conferring risk of schizophreniaHum Mol Genet201120204076408121791550
- WangKSLiuXFAragamNA genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorderSchizophr Res20101241–319219920889312
- WilliamsHJNortonNDwyerSFine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorderMol Psychiatry201116442944120368704
- GlessnerJTReillyMPKimCEStrong synaptic transmission impact by copy number variations in schizophreniaProc Natl Acad Sci U S A201010723105841058920489179
- ArranzMJRiveraMMunroJCPharmacogenetics of response to antipsychotics in patients with schizophreniaCNS Drugs2011251193396922054119
- CacabelosRHashimotoRTakedaMPharmacogenomics of antipsychotics efficacy for schizophreniaPsychiatry Clin Neurosci201165131921265934
- HodgkinsonKAMurphyJO’NeillSBrzustowiczLBassettASGenetic counselling for schizophrenia in the era of molecular geneticsCan J Psychiatry200146212313011280080
- AllenNCBagadeSMcQueenMBSystematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene databaseNat Genet200840782783418583979
- GirardSLGauthierJNoreauAIncreased exonic de novo mutation rate in individuals with schizophreniaNat Genet201143986086321743468
- XuBRoosJLDexheimerPExome sequencing supports a de novo mutational paradigm for schizophreniaNat Genet201143986486821822266
- CookEHJrSchererSWCopy-number variations associated with neuropsychiatric conditionsNature2008455721591992318923514
- IafrateAJFeukLRiveraMNDetection of large-scale variation in the human genomeNat Genet200436994995115286789
- LeeCSchererSWThe clinical context of copy number variation in the human genomeExpert Rev Mol Med201012e820211047
- RedonRIshikawaSFitchKRGlobal variation in copy number in the human genomeNature2006444711844445417122850
- SebatJLakshmiBTrogeJLarge-scale copy number polymorphism in the human genomeScience2004305568352552815273396
- BassettASChromosomal aberrations and schizophrenia. AutosomesBr J Psychiatry19921613233341393302
- IngasonAKirovGGieglingIMaternally derived microduplications at 15q11-q13: implication of imprinted genes in psychotic illnessAm J Psychiatry2011168440841721324950
- GrozevaDKirovGIvanovDRare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophreniaArch Gen Psychiatry201067431832720368508
- International Schizophrenia ConsortiumRare chromosomal deletions and duplications increase risk of schizophreniaNature2008455721023724118668038
- KirovGGrozevaDNortonNSupport for the involvement of large copy number variants in the pathogenesis of schizophreniaHum Mol Genet20091881497150319181681
- KirovGPocklingtonAJHolmansPDe novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophreniaMol Psychiatry201217214215322083728
- MagriCSacchettiETraversaMNew copy number variations in schizophreniaPLoS One2010510e1342220967226
- MelhemNMiddletonFMcFaddenKCopy number variants for schizophrenia and related psychotic disorders in oceanic palau: risk and transmission in extended pedigreesBiol Psychiatry201170121115112121982423
- StefanssonHRujescuDCichonSLarge recurrent microdeletions associated with schizophreniaNature2008455721023223618668039
- Moreno-De-LucaDMulleJGKaminskyEBDeletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophreniaAm J Hum Genet201087561863021055719
- LevinsonDFDuanJOhSCopy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplicationsAm J Psychiatry2011168330231621285140
- IngasonARujescuDCichonSCopy number variations of chromosome 16p13.1 region associated with schizophreniaMol Psychiatry2011161172519786961
- Brunetti-PierriNBergJSScagliaFRecurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalitiesNat Genet200840121466147119029900
- DoornbosMSikkema-RaddatzBRuijvenkampCANine patients with a microdeletion 15q11.2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbancesEur J Med Genet2009522–310811519328872
- GreenwaySCPereiraACLinJCDe novo copy number variants identify new genes and loci in isolated sporadic tetralogy of FallotNat Genet200941893193519597493
- HannesFDSharpAJMeffordHCRecurrent reciprocal deletions and duplications of 16p13.11: the deletion is a risk factor for MR/MCA while the duplication may be a rare benign variantJ Med Genet200946422323218550696
- HarvardCStrongEMercierEUnderstanding the impact of 1q21.1 copy number variantOrphanet J Rare Dis201165421824431
- de KovelCGTrucksHHelbigIRecurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsiesBrain2010133Pt 1233219843651
- MeffordHCClauinSSharpAJRecurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsyAm J Hum Genet20078151057106917924346
- MeffordHCSharpAJBakerCRecurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypesN Engl J Med2008359161685169918784092
- MeffordHCCooperGMZerrTA method for rapid, targeted CNV genotyping identifies rare variants associated with neurocognitive diseaseGenome Res20091991579158519506092
- NagamaniSCErezAShenJClinical spectrum associated with recurrent genomic rearrangements in chromosome 17q12Eur J Hum Genet201018327828419844256
- UllmannRTurnerGKirchhoffMArray CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardationHum Mutat200728767468217480035
- WilliamsNMZaharievaIMartinARare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysisLancet201037697501401140820888040
- BassettASParental origin, DNA structure, and the schizophrenia spectrumAm J Psychiatry2011168435035321474594
- HorowitzAShifmanSRivlinNPisanteADarvasiAA survey of the 22q11 microdeletion in a large cohort of schizophrenia patientsSchizophr Res2005732–326326715653270
- WalshTMcClellanJMMcCarthySERare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophreniaScience2008320587553954318369103
- SpornAAddingtonAReissAL22q11 deletion syndrome in childhood onset schizophrenia: an updateMol Psychiatry20049322522614699434
- RapoportJLAddingtonAMFrangouSPsychMRThe neurodevelopmental model of schizophrenia: update 2005Mol Psychiatry200510543444915700048
- GirirajanSEichlerEEPhenotypic variability and genetic susceptibility to genomic disordersHum Mol Genet201019R2R176R18720807775
- CostainGChowEWRayPNBassettASCaregiver and adult patient perspectives on the importance of a diagnosis of 22q11.2 deletion syndromeJ Intellect Disabil Res2011
- HodgkinsonKPullmanDDuty to warn and genetic diseaseCan J Cardiovasc Nurs2010201121520301857
- PigginsHDSchizophrenia: zooming in on a geneNature2011471733945545621430769
- GreenEDGuyerMSCharting a course for genomic medicine from base pairs to bedsideNature2011470733320421321307933
- SchererSWDawsonGRisk factors for autism: translating genomic discoveries into diagnosticsHum Genet2011130112314821701786
- Family history and BRCA 1/2 testing for identifying women at risk for inherited breast/ovarian cancer: AACE review http://www.cdc.gov/genomics/gtesting/ACCE/fbr.htm
- MillerFAHayeemsRZBytautasJPWhat is a meaningful result? Disclosing the results of genomic research in autism to research participantsEur J Hum Genet201018886787120234389
- BassettASBuryAHodgkinsonKAHonerWGReproductive fitness in familial schizophreniaSchizophr Res19962131511608885043
- BundyHStahlDMacCabeJHA systematic review and meta-analysis of the fertility of patients with schizophrenia and their unaffected relativesActa Psychiatr Scand201112329810620958271
- MitchellPBMeiserBWildeAPredictive and diagnostic genetic testing in psychiatryClin Lab Med201030482984620832655
- TsuangDWFaraoneSVTsuangMTGenetic counseling for psychiatric disordersCurr Psychiatry Rep20013213814311276409
- FeretHConwayLAustinJCGenetic counselors’ attitudes towards individuals with schizophrenia: desire for social distance and endorsement of stereotypesPatient Educ Couns2011821697320211537
- MonacoLCConwayLValverdeKAustinJCExploring genetic counselors’ perceptions of and attitudes towards schizophreniaPublic Health Genomics2010131212619321939
- FinucaneBGenetic counseling for women with intellectual disabilitiesLeRoyBSVeachPMBartelsDMGenetic Counseling Practice: Advanced Concepts and SkillsHobokenWiley-Blackwell2010470507
- TaylorMREdwardsJGKuLLost in transition: challenges in the expanding field of adult geneticsAm J Med Genet C Semin Med Genet2006142C429430317024669
- FarwigKHarmonAGFontanaKMMervisCBMorrisCAGenetic counseling of adults with Williams syndrome: a first studyAm J Med Genet C Semin Med Genet2010154C230731520425790
- LeeCIafrateAJBrothmanARCopy number variations and clinical cytogenetic diagnosis of constitutional disordersNat Genet2007397 SupplS48S5417597782
- AustinJCPeayHLApplications and limitations of empiric data in provision of recurrence risks for schizophrenia: a practical review for healthcare professionals providing clinical psychiatric genetics consultationsClin Genet200670317718716922717
- PeayHLAustinJCHow to Talk with Families About Genetics and Psychiatric IllnessNew YorkWW Norton and Company2011
- SimpsonSABessonJAlexanderDAllanKJohnstonAWOne hundred requests for predictive testing for Huntington’s diseaseClin Genet19924163263301535837
- TylerAMorrisMLazarouLMeredithLMyringJHarperPPresymptomatic testing for Huntington’s disease in Wales 1987–1990Br J Psychiatry19921614814881393334
- XuBWoodroffeARodriguez-MurilloLElucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scansProc Natl Acad Sci U S A200910639167461675119805367
- Al-ChalabiALewisCMModelling the effects of penetrance and family size on rates of sporadic and familial diseaseHum Hered201171428128821846995
- YangJVisscherPMWrayNRSporadic cases are the norm for complex diseaseEur J Hum Genet20101891039104319826454
- ShulmanJMDe JagerPLFeanyMBParkinson’s disease: genetics and pathogenesisAnnu Rev Pathol2011619322221034221
- GuttmacherAECollinsFSCarmonaRHThe family history – more important than everN Engl J Med2004351222333233615564550
- PapadimitriouGNDikeosDGHow does recent knowledge on the heredity of schizophrenia affect genetic counseling?Curr Psychiatry Rep20035423924012857525
- ReveleyAGenetic counselling for schizophreniaBr J Psychiatry19851471071124041685
- TsuangMTGenetic counseling for psychiatric patients and their familiesAm J Psychiatry19781351214651475717559
- HunterMJHippmanCHonerWGAustinJCGenetic counseling for schizophrenia: a review of referrals to a provincial medical genetics program from 1968 to 2007Am J Med Genet A2010152A114715220034078
- LyusVLThe importance of genetic counseling for individuals with schizophrenia and their relatives: potential clients’ opinions and experiencesAm J Med Genet B Neuropsychiatr Genet2007144B81014102117525978
- AustinJCHonerWGPsychiatric genetic counselling for parents of individuals affected with psychotic disorders: a pilot studyEarly Interv Psychiatry200822808921352137
- TorreyEFSurviving Schizophrenia: A Manual for Families, Patients, and Providers5th edNew York, NYCollins2006
- BruderCEPiotrowskiAGijsbersAAPhenotypically concordant and discordant monozygotic twins display different DNA copy-number- variation profilesAm J Hum Genet200882376377118304490
- DempsterELPidsleyRSchalkwykLCDisease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorderHum Mol Genet201120244786479621908516
- KatoTIwamotoKKakiuchiCKuratomiGOkazakiYGenetic or epigenetic difference causing discordance between monozygotic twins as a clue to molecular basis of mental disordersMol Psychiatry200510762263015838537
- MaitiSKumarKHCastellaniCAO’ReillyRSinghSMOntogenetic de novo copy number variations (CNVs) as a source of genetic individuality: studies on two families with MZD twins for schizophreniaPLoS One201163e1712521399695
- HustedJAAhmedRChowEWBrzustowiczLMBassettASChildhood trauma and genetic factors in familial schizophrenia associated with the NOS1AP geneSchizophr Res20101211–318719220541371
- van OsJKenisGRuttenBPThe environment and schizophreniaNature2010468732120321221068828
- KurnitDMLaytonWMMatthysseSGenetics, chance, and morphogenesisAm J Hum Genet19874169799953687945
- RuvinskyAGenetics and RandomnessBoca Raton, FLCRC Press2010
- LichtensteinPBjorkCHultmanCMScolnickESklarPSullivanPFRecurrence risks for schizophrenia in a Swedish national cohortPsychol Med200636101417142516863597
- SlatkinMGenotype-specific recurrence risks as indicators of the genetic architecture of complex diseasesAm J Hum Genet200883112012618597734
- WrayNRGoddardMEVisscherPMPrediction of individual genetic risk to disease from genome-wide association studiesGenome Res200717101520152817785532
- SlatkinMEpigenetic inheritance and the missing heritability problemGenetics2009182384585019416939
- LanderESInitial impact of the sequencing of the human genomeNature2011470733318719721307931
- DrmanacRThe advent of personal genome sequencingGenet Med201113318819021311341
- GreenspanSIBrazeltonTBCorderoJGuidelines for early identification, screening, and clinical management of children with autism spectrum disordersPediatrics2008121482883018381546
- MilesJHAutism spectrum disorders – a genetics reviewGenet Med201113427829421358411
- KjeldsenMJKyvikKOChristensenKFriisMLGenetic and environmental factors in epilepsy: a population-based study of 11900 Danish twin pairsEpilepsy Res2001442–316717811325572
- MulleyJCDibbensLMGenetic variations and associated pathophysiology in the management of epilepsyThe Application of Clinical Genetics20114113125
- PalDKPongAWChungWKGenetic evaluation and counseling for epilepsyNat Rev Neurol20106844545320647993
- PoduriALowensteinDEpilepsy genetics – past, present, and futureCurr Opin Genet Dev201121332533221277190
- SharmaKGenetic epidemiology of epilepsy: a twin studyNeurol India2005531939815805664
- DoCBTungJYDorfmanEWeb-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s diseasePLoS Genet201176e100214121738487
- ElbazABowerJHMaraganoreDMRisk tables for parkinsonism and Parkinson’s diseaseJ Clin Epidemiol2002551253111781119
- SchulteCGasserTGenetic basis of Parkinson’s disease: inheritance, penetrance, and expressionThe Application of Clinical Genetics2011467–8067
- ToftMRossOACopy number variation in Parkinson’s diseaseGenome Med2010296220828427
- BallardCGauthierSCorbettABrayneCAarslandDJonesEAlzheimer’s diseaseLancet201137797701019103121371747
- BertramLLillCMTanziREThe genetics of Alzheimer disease: back to the futureNeuron201068227028120955934
- GatzMReynoldsCAFratiglioniLRole of genes and environments for explaining Alzheimer diseaseArch Gen Psychiatry200663216817416461860
- GoldmanJSHahnSECataniaJWGenetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic CounselorsGenet Med201113659760521577118
- CoulterMEMillerDTHarrisDJChromosomal microarray testing influences medical managementGenet Med201113977077621716121
- Basel-VanagaiteLGoldberg-SternHMimouni-BlochAShkalimVBohmDKohlhaseJAn emerging 1q21.1 deletion-associated neurodevelopmental phenotypeJ Child Neurol201126111311621212457
- BrunetAArmengolLHeineDBAC array CGH in patients with Velocardiofacial syndrome-like features reveals genomic aberrations on chromosome region 1q21.1BMC Med Genet20091014420030804
- ChristiansenJDyckJDElyasBGChromosome 1q21.1 contiguous gene deletion is associated with congenital heart diseaseCirc Res200494111429143515117819
- IkedaMAleksicBKirovGCopy number variation in schizophrenia in the Japanese populationBiol Psychiatry201067328328619880096
- SahooTTheisenARosenfeldJACopy number variants of schizophrenia susceptibility loci are associated with a spectrum of speech and developmental delays and behavior problemsGenet Med2011131086888021792059
- VuTHCoccaroEFEichlerEEGirirajanSGenomic architecture of aggression: rare copy number variants in intermittent explosive disorderAm J Med Genet B Neuropsychiatr Genet2011156B780881621812102
- BallifBCTheisenACoppingerJExpanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplicationMol Cytogenet20081818471269
- Clayton-SmithJGiblinCSmithRADunnCWillattLFamilial 3q29 microdeletion syndrome providing further evidence of involvement of the 3q29 region in bipolar disorderClin Dysmorphol201019312813220453639
- DigilioMCBernardiniLMingarelliR3q29 Microdeletion: a mental retardation disorder unassociated with a recognizable phenotype in two mother–daughter pairsAm J Med Genet A2009149A81777178119610115
- MulleJGDoddAFMcGrathJAMicrodeletions of 3q29 confer high risk for schizophreniaAm J Hum Genet201087222923620691406
- Quintero-RiveraFSharifi-HannauerPMartinez-AgostoJAAutistic and psychiatric findings associated with the 3q29 microdeletion syndrome: case report and reviewAm J Med Genet A2010152A102459246720830797
- VacicVMcCarthySMalhotraDDuplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophreniaNature2011471733949950321346763
- WillattLCoxJBarberJ3q29 microdeletion syndrome: clinical and molecular characterization of a new syndromeAm J Hum Genet200577115416015918153
- BrowneCEDennisNRMaherEInherited interstitial duplications of proximal 15q: genotype-phenotype correlationsAm J Hum Genet1997616134213529399882
- ChamberlainSJLalandeMNeurodevelopmental disorders involving genomic imprinting at human chromosome 15q11-q13Neurobiol Dis2010391132020304067
- CookEHJrLindgrenVLeventhalBLAutism or atypical autism in maternally but not paternally derived proximal 15q duplicationAm J Hum Genet19976049289349106540
- GurrieriFBattagliaATorrisiLPervasive developmental disorder and epilepsy due to maternally derived duplication of 15q11-q13Neurology19995281694169710331703
- HogartAWuDLaSalleJMSchanenNCThe comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13Neurobiol Dis201038218119118840528
- MichelsonMEdenAVinklerCFamilial partial trisomy 15q11-13 presenting as intractable epilepsy in the child and schizophrenia in the motherEur J Paediatr Neurol201115323023321145272
- ThomasJAJohnsonJPeterson KraaiTLGenetic and clinical characterization of patients with an interstitial duplication 15q11-q13, emphasizing behavioral phenotype and response to treatmentAm J Med Genet A2003119A211112012749048
- Ben-ShacharSLanpherBGermanJRMicrodeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disordersJ Med Genet200946638238819289393
- van BonBWMeffordHCMentenBFurther delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcomeJ Med Genet200946851152319372089
- CubellsJFDeoreoEHHarveyPDPharmaco-genetically guided treatment of recurrent rage outbursts in an adult male with 15q13.3 deletion syndromeAm J Med Genet A2011155A480581021594999
- DibbensLMMullenSHelbigIFamilial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: precedent for disorders with complex inheritanceHum Mol Genet200918193626363119592580
- HelbigIMeffordHCSharpAJ15q13.3 microdeletions increase risk of idiopathic generalized epilepsyNat Genet200941216016219136953
- Masurel-PauletAAndrieuxJCallierPDelineation of 15q13.3 microdeletionsClin Genet201078214916120236110
- MillerDTShenYWeissLAMicrodeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disordersJ Med Genet200946424224818805830
- PagnamentaATWingKSadighi AkhaEA 15q13.3 microdeletion segregating with autismEur J Hum Genet200917568769219050728
- SharpAJMeffordHCLiKA recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizuresNat Genet200840332232818278044
- ShinawiMSchaafCPBhattSSA small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypesNat Genet200941121269127119898479
- BedoyanJKKumarRASudiJDuplication 16p11.2 in a child with infantile seizure disorderAm J Med Genet A2010152A61567157420503337
- FernandezBARobertsWChungBPhenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorderJ Med Genet201047319520319755429
- LionelACCrosbieJBarbosaNRare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHDSci Transl Med201139595ra75
- McCarthySEMakarovVKirovGMicroduplications of 16p11.2 are associated with schizophreniaNat Genet200941111223122719855392
- MarshallCRNoorAVincentJBStructural variation of chromosomes in autism spectrum disorderAm J Hum Genet200882247748818252227
- RosenfeldJACoppingerJBejjaniBASpeech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplicationsJ Neurodev Disord201021263821731881
- SchaafCPGoin-KochelRPNowellKPExpanding the clinical spectrum of the 16p11.2 chromosomal rearrangements: three patients with syringomyeliaEur J Hum Genet201119215215620959866
- ShenYDiesKAHolmIAClinical genetic testing for patients with autism spectrum disordersPediatrics20101254e727e73520231187
- ShinawiMLiuPKangSHRecurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head sizeJ Med Genet201047533234119914906
- WeissLAShenYKornJMAssociation between microdeletion and microduplication at 16p11.2 and autismN Engl J Med2008358766767518184952
- GoodshipJCrossILiLingJWrenCA population study of chromosome 22q11 deletions in infancyArch Dis Child19987943483519875047
- ReesEMoskvinaVOwenMJO’DonovanMCKirovGDe novo rates and selection of schizophrenia-associated copy number variantsBiol Psychiatry201170121109111421855053
- VassosECollierDAHoldenSPenetrance for copy number variants associated with schizophreniaHum Mol Genet201019173477348120587603
