Abstract
Acute intermittent porphyria (AIP) is due to a deficiency of the third enzyme, the hydroxymethylbilane synthase, in heme biosynthesis. It manifests with occasional neuropsychiatric crises associated with overproduction of porphyrin precursors, aminolevulinic acid and porphobilinogen. The clinical criteria of an acute attack include the paroxysmal nature and various combinations of symptoms, such as abdominal pain, autonomic dysfunction, hyponatremia, muscle weakness, or mental symptoms, in the absence of other obvious causes. Intensive abdominal pain without peritoneal signs, acute peripheral neuropathy, and encephalopathy usually with seizures or psychosis are the key symptoms indicating possible acute porphyria. More than fivefold elevation of urinary porphobilinogen excretion together with typical symptoms of an acute attack is sufficient to start a treatment. Currently, the prognosis of the patients with AIP is good, but physicians should be aware of a potentially fatal outcome of the disease. Mutation screening and identification of type of acute porphyria can be done at the quiescent phase of the disease. The management of patients with AIP include following strategies: A, during an acute attack: 1) treatment with heme preparations, if an acute attack is severe or moderate; 2) symptomatic treatment of autonomic dysfunctions, polyneuropathy and encephalopathy; 3) exclusion of precipitating factors; and 4) adequate nutrition and fluid therapy. B, during remission: 1) exclusion of precipitating factors (education of patients and family doctors), 2) information about on-line drug lists, and 3) mutation screening for family members and education about precipitating factors in mutation-positive family members. C, management of patients with recurrent attacks: 1) evaluation of the lifestyle, 2) evaluation of hormonal therapy in women, 3) prophylactic heme therapy, and 4) liver transplantation in patients with severe recurrent attacks. D, follow-up of the AIP patients for long-term complications: chronic hypertension, chronic kidney insufficiency, chronic pain syndrome, and hepatocellular carcinoma.
Introduction
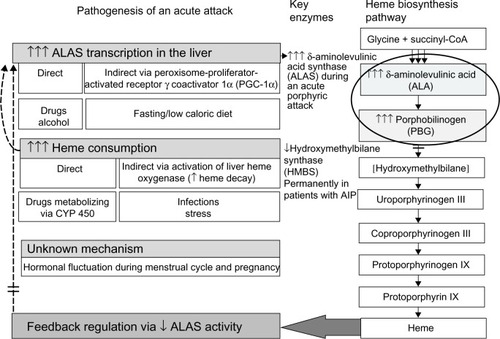
Acute intermittent porphyria (AIP) is a rare inherited metabolic disease due to a deficiency of the hydroxymethylbilane synthase (HMBS) in heme biosynthesis.Citation1 AIP manifests after the puberty with occasional neuropsychiatric crises associated with accumulation of porphyrin precursors such as δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) which are released from the liver into the circulationCitation1,Citation2 (). The diagnosis is often delayed, because clinical manifestations are unspecificCitation2 and commonly mimic acute encephalopathy or abdominal crisis of other origin.Citation3
Figure 1 Precipitating factors and pathogenesis of an acute attack in AIP.
Abbreviation: AIP, acute intermittent porphyria.
AIP is the most common type of acute porphyrias in most of the countries worldwide.Citation1 The major clinical manifestation of AIP is an acute attack, which is clinically indistinguishable from those caused by other acute porphyrias: variegate porphyria (VP) and hereditary coproporphyria (HCP).Citation2 The management of an acute attack in each disease is similar,Citation2 and thus, more specific classification of an acute porphyria can be done in remission. Photosensitivity and skin fragility found in patients with cutaneous porphyrias, VP and HCP, occur independently of acute attacks and do not occur in AIP.Citation4,Citation5
When acute porphyria is suspected, biochemical plasma or urinalyses of porphyrin precursors are mandatory to confirm the diagnosis of an acute porphyric attack.Citation2 More than five-fold elevation of urinary PBG excretion together with typical symptoms of an acute attack is sufficient to start a treatment,Citation6 but in each case other causes of abdominal crisis must be excluded before a specific treatment of an acute attack is administered. Plasma porphyrin spectrum is a valuable tool to confirm the diagnosis in the early phase and helps to identify different subtypes of acute porphyrias during an acute attack.Citation7 In AIP emission peak is only transient, and if taken late, may be negative leading to misdiagnosis. AIP and HCP cannot be distinguished by plasma analysis. Mutation screening can be done at the quiescent phase of the disease ().
Table 1 The laboratory investigations to confirm AIP and other acute porphyrias
Currently, the prognosis of patients with AIP is good even in severe attacks,Citation3,Citation8–Citation12 but physicians should be aware of a potentially fatal outcome of the disease.Citation10 During remission the majority of the patients experience no clinical symptoms. Hypertension, chronic kidney insufficiency, chronic pain syndromes, and hepatocellular carcinoma (HCC) may be long-term complications of AIP.Citation3,Citation8,Citation13–Citation15
The management of patients with AIP include following strategies:
During an acute attack: 1) treatment with heme preparations, if an acute attack is severe or moderate; 2) symptomatic treatment of autonomic dysfunctions, sensorimotor neuropathy and encephalopathy; 3) exclusion of precipitating factors; and 4) adequate nutrition and fluid therapy.
During remission: 1) exclusion of precipitating factors (education of patients and family doctors), 2) information about on-line drug lists, and 3) mutation screening for family members and education about precipitating factors in mutation-positive family members which can diminish mortality and prevent subsequent attacks among them.
Management of patients with recurrent attacks: 1) evaluation of the lifestyle, 2) evaluation of hormonal therapy in women, 3) prophylactic heme therapy, and 4) liver transplantation in AIP patients with severe recurrent attacks.
Follow-up of the AIP patients for long-term complications: chronic hypertension, chronic kidney insufficiency, chronic pain syndrome, and HCC.
Clinical manifestations and pathogenesis of an acute attack
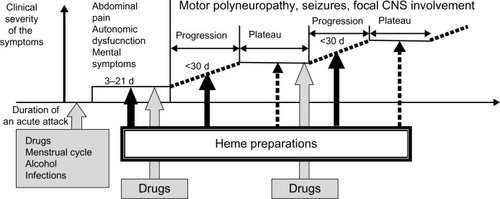
The majority of acute attacks manifest as a combination of abdominal pain, mild mental symptoms, and autonomic dysfunction.Citation2,Citation6 Both acute peripheral neuropathy and severe encephalopathy may develop, if an acute attack proceedsCitation10,Citation11,Citation16 (). It is usually iatrogenic, mainly due to administration of porphyrinogenic drugs when the diagnosis of acute porphyria is delayed.Citation10,Citation11,Citation16 Both endogenous and exogenous factors, such as certain medications, alcohol, infections, low caloric intake, or changes in sex hormone balance during the menstrual cycle or pregnancy, can provoke clinical manifestations in AIPCitation8,Citation17 ( and ). All these factors induce heme synthesis either directly or indirectly via activation of ALA synthase in the liverCitation18 resulting in accumulation of porphyrins and their precursors in the tissues and circulation.
Figure 2 Staging of an acute attack in connection with precipitating factors and recommendations of heme therapy.
Abbreviations: CNS, central nervous system; d, days.
Excess of ALA is the most potential candidate to cause neuronal damage and could be responsible for autonomic and peripheral neuropathy and encephalopathy via multiple mechanisms.Citation19 Results from both the experimental and clinical data support the direct neurotoxicity of ALA, but also modification of γ-aminobutyric acid (GABA)-ergic system due to the structural similarity of ALA and GABA/glutamate,Citation20 as well as formation of free radicals and reactive oxygen species from ALACitation21 may play a role in the pathogenesis of an acute attack.
Heme preparations, in the current treatment, lead to a rapid decrease of synthesis of porphyrin precursors via negative feedback ().Citation6 Reduced transcription of ALA synthase in the liver achieved by heme results in cessation of an acute attack within few days.Citation6 Dose-dependent administration of glucose has also been shown to downregulate ALA synthase in experimental conditions via peroxisome-proliferator-activated receptor γ coactivator 1α (PGC-1α), a protein which directly induces transcription of ALA synthase 1.Citation22 Subsequently, glucose infusions have been used to prevent fasting and may be sufficient in mild attacks. Despite the fact that recombinant human-HMBS-enzyme (rh-HMBS) therapy decreased the plasma level of PBG rapidly, it had no effect on the ALA levelCitation23 or the patients’ acute symptoms. In contrast, liver transplantation immediately corrects porphyrin metabolism to normal demonstrating the dominant role of liver as a source of ALA.Citation24
The exact mechanism of cyclic attacks in women is unknown. Despite the level of sex hormones is at the highest during the second and third trimester, acute attacks are rare during pregnancy.Citation3,Citation8,Citation17,Citation25 Although, especially, progesterone is known to be a potent inducer of ALA synthase,Citation26 the direct role of sex hormones as sole precipitating factors is unlikely.Citation27 Moreover, cyclical attacks occur mainly in premenstruum when the levels of estrogen and progesterone fluctuate the most, and usually are resolved during early menstruation.Citation8,Citation17 Individual variation in the progesterone metabolism may play a role in clinical manifestations of AIP.Citation28 Cytochrome-P450 activities in the liver also vary individually and can result in an abnormal level or ratio of sex hormones affecting the feedback mechanism to hypothalamus. Several neurotransmitters control the menstrual cycle through the regulation of pulsatile release of gonadotropin-releasing hormone (GnRH) and other clock mechanisms in the hypothalamus.Citation29 This interaction may activate abnormal liver metabolism,Citation30 and consequently precipitate acute attacks by the central mechanism making AIP a central nervous system disorder in addition to a liver disease.Citation27
Diagnosis of AIP
The clinical criteria of an acute attack include the paroxysmal nature of the symptoms with abdominal or back pain associated with one or more signs of autonomic dysfunction, hyponatremia, muscle weakness, or mental symptomsCitation6 ().
Table 2 The key symptoms indicating acute porphyria
The biochemical criteria of an acute attack include more than a fivefold increase of urinary PBG excretion (), which can be detected by a simple Watson-Schwartz or Hoesch qualitative test.Citation31 The results should be confirmed by a quantitative measurement of urinary PBG, since false positive results in these screening tests are possible, especially, if perchloric acid instead of amyl alcohol is used as an extract.Citation31–Citation33 If the urine samples are not sheltered from the light, urinalysis may become false negative.Citation31–Citation33
Urinary excretion of PBG is elevated in 88% of the patients with AIP in remission.Citation34 During an acute attack PBG excretion increases commonly at least two- to fourfold from the values found in remission.Citation34 Urinary ALA is always increased during an acute attack but remains elevated only in 61% of AIP cases in remission.Citation34 In AIP, urinary excretion of uroporphyrins is increased, including both I and III isomers, and exceeds that of coproporphyrins I and III.Citation34
Of note, abnormal metabolism of porphyrin and their precursors may also be detected in patients with hepatopathy or heavy metal intoxications.Citation31,Citation35 The clinical manifestations may even resemble AIP,Citation36 but biochemically only a mild to moderate coproporphyrinuria is present, and porphyrin precursors are commonly only transiently elevated.Citation35,Citation36 If urinary ALA level exceeds that of PBG significantly, lead intoxication should be excluded.Citation31
In AIP, plasma porphyrin emission spectrum test with excitation wave length of 405 nm shows a peak at 615–620 nm during an acute attack similar to HCP but it can be less frequently found in remission.Citation32,Citation34 Emission peak is due to porphyrins’ ability to absorb light at wave length around 400 nm and their emission as red fluorescence, at around 600 nm.Citation37 Plasma emission spectrum test is used mainly to exclude symptomatic V P, since it has a unique 624–627 nm spectrum due to protein-associated plasma porphyrins.Citation31,Citation32,Citation38 Of note, emission peak is commonly negative at the asymptomatic phase of VP.
Around 20% of the patients with AIP have moderately increased excretion of fecal protoporphyrin, which is less prominent than that of VP patients.Citation5,Citation34 Since fecal coproporphyrin level is usually normal in AIP,Citation34 more than tenfold excretion of coproporphyrin (isoform III:I >2) in feces together with protoporphyrin suggests HCP.Citation4
In 84%–95% of patients with AIP,Citation34,Citation39 erythrocyte HMBS (Erc-HMBS) activity has been lower than normal. In the variant form of AIP (5%–16% of all patients),Citation34,Citation39 Erc-HMBS activity was normal due to an alternative splicing of the HMBS gene in erythroid cells.Citation40 HMBS activity should be assayed in remission, since erythropoiesis may be enhanced during an attack as well as in hypochromic or hemolytic anemias and hepatopathy.Citation33,Citation41 In contrast, it can be decreased in non-porphyric individuals with sideropenia.Citation33
DNA analysis is the most reliable method to confirm AIP in the patients and their symptom-free relatives.Citation34,Citation42 The direct sequencing of the HMBS gene is used to identify a mutation in the proband and the asymptomatic gene carriers among the family members.Citation42 The sensitivity of the mutation analysis is 90%–100%.Citation34,Citation43,Citation44 To date, 391 mutations have been reported in the HMBS gene,Citation45 and therefore DNA testing in an index case of a family is perhaps more laborious and time consuming, but afterward mutation analysis may easily reveal several family members at risk.
Treatment of an acute attack
Current treatment options include heme preparations during an acute attack, which may be life-saving, especially if encephalopathy or polyneuropathy develop.Citation2 The treatment should be started immediately during a severe or moderate acute attack after the demonstration of typical symptoms of acute porphyria and more than fivefold elevation of urine PBG shown by qualitative tests.Citation6 Other causes of abdominal crises and neuropsychiatric symptoms often demanding other specific and rapid interventions should always be excluded.
Only around 30%–50% of the patients with a mutation in the HMBS gene have mild or moderate clinical symptoms of AIP during their life span.Citation8,Citation17,Citation46 Less commonly, around 3%–5% of the patients with AIPCitation17,Citation47 have recurrent severe attacks, and no time for neuronal recovery. These patients are at a high risk for chronic pain syndrome. The onset and clinical outcome of an attack is commonly influenced by several exogenous factors simultaneously, and endogenous factors, such as the residual activity of a mutated protein, individual differences in other metabolic pathways in the liver and in neuronal protection capacity, may modify the clinical outcome.Citation12,Citation34
Heme
Heme preparations have been used for acute attacks for more than three decades without tolerance.Citation3,Citation6,Citation48,Citation49 Hemin is isolated and purified from human red cell concentrates. In Europe, Asia, and South Africa, hemin is commercially available as heme arginate (Normosang®, Orphan Europe SARL, Puteaux, France) and in Northern America as lyophilized hematin (Panhematin®, Ovation Pharmaceuticals Inc., Deerfield, IL, USA).
In an open series of 22 patients, the patients treated with heme arginateCitation6 recovered more rapidly in comparison with those treated with glucose infusions in the earlier series.Citation50–Citation53 Safety and efficiency of lyophilized hematin has also been demonstrated in six open-labeled studies involving over 200 AIP patients.Citation48 The only study using a placebo-controlled series found insignificant benefit of heme arginate for the analgesic requirement, pain score, and duration of the hospital stay.Citation54 The validity of the trial has been questionedCitation6 due to small number of patients (eleven patients treated with heme and ten with a placebo), a high proportion of the patients with peripheral neuropathy in this series (43%), delayed administration of preparation (>2 days after admission), and difficulties in arranging a placebo, which resembles heme arginate.
Heme arginate and lyophilized hematin are usually infused daily (3–4 mg/kg) into a large peripheral vein or venous access port for 3–4 consequent days, but a repetitive course may be required if porphyric symptoms are still progressingCitation6,Citation48 (). Concentrated solution of heme arginate is mixed with 100 mL physiological saline, and lyophilized hematin is reconstituted with sterile water before infusion.Citation6,Citation48,Citation55 Lyophilized hematin should be used immediately after reconstitution. Heme arginate should also be used soon after dilution, since it becomes unstable and may aggregate. In both cases, addition of human serum albumin may be beneficial in order to diminish the risk of phlebitis at the site of infusion. After infusion, the vein should be washed with saline for 10–15 minutes. For the long-term use of heme, a central access catheter (tunneled catheter or portacat) may be useful. Low molecular weight heparin can be used subcutaneously to prevent or treat thrombophlebitis.
Table 3 Clinical manifestations and treatment of an acute attack
The treatment with heme preparations should be started without delay, but even in the late stage of progressing motor neuropathy, it is efficient.Citation10 If a patient’s neurological condition has stabilized (plateau phase, ), an additional treatment with heme is rarely necessary but other causes such as infections may deteriorate the patient’s clinical condition and should be treated properly.
The reported side effects include mild coagulopathy,Citation56 thrombophlebitis,Citation6 and anaphylactic shock in one case.Citation57
High carbohydrate loading and supportive treatment
The treatment of acute attacks with high carbohydrate diet or infusions (300–500 g/day) has been in use in order to downregulate the activity of ALA synthase and prevent fasting.Citation58 High dose of glucose should be infused continuously 24 hours per day with an automatic syringe, and blood sugar level should be monitored regularly to avoid hyper- or hypoglycemia causing additional neurological complications. If a patient is able to eat, carbohydrate rich meals may similarly have a beneficial effect.
Currently, the use of glucose is limited only to mild attacks (ie, mild pain, no paresis, seizures, or hyponatremia) according to the guidelines for the treatment of an acute attack in the USA and South Africa, or if heme arginate or hematin are not available locallyCitation11,Citation59 (). In mild cases, the dose of glucose can be lower than discussed earlier. If glucose infusions do not result in clinical remission within a day or two, or if an acute attack is severe at the onset, heme preparations should be used. Mild or clearly resolving attacks with minor pain or anxiety may be treated symptomatically.Citation60
Low to moderate dose of glucose and saline infusions should be used as supportive treatment to prevent dehydration and during fasting if the patient is unable to drink.Citation49 The amount of daily fluids may vary from restricted fluid intake in a case of inappropriate antidiuretic hormone secretion to rapid restoration of intravascular volume and correction of electrolyte disturbances in rhabdomyolysis-induced renal failure.Citation61 Thus, fluid restoration must be tailored individually and careful monitoring of water and electrolyte balance, including sodium, potassium, and magnesium, and renal function should be done (). Mild to severe hyponatremia is a rather common phenomenon (25%–60%) during an acute attack,Citation10,Citation11,Citation50–Citation53 and should be corrected slowly (<12 mmol/L/24 h)Citation61,Citation62 because of potential pontine myelinolysis, a condition also described in a patient with AIP.Citation62
Table 4 Signs, symptoms, and metabolites followed during an acute attack
Rhabdomyolysis during an acute attack is often neglected,Citation61 but can be easily diagnosed by measurement of plasma myoglobin or creatinine kinase level and should be treated according to the guidelines. Acute kidney insufficiency is a rare but a severe complication of an acute attack, and may proceed to hemodialysis.Citation11,Citation15,Citation63
Elimination of precipitating factors
All potentially precipitating factors such as drugs, smoking, and alcohol should be eliminated during an acute attack. Infections should be treated promptly and caloric intake of a patient should be sufficient to avoid fasting.Citation2,Citation49,Citation59 Administration of porphyrinogenic drugs commonly results in proceeding of an acute attack to neuropathy or encephalopathy (). Several lists of potentially safe and unsafe drugs are available on the Internet.Citation64–Citation67
Some drugs, such as barbiturates and sulfonamides, are strictly forbidden since their use has been associated with several severe attacks. Most of the drugs, however, are classified as potentially porphyrinogenic, since clinical data about their safety in acute porphyria is lacking. The majority of the patients, especially asymptomatic, tolerate many drugs well; thus, total avoidance of drugs for the safety reasons leads to inappropriate treatment of patients’ other diseases. Antibiotics excluding sulfonamides, drugs for cardiovascular diseases, and pain killers are usually well tolerated in addition to preparations used in oncology. The follow-up of urinary excretion of porphyrin precursors may elucidate the effect of a drug on the heme biosynthesis but increased excretion of porphyrin metabolites without clinical symptoms should not solely determine its use. In each case the potential risk and advantage of a drug should be evaluated by a clinician.
Symptomatic therapy
Symptomatic therapy for pain, hypertension, tachycardia, nausea, and vomiting is commonly required ().Citation2,Citation49 Abdominal pain is usually intensive (visual analog scale, VAS >7 cm, scale from 0 to 10 cm)Citation68 and opiates are needed. Morphine, pethidine, oxycodone, tramadol, and fentanyl have been used without complication. Paracetamol and anti-inflammatory drugs can be used in mild cases.
Beta blockers are commonly used for tachycardia and to prevent arrhythmia. If a hypertensive crisis develops, it can be treated with beta blockers or clonidine or other drugs recommended by the current guidelines.Citation69 Nausea and vomiting can be controlled by olanzapine, lorazepam, or prochlorperazine.Citation2,Citation49 Domperidone has been used during an acute attack, but interactions with opiates and other drugs increasing the risk for arrhythmias may prevent its use. Metoclopramide has been associated with neuropathy and encephalopathy during a few acute attacks,Citation70 but some patients have used it without complications.Citation66 Urinary retention is quite common and can be treated with catheterization. Blood pressure, heart rate, pain score by visual analog scale,Citation68 and severity of the muscle weakness should be evaluated daily at the bed side. Bulbar paresis, proceeding muscle weakness, and arrhythmia are signs of progression of an acute attack and the patient should be transferred to the intensive care unit.Citation10 Respiratory insufficiency as a sign of motor neuropathy increases risk of pneumonia and may require early mechanical ventilation. Patients with paresis due to neuropathy or encephalopathy require rehabilitation therapy even in the early phase.
Epileptic seizures, which are usually generalized, can be treated with intravenous diazepam, gabapentin, levetiracetam, or propofol if status epilepticus develops.Citation2,Citation49,Citation59 Usually there is a single or few transient seizures, which associate with acute encephalopathy visualized as posterior reversible syndrome in brain magnetic resonance imaging (MRI) and does not require anticonvulsive treatment in the follow-up.Citation16 Correction of hyponatremia may be beneficial in patients with seizures ( and ). Insomnia and anxiety are usually mild and do not require additional medications but can be treated with benzodiazepines, such as lorazepam.Citation49 Hallucinations are also signs of acute encephalopathy and should be treated with phenothiazine or olanzapine.
Prognosis of an acute attack
Duration of the diagnostic delay of an acute attack correlates with a fatal outcomeCitation10 mainly due to the administration of drugs known to precipitate AIP for a misdiagnosed attack. The causes of death are related to complications of prolonged ventilation and cardiac arrest.Citation10,Citation11,Citation50,Citation51,Citation53 The majority of patients display full functional recovery even after a severe attack.Citation10
The mortality has decreased dramatically during the last decades among diagnosed AIP patients by 5%–20% during an acute attack,Citation3,Citation8–Citation11 but it is still a potentially fatal disease.
Prevention of acute attacks
Avoidance of precipitating factors
In remission, patients may tolerate medications classified as potentially unsafe and alcohol, but if porphyric symptoms appear their use should be restricted.Citation8,Citation17,Citation49 The education of patients and their family doctors about precipitating factors, including the information of safe and unsafe drugs, necessity of prompt treatment of infections, healthy lifestyle with regular normocaloric diet, avoidance of alcohol, if porphyric symptoms occur, and smoking, reduction of excessive stress are essential for patients’ prognosis.Citation8,Citation17,Citation49 In the treatment of obesity, the patients with AIP should include carbohydrates into their diet, and the weight reduction should be done slowly.
DNA diagnostics among family members is recommended before the adulthood, since it decreases the likelihood of an acute attack to 5% in patients diagnosed at the presymptomatic phase.Citation12 The prenatal screening in families with AIP is not recommended because of a low risk of severe attacks among mutation carriersCitation12 and good therapy options even if acute attacks would develop.Citation49
Prevention of cyclical recurrent attacks
In women with AIP, the menstrual cycle is the most common precipitating factor (10%–39%) manifesting usually with 1–3 months interval in premenstruum.Citation8,Citation17 In the majority cases, these attacks are mild and do not require hospitalization. Irregular cycles due to hormonal imbalance may predispose to cyclical attacks. However, in 3%–5% of the women cyclical attacks are severe and frequent (up to 2–4 weeks interval),Citation17,Citation47 which disable their lives. If the patient has recurrent attacks preventive therapy is needed.
Evaluation of the lifestyle
Usually these women have several precipitating factors simultaneously. It is important to first correct lifestyle, indicating cessation of smoking and use of alcohol or any medication or homeopathic drug which may be porphyrinogenic. Since body mass index (BMI) is commonly at the low or low normal level among women with recurrent attacks, gaining weight, at least a few kilos, may be beneficial to balance energy metabolism. Patients should avoid fasting and potential hypoglycemia by regular eating habits.
Evaluation of hormonal therapy in women
The exogenous hormonal therapy can be used, if lifestyle changes are insufficient to prevent attacks. Contraceptive pills have been used for prophylaxis of recurrent acute attacks for many years,Citation8,Citation71 but not all women respond to this therapy even after 3 months of use. We have used ethinyl estradiol-levonorgestrel preparation successfully, and the responders have tolerated them well, even for years. About 46%–58% of the Finnish and Swedish women with AIP and VP have used hormonal pills for contraception,Citation8,Citation17 and the majority of them have had no complications in remission despite use of various progesterone compounds in combination with estrogen preparations. Intrauterine devices including levonorgestrel have not been reported to cause porphyric symptoms.Citation17 Based on our results, we have let our patients use hormonal contraception under supervision.
Of note, contraceptive pills can also provoke acute attacks in 5%–14% of the women with both latent and manifest AIP (24% of selected women with previously manifest AIP),Citation8,Citation17 and as such many guidelines recommend not to use them.Citation49,Citation66,Citation67 Tolerance to progesterone preparations may vary individually but estrogen preparations, especially used for menopausal symptoms, do not usually precipitate porphyric symptoms.Citation17,Citation71 Thus, it is important that the risk for potential complications and benefits of hormonal therapy are evaluated individually by a clinician. Excretion of porphyrin precursors should be controlled and hormonal preparations should be stopped, if symptoms suggestive for acute porphyria occur during their use.
In contrast, GnRH analogs inducing ovarian suppression are not porphyrinogenic and have been reported to diminish the severity and frequency of attacks in 60%–80% of the women.Citation72–Citation75 The responders have more frequently had regular menstrual cycles and pronounce decrease in the estradiol level after the treatment with GnRH analogs when compared to the women who have been nonresponders or have responded only to a high dose of the preparation.Citation72,Citation73 Drug-induced menopause decreased the excretion of porphyrin precursors to 60% of the previous values in both groups.
The treatment should first be continued up to a few months, and thereafter its efficiency should be evaluated. If a longer period for treatment is needed, GnRH agonist can be combined with estrogen preparations to avoid osteopenia and other menopausal symptoms. Estrogen patches are preferred to avoid the first passage metabolism in the liver. Progesterone should be administered regularly to avoid increased risk of endometrial cancer during the postmenopausal estrogen therapy, but they may also induce acute attacks during the combination treatment.Citation74 Intrauterine device with levonorgestrel has been used without complications in this compound hormonal therapy,Citation74 and another option is to lower the dose of GnRH analogs to sustain natural sex-hormone level, which could prevent the long-term side effects of the GnRH agonist.Citation17,Citation74 The patients should be followed carefully and efficiently, and side effects and need for the combination therapy should be evaluated at regular intervals. Women with cyclical attacks may also become asymptomatic after their natural menopause.Citation17 Screening of urinary excretions of PBG and ALA may elucidate the biochemical activity of the disease, and maybe useful to follow.
Prophylactic heme therapy
Regular heme infusions commonly alleviate the severity and frequency of recurrent attacks.Citation2,Citation3,Citation49 The aim of this treatment is to decrease substantially the level of porphyrin precursors in plasma. The majority of patients respond well but the long-term treatment may induce dependence on the exogenic heme. As a result, a patient may have increased need for heme from monthly to twice a week infusions and withdrawal of the treatment is difficult due to severe porphyric symptoms. Frequent administrations of heme preparations may lead to thrombotic complications of superficial veins, and assessment of a permanent central venous catheter may be necessary. Moreover, long-term treatment of heme may lead to iron overload and organ damage due to hemosiderosis. Progressing hepatopathy, heart failure, and endocrinopathies may develop. Follow-up of plasma ferritin and transferrin saturation levels is necessary, and computed tomography (CT) or MRI reveal iron load in organs. Venesections are usually poorly tolerated but iron chelates may be used.
Liver transplantation in AIP patients with severe recurrent attacks
If the standard treatment is unsuccessful or quality of life is unbearable, liver transplantation is an option for severely affected patients.Citation24,Citation49 Currently, more than ten AIP patients have undergone liver (or liver-kidney) transplantation since 2004.Citation24,Citation49,Citation76 It has been successful in the majority of cases resulting in immediate correction of abnormal porphyrin metabolism and cessation of attacks and chronic pain syndrome.Citation24 Immunosuppressive drugs have been tolerated well by the patients, and their quality of life has improved dramatically. A few patients have died soon after liver transplantation mainly due to infections. Timing is also important in the liver transplantation. Patients should not wait too long in a too poor condition since the recovery from the operation and immunosuppression may associate with early complications such as infections or thromboembolic complications.Citation76 If chronic motor neuropathy and encephalopathy have been present for years, the full recovery of neuronal damage may not be reached even after total normalization of porphyrin metabolism. Of note, partial liver transplantation has not corrected biochemical abnormalities or clinical manifestations permanently and should not be done.Citation77 Moreover, transplantation of the HMBS deficient liver induced an abnormal porphyrin metabolism in a recipient.Citation77
Management of pregnancies
Previously, pregnancies were commonly complicated by acute attacks mainly during the first trimester and postpartum, and women with AIP were advised to avoid pregnancy. Currently, overall prognosis for pregnancy in AIP is good, especially if the diagnosis is known in advance and no porphyrinogenic drugs are used.Citation8,Citation17,Citation25
Cyclical attacks do not predict attacks during pregnancy, and pregnancy nowadays seldom precipitates acute attacks.Citation8,Citation17,Citation25 Heme preparations have been used during pregnancy without fetal or maternal complications.Citation11,Citation27,Citation49,Citation78 In some women, who have experienced cyclical attacks for many years, recurrent attacks have stopped after the first trimester of pregnancy, and these women have been asymptomatic thereafter.Citation17,Citation27 Thus, pregnancy may act as a hormonal therapy and could be even considered as an option for the women with cyclical attacks.
Long-term complications of AIP
Hypertension and renal insufficiency
The prevalence of hypertension is significantly higher in patients with manifest AIP than in general population.Citation3,Citation8,Citation79,Citation80 Thus, early monitoring of blood pressure and efficient treatment with antihypertensive drugs is mandatory to avoid organ complications. This is especially crucial if renal failure is already present.
Monitoring of creatinine level in the follow-up of patients with manifested AIP shows mild to moderate elevation in 10%–50% of the cases.Citation8,Citation79,Citation80 This is significantly more common than in general population.Citation3,Citation8,Citation9,Citation15,Citation63 Tubulointerstitial kidney disease has been the main presentation in a biopsy of the investigated cases.Citation8,Citation15,Citation63 It has been commonly associated with recurrent acute attacks, hypertension, and use of anti-inflammatory drugs.Citation8,Citation15,Citation63,Citation80 The main recommendation for renal protection include adequate drinking regimen, blood pressure control, and avoidance of anti-inflammatory or other nephrotoxic drugs according to the general guidelines. Patients with AIP and nephropathy, even mild, have an increased risk of deterioration of renal functions during an attack, and should be treated carefully with fluid therapy and hemodialysis if necessary.
Chronic hemodialysis may induce cutaneous symptoms in patients with AIP mimicking porphyria cutanea tarda (PCT).Citation81 Since mainly ALA and PBG but not porphyrins have been filtered during dialysis, recurrent attacks were transformed to cutaneous manifestations in one patient.Citation81 Use of high-flux hemodialysis and erythropoietin substitution might be of help since they remove plasma porphyrins and have alleviated clinical manifestation in PCT patients with end-stage renal failure.Citation82,Citation83 Kidney transplantationCitation84 or combined liver and kidney transplantationCitation85 have been done successfully and should be done whenever needed. The patients who have undergone kidney or liver transplantation should be followed up regularly because of increased risks of renal failure, hypertension, and cardiovascular complications. Metabolic changes and osteoporosis may also develop. Skin cancers and lymphomas are more common than in the general population and thus, extensive exposure to the sunlight and smoking should be avoided. Long-term complications are often multifactorial but commonly related to immunosuppressive drugs in use.Citation86–Citation88
Hepatocellular carcinoma
Several population-based studies of AIP patients have shown an evidence of a significantly increased incidence of HCC in AIP compared to general population.Citation14,Citation15,Citation89–Citation91 The highest incidence of HCC (23%–27%, more than 60-fold increased risk compared to general population) was shown in the Finnish and Swedish patients with AIP.Citation13,Citation89,Citation91 In contrast to renal insufficiency, HCC has developed in patients with both symptomatic and asymptomatic AIP, although the patients with manifested AIP are likely at higher risk. Only in one-third of the cases, HCC has coexisted with liver cirrhosis or well-known risk factors for HCC, such as viral hepatitis or heavy alcohol consumption.Citation8,Citation13–Citation15,Citation85,Citation89 Hepatoma should always be considered if a patient develops acute attacks or an abdominal pain after long-term remission or abnormally high porphyrin excretion pattern even at the old age.Citation13–Citation15 Hepatomas can be solitary or multiple in AIP patients,Citation13,Citation91 and should be treated according to current guidelines (ie, hepatic resection, local ablation therapies, multikinase inhibitors) whenever possible.Citation13,Citation15,Citation92
Since 10% of the patients with AIP die of hepatoma,Citation91 the patients with AIP most likely benefit from regular screening for HCC after 50 years of age.Citation13–Citation15 Early diagnosis of HCC is crucial for the prognosis and can be achieved only by accurate radiological imaging.
Chronic pain
Among AIP patients with recurrent acute attacks, chronic neuropathic, myalgic, or abdominal pain usually accompanied by fatigue is common (18%–22%).Citation3,Citation17 This could be due to repetitive autonomic and peripheral nerve damage during acute attacks, and the long-term hypocaloric nutrition inducing catabolic stage and muscle atrophy. The mouse model of AIP, which is in contrast to human autosomal-dominant AIP caused by compound heterozygous mutations in HMBS gene (HMBS -/-) and severe deficiency of HMBS enzyme, has developed chronic axonal motor neuropathy even in the absence of acute attacks and elevated ALA levels.Citation19 In humans, a homozygous AIP patient has developed a chronic sensorimotor neuropathy associated with severe neurodegenerative encephalopathy and mental retardation at his early childhood but no chronic pain syndrome has been present.Citation93
The treatment options include prevention of acute attacks as described earlier and prolonged treatment with gabapentinCitation94 or antidepressants, such as fluoxetine.Citation49 Prophylactic heme infusions commonly alleviate the chronic pain syndrome.Citation3,Citation49 Regular use of opiates induces tolerance to these drugs and high doses may increase the risk of side effects such as addiction, somnolence, and apnea.
Future therapies
rh-HMBS preparation has been shown to lower plasma PBG levels in symptom-free patients with increased excretion of urinary PBG.Citation23 The similar biochemical effect of rh-HMBS therapy was demonstrated in the HMBS (−/−) mouse model.Citation95 The rh-HMBS-enzyme replacement therapy, however, had no effect on the patients’ symptoms during acute attacks mainly due to constantly high plasma ALA levels.Citation23 This may be due to the fact that the rh-HMBS included only the erythroid specific form of the enzyme and was not targeted to the liver, the main site of ALA overproduction. The potential development of rh-HMBS-enzyme replacement therapy should be focused on the delivery of an active enzyme into the liver restoring the normal HMBS activity and heme biosynthesis.
Both adenoviral-mediated and nonviral HMBS gene transfers corrected the metabolic defect in the cell lines of AIP patients and HMBS (−/−) mice.Citation96–Citation98 Only virus-mediated DNA transfer into the liver was successful and corrected metabolic defect in HMBS (−/−) mice at least for a month.Citation96,Citation99 Adenoviral cytotoxicity, especially, for hepatocytes, the immunological response to viral antigens, and only transient transduction of the therapeutic gene limit potential applications of this method.Citation100 Adeno-associated viral (AAV) vector encoding HMBS and driven by liver-specific regulatory elements, such as α1-microglobulin enhancer and α1-antitrypsin promoter, is a promising alternative to the first-generation adenoviral vectors, since it demonstrated long-term transgene expression of HMBS in the liver with reduced risk of side effects.Citation101 AAV vector encoding HMBS (recombinant AAV serotype 5-codon-optimized human HMBS, rAAV5-cohHMBS) has been used in macaques.Citation102 The safety and efficiency of AAV vector containing the HMBS gene is currently investigated in the clinical trial (Phase I).Citation103
Small interfering RNA targeting liver ALA synthase results in reduction of ALA synthase mRNA and prevents acute attacks in AIP mouse model, and it is under development for human studies.Citation104 This demonstrates that downregulation of liver heme biosynthesis via direct ALA synthase inhibitor could result in a promising future therapy.
Conclusion
Before the glucose and hematin treatment of an acute attack has been introduced, the mortality rate during an acute attack of AIP was up to 66%.Citation105 Currently, the improved diagnostics, the early and effective treatment with heme preparations, and the prevention of acute attacks have decreased the mortality substantially. However, the morbidity and mortality (5%–20%) of an acute attack is still significant if the diagnosis of AIP is delayed.Citation3,Citation8,Citation10,Citation11 Thus, it is important that AIP patients are treated in the experienced centers with facilities that enable monitoring of their clinical and biochemical profile.
Current options to prevent acute attack include patients and family doctor education and family screening for HMBS mutation carriers. In patients with recurrent attacks, ovarian suppression using hormonal interventions, prophylactic heme therapy, or liver transplantation should be considered to improve the prognosis and quality of their lives. Future therapies, such as enzyme or gene delivery, should demonstrate clinical efficiency and safety before they can be applied to the clinical use. In the long-term follow-up, impaired renal function and hypertension should be treated carefully, and screening for hepatomas should be considered after 50 years of age.
Disclosure
The authors have participated in clinical trials organized by Zymenex, Alnylam, Clinuvel, and Boehringer Ingelheim, and as speakers in seminars organized by Orphan Europe. The authors report no other conflicts of interest in this work.
References
- AndersonKESassaSBishopDFDesnickRJDisorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyriasScriverCRBeaudetASlyWSValleDThe Metabolic and Molecular Bases of Inherited Disease28th edNew YorkMcGraw-Hill200129913062
- KauppinenRPorphyriasLancet200536524125215652607
- BonkovskyHLMaddukuriVCYaziciCAcute porphyrias in the USA: ceatures of 108 subjects from porphyrias consortiumAm J Med2014127121233124125016127
- KuhnelAGrossUDossMOHereditary coproporphyria in Germany: clinical-biochemical studies in 53 patientsClin Biochem200033646547311074238
- von und zu FraunbergMTimonenKMustajokiPKauppinenRClinical and biochemical characteristics and genotype-phenotype correlation in Finnish variegate porphyria patientsEur J Hum Gen200210649657
- MustajokiPNordmannYEarly administration of Heme Arginate for acute porphyric attacksArch Int Med199315317200420088357285
- SardhEHarperPAnderssonDEFloderusYPlasma porphobilinogen as a sensitive biomarker to monitor the clinical and therapeutic course of acute intermittent porphyria attacksEur J Int Med2009202201207
- KauppinenRMustajokiPPrognosis of acute porphyria: occurrence of acute attacks, precipitating factors, and associated diseasesMedicine19927111131549056
- JeansJBSavikKGrossCRMortality in patients with acute intermittent porphyria requiring hospitalization: a United States case seriesAm J Med Genet19966542692738923933
- PischikEBulyanitsaAKazakovVKauppinenRClinical features predictive of a poor prognosis in acute porphyriaJ Neurol2004251121538154115645362
- HiftRJMeissnerPNAn analysis of 112 acute porphyric attacks in Cape Town, South Africa: Evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severityMedicine (Baltimore)2005841486015643299
- von und zu FraunbergMPischikEUddLKauppinenRClinical and biochemical characteristics and genotype-phenotype correlation in 143 Finnish and Russian patients with acute intermittent porphyriaMedicine (Baltimore)2005841354715643298
- SardhEWahlinSBjornstedtMHarperPAnderssonDEHigh risk of primary liver cancer in a cohort of 179 patients with Acute Hepatic PorphyriaJ Inherit Metab Dis20133661063107123344888
- InnalaEAnderssonCScreening for hepatocellular carcinoma in acute intermittent porphyria: a 15-year follow-up in northern SwedenJ Int Med20112695538545
- StewartMFReview of hepatocellular cancer, hypertension and renal impairment as late complications of acute porphyria and recommendations for patient follow-upJ Clin Pathol2012651197698022851509
- PischikEKauppinenRNeurological manifestations of acute intermittent porphyriaCell Mol Biol (Noisy-le-grand)2009551728319268005
- AnderssonCInnalaEBackstromTAcute intermittent porphyria in women: clinical expression, use and experience of exogenous sex hormones. A population-based study in northern SwedenJ Int Med20032542176183
- FraserDJPodvinecMKaufmannMRMeyerUADrugs mediate the transcriptional activation of the 5-aminolevulinic acid synthase (ALAS1) gene via the chicken xenobiotic-sensing nuclear receptor (CXR)J Biol Chem200227738347173472612121995
- MeyerUASchuurmansMMLindbergRLAcute porphyrias: pathogenesis of neurological manifestationsSemin Liver Dis199818143529516677
- BrennanMJCantrillRCDelta-Aminolaevulinic acid and amino acid neurotransmittersMol Cell Biochem198138Pt 149586117007
- PischikEKauppinenRPotential role of oxidative damage in neurological manifestations of acute intermittent porphyriaGadothNGöbelHHOxidative Stress and Free Radical Damage in NeurologyVIIITotowa, NJHumana Press2011293311
- HandschinCLinJRheeJNutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alphaCell2005122450551516122419
- SardhERejkjaerLAnderssonDEHarperPSafety, pharmacokinetics and pharmocodynamics of recombinant human porphobilinogen deaminase in healthy subjects and asymptomatic carriers of the acute intermittent porphyria gene who have increased porphyrin precursor excretionClin Pharmacokinet200746433534917375984
- SingalAKParkerCBowdenCThaparMLiuLMcGuireBMLiver transplantation in the management of porphyriaHepatology20146031082108924700519
- MarsdenJTReesDCA retrospective analysis of outcome of pregnancy in patients with acute porphyriaJ Inh Metab Dis2010335591596
- RifkindABGillettePNSongCSKappasAInduction of hepatic delta-amino-levulinic acid synthetase by oral contraceptive steroidsJ Clin Endocrinol Metab19703033303354189570
- PischikEKauppinenRCan pregnancy stop cyclical attacks of porphyria?Am J Med20061191889016431203
- InnalaEBackstromTPoromaaISAnderssonCBixoMWomen with acute intermittent porphyria have a defect in 5alpha-steroid production during the menstrual cycleActa Obstet Gynecol Scand201291121445145222924787
- ChristianCAMoenterSMThe neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surgesEndocr Rev201031454457720237240
- KalsbeekALa FleurSVan HeijningenCBuijsRMSuprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liverJ Neurosci200424357604761315342726
- BonkovskyHLBarnardGFDiagnosis of porphyric syndromes: a practical approach in the era of molecular biologySemin Liver Dis199818157659516679
- DeaconACElderGHACP Best Practice No 165: front line tests for the investigation of suspected porphyriaJ Clin Pathol200154750050711429419
- ThunellSHarperPBrockAPetersenNEPorphyrins, porphyrin metabolism and porphyrias. II. Diagnosis and monitoring in the acute porphyriasScand J Clin Lab Invest200060754155911202049
- KauppinenRvon und zu FraunbergMMolecular and biochemical studies of acute intermittent porphyria in 1 96 patients and their familiesClin Chem200248111891190012406973
- DaniellWEStockbridgeHLLabbeRFEnvironmental chemical exposures and disturbances of heme synthesisEnviron Health Perspect1997105Suppl 137539114276
- PischikEKazakovVKauppinenRIs screening for urinary porphobilinogen useful among patients with acute polyneuropathy or encephalopathy?J Neurol2008255797497918574620
- MooreMRMcCollKERimingtonCGoldbergADisorders of Porphyrin MetabolismNew YorkPlenum Publishing Corporation1987
- Poh-FitzpatrickMBLamolaAADirect spectrofluorometry of diluted erythrocytes and plasma: a rapid diagnostic method in primary and secondary porphyrinemiasJ Lab Clin Med19768723623701245797
- WhatleySDRobertsAGLlewellynDHBennettCPGarrettCElderGHNon-erythroid form of acute intermittent porphyria caused by promoter and frameshift mutations distant from the coding sequence of exon 1 of the HMBS geneHum Genet2000107324324811071386
- GrandchampBPicatCMignotteVTissue-specific splicing mutation in acute intermittent porphyriaProc Natl Acad Sci USA19898626616642563167
- KostrzewskaEGregorAIncreased activity of porphobilinogen deaminase in erythrocytes during attacks of acute intermittent porphyriaAnn Clin Res19861841951983789651
- KauppinenRMolecular diagnostics of acute intermittent porphyriaExp Rev Mol Diag200442243249
- PuyHDeybachJCLamorilJMolecular epidemiology and diagnosis of PBG deaminase gene defects in acute intermittent porphyriaAm J Hum Genet1997606137313839199558
- WhatleySDWoolfJRElderGHComparison of complementary and genomic DNA sequencing for the detection of mutations in the HMBS gene in British patients with acute intermittent porphyria: identification of 25 novel mutationsHum Genet1999104650551010453740
- The Human Gene Mutation Database [database on the Internet]Cardiff, UKInstitute of Medical Genetics in Cardiff, Cardiff University Available from: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=HMBSAccessed June 13, 2015
- BylesjoIWikbergAAnderssonCClinical aspects of acute intermittent porphyria in northern Sweden: a population-based studyScand J Clin Lab Invest200969561261819401933
- ElderGHarperPBadmintonMSandbergSDeybachJCThe incidence of inherited porphyrias in EuropeJ Inh Metab Dis2013365849857
- AndersonKECollinsSOpen-label study of hemin for acute porphyria: clinical practice implicationsAm J Med20061199801.e819e82416945618
- SteinPBadmintonMBarthJReesDStewartMFBest practice guidelines on clinical management of acute attacks of porphyria and their complicationsAnn Clin Biochem201350Pt 321722323605132
- GoldbergAAcute intermittent porphyria. A study of 50 casesQ J Med19592818320913658350
- RidleyABThe neuropathy of acute intermittent porphyriaQ J Med1969381513073335343605
- SteinJATschudyDPAcute intermittent porphyria: a clinical and biochemical study of 46 patientsMedicine19704911164907358
- MustajokiPKoskeloPHereditary hepatic porphyrias in FinlandActa Med Scand19762003171178970225
- HerrickALMcCollKEMooreMRCookAGoldbergAControlled trial of haem arginate in acute hepatic porphyriaLancet198918650129512972566827
- AndersonKEBonkovskyHLBloomerJRShedlofskySIReconstitution of hematin for intravenous infusionAnn Int Med2006144753753816585674
- SimionattoCSCabalRJonesRLGalbraithRAThrombophlebitis and disturbed hemostasis following administration of intravenous hematin in normal volunteersAm J Med19888545385403177402
- DaimonMSusaIgarashiMKatoTKamedaWAdministration of heme arginate, but not hematin, caused anaphylactic shockAm J Med2001110324011221635
- TschudyDPWellandFHCollinsAThe effect of carbohydrate feeding on the induction of delta-aminolevulinic acid synthetaseMetabolism19641339640614169218
- AndersonKEBloomerJRBonkovskyHLRecommendations for the diagnosis and treatment of the acute porphyriasAnn Int Med2005142643945015767622
- ElderGHHiftRJTreatment of acute porphyriaHosp Med200162742242511480131
- YrjonenAPischikEMehtalaSKauppinenRA novel 19-bp deletion of exon 15 in the HMBS gene causing acute intermittent porphyria associating with rhabdomyolysis during an acute attackClin Genet200874439639818647325
- SusaSDaimonMMoritaYAcute intermittent porphyria with central pontine myelinolysis and cortical laminar necrosisNeuroradiology1999411183583910602858
- MarsdenJTChowdhuryPWangJAcute intermittent porphyria and chronic renal failureClin Nephrol200869533934618538096
- Porphyria South Africa [homepage on the Internet] Available from: http://www.porphyria.uct.ac.za/Accessed June 13, 2015
- Epnet-European Porphyria Network [homepage on the Internet] Available from: http://www.porphyria-europe.comAccessed June 13, 2015
- The Drug Database for Acute Porphyria [homepage on the Internet] Available from: http://www.drugs-porphyria.orgAccessed June 13, 2015
- American Porphyria Foundation [homepage on the Internet] Available from: http://www.porphyriafoundation.com/drug-databaseAccessed June 13, 2015
- JensenMPKarolyPSelf-report scales and procedures for assessing pain in adultsMelzackRHandbook of Pain AssessmentNew YorkGuildford Press1992135151
- MuiesanMLSalvettiMAmadoroVAn update on hypertensive emergencies and urgenciesJ Cardiovasc Med (Hagerstown)201516537238225575271
- ShenhavSGemerOSassoonESegalSAcute intermittent porphyria precipitated by hyperemesis and metoclopramide treatment in pregnancyActa Obstet Gynecol Scand19977654844859197454
- GrossUHoncampMDaumeEFrankMDusterbergBDossMOHormonal oral contraceptives, urinary porphyrin excretion and porphyriasHorm Metab Res19952783793837590628
- De BlockCELeeuwIHGaalLFPremenstrual attacks of acute intermittent porphyria: hormonal and metabolic aspects – a case reportEur J Endocrinol19991411505410407223
- HerrickALMcCollKEWallaceAMMooreMRGoldbergALHRH analogue treatment for the prevention of premenstrual attacks of acute porphyriaQ J Med1990752763553632117297
- InnalaEBackstromTBixoMAnderssonCEvaluation of gonadotropin-releasing hormone agonist treatment for prevention of menstrual-related attacks in acute porphyriaActa Obstet Gynecol Scand20108919510020021268
- AndersonKESpitzIMBardinCWKappasAA gonadotropin releasing hormone analogue prevents cyclical attacks of porphyriaArch Int Med19901507146914742196028
- DowmanJKGunsonBKMirzaDFBramhallSRBadmintonMNNewsomePNLiver transplantation for acute intermittent porphyria is complicated by a high rate of hepatic artery thrombosisLiver Transplant2012182195200
- DowmanJKGunsonBKBramhallSBadmintonMNNewsomePNLiver transplantation from donors with acute intermittent porphyriaAnn Int Med2011154857157221502660
- BadmintonMNDeybachJCTreatment of an acute attack of porphyria during pregnancyEur J Neurol200613666866916796597
- ChurchSEMcCollKEMooreMRYoungsGRHypertension and renal impairment as complications of acute porphyriaNephrol Dial Transplant19927109869901331893
- AnderssonCLithnerFHypertension and renal disease in patients with acute intermittent porphyriaJ Int Med19942362169175
- SardhEAnderssonDEHenrichsonAHarperPPorphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimesCell Mol Biol (Noisy-le-grand)2009551667119268004
- CarsonRWDunniganEJDuBoseTDJrGoegerDEAndersonKERemoval of plasma porphyrins with high-flux hemodialysis in porphyria cutanea tarda associated with end-stage renal diseaseJ Am Soc Nephrol199229144514501627767
- AndersonKEGoegerDECarsonRWLeeSMSteadRBErythropoietin for the treatment of porphyria cutanea tarda in a patient on long-term hemodialysisN Engl J Med199032253153172104958
- WarholmCWilczekHRenal transplantation in a case of acute intermittent porphyriaJ Clin Pharmacol200343101158116014517198
- WahlinSHarperPSardhEAnderssonCAnderssonDEEriczonBGCombined liver and kidney transplantation in acute intermittent porphyriaTransplant Int2010236e18e21
- ÅbergFJulaAHöckerstedtKIsoniemiHCardiovascular risk profile of patients with acute liver failure after liver transplantation when compared with the general populationTransplantation201089616820061920
- ÅbergFKoivusaloAMHöckerstedtKIsoniemiHRenal dysfunction in liver transplant patients: comparing patients transplanted for liver tumor or acute or chronic diseaseTranspl Int20072059159917425724
- ÅbergFPukkalaEHöckerstedtKSankilaRIsoniemiHRisk of malignant neoplasms after liver transplantation: a population-based studyLiver Transpl2008141428143618825704
- AnderssonCBjersingLLithnerFThe epidemiology of hepatocellular carcinoma in patients with acute intermittent porphyriaJ Int Med19962404195201
- LithnerFWetterbergLHepatocellular carcinoma in patients with acute intermittent porphyriaActa Med Scand198421532712746328897
- KauppinenRMustajokiPAcute hepatic porphyria and hepatocellular carcinomaBr J Cancer19885711171202831925
- ColomboMSangiovanniATreatment of hepatocellular carcinoma: beyond international guidelinesLiver Int201535Suppl 112913825529098
- SolisCMartinez-BermejoANaidichTPAcute intermittent porphyria: studies of the severe homozygous dominant disease provides insights into the neurologic attacks in acute porphyriasArch Neurol200461111764177015534187
- LinTCLaiSLHsuSPRoLSTreatment of neuropathic pain in acute intermittent porphyria with gabapentinJ Formos Med Assoc2013112957857923735466
- JohanssonAMollerCFoghJHarperPBiochemical characterization of porphobilinogen deaminase-deficient mice during phenobarbital induction of heme synthesis and the effect of enzyme replacementMol Med200399–1219319915208740
- JohanssonANowakGMollerCBlombergPHarperPAdenoviral-mediated expression of porphobilinogen deaminase in liver restores the metabolic defect in a mouse model of acute intermittent porphyriaMol Ther200410233734315294180
- JohanssonAMollerCHarperPCorrection of the biochemical defect in porphobilinogen deaminase deficient cells by non-viral gene deliveryMol Cell Biochem20032501–2657112962144
- JohanssonAMollerCGellerforsPHarperPNon-viral mediated gene transfer of porphobilinogen deaminase into mammalian cellsScand J Clin Lab Invest200262210511312004925
- JohanssonANowakGMollerCHarperPNon-viral delivery of the porphobilinogen deaminase cDNA into a mouse model of acute intermittent porphyriaMol Gen Metab20048212026
- TarnerIHMuller-LadnerUFathmanCGTargeted gene therapy: frontiers in the development of ‘smart drugs’Trends Biotechnol200422630431015158060
- YasudaMBishopDFFowkesMChengSHGanLDesnickRJAAV8-mediated gene therapy prevents induced biochemical attacks of acute intermittent porphyria and improves neuromotor functionMol Ther2010181172219861948
- PanedaALopez-FrancoEKaeppelCSafety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman primates: a potential therapy for acute intermittent porphyriaHum Gene Ther201324121007101724070415
- Augmenting PBGD expression in the liver as a novel gene therapy for acute intermittent porphyria (AIPgene)Hum Gene Ther Clin Dev2014252616324933563
- YasudaMGanLChenBRNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria miceProc Natl Acad Sci USA2014111217777778224821812
- WaldenströmJStudien uber Porphyrie [Studies of porphyria]Acta Med Scand193792Suppl1254 German

