Abstract
The burden of uncontrolled asthma in children and adolescents is high. Treatment options for pediatric patients (aged under 18 years) with asthma are largely influenced by the Global Initiative for Asthma recommendations. Algorithms for adolescents (12–18 years) and adults are identical, but recommendations for children aged under 6 years and 6–11 years differ. Although the goals of treatment for pediatric patients with asthma are similar to those for adults, relatively few new therapies have been approved for this patient population within the last decade. Designing clinical trials involving children presents several challenges, notably that children are often less able to perform lung function tests, and traditional endpoints used in clinical trials with adults, such as forced expiratory volume in 1 second, asthma exacerbations and questionnaires, have limitations associated with their use in children. There are also ethical considerations related to the performance of longer placebo-controlled exacerbation trials. This review considers additional clinical endpoints to those traditionally reported, including forced expiratory flow at 25%–75% of forced vital capacity, which may help shed light on which treatments are most effective for use in pediatric patients with asthma. The pros and cons of specific and potentially clinically relevant endpoints are considered, along with device considerations and patient preferences that may enhance adherence and quality of life. Recent advances in the management of children and adolescents, including the US Food and Drug Administration and European Medicines Agency approval of tiotropium in patients with asthma aged 6 years and over, are also discussed.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Asthma remains one of the most common chronic diseases of childhood and adolescence.Citation1,Citation2 Much of the understanding of the epidemiology of pediatric asthma is derived from data collected in the International Study of Asthma and Allergies in Childhood (ISAAC).Citation3,Citation4 Phase III of ISAAC involved around 1.2 million children from 233 centers in 98 countries, and found that the global prevalence of asthma was 14% and 12% in 13- to 14-year-olds and 6- to 7-year-olds, respectively.Citation5 ISACC has, however, reported substantial differences (of up to 13-fold) between countries in the prevalence of asthma symptoms (specifically wheeze in the past 12 months) in children.Citation6 The 2014 Global Asthma Report described an increase in the frequency of asthma symptoms in children in many low- and middle-income countries between 1993 and 2003, while prevalence remained the same or decreased in high-income countries.Citation7
The burden associated with asthma in children and adolescents is considerable. In 2010, asthma was the eighth highest ranked cause of disability-adjusted life years in children aged 5–9 years and the third highest for children aged 10–14 years worldwide.Citation6,Citation8 Recurrent symptoms such as wheeze, cough and shortness of breath can also have a marked negative impact on the quality of life of children.Citation9 Furthermore, asthma exacerbations are the leading cause of hospitalization in childrenCitation10 and of the children who attend an emergency department with an asthma exacerbation, more than half are aged between 2 and 7 years old.Citation11 These findings make asthma a leading cause of childhood morbidity from chronic diseases.Citation12 The global socioeconomic burden of pediatric asthma is further increased by school absencesCitation9 and the relatively high cost of asthma care.Citation6
Alarmingly, it is estimated that asthma control is inadequate in more than half of pediatric patients with asthma despite currently available treatment and guidelines.Citation9,Citation13 Clearly, there is a pressing need to alleviate the burden of uncontrolled asthma in pediatric patients, and effective treatment is paramount to achieving this. As will be discussed, treatment recommendations outlined by the Global Initiative for Asthma (GINA) consist of an inhaled corticosteroid (ICS) backbone with or without additional reliever therapy.Citation13 Evidence has recently emerged supporting the use of anti-cholinergics in asthma,Citation14 and tiotropium has become the first long-acting muscarinic receptor antagonist (LAMA) to be approved for the treatment of asthma.Citation15 The aim of this review is to report on the use of anticholinergic therapies in pediatric patients with asthma and to discuss the difficulties in investigating new therapies for this population.
GINA Report recommendations
The GINA Report is intended to be a useful resource for the management of asthma, and provides recommendations that should be adjusted in line with local practices and available health care resources.Citation13 The principle aims of these treatment recommendations are control of asthma symptoms and the minimization of future risk of exacerbations, fixed airflow limitation and treatment side effects. In pediatric patients, additional consideration is given to avoiding reduced lung growth and minimizing adverse effects, given the potential capacity of ICS treatment to negatively impact development.Citation14,Citation16 The GINA strategy recommends a stepwise approach towards asthma treatment, building on a framework of ICS controller medication with or without a reliever therapy.Citation13
Algorithms for treatment of adult and adolescent (12–18 years) patients with asthma are the same (). For adolescents, the current GINA recommendations are low-dose ICS treatment followed by a stepwise increase in ICS dose and/or additional (or a second class of) maintenance therapy, such as a long-acting β2-agonist (LABA) or leukotriene receptor antagonist (LTRA), if control has not been achieved. Add-on treatment (eg, with tiotropium) is then suggested if control is still not attained. There are key differences in the recommendations for children. In children aged 6–11 years, increasing the ICS dose is preferred over combination ICS/LABA. If this is ineffective, then it is suggested that the child be referred for expert assessment and advice. In children under 6 years of age, the preferred asthma control medication is low-dose ICS, such as 200 µg budesonide or equivalent (). Due to lack of data, treatment options are limited in children under 6 years of age whose asthma symptoms are poorly controlled with ICS monotherapy.Citation13 Therefore, data for additional second-line controller medications, such as theophylline, oral corticosteroids and anticholinergics, are particularly valuable to this patient subset.
Figure 1 Stepwise approach for the treatment of asthma in (A) patients aged 6 years and over and (B) children under 6 years of age, as recommended in the GINA Report.
Abbreviations: FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; HDM, house dust mite; ICS, inhaled corticosteroids; IgE, immunoglobulin E; Inc., increase; intermitt, intermittent; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; med, medium dose; OCS, oral corticosteroids; SABA, short-acting β2-agonist; SLIT, sublingual immunotherapy; theoph, theophylline.
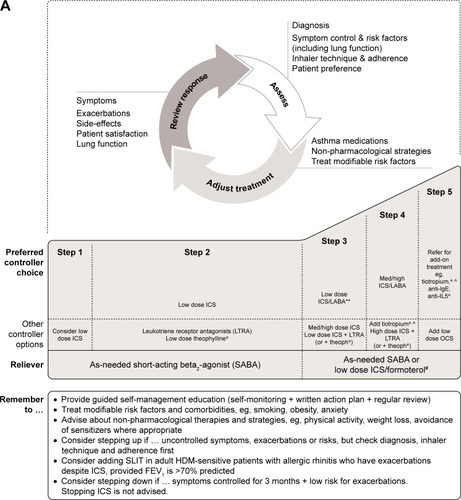
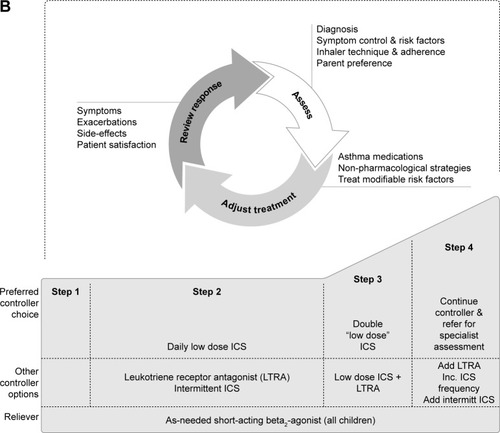
While the GINA Report and its recommendations are regularly updated (most recently in 2018), there have been no updated recommendations from the National Heart, Lung and Blood Institute on asthma diagnosis/management since 2007.
Anticholinergic therapies
Anticholinergic agents target the function of acetylcholine, a neurotransmitter that plays a key role in the pathophysiology of asthma by way of driving bronchial smooth muscle contraction, mucus secretion, vasodilation and increasing airway tone. Further, cholinergic activity is believed to be the predominant driver of bronchoconstriction.Citation14,Citation17 There are two categories of acetylcholine receptors: nicotinic and muscarinic. Of these, the muscarinic M1, M2 and M3 subtypes are believed to be primarily involved in regulating broncho-constriction.Citation14 Better understanding of muscarinic receptor agonists over the past two decades has led to investigation of both short- and long-acting anticholinergics in asthma. Several studies in children with severe asthma exacerbations suggest that the addition of the short-acting anticholinergic agent ipratropium to a β2-agonist may reduce hospital admissions and improve lung function.Citation18–Citation21 A 2012 meta-analysis of short-acting anticholinergics as bronchodilators in children aged $2 years with acute asthma exacerbations suggested that short-acting anticholinergics were less efficacious than β2-agonists.Citation22 However, the safety of anticholinergics in children, combined with the lack of additional controller medications licensed for this population, has sustained an interest in developing anticholinergics for use in this patient population.
In recent years, evidence has emerged supporting the use of long-acting anticholinergic agents.Citation14 Of note, not all LAMAs have equivalent potency as bronchodilators due to their differential effects on the muscarinic receptor subtypes M1–M3.Citation23 The different biological functions of M1–M3 receptors in the airways distinguish their potential as therapeutic targets in asthma. M3 is the primary receptor subtype involved in bronchial and tracheal muscle contraction, and acts alongside M1 to increase smooth muscle tone in the airways.Citation24,Citation25 Conversely, M2 receptors inhibit the release of acetylcholine from parasympathetic nerves and decrease smooth muscle tone. Consequently, LAMAs that primarily mediate blockade of the M1 and M3 receptors are the most attractive candidate therapies for asthma treatment.Citation23,Citation26
Tiotropium for pediatric asthma
Evidence of tiotropium efficacy in pediatric patients with asthma
Tiotropium is a once-daily LAMA that has been approved for the treatment of chronic obstructive pulmonary disease for over a decade.Citation27 Of note, tiotropium dissociates slowly from the M1 and M3 muscarinic receptors, but does so rapidly from the M2 receptor, making it a highly potent bronchodilator.Citation28 A number of Phase III clinical trials in adult patients with symptomatic asthma recently led to tiotropium becoming the first LAMA to be approved for the treatment of asthma.Citation29–Citation32 These studies demonstrated that tiotropium improves lung function and asthma control in patients with a range of asthma severities, with a safety profile similar to that of placebo. The 2018 GINA Report recommends tiotropium as an add-on therapy in patients aged $12 years with a history of asthma exacerbations at Steps 4 and 5 of the stepwise approach ().Citation13
Further investigation of tiotropium in asthma has demonstrated the effectiveness of tiotropium add-on therapy across all age groups.Citation33 In Phase II dose-escalation studies of tiotropium in children and adolescents with symptomatic asthma, similar safety and tolerability profiles to placebo were observed, with early indications of efficacy.Citation34,Citation35 As a consequence of these positive early findings, five Phase III, randomized, double-blind, placebo-controlled, parallel-group trials in children and adolescents have recently investigated the efficacy and safety of once-daily tiotropium as an add-on therapy to ICS with or without other controllers ().Citation36–Citation43
Table 1 Key results of Phase III studies with 2.5 and 5 µg tiotropium in children and adolescents with asthma
Two of these trials were performed in adolescents aged 12–17 years ().Citation37,Citation41 The first of these, RubaTinA-asthma® (NCT01257230), was performed in patients with moderate symptomatic asthma. Here, the efficacy and safety of once-daily tiotropium (5 or 2.5 µg) or placebo administered via Respimat® (Boehringer Ingelheim Pharma, Ingelheim am Rhein, Germany) were assessed as an add-on therapy to ICS (200–800 µg or 400–800 µg budesonide or equivalent/day for patients aged 12–14 years or 15–17 years, respectively) with or without an LTRA over 48 weeks. Both doses of tiotropium were safe and well tolerated, and conferred a significantly greater improvement in peak forced expiratory volume in 1 second (FEV1) within 3 hours after dosing (FEV1(0–3h)) at Week 24 compared with placebo.Citation37 At Week 24, both doses were associated with significant improvements in forced expiratory flow between 25% and 75% of forced vital capacity (FEF25–75) compared with placebo at most time points.Citation37
PensieTinA-asthma® (NCT01277523) was a Phase III study in adolescent patients with severe symptomatic asthma. Once-daily tiotropium (5 or 2.5 µg) or placebo, both administered by the Respimat®, was added to high-dose ICS (>400 µg or 800–1,600 µg budesonide or equivalent/day for patients aged 12–14 years or 15–17 years, respectively) plus at least one controller therapy (LABA or LTRA) or medium-dose ICS (200–400 µg budesonide or equivalent in patients aged 12–14 years and 400–800 µg budesonide or equivalent in patients aged 15–17 years) plus two or more other controller therapies (LABA and/or LTRA and/or sustained-release theophylline) over 12 weeks. In this study of adolescents with severe asthma, numerical improvements in measures of lung function and asthma control were reported with once-daily tiotropium add-on therapy, while the safety and tolerability of tiotropium were comparable with those of placebo.Citation41
Two Phase III trials were performed in children aged 6–11 years ().Citation38,Citation39 In the first of these, VivaTinA-asthma® (NCT01634152), participants had severe symptomatic asthma. The efficacy and safety of tiotropium (5 or 2.5 µg) or placebo, both administered via the Respimat®, were assessed as an add-on therapy to high-dose ICS (>400 µg budesonide or equivalent per day) with one or more controller medications (LABA or LTRA) or medium-dose ICS (200–400 µg budesonide or equivalent per day) with two or more controller medications (LABA and/or LTRA and/or sustained-release theophylline) over 12 weeks. Compared with placebo, 5 µg tiotropium had a similar safety and tolerability profile and significantly improved peak FEV1(0–3h) at Week 12; both doses were associated with a significant improvement in FEF25–75 at Weeks 12 and 24.Citation39 Add-on tiotropium in children aged 6–11 years was also evaluated in moderate symptomatic asthma in the CanoTinA-asthma® trial. Tiotropium (5 or 2.5 µg) versus placebo, both delivered via the Respimat®, was evaluated as an add-on therapy to medium-dose ICS (200–400 µg budesonide or equivalent/day) with or without an LTRA over 48 weeks. Both doses of tiotropium were well tolerated, and the 5 µg dose conferred a statistically significant improvement in FEV1(0–3h) at Week 24; both doses were associated with a significant improvement in FEF25–75 at the majority of time points throughout the 48-week study period.Citation38 Finally, the NinoTinA-asthma® (NCT01634113) trial was conducted in preschool children aged 1–5 years with persistent asthma symptoms (). Once-daily tiotropium (5 or 2.5 µg) or placebo, delivered via the Respimat®, was given as an add-on therapy to ICS at a stable dose with or without additional controller medication for 12 weeks. In this study, tiotropium was shown to be safe and reduced the risk of asthma exacerbations reported as adverse events by more than 50% in very young patients.Citation43
Overall, these studies demonstrate tiotropium to be efficacious in children and adolescents with asthma, irrespective of disease severity. In support of this, a pooled analysis of the RubaTinA-asthma® and CanoTinA-asthma® studies supported the use of tiotropium as an add-on therapy to the usual background medication of pediatric patients aged 6–11 years with symptomatic persistent asthma.Citation44 Furthermore, in a systematic review of three studies of tiotropium in school-age symptomatic asthmatics, tiotropium was associated with significant improvements in FEV1 peak responses and a reduction in asthma exacerbation frequency compared with placebo. This review concluded tiotropium to be an efficacious and well-tolerated add-on to ICS plus one or more controller medications in this population.Citation45
Efficacy of tiotropium in allergic asthma
Allergic asthma is the most common asthma phenotype.Citation46 In childhood, asthma is frequently associated with atopy, a condition that predisposes individuals to develop immunoglobulin E (IgE) against specific allergens.Citation47 Studies have been performed to investigate whether baseline IgE levels or blood eosinophil counts can influence response to tiotropium add-on therapy in patients aged 6–17 years with asthma. Data were pooled from moderate symptomatic patients in the CanoTinA-asthma®38 and RubaTinA-asthma®37 trials and from severe symptomatic patients in the VivaTinA-asthma®39 and PensieTinA-asthma®41 trials.Citation36,Citation48,Citation49 In these pooled studies, tiotropium improved lung function irrespective of baseline IgE levels and blood eosinophil counts. Overall, these data demonstrate that tiotropium is effective in children and adolescents without the need for phenotyping of allergic status according to IgE levels and blood eosinophil counts.
Pharmacokinetics and pharmacogenomics of tiotropium
Age and developmental status are major influences on numerous factors, including anatomy, physiology and pathology, that could affect pharmacodynamics, kinetics and drug metabolism.Citation47 Therefore, it is pertinent to compare the pharmacokinetics of tiotropium when administered to patients with asthma from different age groups. The pharmacokinetic properties of tiotropium are well established in adult patients with symptomatic asthma. Pharmacokinetic parameters of tiotropium demonstrate its fast absorption and long elimination time, with maximum plasma concentrations reached within 5 minutes of post-inhalation and a half-life of approximately 30 hours.Citation50 Furthermore, pharmacokinetics of tiotropium in adult patients with asthma are dose proportional up to 5 µg once daily.Citation51
Plasma and urine samples from subsets of symptomatic patients with asthma aged 6–11 yearsCitation35 and 12–17 yearsCitation34 treated with tiotropium in Phase II trials were used to evaluate the pharmacokinetic properties of tiotropium in these age groups.Citation52,Citation53 These studies found the pattern of absorption, exposure and clearance of tiotropium at steady state to be consistent with those reported in adults. The pharmacokinetic characteristics of tiotropium in children aged 1–5 years were also studied in the Phase III NinoTinA-asthma® study. Here, exposure to tiotropium, when normalized for body surface area, was comparable to older age groups based on urinary excretion data.Citation40,Citation43
Tiotropium pharmacokinetic data from patients with asthma of different ages were summarized in a clinical pharmacology review carried out by the US Food and Drug Administration ().Citation54 In agreements with the aforementioned analyses, exposure of tiotropium in urine and plasma samples from patients with asthma is comparable in children aged 6–11 years, adolescents and adults.
Table 2 Pharmacokinetics of tiotropium in asthma patients across age groups
Although no formal drug interaction studies have been conducted, tiotropium has been used with other drugs without clinical evidence of interactions.Citation55 Concomitant treatment with other asthma medications such as LABA, ICS+LABA, oral corticosteroids and leukotriene modifiers does not affect exposure to tiotropium.Citation54
Pharmacogenomics is another factor that may impact response to therapy. In this respect, a study performed in adults with severe asthma found that the presence of Arg16Gly in ADRB2 may predict response to tiotropium.Citation56 However, similar studies are yet to be performed in children and adolescents.
Clinical trials with other LAMAs in asthma
Of the clinical trials assessing the safety and efficacy of LAMAs in the treatment of asthma, the tiotropium program has thus far been the most advanced across all age groups.Citation27 A number of studies evaluating the potential of umeclidinium bromide and glycopyrronium in adult asthma have been completed or are ongoing (), although neither agent is approved for the treatment of asthma.Citation27,Citation57–Citation63 Aside from tiotropium, other LAMAs are yet to be studied in children or adolescents with asthma. These pediatric trials are unlikely to be conducted unless their efficacy and safety are first demonstrated in adults. At the time of this publication, no studies are known to be completed or ongoing with aclidinium bromide in asthma.
Table 3 Asthma clinical trials in adults with LAMAs other than tiotropium
Umeclidinium is a potent anticholinergic that demonstrates slow functional reversibility at the human M3 muscarinic receptor compared with the M2 receptor.Citation64 Once-daily umeclidinium bromide combined with fluticasone furoate, administered via a dual-strip dry powder inhaler (DPI), has been investigated in adults with asthma who were symptomatic despite maintenance ICS treatment. This study reported significant increases in trough FEV1 in response to combination treatment, but not with fluticasone furoate alone.Citation59 Furthermore, umeclidinium monotherapy (delivered via DPI) in patients with asthma not requiring ICS treatment did not provide a therapeutic benefit, given that it conferred modest, inconsistent, dose-independent improvements in trough FEV1.Citation65 Other ongoing and completed studies in adult patients are summarized in .
As with tiotropium, glycopyrronium is an anticholinergic with higher selectivity for M3 receptors than for M2 receptors, and dissociates more slowly from the M3 receptors than from the M2 receptors.Citation66 The only study of glycopyrronium to be published thus far compared the effects of glycopyrronium (administered via Breezhaler) and tiotropium (administered via Respimat®) on methacholine-induced bronchoconstriction in adults with symptomatic asthma. Here, tiotropium provided statistically superior bronchoprotection at both 24 and 72 hours compared with glycopyrronium.Citation62 An additional study of glycopyrronium in adults has been completed and results are awaited ().
Challenges for future clinical trials in pediatric asthma
Several challenges are posed by the design of clinical trials in asthma involving children as participants, particularly the choice of endpoints to measure clinical efficacy. The endpoints traditionally used to measure drug efficacy in clinical trials of asthma in adults may not be the most appropriate for use with pediatric patients. These challenges need to be carefully considered in future trials in pediatric patients with asthma.
Clinical trial endpoints
The three attainable objectives of drug development across all age groups in asthma are 1) prevention and treatment of symptoms, 2) exacerbations, and 3) further complications.Citation47 Clinical trials to investigate candidate drugs therefore have endpoints designed to quantify achievement of these goals (). To demonstrate the symptom-modifying effect of a reliever drug, there must be an improvement in an objective measure of airflow. For a drug to be registered as a symptom-preventing agent, there must be a demonstration of benefit in symptomatology, disease control, lung function, exacerbations and/or quality of life.Citation47
Table 4 Traditional and alternative clinical trial endpoints
It is widely recognized that clinical research in asthma lacks standardization in outcomes.Citation67 To address this, an Asthma Outcomes workshop was convened with the specific purpose of stratifying core, supplemental and emerging asthma outcomes.Citation67 Importantly, this workshop differentiated between recommended endpoints in adult and pediatric patients. As will be discussed, measures of asthma outcomes frequently used in adult patients are often less appropriate for use in pediatric patients. Alternative endpoints to those traditionally reported in clinical trials may help to shed light on which treatments are most effective in children. More details on these traditional and alternative endpoints are provided in the following text and in .
Traditional endpoints
Objective measurements such as FEV1 and peak expiratory flow (PEF) play an important role in the evaluation of airflow in patients with asthma during clinical trials.Citation47 In the assessment of efficacy of potential bronchodilators, FEV1 is the gold standard measurement of lung function.Citation68 Although FEV1 has numerous advantages, including robustness, repeatability, standardization and ease of measurement,Citation47 there are limitations to its use as a clinical endpoint. For example, FEV1 does not always confer a complete representation of the disease processes that occur in asthma, such as hyperinflation and airway plugging.Citation69 In clinical trials with pediatric patients, the suitability of FEV1 as an endpoint has been questioned given that FEV1 does not correlate with asthma severity in children. In fact, FEV1 values fall within the normal range in the majority of pediatric patients with asthma, even when patients are markedly symptomatic.Citation47,Citation68,Citation70 Also, efficient and reliable spirometry testing can be difficult in preschool children, especially in the younger age groups. In a study involving 355 children aged 3–5 years, only around half were able to perform effective spirometry.Citation71 Another traditional endpoint – PEF – has also been shown not to correlate with asthma severity in children. Instead, PEF correlates with FEV1, suggesting this test may also have limitations as an asthma outcome measurement in children with asthma.Citation72 In addition, portable PEF meters allow for self-monitoring at home, which can be beneficial for children, although all patients need to be observed carefully, as it has been reported that many patients, both adults and children, do not use the PEF meters correctly.Citation73
Frequency of asthma exacerbations is a commonly used subjective measurement, and is recommended as both a core and supplemental asthma outcome in children.Citation67 These episodes of airway narrowing are most common in infants and young children.Citation74 Of note in children, their recurrence in early years is associated with progressive loss of lung function, making the prevention of exacerbations an especially important aspect of asthma control.Citation75,Citation76 However, the exact definition of what constitutes an exacerbation in a clinical trial has remained elusive. Most clinicians are able to identify asthma exacerbations through the observation of symptoms and medication changes, but the subjective nature of this assessment can lead to variations between studies and investigators.Citation74,Citation77 To rectify this issue, experts in the Asthma Outcomes workshop coined a cross-age definition of asthma exacerbations as “worsening of asthma requiring the use of systemic corticosteroids to prevent serious outcomes”.Citation78 There are also ethical considerations around the use of exacerbations as an endpoint in placebo-controlled trials conducted in children. The chance of being assigned to the placebo group could deter parents from enrolling their child onto such a trial.Citation79
Questionnaires also play a role in asthma control by quantifying composite scores for outcome measures. The Asthma Control Questionnaire (ACQ) and Asthma Control Test (ACT) are commonly employed to assess asthma control.Citation80 There are obvious difficulties in completion of questionnaires by children, and assistance given by caregivers can prevent a true representation of the patient’s disease state.Citation74,Citation80 The episodic nature of asthma in young children also confounds the use of questionnaires.Citation81 There are no core questionnaires recommended for efficacy studies in children. The Childhood ACT (cACT), completed by children and caregivers, is recommended for baseline characterization of children aged 4–11 years.Citation80 cACT was recommended by the Asthma Outcomes workshop both as a core baseline and observational outcome, and as a supplemental outcome for efficacy.Citation67 Further questionnaires for use in pediatric patients with asthma, such as the Test for Respiratory and Asthma Control in Kids instrument for infants aged 0–4 years,Citation82 are in development but face ongoing challenges associated with language and the capabilities of the respondent.Citation74,Citation80 An interviewer-administered ACQ test has been used in some studies with children.Citation38,Citation39
Alternative endpoints
Objective measurements of asthma control are at the center of clinical trials testing the efficacy of asthma medications.Citation68 Spirometry plays an important part in this, and is recommended as a core asthma outcome for children across all aspects of prospective clinical trials.Citation67 However, the limitations of traditional spirometrical measurements (eg, FEV1) in children highlight a need for alternatives. One such alternative, FEF25–75, is a sensitive measure of small airway obstruction that quantifies the maximum mid-expiratory flow rate. FEF25–75 has been purported as more reflective of small airways function than FEV1Citation83 and in a large cohort of patients it was significantly associated with asthma control.Citation84 Unlike FEV1, FEF25–75 has been linked with acute wheezing, response to bronchodilators and ventilation defects, and is predictive of asthma severity in children.Citation85 However, some investigators have questioned its contribution to clinical decision making; one database study found that although abnormally low FEF25–75 values were more prevalent in children than in adults, only in a very small number of patients did FEF25–75 detect an impairment that FEV1 and FVC measures did not.Citation86
There is a keen interest in the use of alternative acceptable measures of lung function in children who cannot perform spirometry, such as impulse oscillometry (IOS). IOS is a type of airway mechanic measurement performed by non-invasively superimposing pressure fluctuations on the airway during spontaneous breathing of the subject.Citation87 A comparison of IOS and spirometry as measures of pediatric asthma control found that IOS complemented information gained by FEV1. Furthermore, IOS offered some additional insights into alterations in airway mechanics in response to therapy.Citation88 However, there are cost considerations associated with IOS due to the high cost of the machines.
Biomarkers are another non-invasive objective measurement of asthma control, but are lacking in children, and utility as a broad measure of asthma control across ages and phenotypes seems unlikely.Citation89
Patient considerations when choosing an anticholinergic for pediatric asthma
In children with asthma, poor adherence to medication has been shown to lead to increased morbidity.Citation90 When selecting a treatment for pediatric asthma, patient preferences must be considered to help maintain therapeutic adherence. In children, a particularly relevant aspect of this is drug delivery, which poses significant challenges in young patients.Citation74 Inhalers are the main device used in asthma treatment, and the GINA Report recommends they should reproducibly deliver a predetermined dose to the lungs with minimal deposition elsewhere in the body.Citation13 Administration of ICS is most commonly performed using a pressured metered-dose inhaler (pMDI) device connected to a spacer, yet only a small fraction of the dose from these devices reach the distal airways.Citation74 Anticholinergics can also be administered by inhalation, using pMDIs, nebulizers and DPIs.Citation91
Patient satisfaction was a priority in the development of the inhaler used to administer tiotropium in pediatric clinical trials (Respimat® Soft Mist™ Inhaler, Boehringer Ingelheim Pharma). This pocket-sized inhaler has been shown to reproducibly deliver a single-breath, metered dose of inhalable aerosol from an aqueous drug solution in an environmentally friendly manner.Citation92 Optimization of delivery to the lungs and minimal inspiratory effort were key in the design of this device,Citation93 and likely explain why patients favored this inhaler over the commonly used Turbuhaler® (AstraZeneca UK Ltd, Luton, UK) DPI in a clinical trial. The two devices were compared in a randomized, placebo-controlled study, which found that 74% of patients preferred Respimat® compared with 17% who preferred Turbuhaler® and 9% who had no preference.Citation94 Among pediatric patients with asthma, the benefits and ease of use of this innovative tiotropium inhaler could help to maximize patient adherence.
Conclusion
Pediatric asthma is a chronic disease leading to significant morbidity and health care utilization. As many as 50% of patients remain uncontrolled despite national and international guidelines designed to improve control. As this review reveals, new add-on therapy options to ICS/LABA are required, particularly for children and adolescents. However, developing such treatments is challenging. There are limitations to the conclusions that we can draw from the data sources used in this review, for example to the paucity of pediatric trials, the smaller sample sizes than in adult trials and the lack of information on LAMAs other than tiotropium in pediatric populations. It is also clearly essential that clinical trial design continues to consider alternative clinical endpoints, such as FEF25–75 and IOS, that are likely to be more suitable for use in clinical trials of pediatric asthma.
Tiotropium Respimat® is approved in asthma for adult patients (aged $18 years) in Singapore, patients aged $15 years in Japan and, most recently, approved for patients aged $6 years in the US and EU.Citation27,Citation95 The clinical trials of tiotropium in pediatric asthma summarized in this review support the safety and efficacy of tiotropium in children and adolescents, irrespective of disease severity and phenotype. At present, tiotropium is considered as an option for add-on to ICS/LABA combination. However, data suggest that it could be considered as an alternative to adding a LABA to ICS, and this may prove an attractive option for future therapeutic strategies.
Acknowledgments
Editorial support (writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Wemimo Omotosho, PhD (MediTech Media, London, UK) and was funded by Boehringer Ingelheim. The author would like to thank Kjeld Hansen, a member of the Patient Ambassador Group for the European Lung Foundation, for his input to the video summary for this manuscript.
Disclosure
The author reports no conflicts of interest in this work.
References
- Centres for Disease Control and PreventionNational Health Interview Survey, US children2015 Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2015_SHS_Table_C-1.pdfAccessed August 31, 2017
- Asthma UKAsthma facts and statistics2017 Available from: https://www.asthma.org.uk/about/media/facts-and-statistics/Accessed August 26, 2017
- AsherMIKeilUAndersonHRInternational Study of Asthma and Allergies in Childhood (ISAAC): rationale and methodsEur Respir J1995834834917789502
- EllwoodPAsherMIBeasleyRClaytonTOStewartAWISAAC Steering CommitteeThe international study of asthma and allergies in childhood (ISAAC): phase three rationale and methodsInt J Tuberc Lung Dis200591101615675544
- MallolJCraneJvon MutiusEOdhiamboJKeilUStewartAISAAC Phase Three Study GroupThe International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesisAllergol Immunopathol (Madr)2013412738522771150
- AsherIPearceNGlobal burden of asthma among childrenInt J Tuberc Lung Dis2014181269127825299857
- The Global Asthma NetworkThe Global Asthma Report 2014 Available from: http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdfAccessed August 26, 2017
- MurrayCJVosTLozanoRDisability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592197222323245608
- SchmierJKManjunathRHalpernMTJonesMLThompsonKDietteGBThe impact of inadequately controlled asthma in urban children on quality of life and productivityAnn Allergy Asthma Immunol200798324525117378255
- MurrayCSPolettiGKebadzeTStudy of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in childrenThorax2006611537638216384881
- GuttmannAZagorskiBAustinPCEffectiveness of emergency department asthma management strategies on return visits in children: a population-based studyPediatrics20071206e1402e141018055658
- PedersenSEHurdSSLemanskeRFJrGlobal Initiative for AsthmaGlobal strategy for the diagnosis and management of asthma in children 5 years and youngerPediatr Pulmonol201146111720963782
- Global Initiative for AsthmaGINA Report, Global strategy for asthma management and prevention2018 Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed March 25, 2018
- PriceDFromerLKaplanAvan der MolenTRomán-RodríguezMIs there a rationale and role for long-acting anticholinergic bronchodilators in asthma?NPJ Prim Care Respir Med2014241402325030457
- McIvorERMcIvorRAThe evolving role of tiotropium in asthmaJ Asthma Allergy20171023123628860828
- PhilipJThe effects of inhaled corticosteroids on growth in childrenOpen Respir Med J20148667325674176
- BarnesPJNeural mechanisms in asthmaBr Med Bull19924811491681352167
- QureshiFZaritskyALakkisHEfficacy of nebulized ipratropium in severely asthmatic childrenAnn Emerg Med19972922052119018183
- GriffithsBDucharmeFMCombined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in childrenCochrane Database Syst Rev20138CD000060
- SchuhSJohnsonDWCallahanSCannyGLevisonHEfficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthmaJ Pediatr199512646396457699549
- ZorcJJPusicMVOgbornCJLebetRDugganAKIpratropium bromide added to asthma treatment in the pediatric emergency departmentPediatrics19991034 Pt 174875210103297
- TeohLCatesCJHurwitzMAcworthJPvan AsperenPChangABAnticholinergic therapy for acute asthma in childrenCochrane Database Syst Rev20124CD003797
- BarnesPJMuscarinic receptor subtypes in airwaysEur Respir J199363283318472820
- GosensRZaagsmaJMeursHHalaykoAJMuscarinic receptor signaling in the pathophysiology of asthma and COPDRespir Res200677316684353
- BusseWWDahlRJenkinsCCruzAALong-acting muscarinic antagonists: a potential add-on therapy in the treatment of asthma?Eur Respir Rev201625139546426929422
- BelmonteKECholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary diseaseProc Am Thorac Soc200524297304 discussion 311–31216267352
- AalbersRParkHSPositioning of long-acting muscarinic antagonists in the management of asthmaAllergy Asthma Immunol Res20179538639328677351
- BarnesPJThe pharmacological properties of tiotropiumChest20001172 Suppl63S66S10673478
- KerstjensHAEngelMDahlRTiotropium in asthma poorly controlled with standard combination therapyN Engl J Med2012367131198120722938706
- KerstjensHACasaleTBBleeckerERTiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trialsLancet Respir Med20153536737625682232
- OhtaKIchinoseMTohdaYLong-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled studyPLoS One2015104e012410925894430
- PaggiaroPHalpinDMBuhlRThe effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trialJ Allergy Clin Immunol Pract201641104.e2113.e226563670
- RaissyHHKellyHWTiotropium bromide in children and adolescents with asthmaPaediatr Drugs201719653353828808948
- VogelbergCEngelMMoroni-ZentgrafPTiotropium in asth matic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging studyRespir Med201410891268127625081651
- VogelbergCMoroni-ZentgrafPLeonaviciute-KlimantavicieneMA randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroidsRespir Res2015162025851298
- HamelmannEVogelbergCVoelkerBTiotropium add-on therapy improves lung function in children and adolescents with moderate and severe symptomatic asthma, independent of markers of allergic statusAllergy201772S103:413
- HamelmannEBatemanEDVogelbergCTiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trialJ Allergy Clin Immunol2016138244145026960245
- VogelbergCEngelMLakiITiotropium add-on therapy improves lung function in children with symptomatic moderate asthmaJ Allergy Clin Immunol Pract20186621602162.e929751157
- SzeflerSJMurphyKHarperT3rdA phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthmaJ Allergy Clin Immunol201714051277128728189771
- BisgaardHVandewalkerMGrahamLMSafety of tiotropium in pre-school children with symptomatic persistent asthmaEur Respir J201648Suppl 60PA315
- HamelmannEBernsteinJAVandewalkerMA randomised controlled trial of tiotropium in adolescents with severe symptomatic asthmaEur Respir J2017491 pii: 1601100
- VogelbergCEngelMLakiITiotropium add-on therapy improves lung function in children with symptomatic moderate asthmaJ Allergy Clin Immunol Pract2018
- VrijlandtEJLEEl AzziGVandewalkerMSafety and efficacy of tiotropium in children aged 1–5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trialLancet Respir Med20186212713729361462
- VogelbergCHamelmannESzeflerSOnce-daily tiotropium respimat add-on therapy improves lung function and control in adolescents and children with moderate symptomatic asthmaJ Allergy Clin Immunol20171392AB95
- RodrigoGJNeffenHEfficacy and safety of tiotropium in school-age children with moderate–to-severe symptomatic asthma: a systematic reviewPediatr Allergy Immunol201728657357828692145
- SchatzMRosenwasserLThe allergic asthma phenotypeJ Allergy Clin Immunol Pract201426645648 quiz 64925439351
- HolgateSTBousquetJChungKFGroup for the Respect of Ethics and Excellence in Science: Asthma sectionSummary of recommendations for the design of clinical trials and the registration of drugs used in the treatment of asthmaRespir Med200498647948715191031
- VandewalkerMVogelbergCHamelmannETiotropium Respimat® add-on therapy improves lung function in adolescents and children with moderate symptomatic asthma, irrespective of IgE levels and eosinophil countPoster P521 presented at: the American Thoracic Society International ConferenceMay 19–24; 2016Washington, DC
- GoldsteinSVogelbergCHamelmannETiotropium Respimat® add-on therapy is effective in children and adolescents with severe symptomatic asthma, irrespective of immunoglobulin E levels and eosinophil countPoster P520 presented at: the American Thoracic Society International ConferenceMay 19–24; 2016Washington, DC
- BeehKMKirstenAMDusserDPharmacodynamics and pharmacokinetics following once-daily and twice-daily dosing of tiotropium Respimat® in asthma using standardized sample-contamination avoidanceJ Aerosol Med Pulm Drug Deliv201629540641526859538
- SharmaAKerstjensHAAalbersRMoroni-ZentgrafPWeberBDahlRPharmacokinetics of tiotropium administered by Respimat((R)) in asthma patients: analysis of pooled data from Phase II and III clinical trialsPulm Pharmacol Ther201742253228039077
- SharmaAWeiseSSzeflerSPharmacokinetics of tiotropium in patients aged 6–11 years with moderate asthma following administration via the Respimat® inhalerAbstract presented at EAACI PAAM Eur Acad Allergy Clin Immunol CongOctober 26–28, 2017London, UK
- SharmaAAalbersRHamelmannEPharmacokinetics of tiotropium administered by Respimat® in adolescent asthma patientsEur Resp J201750S61:PA585
- US Food and Drug AdministrationTiotropium bromide clinical pharmacology review2016 Available from: https://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm549091.pdfAccessed August 30, 2017
- Boehringer Ingelheim LimitedSpiriva Respimat 2.5 microgram, inhalation solution – Summary of Product Characteristics2017
- ParkHWYangMSParkCSAdditive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict responseAllergy200964577878319183167
- GlaxoSmithKlineA phase III parallel group study, comparing the efficacy, safety and tolerability of the fixed dose combination (FDC) of fluticasone furoate+umeclidinium bromide+vilanterol (FF/UMEC/VI) with the FDC of FF/VI in subjects with inadequately controlled asthma Available from: https://clinicaltrials.gov/ct2/show/NCT02924688NLM identifier: NCT02924688Accessed August 30, 2017
- YangSGoyalNBeeraheeMTrivediRLeeLPascoeSDose-response modelling of umeclidinium and fluticasone furoate/umeclidinium in asthmaEur J Clin Pharmacol20157191051105826174114
- LeeLAYangSKerwinETrivediREdwardsLDPascoeSThe effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose-ranging studyRespir Med20151091546225452139
- Pearl Therapeutics, IncChronic dosing cross-over study to assess the efficacy and safety of glycopyrronium (PT001) in adult subjects with intermittent asthma or mild to moderate persistent asthma Available from: https://clinicaltrials.gov/ct2/show/results/NCT02433834NLM identifier: NCT02433834Accessed August 30, 2017
- Chiesi Farmaceutici S.p.A.Efficacy of LAMA added to ICS in treatment of asthma (ELITRA) Available from: https://clinicaltrials.gov/ct2/show/NCT02296411NLM identifier: NCT02296411Accessed August 30, 2017
- BlaisCMDavisBECockcroftDWDuration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmaticsRespir Med20161189610127578477
- University of SaskatchewanEffect of a LAMA and a uLABA on the methacholine dose-response curve Available from: https://clinical-trials.gov/ct2/show/NCT02953041NLM identifier: NCT02953041Accessed August 30, 2017
- SalmonMLuttmannMAFoleyJJPharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseasesJ Pharmacol Exp Ther2013345226027023435542
- LeeLABriggsAEdwardsLDYangSPascoeSA randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthmaRespir Med20151091637325464907
- AlaghaKPalotASofalviTLong-acting muscarinic receptor antagonists for the treatment of chronic airway diseasesTher Adv Chronic Dis201452859824587893
- BusseWWMorganWJTaggartVTogiasAAsthma outcomes workshop: overviewJ Allergy Clin Immunol20121293 SupplS1S822386504
- SpahnJClinical trial efficacy: what does it really tell you?J Allergy Clin Immunol20031125 SupplS102S10614586394
- StåhlECorrelation between objective measures of airway calibre and clinical symptoms in asthma: a systematic review of clinical studiesRespir Med200094873574110955747
- YawnBPBrennemanSKAllen-RameyFCCabanaMDMarksonLEAssessment of asthma severity and asthma control in childrenPediatrics2006118132232916818581
- CrenesseDBerliozMBourrierTAlbertiniMSpirometry in children aged 3 to 5 years: reliability of forced expiratory maneuversPediatr Pulmonol2001321566111416877
- FonsecaACFonsecaMTRodriguesMELasmarLMCamargosPAPeak expiratory flow monitoring in asthmatic childrenJ Pediatr (Rio J)200682646546917171206
- SelfTHGeorgeCMWallaceJLPattersonSJFinchCKIncorrect use of peak flow meters: are you observing your patients?J Asthma201451656657224720711
- SzeflerSJChmielJFFitzpatrickAMAsthma across the ages: knowledge gaps in childhood asthmaJ Allergy Clin Immunol20141331313 quiz 1424290281
- O’BrianALLemanskeRFJrEvansMDGangnonREGernJEJacksonDJRecurrent severe exacerbations in early life and reduced lung function at school ageJ Allergy Clin Immunol201212941162116422236729
- O’ByrnePMPedersenSLammCJTanWCBusseWWSTART Investigators GroupSevere exacerbations and decline in lung function in asthmaAm J Respir Crit Care Med20091791192418990678
- CabanaMDKunselmanSJNyenhuisSMWechslerMEResearching asthma across the ages: insights from the National Heart, Lung, and Blood Institute’s Asthma NetworkJ Allergy Clin Immunol20141331273324369796
- FuhlbriggeAPedenDApterAJAsthma outcomes: exacerbationsJ Allergy Clin Immunol20121293 SupplS34S4822386508
- CaldwellPHMurphySBButowPNCraigJCClinical trials in childrenLancet2004364943680381115337409
- CloutierMMSchatzMCastroMAsthma outcomes: composite scores of asthma controlJ Allergy Clin Immunol20121293 SupplS24S3322386507
- BacharierLBGuilbertTWDiagnosis and management of early asthma in preschool-aged childrenJ Allergy Clin Immunol20121302287296 quiz 297–29822664162
- MurphyKRZeigerRSKosinskiMTest for respiratory and asthma control in kids (TRACK): a caregiver-completed questionnaire for preschool-aged childrenJ Allergy Clin Immunol20091234833.e9839.e919348922
- LipworthBTargeting the small airways asthma phenotype: if we can reach it, should we treat it?Ann Allergy Asthma Immunol2013110423323923535085
- CiprandiGCirilloIPasottiFRicciardoloFLFEF25-75: a marker for small airways and asthma controlAnn Allergy Asthma Immunol2013111323323987209
- GibbEKaplanDThyneSMLyNPFef 25-75 as a predictor of asthma severity in childrenPaper presented at: American Thoracic Society International ConferenceMay 21; 2012San Francisco, CA
- QuanjerPHWeinerDJPrettoJJBrazzaleDJBorosPWMeasurement of FEF25–75% and FEF75% does not contribute to clinical decision makingEur Respir J20144341051105824072211
- KomarowHDMylesIAUzzamanAMetcalfeDDImpulse oscillometry in the evaluation of diseases of the airways in childrenAnn Allergy Asthma Immunol2011106319119921354020
- LarsenGLMorganWHeldtGPChildhood Asthma Research and Education Network of the National Heart, Lung, and Blood InstituteImpulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthmaJ Allergy Clin Immunol20091234861.e1867.e119070356
- JamesAHedlinGBiomarkers for the phenotyping and monitoring of asthma in childrenCurr Treat Options Allergy20163443945227942431
- McQuaidELKopelSJKleinRBFritzGKMedication adherence in pediatric asthma: reasoning, responsibility, and behaviorJ Pediatr Psychol200328532333312808009
- Palo Alto Medical FoundationInhaled medications: anticholinergics2015 Available from: http://www.pamf.org/asthma/medications/inhaled/atrovent.htmlAccessed August 30, 2017
- DalbyRNEicherJZierenbergBDevelopment of Respimat(®) Soft Mist™ Inhaler and its clinical utility in respiratory disordersMed Devices (Auckl)2011414515522915941
- WachtelHKattenbeckSDunneSThe Respimat® development story: Patient-centered innovationPulm Ther2017311930
- HodderRReesePRSlatonTAsthma patients prefer Respimat Soft Mist Inhaler to TurbuhalerInt J Chron Obstruct Pulmon Dis2009422523219554196
- Boehringer IngelheimAsthma: Expanded indication for SPIRIVA Respimat for people 6 years and older Available at: https://www.boehringer-ingelheim.com/press-release/expanded-asthma-indication-spiriva-respimat-euAccessed May 24, 2018

