Abstract
Background
Biological agents provide an important therapeutic alternative for rheumatoid arthritis patients refractory to conventional disease-modifying antirheumatic drugs. Few head-to-head comparative trials are available.
Purpose
The aim of this meta-analysis was to compare the relative efficacy of different biologic agents indicated for use as monotherapy in rheumatoid arthritis.
Methods
A systemic literature search was performed on electronic databases to identify articles reporting double-blind randomized controlled trials investigating the efficacy of biologic agents indicated for monotherapy. Efficacy was assessed using American College of Rheumatology (ACR) 20, 50, and 70 criteria at 16–24 weeks. Relative efficacy was estimated using Bayesian mixed-treatment comparison models. Outcome measures were expressed as odds ratio and 95% credible intervals.
Results
Ten randomized controlled trials were selected for data extraction and analysis. Mixed-treatment comparison analysis revealed that tocilizumab offered 100% probability of being the best treatment for inducing an ACR20 response versus placebo, methotrexate, adalimumab, or etanercept. Likewise, for ACR50 and ACR70 outcome responses, tocilizumab had a 99.8% or 98.7% probability of being the best treatment, respectively, compared to other treatments or placebo. Tocilizumab increased the relative probability of being the best treatment (vs methotrexate) by 3.2-fold (odds ratio: 2.1–3.89) for all ACR outcomes.
Conclusion
Tocilizumab offered the greatest possibility of obtaining an ACR20, ACR50, and ACR70 outcome vs other monotherapies or placebo.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that according to recent estimates affects approximately 1% of the adult population in developed countries.Citation1,Citation2 Conventional disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX) are given as first-line treatment alone or in combination with another DMARD.Citation3 For the past 20 years, biological DMARDs (bDMARDs) have become available that target specific parts of the immune system and offer an important alternative for patients refractory to or intolerant to conventional DMARDs, or where continued therapy with a DMARD is inappropriate/contraindicated.Citation3 Although most patients who are eligible for biological therapy maintain treatment with MTX or another synthetic DMARD, up to 40% discontinue or show poor adherence, due to side effectsCitation4 or preference.Citation5 As a consequence, approximately a third of patients take biologics as monotherapy (according to data from biologic registries and US claims databases).Citation6–Citation13 Among the different biological therapies available, only the tumor necrosis factor-α (TNF-α) inhibitors certolizumab pegol, etanercept, and adalimumab are currently approved as monotherapy for patients with RA in Europe and USA.Citation14–Citation16 In addition, in Europe and USA, the interleukin-6 inhibitor tocilizumab is licensed for use as monotherapy.Citation17 Other biologics such as infliximab and golimumab (both TNF-α inhibitors) and the CD-20 inhibitor rituximab are approved only with MTX.Citation18–Citation20 Other non-TNF-α inhibitors tofacitinib/anakinra and abatacept are only approved as monotherapy in USA.Citation21,Citation22
While it is true that many pivotal RCTs have already demonstrated superior efficacy of these biological agents compared to placebo or conventional DMARDs, there are currently limited head-to-head RCTs for these biological agents. Regarding biologics indicated for monotherapy use, only one trial has specifically examined the superiority of a biological drug directly compared to another.Citation23 The ADACTA trial, a multicentric, randomized double-blind controlled trial included 325 patients and examined the efficacy and safety of tocilizumab compared to adalimumab at 24 weeks. Tocilizumab was shown to be superior as measured by disease activity score in 28 joints (DAS-28), while safety profiles remained similar between the two treatments.Citation23 While this trial has shown superiority of tocilizumab compared with adalimumab in monotherapy in the setting of RA, no published RCT provides head-to-head efficacy evidence comparing all biological agents indicated for monotherapy in RA patients. In the absence of these trials, which would also be difficult to justify due to the cost and time involved, the mixed-treatment comparison (MTC) statistical method allows to estimate through direct and indirect comparisons, the efficacy of different drugs from several trials.Citation24,Citation25 Different MTC methods have been considered in the literature, and one is based on Bayesian principles. The Bayesian MTC approach is recognized for having greater flexibility and capacity for handling complex modeling structures compared to other non-Bayesian approaches.Citation26,Citation27
While several recent systematic reviews and meta-analyses (including Bayesian MTC analyses) have examined the efficacy of biologic therapies in the treatment of RA, some studies have either examined the anti-TNF-α class of bio-logics only,Citation28–Citation30 or included studies using doses prescribed in the US,Citation31 while the majority of these reviews did not compare the effect of biologics administered as monotherapy only.Citation24,Citation25,Citation28–Citation34 In almost all of these studies, the American College of Rheumatology (ACR) criteria outcome measure was chosen to express relative efficacy between treatments.Citation35 A recent previous systematic review and meta-analysis has compared the relative efficacy of EU-licensed biologic combination therapy or monotherapy for patients intolerant of or contraindicated to continue MTX.Citation36 However, this study reported results on the efficacy of biologic agents used in monotherapy against placebo and not against MTX. It is already known that biologic agents are more efficacious than placebo, however, and more importantly, we want to know if and to what extent they are more effective than MTX. In addition, this study did not include the recent ADACTA study, the only head-to-head RCT trial performed to date comparing biologics indicated for use as monotherapy.Citation23
In the present analysis, we used a Bayesian MTC methodology to determine the best choice of treatment among currently available biologic therapies at common doses (prescribed within the EU), administered as monotherapy and assessed by ACR response (ACR20, ACR50, and ACR70) at 16–24 weeks compared to MTX.
Methods
Search and selection
The authors advise that since ethics approval was already obtained for each study included in this meta-analysis, no formal ethics approval was required to undertake this analysis. A literature search was only performed on articles published in peer-reviewed journals, to improve the methodological quality of studies examined and conclusions drawn. A systematic electronic search was performed for the period using the following databases: PubMed/Medline, INIST (Institut de l’Information Scientifique et Tecnique), Science Direct, Google Scholar, and Cochrane Library between July and September 2013. Text words that were applied to the search field included “rheumatoid arthritis” AND (etanercept OR certolizumab OR adalimumab OR tocilizumab). The search was repeated, filtering for “randomized controlled trials”. We only included clinical trials published in English language and excluded reviews, letters, and abstracts.
Study eligibility criteria
Criteria for inclusion of studies in the present MTC analysis included double-blind RCTs, with primary outcomes of ACR20, ACR50, or ACR70 response to treatment at 16 weeks or greater. Subjects included adults aged 18 years or older who met the 1987 revised ACR criteria for RA. Interventions included any biologic agent licensed for use as monotherapy in the EU in case of intolerance to MTX or previous biological treatment. Comparator drugs included DMARDs (eg, MTX), bDMARDs, or placebo. ACR20, ACR50, or ACR70 response was defined as a 20%, 50%, or 70% improvement in tender and swollen joints, and same level of improvement in at least three of the following five disease parameters: patient’s global assessment of disease activity, physician’s global assessment of disease activity, patient’s assessment of pain, patient’s assessment of physical disability (measured by the Health Assessment Questionnaire-Disability Index), and level of acute-phase reactants.Citation35 RCTs having continuous clinical values as primary endpoints, such as DAS-28 or radiological outcome, were excluded as were studies comparing different dosing regimens (not used in clinical practice) of the same agent or studies examining treatments in combination (not monotherapy).
Statistical analysis
Bayesian MTC meta-analysis was conducted on the primary trial endpoints ACR20, ACR50, and ACR70 in all studies that met inclusion criteria after careful assessment of heterogeneity across trials, in terms of subject characteristics, trial methodologies, and treatment protocols. WinBUGS 1.4 statistical softwareCitation37,Citation38 (MRC Biostatistics Unit, Cambridge, UK) was used to perform MTC based on Markov Chain Monte Carlo (MCMC) methods. The MTC method represents a generalization of meta-analysis, whereby possible comparisons not addressed within the individual primary trials can be performed.Citation39 This method preserves within-trial randomization and enables all available direct and indirect comparisons between treatments to be made in one analysis.Citation40 Results of all trials were analyzed simultaneously by a fixed-effect model.Citation41,Citation42 Primary outcomes were expressed as odds ratios (OR) and corresponding 95% credible intervals (CrI) (Bayesian equivalent of confidence interval) comparing the different treatments. For all analyses, we chose proper noninformative prior distributions for parameters included in the model. Analysis was implemented using Gibbs sampler algorithm through WinBUGS statistical software, based on 30,000 iterations after a burn-in of 2000. The value taken as the MCMC estimate was the mean over iteration sampled, starting with the first iteration following burn-in. Satisfactory convergence was verified by trace plots, monitoring Monte Carlo errors, and with Gelman–Rubin diagnostics. Differences in baseline demographic and clinical characteristics for treatment groups were analyzed by one-way ANOVA using Instat software (GraphPad, La Jolla, CA, USA); a P-value of ≤0.05 was considered statistically significant.
Results
Trial flow and study characteristics
shows a flow diagram of the selection process. Our initial search returned 994 distinct results, of which only 47 were potentially relevant based on reading their title and abstract. A further 37 studies were excluded because they did not meet inclusion criteria or did not examine RA patients or had follow-up periods <16 weeks. Data regarding certolizumab pegol were not included in the analysis as the study reported data on a 12-week, double-blind phase, followed by another 12 weeks performed in open label, while all other included studies reported data on the basis of a 24-week (apart from one study, which had a follow-up period of 16 weeks) double-blind follow-up. In RCTs, where the efficacy of tocilizumab not associated to MTX vs tocilizumab associated to MTX was tested, they were not included in the analysis as the comparator was represented by an association of a biologic agent with a DMARD, and in the present analysis, only monotherapies of biologic agents were included. Ten RCTs met the selection criteria and were included in the final meta-analysis.Citation23,Citation43–Citation51 Characteristics of the ten studies are presented in . All ten studies were randomized double-blind and conducted between 1999 and 2013. Study duration ranged from 24 weeks to 2 years, and sample size (intention-to-treat population) ranged from 102 to 531 patients. All studies had a follow-up period of at least 16 weeks, with two studies having a follow-up of 1Citation46 and 2 years,Citation47 respectively. Five studies examined the effect of tocilizumab (8 mg/kg, intravenous, every 4 weeks) to comparator (MTX, placebo, or adalimumab), while three studies examined the effect of etanercept (25 mg, subcutaneous, twice-weekly) to a comparator (placebo or MTX), and two studies compared the efficacy of adalimumab (40 mg, subcutaneous, every second week) to either MTX or placebo. Treatment doses of etanercept, adalimumab, and tocilizumab were standard recommended doses, and MTX doses ranged from 6.9 to 20 mg/wk.
Figure 1 Selection process for studies included in meta-analysis.
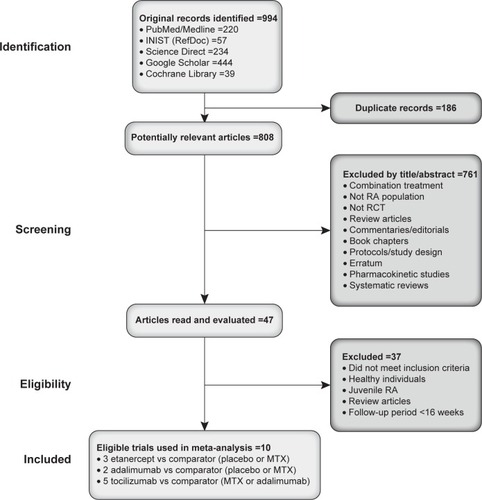
Table 1 Characteristics of trials included in meta-analysis
Patient characteristics
Baseline clinical characteristics for patients (N=3,210) in the ten trials are presented in . The proportion of female patients ranged from 74% to 83%, and mean age ranged from 50 to 54 years. Disease duration and markers of disease activity (erythrocyte sedimentation rate and C-reactive protein) showed normal variation between the ten studies. Pooled analysis of baseline characteristics by treatment arm showed no difference in the proportion of female patients or mean age, whereas a statistically significant difference in disease duration and disease activity markers emerged. However, these differences were mainly attributed to higher values for placebo-treated patients, based on two studies only.Citation43,Citation45 DAS-28, erythrocyte sedimentation rate, and C-reactive protein values for biologic treatments were not significantly different from each other (). The magnitude of ACR response in treatment arms was similar between the ten studies, ranging from 50% to 80% for ACR20, 15% to 30% for ACR50, and 15% to 30% for ACR70 (). ACR response was also similar in studies including early RA patients (four studies, mean: 1 year disease duration) vs studies including patients with established RA (six studies, mean: 8.4 years disease duration) (data not shown).
Table 2 Baseline demographic and clinical characteristics
Table 3 ACR20/50/70 response in trials included in meta-analysis
MTC analysis of ACR response
MTC analysis revealed that for ACR20, tocilizumab had a probability of 100% of being the best treatment in terms of producing an ACR20 response at 24 weeks compared with placebo, MTX, adalimumab, or etanercept (). This equated to a relative odds of being the best treatment of approximately 3-fold greater for tocilizumab compared to either MTX (OR =3.19, 95% CrI =2.46–4.09), etanercept (OR =3.89, 95% CrI =2.46–5.81), or adalimumab (OR =2.49, 95% CrI =1.61–3.71) (). As expected, placebo did not offer significant probability of attaining ACR20 response over other treatments (), and etanercept only offered marginal advantage compared to MTX. A similar benefit was also observed for ACR50 response, whereby tocilizumab had a 99.8% probability of being the best treatment compared to etanercept, which had a probability of 0.14%, and adalimumab, a probability of 0.02% (). The relative odds of being the best treatment was approximately 2.5-fold greater for tocilizumab compared to MTX (OR =3.1, 95% CrI =2.33–3.97), etanercept (OR =2.11, 95% CrI =1.27–3.31), or adalimumab (OR =2.25, 95% CrI =1.39–3.47) (). Interestingly, little difference was noted between adalimumab and etanercept, while both etanercept and adalimumab fared better than MTX (). With regard to ACR70, tocilizumab showed a probability of 98.7% of being the best treatment in inducing ACR70 remission compared to etanercept, which had a probability of 1.2%, and adalimumab, which had a probability of 0.12% (). The relative odds of being the best treatment in inducing ACR70 response was approximately 2.5-fold greater for tocilizumab compared to MTX (OR =3.56, 95% CrI =2.51–4.92), etanercept (OR =2.14, 95% CrI =1.11–3.74), or adalimumab (OR =2.27, 95% CrI =1.32–3.73) (). Similar to ACR50 outcome, both etanercept and adalimumab offered greater probability of attaining ACR70 response compared to MTX (). For all ACR outcomes, tocilizumab increased the relative probability of being the best treatment by approximately 3-fold (OR =2.1–3.89) compared to other treatments, (data not shown).
Figure 2 Relative odds of different drug comparisons being the most effective treatment for obtaining improvement in ACR outcome.
Abbreviations: A, adalimumab; ACR, American College of Rheumatology; E, etanercept; M, methotrexate; P, placebo; T, tocilizumab.
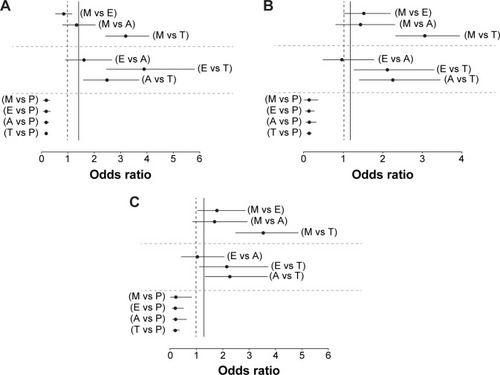
Table 4 Results of mixed-treatment comparison for each drug in being the most effective treatment for obtaining ACR20 improvement
Table 5 Results of mixed-treatment comparison for each drug in being the most effective treatment for obtaining ACR50 improvement
Table 6 Results of mixed-treatment comparison for each drug in being the most effective treatment for obtaining ACR70 improvement
Discussion
This meta-analysis included ten double-blind randomized controlled trials including 3,210 patients comparing a biologic to placebo or MTX and another biologic (in one trial only), having the same endpoint since only one head-to-head trial’s evidence is currently available.Citation23 Since our main aim in the present study was to examine practical parameters that can impact upon real-life decisions within the rheumatology clinic, we specifically focused on the relative efficacy of currently licensed doses of commonly used biologic treatments administered as monotherapy for RA within the European Union.
All studies included in the MTC analysis were similar with regard to average age of patients, disease duration, and baseline disease severity, excluding these factors as sources of heterogeneity across trials and, therefore, bias in the indirect estimates. The main findings from this MTC analysis demonstrate that tocilizumab grants the largest possibility (approximately 3-fold greater) of obtaining ACR20, ACR50, and ACR70 outcome compared to all other drugs, when administered as monotherapy.
The design of this analysis granted the possibility of confirming that all biologic drugs are better than placebo; however, for etanercept and adalimumab, a statistically significant difference with MTX was not observed, while tocilizumab was found to be statistically superior to MTX for all three ACR endpoints. Etanercept and adalimumab were found to be comparable to MTX, in terms of granting an ACR20 response, but were then slightly better than MTX for ACR50 and ACR70 and always found to be better than placebo. Tocilizumab was always superior to placebo, MTX, etanercept, and adalimumab for all endpoints.
Several recent systematic reviews and meta-analysis (including Bayesian MTC analyses) have already compared the efficacy of biologic therapies for the treatment of RA.Citation24,Citation25,Citation28–Citation34 However, while some studies have either examined the anti-TNF-α class of biologics only,Citation28–Citation30 virtually all of these studies based their analysis on the efficacy of biologics used in combination with conventional DMARDs such as MTX.Citation24,Citation25,Citation28–Citation34 Since it has been established that up to one-third of RA patients take biologics as monotherapy, as they are either refractory or intolerant to MTX or where a conventional DMARD is contraindicated,Citation5–Citation13 it is essential to have an awareness of the best treatment options for these patients.
In the few MTC analyses that specifically examined the efficacy of tocilizumab, this IL-6 inhibitor was as good if not better than other biologics examined for ACR response.Citation25,Citation32–Citation34,Citation36 Likewise, findings from our MTC analysis indicate a probability of 98.7% for tocilizumab being the best treatment for ACR70 compared to <1% for other treatments. We have also calculated credible sets for best treatment ranks and noted that they did not overlap and were not wide ranging (data not shown). It is worth noting that the ACR70 response in studies included in the present analysis was approximately 30%, double that of comparator treatments, and higher than other biologic treatments. In the only Bayesian network meta-analysis that has compared the efficacy of biologic agents as monotherapy, tocilizumab was found to have a probability of being the best treatment of 69.2% for all three ACR endpoints vs placebo,Citation36 while our study also investigated a comparison against MTX. In real life, we only have the possibility to treat patients with a biologic or MTX, not with placebo. This is the added value of our study, since we already know that active treatment is better than placebo (as monotherapy). In this comprehensive analysis by Orme et alCitation36 the follow-up period for selection was 12–30 weeks, and the only head-to-head trial examining tocilizumab compared to another biologicCitation23 was not included in their analysis. Furthermore, in that study, comparison was only made to placebo. A recent update on the 2013 European League Against Rheumatism (EULAR) recommendationsCitation3 for the management of RA regarding efficacy, bDMARDs confirmed the efficacy of bDMARDs and conventional synthetic DMARDs vs conventional DMARDs alone (level 1B evidence).Citation52 It also emerged from this systematic review (conducted from 2009 to 2013) that biological DMARDs and MTX combination therapy was more efficacious compared to all biological DMARD classes (1B). Regardless, although the majority of patients are eligible for biological therapy with MTX or another synthetic DMARD, that has proven efficacy, as many as 40% discontinue due to poor adherence, side effects,Citation4 or preference.Citation5 Consequently, about a third of patients will be treated with biologics as monotherapy.Citation6–Citation13
If results from the present MTC are taken into consideration for decision making, patients should be treated with MTX if patients were not previously declared intolerant to MTX or MTX nonresponders; while for those patients who are intolerant to MTX or not adherent, they should be switched to tocilizumab-based therapy in an attempt to achieve ACR20, ACR50, and ACR70 for a greater number of patients. For new patients who cannot undergo MTX-based therapy, tocilizumab should represent the first choice in monotherapy treatment. Considering these three biologics (etanercept, adalimumab, and tocilizumab), in absence of data from this MTC analysis, if a new patient was required to initiate a biologic monotherapy, clinicians would have a 66% chance of making the wrong decision in giving any of these treatments in an attempt to administer the best treatment for achieving clinical improvement, as established by ACR criteria. In contrast, following this MTC, clinicians now have a 98% of probability of administering the best treatment for achieving ACR improvement criteria when administering tocilizumab (corresponding to a 2% chance of making the wrong decision).
Aside from assessing the relative therapeutic efficacy of one biologic compared to another, the issue of which choice of therapy is cost-effective is also an important consideration. Two recent meta-analyses were specifically designed to evaluate the cost-utility and value of reducing the uncertainty associated with the decision to use first-line biologic treatment after failure or inadequate response to DMARDs in moderate-to-severe RA.Citation53,Citation54 Both of these studies concluded that replacing another biologic therapy with tocilizumab or adding tocilizumab to current standard care was a cost-effective strategy in the treatment of RA patients, equating to €17,100 and €20,000 quality-adjusted life years for the two studies, respectively.Citation53,Citation54 Furthermore, a recent network analysis by Jansen et alCitation55 compared biologics as mono-therapy or in combination with MTX in terms of patient reported outcomes in RA patients who had an inadequate response to conventional DMARDs. This analysis revealed that tocilizumab was associated with a greater improvement in pain and self-reported disease activity compared to anti-TNF inhibitors. In patients intolerant to MTX, tocilizumab appears to offer a greater possibility of improved patient reported outcomes compared to anti-TNF monotherapy and may therefore represent an attractive therapeutic option in this patient population. In addition to efficacy, tolerability and safety of different biologics are important considerations for the choice of therapy in RA patients. This component was not assessed in the present analysis, but has been extensively examined in numerous clinical trialsCitation23,Citation43–Citation51 and subsequently in recent reviewsCitation56 and meta-analyses.Citation57–Citation60 One meta-analysis by Burmester et alCitation57 compared the safety profile of tocilizumab to other biological agents and showed similar rates of serious adverse events, serious infections, lymphoma, and congestive heart failure. In addition, an indirect comparison of abatacept, golimumab, and rituximab with tocilizumab in patients with RA, following inadequate response to TNF inhibitors, showed similar safety profiles.Citation58 Findings from these meta-analyses and other studies have since been consolidated in recent EULAR consensus statements.Citation59,Citation60
The present MTC does have some potential limitations. The present MTC analysis used ACR response (between 16 and 24 weeks) as the outcome criteria. Although the inclusion of other endpoint measures (such as erosion or structural damage assessed by radiographic scores) were considered a priori, this was not possible due to inconsistency of these measures in RCTs examined. Further indirect analysis specifically examining whether the relative advantage by tocilizumab in terms of ACR outcome measures can also be extended to these other hard-endpoints at not only 24 weeks, but where possible, longer follow-up periods (eg, 1 year) would be worth investigating. Less heterogeneity among the included studies with regard to disease duration and follow-up period would have been desired. However, careful assessment of trial methodologies, treatment protocols, and patient characteristics did not reveal any association with extent of outcome that was not attributed to treatment alone. The ACR20 response is chosen as a primary endpoint in most RA clinical trials, and ACR50 and ACR70 responses are usually also reported. As previously described, these measures are a binomial reduction built on the underlying continuous distribution of response.Citation32 Although this outcome measure has been frequently used in other MTC analyses,Citation24,Citation25,Citation28–Citation34,Citation36 it has more recently come under criticism for being limited by its lack of sensitivity to change in binary outcome measures.Citation29 Additional continuous outcome criteria (eg, the Health Assessment Questionnaire multiplier) may increase sensitivity and complement binary measures.Citation61 It is also important to mention that the ADACTA trialCitation23 was performed more recently compared to other older trials included in our analysisCitation43–Citation51 and this time difference may result in unforeseen differences in patient clinical characteristics. The present analysis compared the relative efficacy of different biologic agents administered as monotherapy for the treatment of RA. This analysis did not compare the safety profile of these biological agents, which is an essential component when choosing the best therapeutic option for a patient. One perceived weakness of this analysis is the range in difference in MTX doses among the different studies included. However, the MTX groups of all analyzed biologic agents were pooled together in the Bayesian meta-analysis, thus granting an acceptable approximation of data. Therefore, this analysis was not based on the direct confrontation of a single biologic agent with its control group, but on the indirect confrontation of all biologic agents with the pooled group of all control arms. For this reason, the difference in the MTX dosage (particularly for the two Japanese studiesCitation49,Citation51 included that had low, fixed MTX doses) for various control groups exerts only a small effect on the analysis performed. To verify this, analysis was repeated without the inclusion of the two Japanese studies, and although the OR was marginally reduced, tocilizumab still emerged as the best treatment in obtaining ACR20, ACR50, and ACR70 outcome compared to all other drugs (data not shown).
This analysis includes indirect comparisons across different clinical trials and is not a replacement for head-to-head data. Findings from the present analysis should be interpreted with caution, taking into consideration the aforementioned limitations associated with MTC. However, in the absence of these trials, Bayesian MTC models are recognized as accepted methodologies for the comparison of therapies and essential for therapeutic decision-making.
Conclusion
Findings derived from the present analysis indicate that treatment with tocilizumab allows a significantly greater proportion of patients to attain clinical benefit within 6 months compared to other biologic therapies. Although most patients who are eligible for biological therapy will still continue treatment with MTX or another synthetic DMARD, a significant proportion of patients at some stage will require monotherapy. Tocilizumab may well be considered as a first-choice, cost-effective treatment in these patients. Further comparative efficacy trials to directly compare targeted treatments for RA are clearly required. For the moment, MTC analysis, as presented here, will play an important role in aiding day-to-day decision-making in clinical practice.
Author contributions
AM designed the study, and participated in the interpretation of data and writing of the manuscript. MB and LP performed MTC meta-analysis and participated in the interpretation of data. CGE performed data analysis (trial and patient clinical characteristics) and wrote the article. EB participated in the design of the study and interpretation of data. All authors contributed to drafting the manuscript, and read and approved the final manuscript.
Acknowledgments
The authors thank Primula Multimedia SRL, Italy, for editorial assistance in the preparation of this manuscript. This assistance was sponsored by Roche S.p.A., Italy.
Disclosure
Roche S.p.A. did not play any role in the design, interpretation, and eventual writing of the paper. The authors report no conflicts of interest in this work.
References
- KvienTKEpidemiology and burden of illness of rheumatoid arthritisPharmacoeconomics2004222 Suppl 111215157000
- HelmickCGFelsonDTLawrenceRCEstimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part IArthritis Rheum2008581152518163481
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201473349250924161836
- SalliotCVan Der HeijdeDLong term safety of methotrexate monotherapy in rheumatoid arthritis patients: a systematic literature researchAnn Rheum Dis20096871100110419060002
- de ThurahANorgaardMHarderIStengaard-PedersenKCompliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients’ beliefs about the medicine: a prospective cohort studyRheumatol Int201030111441144819823840
- ListingJStrangfeldARauRClinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low – results from RABBIT, the German biologics registerArthritis Res Ther20068R6616600016
- AsklingJForedCMBrandtLTime-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonistsAnn Rheum Dis200766101339134417261532
- HeibergMSKoldingsnesWMikkelsenKThe comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter studyArthritis Rheum200859223424018240258
- LeeSJChangHYaziciYGreenbergJDKremerJMKavanaughAUtilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort studyJ Rheumatol20093681611161719369454
- MarietteXGottenbergJERavaudPCombeBRegistries in rheumatoid arthritis and autoimmune diseases: data from the French registriesRheumatology201150122222921148156
- SolimanMMAshcroftDMWatsonKDLuntMSymmonsDPHyrichKLImpact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics RegisterAnn Rheum Dis201170458358921330639
- PappasDAReedGWSaundersKCharacteristics associated with biologic monotherapy use in biologic-naive patients with rheumatoid arthritis in a US registry populationRheumatol Ther201528596
- YaziciYShiNJohnAUtilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapyBull NYU Hosp Jt Dis2008662778518537774
- Cimzia® (Certolizumab pegol) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdfAccessed November 8, 2013
- Enbrel® (Etanercept) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdfAccessed November 8, 2013
- Humira® (Adalimumab) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdfAccessed November 8, 2013
- Roactemra® (Tocilizumab) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000955/WC500054890.pdfAccessed November 8, 2013
- Remicade® (Infliximab) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdfAccessed November 8, 2013
- Simponi® (Golimumab) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000992/WC500052368.pdfAccessed November 8, 2013
- MabThera® (Rituximab) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdfAccessed November 8, 2013
- Kineret® (Anakinra) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000363/WC500042310.pdfAccessed November 8, 2013
- Orencia® (Abatacept) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000701/WC500048935.pdfAccessed November 8, 2013
- GabayCEmeryPvan VollenhovenRADACTA Study InvestigatorsTocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trialLancet201338198771541155023515142
- NixonRMBansbackNBrennanAUsing mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritisStat Med20072661237125416900557
- DevineEBAlfonso-CristanchoRSullivanSDEffectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approachPharmacotherapy2011311395121182357
- SuttonAJAbramsKRBayesian methods in meta-analysis and evidence synthesisStat Methods Med Res200110427730311491414
- JonasDEWilkinsTMBangdiwalaSFindings of Bayesian Mixed Treatment Comparison Meta-Analyses: Comparison and Exploration Using Real-World Trial Data and Simulation [Internet]Rockville, MDAgency for Healthcare Research and Quality (US)2013
- SalliotCFinckhAKatchamartWIndirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysisAnn Rheum Dis201170326627121097801
- SchmitzSAdamsRWalshCDBarryMFitzGeraldOA mixed treatment comparison of the efficacy of anti-TNF agents in rheumatoid arthritis for methotrexate non-responders demonstrates differences between treatments: a Bayesian approachAnn Rheum Dis201271222523021960560
- AaltonenKJVirkkiLMMalmivaaraAKonttinenYTNordströmDCBlomMSystematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritisPLoS One201271e3027522272322
- HochbergMCBerrySBroglioKMixed treatment comparison of efficacy and tolerability of biologic agents in patients with rheumatoid arthritisCurr Med Res Opin201329101213122223745516
- BergmanGJHochbergMCBoersMWintfeldNKielhornAJansenJPIndirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugsSemin Arthritis Rheum201039642544120223500
- TurkstraENgSKScuffhamPAA mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritisCurr Med Res Opin201127101885189721848493
- Gallego-GalisteoMVilla-RubioAAlegre-del ReyEMárquez-FernándezERamos-BáezJJIndirect comparison of biological treatments in refractory rheumatoid arthritisJ Clin Pharm Ther201237330130721831256
- FelsonDTAndersonJJBoersMAmerican College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritisArthritis Rheum19953867277357779114
- OrmeMEMacgilchristKSMitchellSSpurdenDBirdASystematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70Biologics2012642946423269860
- LunnDJBestNThomasAWakefieldJSpiegelhalterDBayesian analysis of population PK/PD models: general concepts and softwareJ Pharmacokinet Pharmacodyn200229327130712449499
- SpiegelhalterDThomasABestNLunnDWin-BUGS User Manual: Version 1.4Cambridge, MAMRC Biostatistics Unit2003
- SpiegelhalterDJAbramsKRMylesJPBayesian Approaches to Clinical Trials and Health-Care Evaluation (Statistics in Practice)Chichester, EnglandJohn Wiley & Sons Ltd2004
- CaldwellDAdesAHigginsJSimultaneous comparison of multiple treatments: combining direct and indirect evidenceBr Med J2005331752189790016223826
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- WeltonNJCaldwellDMAdamopoulosEVedharaKMixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart diseaseAm J Epidemiol200916991158116519258485
- MorelandLWSchiffMHBaumgartnerSWEtanercept therapy in rheumatoid arthritis. A randomized, controlled trialAnn Intern Med1999130647848610075615
- BathonJMMartinRWFleischmannRMA comparison of etanercept and methotrexate in patients with early rheumatoid arthritisN Engl J Med2000343221586159311096165
- van de PutteLBAtkinsCMalaiseMEfficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failedAnn Rheum Dis200463550851615082480
- KlareskogLvan der HeijdeDde JagerJPTEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigatorsTherapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trialLancet2004363941067568115001324
- BreedveldFCWeismanMHKavanaughAFThe PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatmentArthritis Rheum2006541263716385520
- MainiRNTaylorPCSzechinskiJCHARISMA Study GroupDouble-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexateArthritis Rheum20065492817282916947782
- NishimotoNHashimotoJMiyasakaNStudy of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumabAnn Rheum Dis20076691162116717485422
- JonesGSebbaAGuJComparison of tocilizumab mono-therapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION studyAnn Rheum Dis2010691889619297346
- NishimotoNMiyasakaNYamamotoKStudy of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapyMod Rheumatol2009191121918979150
- NamJLRamiroSGaujoux-VialaCEfficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritisAnn Rheum Dis201473351652824399231
- DiamantopoulosABenucciMCapriSEconomic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in ItalyJ Med Econ201215357658522313326
- SoiniEJHallinenTAPuolakkaKVihervaaraVKauppiMJCost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritisJ Med Econ201215234035122168785
- JansenJPBuckleyFDejonckheereFOgaleSComparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs – a systematic review and network meta-analysisHealth Qual Life Outcomes20141210224988902
- TanakaTHishitaniYOgataAMonoclonal antibodies in rheumatoid arthritis: comparative effectiveness of tocilizumab with tumor necrosis factor inhibitorsBiologics2014814115324741293
- BurmesterGRFeistEKellnerHEffectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA)Ann Rheum Dis20117075575921187298
- SinghJAWellsGAChristensenRAdverse effects of biologics: a network meta-analysis and Cochrane overview (review)Cochrane Database Syst Rev20112CD00879421328309
- SchoelsMMvan der HeijdeDBreedveldFCBlocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statementAnn Rheum Dis201372458358923144446
- RamiroSGaujoux-VialaCNamJLSafety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritisAnn Rheum Dis201473352953524401994
- American College of Rheumatology Committee to Reevaluate Improvement CriteriaA proposed revision to the ACR20: the hybrid measure of American College of Rheumatology responseArthritis Rheum200757219320217330293

