Abstract
Purpose
Erectile dysfunction (ED) is associated with testosterone deficiency and is a symptom of functional hypogonadism. A correlation between ED and cardiovascular disease (CVD) has been recognized, and ED has been proposed as an early marker of CVD. However, the relationship between ED and CVD risk in hypogonadism requires clarification and whether testosterone therapy (TTh) can be a beneficial treatment strategy, but long-term data are limited. This study investigates long-term TTh in men with hypogonadism and ED with a history of CVD.
Methods
Seventy-seven patients with a history of CVD and diagnosed with functional hypogonadism and erectile dysfunction (erectile function domain score <21 on the International Index of Erectile Function questionnaire (IIEF questions 1–5)) were enrolled and TTh effects on anthropometric and metabolic parameters investigated for a maximum duration of 12 years. All men received long-acting injections of testosterone undecanoate at 3-monthly intervals. Eight-year data were analysed. Data collection registry started in November 2004 till January 2015.
Results
In hypogonadal men receiving TTh, IIEF increased by 5.4 (p<0.001). Total weight loss was 23.6 ± 0.6 kg after 8 years. HbA1c had declined by an average of 2.0% (P<0.0001). Total cholesterol levels significantly declined following TTh after only 1 year (P<0.0001), and HDL increased from 1.6±0.5 at baseline to 2±0.5 mmol/L following 8 years of TTh (P<0.0001). SBP decreased from 164±14 at baseline to 133±9 mmHg, signifying a reduction of 33±1 mmHg (P<0.0001).
Conclusion
In hypogonadal men with a history of CVD, TTh improves and preserves erectile function over prolonged periods with concurrent sustained improvements in cardiometabolic risk factors. Measuring ED and testosterone status may serve as an important male health indicator predicting subsequent CVD-related events and mortality and TTh may be an effective add-on treatment in secondary prevention of cardiovascular events in hypogonadal men with a history of CVD.
Introduction
Functional hypogonadism is a common medical condition affecting men, characterized by serum testosterone levels of 12.1 nmol/L (<350 ng/dL) and at least one clinical symptom including sexual dysfunction (difficulty achieving organism, decrease in libido, erectile dysfunction, reduced physiologic erections, absent penile sensation, and decreased ejaculate), reduced stamina, changes in cholesterol levels, irritability, depressed mood, difficulty concentrating, anemia, osteoporosis, and hot flushes.Citation1,Citation2 Ageing males experience a progressive decline in serum testosterone levels leading to increased prevalence of testosterone deficiency and/or hypogonadism.Citation3 Testosterone deficiency is highly prevalent in men with metabolic syndrome (MetS), type 2 diabetes (T2D) and established cardiovascular disease CVD, which also increases with age.Citation3 A negative correlation between testosterone and cardiovascular outcomes has been established in epidemiological studies.Citation4 While these associations do not imply causality, the ablation of testosterone through androgen deprivation therapy (ADT) for the treatment of prostate cancer increases the risk of cardiovascular events, including myocardial infarction (MI), stroke and overall cardiovascular mortalityCitation5 while increasing ED.Citation6
ED is considered a vascular impairment that shares many risk factors with CVD, and has been proposed as an early marker of symptomatic CVD.Citation7 T2D is a CVD risk factor and severity of ED in men with diabetes correlates with glycaemic control and disease duration.Citation8 Additionally, there is a positive correlation between ED and silent CAD in men with seemingly well-controlled T2D.Citation9 This has led to the suggestion that ED may be an early indicator of CVD.Citation7,Citation10,Citation11 Reports have identified ED preceding CAD in about two-thirds of cases, with the time interval from ED to CAD symptoms being 2–3 years and a cardiovascular event 3–5 years.Citation12,Citation13 Furthermore, the severity of ED correlates with the severity of the CAD.Citation14 Therefore, with ED commonly preceding CVD, its diagnosis may offer an opportunistic window for CVD risk mitigation and treating risk factors for ED may improve cardiovascular health.
Importantly, low testosterone levels are considered a predisposing risk for ED and both ED and testosterone deficiency are independently correlated with increased risk of CAD.Citation16 Low testosterone increases the risk of men with erectile dysfunction dying from cardiovascular events.Citation17 Furthermore, hypertension, hyperlipidaemia, diabetes, obesity, and depression are common conditions and risk factors for CVD that are also independently associated with ED and low testosterone.Citation18 Testosterone therapy (TTh), the primary treatment for alleviating symptoms of functional hypogonadism, improves vascular dysfunction, reduces inflammation associated with atherosclerosis and improves clinical surrogate markers of atherosclerosis in hypogonadal men.Citation19–Citation21 Several RCTs have also demonstrated improvements in CVD risk factors following TTh, including insulin resistance, dyslipidemia, central adiposity and glycaemic control in hypogonadal men with T2D and/or MetSCitation22–Citation25; and, despite the complex relationship between testosterone and the cardiovascular system, TTh is considered safe once other comorbidities are addressed.Citation26 Similarly, a number of studies have detailed the benefits and improvement of erectile function in hypogonadal men with ED following TTh.Citation27–Citation29 Furthermore, our previous study showed that long-term TTh for up to 12 years improved erectile function, anthropometric and cardiometabolic risk factors with the benefits of TTh being more pronounced in patients with moderate/severe ED at baseline than in patients with no/mild ED at baseline.Citation30
The role of low testosterone and ED as indicators of CVD still, however, remains unclear as long-term data are lacking. Whether treating functional hypogonadism with long-term TTh improves ED as an indicator of vascular health and subsequently improves CVD risk is not known. Testosterone administration is, however, indicated in the treatment of ED in hypogonadal malesCitation30 and such interventional studies afford an opportunity to investigate the potential therapeutic benefits of TTh on ED and CVD (including cardiovascular risk factors such as MetS and T2D). The aim of this study is to investigate the long-term effectiveness of TTh on improving erectile function as an indicator of cardiometabolic/vascular health in men with functional hypogonadism and a history of CVD, and demonstrating our results as a continuation work of previously published paper,Citation29,Citation56 in which long TTh treatment may decrease the risk of CVD by improving the cardiometabolic parameters in hypogonadal men.
Patients and Methods
Patients
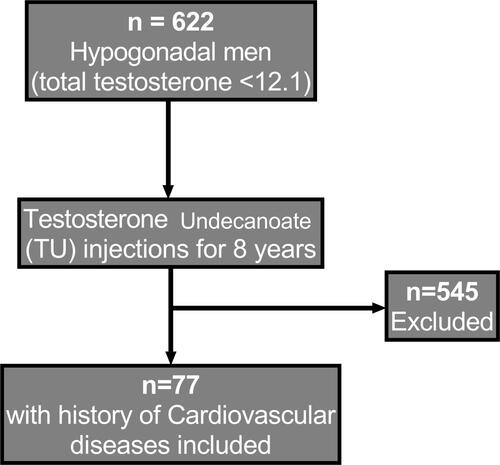
This study represents two prospective, cumulative observational registry studies of 622 hypogonadal men presenting to two urological centers at the Institute of Urology and Andrology, Segeberger Kliniken, Norderstedt-Hamburg, Germany, and Men’s Health Department, Hamad Medical Corporation, Doha, Qatar. Study participants were diagnosed with functional hypogonadism by fulfilling the criteria of having total testosterone <12.1 nmol/L (mean 9.78 ± 1.56 nmol/L) in the presence of hypogonadal symptoms measured by the Aging Males’ Symptoms scale (AMS)) and presenting with ED, having an IIEF-EF of <21 (maximum score: 30). Seventy-seven men with a history of CVD (12.4% of the whole patient cohort), indicated by a previous diagnosis of coronary artery disease (CAD) (n=48) and/or myocardial infarction (MI) (n=40) and/or stroke (n=7), were identified and included in the analysis. Forty-one patients (53%) had T2D (). Additionally, 72 patients (94%) were on anti-hypertensives, 58 (75%) on statins, and 37 (48%) on antidiabetic medications. Patients received continuous testosterone undecanoate (TU) injections (Nebido®; Bayer AG, Leverkusen, Germany) in 3-monthly intervals, following an initial interval of 6 weeks, for up to 8 years. Mean age at baseline of the 77 patients with a history of CVD was 60.65 ± 4.98 years and mean follow-up time was 7.29 ± 1.20 years. Exclusion criteria for TU administration included previous treatment with androgens, prostate cancer, breast cancer, recent angina, or severe untreated sleep apnea. Data collection registry started in November 2004 till January 2015. Ethical considerations were conducted in accordance with declaration of Helsinki, Institutional Review Board (IRB) approval were given from the ethics committee in the German medical association (Ärztekammer) (EK/CH/AU/1/6/2015) for observational studies in patients receiving standard treatment were followed. After receiving an explanation regarding the nature and the purpose of the study, all subjects provided written consent to be included in the registry and have their data analyzed.
Assessment and Follow-Up
Assessments and measurements were taken at each or every second urology visit (between two and four times per year) and the annual average was calculated for each parameter. Erectile function was evaluated by the erectile function domain of the International Index of Erectile Function (IIEF-EF).Citation31 IIEF is a validated, multidimensional, 15-item self-administered questionnaire commonly employed to assess erectile function and therapeutic efficacy thereof.Citation32 Erectile function is specifically addressed by six questions from the “erectile function domain” of the questionnaire. Patients’ height and weight were recorded and body mass index (BMI) was calculated (BMI = weight (kg)/height2 (m2)). Waist circumference (WC) was measured midway between the iliac crest and the costal margin. Systolic blood pressure (SBP), diastolic blood pressure (DPB) and pulse pressure (SBP-DPB) were also measured. Laboratory tests included glycated hemoglobin (HbA1c) measured by high performance liquid chromatography (HPLC), and serum lipid profile including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs) and non-HDL-C measured enzymatically with Alinity c-Module (Abbott).
Statistical Analysis
Statistical values were reported at each time point such as mean, median, standard deviation (SD), range, and sample size. Linear mixed-effects model was used in change of the outcome scores across the study period. To indicate follow-up sessions, time was included as a fixed effect in the model. For the intercept, a random effect was included in the model. Computing the differences in least square means at baseline versus the score at each follow-up visit was used to determine the estimation and test of change in scores.
Results
Effects of Long-Term TTh on Erectile Function
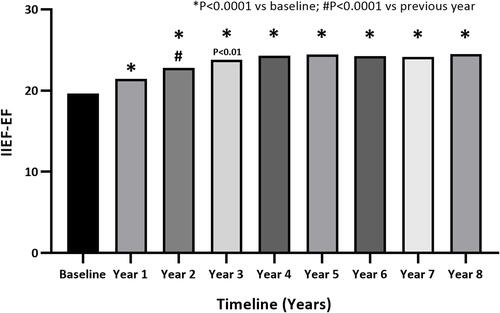
Over the course of TTh, a significant and sustained improvement in erectile function domain score occurred: 19.6±6.34 at baseline, 21.4±5.36 after 1 year, 22.8±4.97 after 2 years, 23.8±4.52 after 3 years, 24.3±4.34 after 4 years, 24.4±4.53 after 5 years, 24.4±4.57 after 6 years, 24.2±4.76 after 7 years and 24.5±4.4 after 8 years (). By the end of the follow-up period, the erectile function domain score had improved by 5.4 (P<0.0001) after model adjustment.
Effects of Long-Term TTh on Weight Loss, WC, and BMI
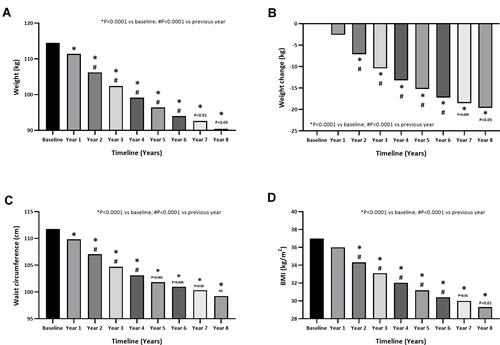
Weight declined progressively from year to year, reducing from 114.5 ± 13.41 to 90.42 ± 8.77 after 8 years of continuous TTh in 77 hypogonadal men () (P<0.0001) and significant weight loss (WL) totalled 23.6 ± 0.6 kg (19.62 ± 5.71%) after 8 years. The percentage of change in weight was progressive and increased with continuous treatment (model adjusted 2.6% after 1 year, 7.1% after 2 years, 10.4% after 3 years, 13.2% after 4 years, 15.4% after 5 years, 17.4% after 6 years, 19.0% after 7 years, and 20.2% after 8 years) (). WL was complemented by a significant gradual decrease in WC. WC reduction in response to TTh was significant from year to year decreasing from 111.8±8.2 at baseline to 99.2 ± 6.5 cm after 8 years of TTh (). WC reduction totalled to 12.5 ± 0.4 cm at the end of the 8-year follow-up period (model adjusted 1.7% after 1 year, 4.4% after 2 years, 6.3% after 3 years, 7.7% after 4 years, 8.8% after 5 years, 9.6% after 6 years, 10.4% after 7 years, and 11.1% after 8 years). BMI also reduced considerably over the entire follow-up period with a mean reduction of 8 kg/m2 ().
Figure 3 Anthropometric parameters in hypogonadal men with a history of cardiovascular disease receiving long-term testosterone therapy.
Effects of Long-Term TTh on CVD Risk Factors
Effects on HbA1c
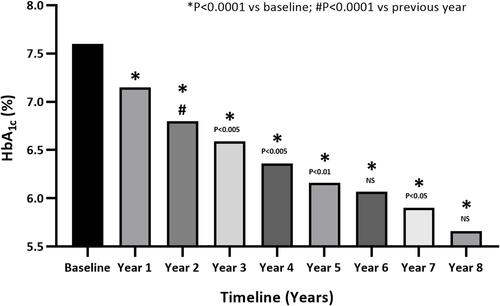
HbA1c levels were significantly reduced year to year following TTh: 7.6% at baseline, 7.2% after 1 year, 6.8% after 2 years, 6.6% after 3 years, 6.4% after 4 years, 6.2% after 5 years, 6.0% after 6 years, 5.9% after 7 years, and 5.7% after 8 years (). HbA1c had declined by an average of 2.0% (P<0.0001) at the end of the follow-up period. The TGs:HDL-C ratio was used as a surrogate marker of insulin resistance and decreased from 5.4±2 to 2.5±0.6 (P<0.0001).
Effects on Lipid Profiles
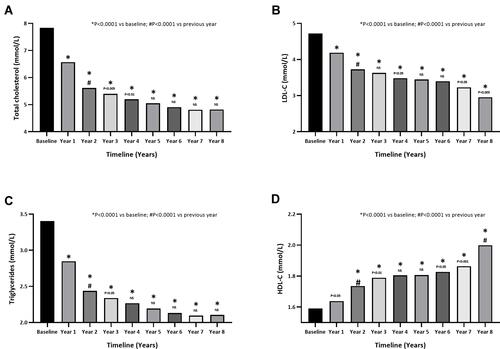
TC levels significantly declined following TTh after only 1 year (P<0.0001) and continued to gradually decline over the 8-year follow-up period (). TC decreased from 7.8±0.9 mmol/L at baseline to 4.8±0.2 mmol/L after 8 years (P<0.0001). Similarly, following 8 years of TTh, LDL-C was reduced from 4.7±0.9 to 3.0±0.7 mmol/L (), and TGs from 3.4±0.7 to 2.1±0.1 mmol/L () (P<0.0001). Furthermore, HDL increased from 1.6±0.5 at baseline to 2±0.5 mmol/L following 8 years of TTh (P<0.0001) (). The TC:HDL ratio declined from 5.5±2.0 at baseline to 2.6±0.7 at 8 years (P<0.0001). Non-HDL cholesterol decreased from 6.2±0.8 at baseline to 2.8±0.5 mmol/L at 8 years) (P<0.0001).
Figure 5 (A) Systolic and (B) diastolic blood pressure (mmHg) in hypogonadal men with a history of cardiovascular disease receiving long-term testosterone therapy. (C) Pulse pressure in hypogonadal men with a history of cardiovascular disease receiving long-term testosterone therapy.
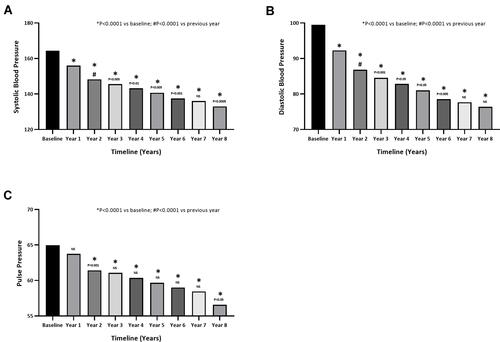
Figure 6 Serum lipids in hypogonadal men with a history of cardiovascular disease receiving long-term testosterone therapy (A) total cholesterol, (B) LDL-cholesterol, (C) triglycerides and (D) HDL-cholesterol. Note: Data are shown as mean. *p<0.0001 vs baseline. #p<0.0001 vs previous year.
Effects on SBP and DBP
Long-term TTh in hypogonadal patients with a history of CVD resulted in marked and significant reduction in blood pressure. SBP decreased from 164±14 at baseline to 133±9 mmHg following 8 years of TTh, signifying a reduction of 33±1 mmHg (P<0.0001) (). The decrease in SBP was gradual but significant over the first 6 years compared to the previous year and gradually declined up to the end of the 8-year follow-up period. Similarly, 8 years of TTh reduced DBP by 24±1 mmHg from 99±11 mmHg at baseline to 77±5 mmHg (P<0.0001) (). Again, a significant decrease of DBP occurred over the first 6 years of treatment compared to the previous year and then remained low for the remainder of the 8-year follow-up period.
Effects on Pulse Pressure
Long-term TTh resulted significant reduction in pulse pressure from 65±6 to 57±8 in hypogonadal patients with a history of CVD. Pulse pressure was gradually and significantly reduced over the course of treatment compared to baseline with a mean change of 9±1 at the end of the follow-up period. Pulse pressure finally dropped to within the normal range after 5 years of TTh ().
Safety and Compliance
There were no major adverse CV events recorded for any patient. No patient had a urological event (prostate cancer or voiding dysfunction). No patient missed a single TU injection. No patient dropped out.
Discussion
This observational registry assessing 77 hypogonadal men with a history of CVD on continuous TTh for up to 8 years demonstrated significant and progressive improvements in IIEF sexual function scores following long-term TU administration accompanied by decreased cardiovascular events compared to non-treated age and health status–matched controls. Furthermore, a gradual yet significant reduction in blood pressure following TTh was maintained over the entire 8-year treatment period. Importantly, 94% of the patients included in this study were on anti-hypertensives to control their blood pressure, although with limited success as baseline blood pressure was elevated prior to TTh. Pulse pressure, indicated as a surrogate marker for arterial stiffness, was also significantly reduced following TTh demonstrating further vascular improvements.
The benefits of TTh have been observed in men with congestive heart failure and cardiac ischemia/angina, including a reduction in carotid intima-media thickness (CIMT), a clinical surrogate marker of atherosclerosis.Citation33 In the present study, we report that there were no CV deaths or major adverse CV events (MACE) recorded for any patient receiving TTh. The lack of MACE in patients with moderate/severe ED is particularly remarkable considering their worse baseline health status and higher mortality risk. Studies have previously shown that mortality in men with low testosterone levels was reduced following TTh.Citation34,Citation35 Shores et alCitation34 observed that men receiving TTh had a lower mortality rate (10.3%) compared with untreated hypogonadal men (20.7%). Muraleedharan et alCitation35 also observed that TTh in hypogonadal men with T2D reduced mortality to 8.4% compared with 19.2% in untreated men. Most importantly, following the 8-years of continuous TTh in the present study, we did not observe any increase in CVD risk in this subset of patients.
A negative correlation between testosterone and hypertension has been demonstrated in men.Citation36 It is suggested that testosterone modulates arterial blood pressure via various mechanisms.Citation37 Testosterone levels have reported being negatively associated with SBP.Citation36 In a study of 206 men from the Baltimore Longitudinal Study of Ageing, serum testosterone levels were an independent negative predictor for developing arterial stiffness.Citation38 This association persisted after adjusting for risk factors such as age, pulse pressure, fasting plasma glucose, BMI and TC, suggesting that hypogonadism contributes to elevated blood pressure and TTh decreases blood pressure.Citation39,Citation40 In the current study, we report that TTh gradually, yet significantly decreases blood pressure, which was maintained over the 8-year follow-up period. Furthermore, pulse pressure (PP) is an indicator for arterial stiffness, which is related to endothelial dysfunction, and increased PP is related to increase risk of cardiovascular events, hence increase mortality.Citation41,Citation42 An association between ED and PP was revealed by the present study. In TTh group, a reduction in PP was noticed to be associated with a significant improvement in ED, whereas no improvement and a progressive deterioration was experienced in PP and ED in the other group. The present study therefore supports beneficial effects of TTh on vascular function reported in previous observational studiesCitation43 and placebo-controlled trialsCitation44 whereby a reduction in arterial stiffness was reported following TTh.
The most common cause of ED is an underlying vascular disease caused mainly by atherosclerosis within the penile arteries, and the prevalence of ED is higher in patients with CVD.Citation45 The severity of ED has been reported to directly correlate with the severity of the CADCitation14 and suggested as a potential indicator of silent CAD in men with uncomplicated T2D and relatively low CAD risk.Citation9 Therefore, ED is considered a manifestation of a generalized vascular disease and has been suggested as an indicator for future cardiovascular events. The artery size hypothesis indicates that vascular disease may be comparative in arteries independent of location and vessel size yet the same level of endothelial dysfunction and atherosclerosis may lead to a more significant reduction of blood flow in erectile tissues compared with that in coronary arteries suggesting ED would precede CAD.Citation11 Several studies show that ED manifests prior to CAD in approximately two-thirds of cases,Citation12–Citation14,Citation46 with the time interval from ED to CAD symptoms being 2–3 years and ED to a cardiovascular event 3–5 years.Citation41 Due to the increased risk of CAD among men with functional hypogonadism and EDCitation16 findings from our previous studyCitation42 and the present study, a role for routinely assessing ED to identify individuals at increased risk of CAD is supported. Indeed, hypogonadism is prevalent in patients with ED, and low testosterone levels are reported in around 23–36% of the patients presenting with ED.Citation15 Low testosterone levels are considered to be a predisposing risk for ED, and both ED and testosterone deficiency are both independently associated with increased risk of CAD.Citation16 Therefore, identifying men at risk of CAD via diagnosis of ED and underlying.
Severe ED additionally functions as a prognostic indicator of cardiovascular risk comorbidities in men with functional hypogonadism.Citation42 Indeed, our previous study suggested that the severity of ED within patients with functional hypogonadism correlated with comorbidities including increased waist circumference, hyperglycemia, hypertriglyceridemia, hyperlipidemia and a history of diabetes mellitus.Citation47 ED was found to be present in 33% of men with uncomplicated T2D and silent CAD, compared to the 5% in T2D men without myocardial ischemia suggesting ED as an indicator of CAD in T2D men.Citation9 Furthermore, obesity and increased adiposity are common clinical features of functional hypogonadism frequently associated with ED.Citation48 In the current study, TTh reduced weight, WC and mean BMI (which was within the obese range at baseline) in a gradual yet sustained manner. Additionally, despite being treated with statins to control their dyslipidemia, most patients exhibited elevated serum lipid profiles at baseline which were significantly improved (reduced TC, LDL-C, TGs and increased HDL-C) following long-term TTh. It worth to mention, that 25% of the patients did not undergone statin treatment, at which they are more susceptible to CAD. HbA1c was also improved by TTh. The shared aetiology between metabolic dysfunction, vascular dysfunction and ED suggests that the improvements in ED and CVD following TTh in this study may be due to the overall improvement in metabolic parameters.
TTh to treat functional hypogonadism in the present investigation and other studies has resulted in improvement of components of MetS and T2D that are considered CVD risk factors, thus improving cardiometabolic functionCitation49 and reducing the risk of CVD.Citation50,Citation51 It is noted that continuous treatment with TTh is required to maintain the long-term benefits of testosterone.Citation52,Citation53 These well-recognized benefits of TTh on metabolic parameters also extend to the improvement of erectile function and quality of life.Citation51,Citation54 Therefore, the IIEF-5 questionnaire may be regarded not only as a clinical indicator to screen for ED but also as a potential diagnostic tool to assess the global CAD risk profile of men with functional hypogonadism allowing early intervention with TTh to improve ED and CVD risk. However, in a cohort of men with erectile dysfunction it was suggested that hypogonadism‐associated CV risk was dependent upon the characteristics of subjects, being more evident in normal weight than in obese patients.Citation55 Conversely, in patients with a previous history of CV events, hypogonadism was associated with a reduced risk of new CV events, even after adjusting for confounders, whereas no relationship was observed in subjects free of previous CV events.Citation56
This observational study is not without inherent limitations. As this was not a randomized placebo-controlled trial, it does not allow direct comparison of TTh versus non-treatment, limiting the scope of interpretation. However, we consider that the cardiovascular health benefits of TTh are time dependent, particularly when related to metabolic improvements, and therefore this real-world evidence study provides valuable data about the true clinical significance of TTh, which cannot be derived from RCTs due to their short-term nature. Long-term treatment is also more reflective of the therapeutic application of TTh for the responding patient, as we have previously demonstrated that treatment interruption can lead to regression of clinical benefits including parameters of cardiovascular health.Citation52,Citation57 Moreover, during long-term clinical trials, there are important ethical considerations of not treating hypogonadal men who presented at our clinic wishing to undergo TTh. Reported cardiovascular disease was the clinical outcome indicating vascular health, but assessing subclinical measures, such as carotid intima media thickness (CIMT) or vascular composition via CT scan, may give a more detailed analysis of disease progression, remission or regression.
Conclusions
The triad of aetiological disparities of low T, ED and CVD, correlate and interact to indicate an underlying negative health status in men and suggests that both ED and testosterone levels could have a predictive capacity for worse cardiovascular outcomes. This study indicates that ED may be an early predictor of CVD and low testosterone may be an early indicator of vascular dysfunction associated with ED and CVD, particularly in the presence of cardiometabolic risk factors. Due to the increased risk of CVD among men with functional hypogonadism,Citation15 findings from our previous studyCitation42 and the present study, we support a role for routinely assessing ED to identify individuals at increased risk of CVD. Furthermore, a diagnosis of ED should prompt a clinical investigation of serum testosterone levels and consequently correlated CVD comorbidities, and TTh considered. Correcting testosterone levels in men via TTh may therefore confer a vascular benefit that leads to improvements in both CVD and ED. Indeed, the beneficial effect of long-term TTh on CVD-related major adverse events was clearly confirmed in the present study with no MI and no stroke reported in the TTh group. We demonstrate that long-term TTh for up to 8 years in men with functional hypogonadism and a history of CVD significantly improves erectile function and protects against CVD events. The present study highlights the importance for patients to remain on TTh consistently for an extended period of time compared to published randomized controlled studies in order to achieve the maximum benefits of TTh in clinical practice. Therefore, ED and testosterone status warrant thorough clinical assessment to identify “at-risk” males for the consideration of long-term TTh with the goal of correcting the biochemical hormonal defect to improve both sexual function and cardiovascular health.
Acknowledgment
Editorial support for this manuscript was provided by Astra-Health, www.astra-health.co.uk.
Disclosure
Prof. Dr Aksam Yassin reports grants from Bayer AG, during the conduct of the study; personal fees from honoraria, generally, outside the submitted work. Prof. Dr Farid Saad reports personal fees from Bayer AG, during the conduct of the study and outside the submitted work; and owns shares of Bayer AG, Berlin, Germany. The authors report no other potential conflicts of interest for this work.
References
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. doi:10.1056/NEJMoa0911101
- Livingston M, Kalansooriya A, Hartland AJ, Ramachandran S, Heald A. Serum testosterone levels in male hypogonadism: why and when to check-A review. Int J Clin Pract. 2017;71(11). doi:10.1111/ijcp.12995
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87(2):589–598. doi:10.1210/jcem.87.2.8201
- Zarotsky V, Huang MY, Carman W, et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2(6):819–834. doi:10.1111/andr.274
- Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68(3):386–396. doi:10.1016/j.eururo.2014.11.039
- Mazzola CR, Mulhall JP. Impact of androgen deprivation therapy on sexual function. Asian J Androl. 2012;14(2):198–203. doi:10.1038/aja.2011.106
- Gandaglia G, Briganti A, Jackson G, et al. A Systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Kapoor D, Clarke S, Channer KS, Jones TH. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl. 2007;30(6):500–507. doi:10.1111/j.1365-2605.2007.00744.x
- Gazzaruso C, Giordanetti S, De Amici E, et al. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004;110(1):22–26. doi:10.1161/01.CIR.0000133278.81226.C9
- Montorsi F, Briganti A, Salonia A, et al. Erectile Dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44(3):360–365. doi:10.1016/S0302-2838(03)00305-1
- Montorsi P, Ravagnani PM, Galli S, et al. Association between erectile dysfunction and coronary artery disease: matching the right target with the right test in the right patient. Eur Urol. 2006;50(4):721–731. doi:10.1016/j.eururo.2006.07.015
- Hodges LD, Kirby M, Solanki J, O’Donnell J, Brodie DA. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61(12):2019–2025. doi:10.1111/j.1742-1241.2007.01629.x
- Inman BA, St. Sauver JL, Jacobson DJ, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clinic Proce. 2009;84(2):108–113. doi:10.4065/84.2.108
- Montorsi P, Ravagnani PM, Galli S, et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J. 2006;27(22):2632–2639. doi:10.1093/eurheartj/ehl142
- Isidori AM, Buvat J, Corona G, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol. 2014;65(1):99–112. doi:10.1016/j.eururo.2013.08.048
- Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21(8):496–503. doi:10.1016/j.tem.2010.03.002
- Corona G, Monami M, Boddi V, et al. Low Testosterone is Associated with an Increased Risk of MACE Lethality in Subjects with Erectile Dysfunction. J Sex Med. 2010;7(4pt1):1557–1564. doi:10.1111/j.1743-6109.2009.01690.x
- Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30(4):328–338. doi:10.1006/pmed.2000.0643
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi:10.1210/jc.2003-031069
- Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871–876. doi:10.1136/hrt.2003.021121
- Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443–449. doi:10.1530/EJE-09-0092
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906. doi:10.1530/eje.1.02166
- Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30(6):726–733. doi:10.2164/jandrol.108.007005
- Cornoldi A, Caminiti G, Marazzi G, et al. Effects of chronic testosterone administration on myocardial ischemia, lipid metabolism and insulin resistance in elderly male diabetic patients with coronary artery disease. Int J Cardiol. 2010;142(1):50–55. doi:10.1016/j.ijcard.2008.12.107
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612. doi:10.1111/j.1365-2265.2010.03845.x
- Sesti F, Pofi R, Minnetti M, Tenuta M, Gianfrilli D, Isidori AM. Late-onset hypogonadism: reductio ad absurdum of the cardiovascular risk-benefit of testosterone replacement therapy. Andrology. 2020;8(6):1614–1627. doi:10.1111/andr.12876
- Hackett G, Cole N, Mulay A, Strange RC, Ramachandran S. Long-term testosterone therapy in type 2 diabetes is associated with decreasing waist circumference and improving erectile function. World J Mens Health. 2020;38(1):68–77. doi:10.5534/wjmh.180052M
- Permpongkosol S, Khupulsup K, Leelaphiwat S, Pavavattananusorn S, Thongpradit S, Petchthong T. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J Sex Med. 2016;13(8):1199–1211. doi:10.1016/j.jsxm.2016.06.003
- Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJ, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7(7):2572–2582. doi:10.1111/j.1743-6109.2010.01859.x
- Saad F, Caliber M, Doros G, Haider KS, Haider A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23(1):81–92. doi:10.1080/13685538.2019.1575354
- Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54(2):346–351. doi:10.1016/S0090-4295(99)00099-0
- Rosen RC, Cappelleri JC, Gendrano N. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226–244. doi:10.1038/sj.ijir.3900857
- English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102(16):1906–1911. doi:10.1161/01.CIR.102.16.1906
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi:10.1001/archinte.166.15.1660
- Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur j Endocrinol. 2013;169(6):725–733. doi:10.1530/EJE-13-0321
- Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur j Endocrinol. 2004;150(1):65–71. doi:10.1530/eje.0.1500065
- Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47–71. doi:10.1530/JOE-12-0582
- Hougaku H, Fleg JL, Najjar SS, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290(2):E234–242. doi:10.1152/ajpendo.00059.2005
- Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity. 2013;21(10):1975–1981. doi:10.1002/oby.20407
- Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int j Obesity. 2016;40(1):162–170. doi:10.1038/ijo.2015.139
- Sai Ravi Shanker A, Phanikrishna B. Association between erectile dysfunction and coronary artery disease and its severity. Indian Heart J. 2013;65(2):180–186. doi:10.1016/j.ihj.2013.02.013
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18(3):186–194. doi:10.3109/13685538.2015.1046044
- Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-Term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22(5):414–433.
- Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur j Endocrinol. 2012;167(4):531–541. doi:10.1530/EJE-12-0525
- Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89(3):251. doi:10.1136/heart.89.3.251
- Ponholzer A, Temml C, Obermayr R, Wehrberger C, Madersbacher S. Is Erectile Dysfunction an Indicator for Increased Risk of Coronary Heart Disease and Stroke? Eur Urol. 2005;48(3):512–518. doi:10.1016/j.eururo.2005.05.014
- Yassin AA, Nettleship JE, Almehmadi Y, Yassin D-J, El Douaihy Y, Saad F. Is there a relationship between the severity of erectile dysfunction and the comorbidity profile in men with late onset hypogonadism? Arab J Urol. 2015;13(3):162–168. doi:10.1016/j.aju.2015.06.003
- Saad F, Gooren LJ, Haider A, Yassin A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl. 2008;29(1):102–105. doi:10.2164/jandrol.107.002774
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318–327. doi:10.1016/j.atherosclerosis.2009.04.016
- Jones TH. Testosterone replacement therapy. Br j Hospital Med. 2007;68(10):547–553. doi:10.12968/hmed.2007.68.10.27326
- Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13(10):1327–1351. doi:10.1517/14740338.2014.950653
- Yassin A, Nettleship JE, Talib RA, Almehmadi Y, Doros G. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19(1):64–69. doi:10.3109/13685538.2015.1126573
- Yassin A, Almehmadi Y, Saad F, Doros G, Gooren L. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf). 2016;84(1):107–114. doi:10.1111/cen.12936
- Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10(6):1612–1627. doi:10.1111/jsm.12146
- Corona G, Rastrelli G, Monami M, et al. Body Mass index regulates hypogonadism‐associated cv risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011;8(7):2098–2105. doi:10.1111/j.1743-6109.2011.02292.x
- Corona G, Rastrelli G, Maseroli E, et al. Low testosterone syndrome protects subjects with high cardiovascular risk burden from major adverse cardiovascular events. Andrology. 2014;2(5):741–747. doi:10.1111/j.2047-2927.2014.00241.x
- Saad F, Yassin A. Effects of interrupting and resuming long-term testosterone replacement therapy (TRT) with testosterone undecanoate injections (TU) on erectile dysfunction (ED) and anthropometric parameters in hypogonadal men in an observational registry study. J Sexual Med. 2015;12:402.