Abstract
The paradigm for the treatment of monoclonal gammopaties has dramatically changed: therapeutic options in multiple myeloma (MM) have evolved from the introduction of melphalan and prednisone in the 1960s, high-dose chemotherapy and stem cell transplantation in the late 1980s and 1990s, to the rapid introduction of small novel molecules within the last seven years. Based on the understanding of the complex interaction of the MM cells with the bone marrow microenvironment and the signaling pathways that are dysregulated in this process, a number of novel therapeutic agents are now available. Specifically, three novel agents with a specific-targeted anti-MM activity, have been FDA-approved for the treatment of this disease, namely Bortezomib, thalidomide, and lenalidomide which are now all playing a key role in the treatment of MM. The success of targeted therapy in MM has since led to the development and investigation of more than 30 new compounds in this disease and in other plasma cell dyscrasias such as Waldenström’s macroglobulinemia and primary amyloidosis, both in the preclinical settings and as part of clinical trials.
Keywords:
Introduction
Monoclonal gammopaties represent a clinically heterogeneous group of diseases generally considered plasma cell dyscrasias and characterized by abnormal production of monoclonal (M) immunoglobulin, also called M-protein or M-component, produced by a clone that developed from a common progenitors in the B lymphocyte lineage. The M-component may be detected by electrophoresis as a band of resctricted migration in the serum or urine. They include monoclonal gammophaty of undetermined significance (MGUS), multiple myeloma (MM), Waldenström’s macroglobulinemia (WM), primary (AL) amyloidosis, heavy chain diseases, cryoglobulinemia type I and type II, and other lymphoproliferative disorders.
In this review, the role of new targeted therapies available for monoclonal gammopaties will be discussed, focusing on MM, WM, and amyloidosis.
After almost forty years, the paradigm for the treatment of monoclonal gammopaties has dramatically changed: for example, therapeutic options in MM have evolved from the introduction of melphalan and prednisone in the 1960s, high-dose chemotherapy and stem cell transplantation in the late 1980s and 1990s, to the rapid introduction of small novel molecules within the last seven years. Based on the understanding of the complex interaction of the MM cells with the bone marrow (BM) microenvironment and the signaling pathways that are dysregulated in this process, a number of novel therapeutic agents are now available. Specifically, three novel agents with a specific-targeted anti-MM activity, have been US Food and Drug Administration (FDA)-approved for the treatment of this disease, namely Bortezomib, thalidomide, and lenalidomide which are now all playing a key role in the treatment of MM. The success of targeted therapy in MM has since led to the development and investigation of more than 30 new compounds in this disease and in WM, both in the preclinical settings and as part of clinical trials.
Immunomodulatory drugs (IMiDs): thalidomide and lenalidomide
Thalidomide
Thalidomide was first used as a sedative and hypnotic drug in the 1950’s. It was withdrawn from the market because of its teratogenic effects. In 1999 a phase II study showed that thalidomide, used as a single agent in patients with relapsed MM, resulted in an overall response rate (ORR) of 25% (CitationSinghal et al 1999). The main activity and efficacy of thalidomide in MM was then elucidated. It has been shown that thalidomide induces in vitro growth arrest, blocks the increased secretion of tumor necrosis factor alpha (TNF-α), and affects the interaction between myeloma cells and BM microenvironment by decreasing the expression of adhesion molecules (E-selectin, L-selectin, ICAM-1, VCAM-1) or inhibiting the paracrine loops of cytokine secretion, such as vascular endothelial growth factor (VEGF) and interleukin (IL)-6; inhibits angiogenesis; and enhances host immune response against MM; interferes with intracellular growth signalling by inhibiting the constitutive activity of nuclear factor kappa B (NFkB) (CitationHideshima et al 2000; CitationDavies et al 2001; CitationMitsiades et al 2002) (). Several studies then tested the combination of thalidomide with other agents such as dexamethasone and chemotherapeutic drugs in patients with relapsed/refractory MM, and this led to response rates as high as 65% (CitationRajkumar et al 2000, Citation2002; CitationWeber et al 2003; CitationKumar et al 2006). After these encouraging results, thalidomide in combination with dexamethasone entered several phase II clinical trials in newly diagnosed MM patients, and demonstrated a RR of ∼ 65% (CitationRajkumar et al 2002; CitationWeber et al 2003a; CitationKumar and Rajkumar 2006). Subsequently, a large phase III clinical trial was performed using thalidomide with dexamethasone versus high-dose dexamethasone alone for newly diagnosed MM patients, resulting in a 63% RR in the thalidomide/dexamethasone arm versus 41% in the dexamethasone arm, although no survival advantage was observed between the two groups (CitationRajkumar et al 2006).
Figure 1 Mechanisms of action of novel agents. Novel molecules can: I) directly inhibit clonal cells; II) inhibit angiogensis; III) inhibit tumor cell adhesion to bone marrow stromal cells (BMSCs); IV) decrease cytokine production from BMSCs; V) increase host anti-tumor immunity.
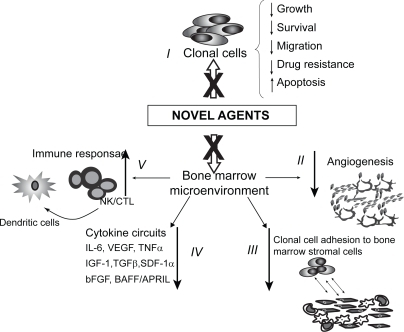
Other phase III trials in elderly patients who were not candidates for autologous stem cells transplant included a randomized study compared melphalan prednisone and thalidomide (MPT) versus melphalan and prednisone (MP), which showed that patients treated with MPT had higher RR (76% versus 48%) and longer event-free survival (EFS) than patients treated with MP alone (54% versus 27%) (CitationPalumbo et al 2006). CitationFacon and colleagues (2006) conducted a large phase III trial of MPT compared to MP or high dose chemotherapy and stem cell transplantation in elderly patients between 65 to 75 years of age and showed that patients treated with MPT had a longer overall survival of 54 months compared to 32 months for MP and 39 months for transplant.
A randomized study has recently investigated the activity of thalidomide in combination with VAD and doxil, compared to VAD-doxil and it resulted in a higher RR in the arm with thalidomide versus the arm without thalidomide (81% versus 66%) (CitationZervas et al 2006). The toxicities of thalidomide correlate both with dose and length of treatment and include neuropathy and deep vein thrombosis. Other important toxicities include fatigue, somnolence, constipation, rash (including Stevens-Johnson syndrome), and hepatic dysfunction (CitationGhobrial and Rajkumar 2003).
In view of its success in the treatment of patients with MM, thalidomide has been tested alone in WM patients, demonstrating partial response in 25% of patients treated with single-agent thalidomide. Adverse effects were common and prevented dose escalation of thalidomide in 75% of patients. In addition, thalidomide in combination with dexamethasone and clarithromycin induced partial response in 10 of 12 (83%) previously treated patients (CitationDimopoulos et al 2003). However, a follow up study of 10 patients with higher doses of thalidomide (200 mg daily) showed only 20% overall response rate (CitationTreon et al 2006a). Several clinical trials using thalidomide in combination with a wide variety of other compounds are ongoing in patients with MM and WM ().
Table 1 Ongoing clinical trials using thalidomide-based regimens in MM and WM (www.clinicaltrials.gov)
High-dose chemotherapy has increased the response rate in patients with MM, but this therapeutical option is not curative and an effective consolidation-maintenance could extend the duration of response. Several studies have evaluated the role of maintenance therapy with thalidomide in MM patients after autologous stem-cell transplantation, which shows that thalidomide improves survival and represents a valid and effective strategy as a maintenance therapy option (CitationAttal et al 2006; Abdelkefi et al 2007; CitationSpencer et al 2007).
Finally, thalidomide has been evaluated also in AL amyloidosis patients where it induced response rates up to 50% when combined with dexamethasone. Unfortunately, the regimen is poorly tolerated, with 50%–65% of patients experiencing grade 3 or 4 toxicities (CitationPalladini et al 2005).
Lenalidomide
Based on the success of thalidomide, lenalidomide (CC-5013; IMiD-3, Celgene Corp), a more potent immunomodulatory derivative of thalidomide was developed. Lenalidomide overcomes growth and survival advantage conferred by the BM-milieu, downregulates VEGF, and exerts antiangiogenic activities. In addition, lenalidomide co-stimulates T cells, enhances antitumor immunity mediated by interferon (IFN)γ and IL-2, and augments natural killer (NK) cell cytotoxicity (CitationHideshima et al 2001; CitationMitsiades et al 2002; CitationDredge et al 2002) ().
Phase I clinical trials using lenalidomide in patients with relapsed and refractory MM, established a dose of 25 mg, and demonstrated a promising RR of 35% (CitationRichardson et al 2006a). Phase II studies followed and established the optimal schedule of 3 weeks on and 1 week off with once daily dosing (Richardson et al 2001, Citation2006b).
Then, two large randomized phase III studies (MM-009, MM-010) compared lenalidomide and dexamethasone to dexamethasone and placebo for patients with relapsed or relapsed and refractory MM. They both showed comparably favorable results, with RR and time to progression with the lenalidomide/dexamethasone combination significantly greater and more than twice the RR seen with dexamethasone alone (CitationDimopoulos 2005a; CitationWeber et al 2006).
Based upon the success of these studies, lenalidomide received FDA-approval for the treatment of relapsed MM in June 2006. A phase II study of the combination of lenalidomide and dexamethasone was performed in 32 newly diagnosed patients with MM and showed an ORR of 91% (CitationRajkumar et al 2005). A recent study demonstrated the efficacy of lenalidomide in combination with melphalan and prednisone which was associated with a RR of 86% (CitationPalumbo et al 2006). Similarly, the combination of lenalidomide with other drugs such as adriamycin and dexamethasone showed a RR of 84% (CitationKnop et al 2006).
A Phase III clinical trial using lenalidomide in combination with dexamethasone in newly diagnosed MM patients has been recently completed and showed that lenalidomide plus low-dose dexamethasone is associated with superior OS compared to lenalidomide plus high-dose dexamethasone (CitationRajkumar et al 2007). The main side effects of lenalidomide include myelosuppression, particularly neutropenia and thrombocytopenia, and deep venous thrombosis especially when it used in combination with high-dose dexamethasone (CitationRajkumar and Blood 2006).
Based on the potent activity of lenalidomide in MM and considered the lack of neuropathy with this agent, a phase II study of lenalidomide 25 mg daily in combination with rituximab is ongoing in patients with relapsed or relapsed/refractory WM.
More than forty clinical trials using lenalidomide in combination with several other compound are actually on going in patients with MM and WM ().
Table 2 Ongoing clinical trials using lenalidomide-based regimens in MM and WM (www.clinicaltrials.gov)
Lenalidomide has also entered a phase II clinical trials for patients with AL amyloidosis. When combined with dexamethasone, lenalidomide induced response rates of nearly 67%, with 29% of hematologic complete response (Sanhorawala et al 2007).
Proteasome inhibitors: Bortezomib and second-generation (NPI-0052; PR171)
Bortezomib
Bortezomib (PS-341, Millennium Pharmaceuticals, Inc) represents the first in class proteasome inhibitor to have progressed into widespread clinical use in MM patients, based on preclinical data showing its in vitro and in vivo anti-tumor activity in MM cells, by inhibiting proliferation, inducing apoptosis and by targeting the BM microenvironment through its antiangiogenic activity and by inhibiting the binding of MM cells to the BM stromal cells (). Bortezomib as single agent has been evaluated in patients with advanced, heavily pretreated MM in the SUMMIT study (Study of Uncrontrolled Multiple Myeloma managed with proteasome Inhibition Therapy) (CitationRichardson et al 2003) which showed an ORR of 35% in 202 patients with relapsed and refractory MM. The CREST (Clinical Response and Efficacy Study of Bortezomib in the Treatment of myeloma) trial, a phase II study randomizing patients to higher (1.3 mg/m3) or lower (1.0 mg/m3) doses of Bortezomib in combination with dexamethasone, revealed positive response rates (33% with low-dose Bortezomib alone, 44% with low-dose Bortezomib/dexamethasone, 50% with high-dose Bortezomib, and 62% with high-dose Bortezomib/dexamethasone) (CitationJagannath et al 2004). Subsequently, the APEX study (Assessment of Proteasome Inhibition for Extending Remission) compared Bortezomib with high-dose dexamethasone in patients with relapsed/refractory MM, and showed an ORR of 38% in the Bortezomib arm, versus 18% obtained in the high-dose dexamethasone. Moreover, Bortezomib demonstrated superiority over dexamethasone in terms of time to progression and survival (CitationRichardson et al 2005a). Based on these encouraging data, Bortezomib was FDA-approved in 2003 with full approval in 2005 and numerous trials using Bortezomib in combination with other agents were built.
Other combinations included chemotherapies and novel agents (CitationRichardson et al 2006c). The combination of Bortezomib, thalidomide and dexamethasone (VTD) in patients with relapsed MM showed an overall response rate of 70% including near complete responses in 16%. High responses were also observed in studies of patients with previously untreated MM. Single agent Bortezomib showed an overall response rate of 40% with 10% complete responses in a phase II study of 66 patients with MM.
The combination of Bortezomib and dexamethasone led to an overall response rate of 66% to 88% in another phase II trial of newly diagnosed MM (CitationJagannath et al 2006; CitationHarousseau et al 2006).
In addition, the combination of Bortezomib (V), melphalan (M) and prednisone (P) (MPV) in nontransplant candidates resulted in an overall response rate of 89% (Mateos et al 2007). Interestingly, a phase III trial randomizing newly diagnosed MM patients to either VMP or MP, has been recently completed and showed that VMP significantly prolongs survival and is superior for all efficacy endpoints: specifically VMP induced rapid and durable responses with unprecedented complete response rate (35%); prolonged time to progression (∼52% reduced risk of progression), time to next therapy/treatment free interval; and overall survival (∼40% reduced risk of death) (CitationSan Miguel et al 2007).
Also the combination of Bortezomib, dexamethasone, and cyclophosphamide was shown to be more effective than Bortezomib either used as single agent or with dexamethasone (CitationDavies et al 2006). These encouraging results were subsequently confirmed by a multicenter randomized phase 3 study comparing the combination of doxil and Bortezomib versus Bortezomib alone (CitationOrlowski et al 2006). Similarly it has been recently demonstrated that liposomal doxorubicin+Bortezomib significantly improves TTP compared to Bortezomib alone, regardless of the number of prior lines of therapy, or anthracycline exposure (CitationBlade et al 2007).
Based on its activity in MM, single agent Bortezomib was tested in WM in phase II trials and achieved 40%–80% responses (CitationDimopoulos et al 2005b). The combination of Bortezomib, dexamethasone and rituximab was recently evaluated in untreated patients with WM. Each cycle of therapy consisted of IV Bortezomib at 1.3 mg/m2 and IV dexamethasone 40 mg on days (1, 4, 8, and 11), and rituximab at 375 mg/m2 (day 11). Patients received four consecutive cycles, followed by a three-month pause, and then 4 more cycles, each given three months apart. The interim analysis of the first 10 patients who received the first 4 cycles of therapy showed partial response in 50% and minor response in the other 50%, with 2 patients (20%) achieving an unconfirmed complete response (CitationTreon et al 2006b).
There are actually several clinical trials ongoing using Bortezomib either alone or in combination with other agents in MM and WM patients ().
Table 3 Ongoing clinical trials using Bortezomib-based regimens in MM and WM (www.clinicaltrials.gov)
Recently the role of proteasome inhibition has been studied in AL amyloidosis, characterized by the overproduction of a destabilized light chain which tends to aggregate and deposit in several tissues (CitationSitia et al 2007; CitationKastritis et al 2007). The process of amyloid deposition induces tissue damage and subsequently organ failure, leading to high mortality. The combination of Bortezomib and dexamethasone has been successfully evaluated in patients with AL amyloidosis who were relapsed or progressed after previous thalidomide-based treatments, and who were ineligible for high-dose melphalan supported by autologous stem cell transplantation: 94% hematologic responses were observed, including 44% complete responses.
New proteasome inhibitor, NPI-0052
Based on the significant anti-MM activity of Bortezomib, a new proteasome inhibitor (NPI-0052; Nereus Pharmaceuticals, CA) with a different structure and different mechanism of action has been developed. NPI-0052 is an oral proteasome inhibitor that has shown significant anti-neoplastic activity in MM and WM (CitationChauhan et al 2005). Importantly, the combination of NPI-0052 and Bortezomib induced significant inhibition of proliferation compared to each agent alone (CitationChauhan et al 2007; CitationRoccaro et al 2008). A phase I clinical trial of NPI-0052 in relapsed MM has recently been initiated.
PR-171
PR-171 is a novel irreversible proteasome inhibitor under investigation for the treatment of hematological malignancies. Two phase I dose-escalation studies have been initiated, aimed at determining the safety, tolerability, and clinical response to PR-17 (CitationO’Connor et al 2006). Patients with multiple myeloma, non-Hodgkin lymphoma, Hodgkin disease, or Waldenström macroglobulinemia who received two or more prior treatments were eligible. Two different dose-intensive schedules were employed in these phase I studies. PR-171 was well-tolerated, and several subjects have achieved long-lasting SD, reduction in paraprotein levels, or symptomatic improvement (CitationO’Connor et al 2006).
Signaling pathway inhibitors
Preclinical data have been demonstrated that monoclonal gammopaties are characterized by disregulation of several signalling pathways, as compared to normal plasma cells (CitationHideshima et al 2004a; CitationHatjiharissi et al 2007; CitationLeleu et al 2007). Moreover there is strong evidence that BM-milieu supports the growth of the clonal cell population. Therefore, this important knowledge has led to the development of several agents that specifically target the neoplastic clone by acting through those upregulated signaling pathways, the BM microenvironment, are able to affect both the clonal cells and the BM-milieu (CitationHideshima et al 2006).
Signaling pathway inhibitors active in both MM and WM
Akt inhibitor: perifosine
Perifosine (NCS-639966; Keryx Biopharmaceuticals, Inc) is an orally-active alkyl-phosphocholine molecule that affects membrane permeability; phospholipid metabolism; as well as mitogenic signaling transduction induced by the PI3/Akt pathway (CitationHideshima et al 2006). It has been recently demonstrated that perifosine has in vitro and in vivo activity against MM and WM cell lines and patient primary tumor cells, even in presence of BM stromal cells which are known to support tumor cell growth and induce resistance to apoptosis. In addition, perifosine showed synergistic activity when used in combination with other agents widely used in MM and WM such as dexamethasone, Bortezomib, doxorubicin, melphalan, and rituximab, specifically for MM and WM, respectively (CitationHideshima et al 2006; CitationLeleu et al 2007). A phase II clinical trial of perifosine with or without dexamethasone in patients with relapsed and refractory MM has recently reported and showed activity, with 69% of patients achieving response and/or stabilization of disease (CitationRicharson et al 2006d).
Another phase II trial of the combination of perifosine with Bortezomib ± dexamethasone is currently underway in MM patients. Similarly, a phase II trial of single agent perifosine in patients with relapsed or relapsed/refractory WM has been initiated using 150 mg oral daily dosing. The preliminary data of 13 patients enrolled on the study, with a median follow up time of 3 months, demonstrated promising activity of this agent. The treatment was well tolerated with minimal side effects. Seven patients were evaluable at the time of analysis and all showed evidence of IgM reduction, with a median IgM reduction of 14% (0%–25%). One patient whose IgM rose in the first month had a 50% reduction from the peak of IgM level at 3 months, indicating a delayed response. These preliminary results indicate that perifosine is a promising agent to be used in combination in future studies both in MM and WM.
Protein kinase C inhibitor: enzastaurin
Enzastaurin[H-Pyrrole-2,5-dione,3-(1-methyl-1H-indol-3-yl)-4-[1-[-1(2pyridinylmethyl)-4-piperidinyl]-1H-indol-3-yl], LY 317615; Eli Lilly and company, (Indianapolis, IN) is an oral PKCβ inhibitor, with downstream inhibition of Akt (CitationPodar et al 2006). In MM, enzastaurin has demonstrated specific inhibition of PKC isoforms and Akt activation along with inducing cytotoxicity and apoptosis in MM and WM cells in vitro and in vivo (CitationPodar et al 2006; CitationMoreau et al 2007). Synergism has been demonstrated when enzastaurin was used in combination with Bortezomib. In addition, enzastaurin inhibted MM and WM cell growth in an in vivo xenograft model of these diseases. Based on these exciting preclinical data, enzastaurin alone and in combination with Bortezomib entered clinical trials in MM, and phase II trial are planned in WM as single agent.
Mammalian target of rapamycin inhibitors: CCI-779, RAD001
mTOR inhibitors such as rapamycin and rapamycin analogues including CCI-779 and RAD001 have demonstrated in vitro and in vivo activity in MM cell lines and animal model (CitationShi et al 2002; CitationMitsiades et al 2004). The combination of rapamycin with active agents in MM such as lenalidomide, Bortezomib and 17-AAG have demonstrated synergistic activity in vitro (CitationRaje et al 2004; CitationFrancis et al 2006). In addition, rapamycin appears to target the BM microenvironment by inhibiting angiogenesis and osteoclast formation in MM in vitro (CitationFrancis et al 2006). Similarly, Preclinical data have demonstrated increased activity of the PI3K/mTOR pathway in WM, and subsequently rapamycin (mTOR inhibitor) has been studied in vitro in WM and showed significant cytotoxicity in WM cells lines, specifically when combined with Bortezomib (unpublished data).
These findings have led to the design of studies using these agents in combination with other active agents in MM and WM. A phase II trial of RAD-001 in combination with lenalidomide, and a phase I/II clinical trial of CCI-779 in combination with Bortezomib are underway in patients with relapsed/refractory MM. In addition, a phase II trial of single agent RAD001 was initiated in aggressive, low grade lymphomas, and rare lymphomas including WM.
Signaling pathway inhibitors active in MM
MEK/ERK inhibitor: AZD-6244
AZD6244 (AstraZeneca, Los Angeles, CA) has been tested in preclinical models in MM and induced inhibition of growth and cytotoxicity in MM cells even in the presence of cytokines/growth factors such as IL-6, IGF-1 that induce MEK/ERK activation (CitationHu et al 2003; CitationHideshima et al 2004a). A phase II trial of single agent AZD6244 is planned in 2007 for patients with relapsed/refractory MM.
p38MAPK inhibitor: SCIO-469
SCIO-469 (Scios, Inc, Mountain View, CA) was first studied in clinical trials in rheumatoid arthritis and has shown in vitro activity in MM cells when co-cultured with BM stromal cells. The combination of SCIO-469 and Bortezomib demonstrated synergistic activity in vitro and in vivo (CitationHideshima et al 2004b). A phase II trial of SCIO-469 alone or in combination with Bortezomib in patients with relapsed MM showed stable disease in 24% with single agent SCIO-469, with its combination with Bortezomib resulting in a response rate of 32%, including response in patients in whom Bortezomib had failed (CitationSiegel et al 2006).
Inhibitors of heat-shock protein 90: 17-AAG, KOS-953, IPI-504
Heat shock protein 90 (HSP90) inhibitors such as geldanamycin and 17-allylamino-17-demethoxygeldanamycin (17-AAG) bind to the N-terminal ATP-binding pocket of Hsp90 and inhibit the stress induced anti-apoptotic response in MM cells and have demonstrated in vitro and in vivo cytotoxic activity alone and in combination with other agents active in MM, specifically Bortezomib (CitationMitsiades CS et al 2006). Phase I clinical trials of KOS 953—a 17AAG derivative—in MM have shown good tolerability with disease stabilization and minor response in patients with relapsed and refractory MM. Other HSP90 inhibitors include IPI-504, which is also being tested in a phase I clinical trial in MM and has excellent tolerability but no responses at doses tested to date (CitationRichardson et al 2005b). Excitingly, KOS-953 combined with Bortezomib has demonstrated responses even in Bortezimib-resistant patients in an ongoing phase I/II trial in patients with relapsed and refractory MM (CitationChanan-Khan et al 2005). Phase III trials of this combination are planned.
Monoclonal antibodies mainly active IN WM
Monoclonal anti-CD20 antibody: rituximab
Rituximab has become one of the main treatment options of patients with WM. Standard rituximab (4 weekly infusions of 375 mg/m2) has demonstrated at least a minor response in 52% of patients (CitationGertz et al 2004). Four weekly rituximab treatments repeated at 3 months triggered response rates of 44%–48% (CitationDimopoulos et al 2003; CitationTreon et al 2005a). Polymorphisms in the FcγRIIIA (CD16) receptor gene may affect response to rituximab in WM. The response to rituximab is delayed in most patients with a median time to partial response of 4 months and a median time to best response of 17 months (CitationTreon et al 2005b). In addition, the IgM level may initially increase in response to rituximab, a phenomenon termed IgM flare that occurs in about 54% of patients (CitationGhobrial et al 2004; CitationTreon et al 2004a). These levels may persist for up to 4 months and do not indicate treatment failure, but may necessitate plasmapheresis to reduce hyper-viscosity. Some patients receive maintenance therapy with rituximab. Although the impact of this regimen on the time to progression has not been determined specifically in WM, it has prolonged time to progression in patients in patients with other low-grade lymphomas who received rituximab maintenance compared to those who did not (Citationvan Oers et al 2006). Rituximab may also be useful in treating patients with IgM autoantibody-related neuropathies (CitationRenaud et al 2006). The use of radioimmunotherapy such as iodine 131I-tositumomab radioimmunotherapy in WM has been limited since the high level of BM involvement precludes their use. However, case reports have shown that these therapies may be effective in patients with WM who have <25% BM involvement (CitationTsai e al 2004).
Combinations of alkylating agents, nucleoside analogs, and rituximab
The addition of alkylating agents to nucleoside analogs is active against WM. For example, the combination of oral cyclophosphamide with subcutaneous cladribine in 37 newly diagnosed patients achieved 84% PR or more, with a median duration of response of 36 months (CitationWeber et al 2003b). The combination of fludarabine and intravenous cyclophosphamide in 11 previously treated patients resulted in 55% overall response. In another study of 49 patients, the combination of fludarabine plus cyclophosphamide induced 78% overall response, with median time to treatment failure was 27 months (CitationTamburini et al 2005). Hematologic toxicity was commonly observed, and 3 patients died of treatment-related toxicities. A phase II clinical trial of 60 patients with WM treated with cyclophosphamide, rituximab, and dexamethasone (DRC) demonstrated an overall response rate of 70%, with 7% complete remission (CitationDimopoulos et al 2006). Treatment was well tolerated and the main toxicity observed was grade 3–4 neutropenia in 20% of the patients. The combination of rituximab, cladribine, and cyclophosphamide was tested in 17 previously untreated patients with WM and achieved at least a partial response in 94% of the patients, with complete response in 18% (CitationWeber et al 2003b). The combination of rituximab and fludarabine was evaluated in 43 WM patients, with an overall response rate of 91% and CR of 7% (CitationTreon et al 2004b). In another study, the combination of fludarabine, cyclophosphamide and rituximab (FCR) was tested in 21 patients with WM who had at least 1–2 prior regimens of therapy; overall response rate was 52%, with 5% complete remissions (CitationTreon et al 2006c). In MM, rituximab is being tested as a single agent or in combination with chemotherapeutic agents with some modest results of ∼64% minor responses and stable disease in one study (CitationMoreau et al 2006).
Other monoclonal antibodies specifically active in WM
Anti-CD52 alemtuzumab-1H (Campath)
CD52 is highly expressed on WM cells in the BM, and alemtuzumab induces cytotoxicity of WM cells in vitro. A phase II study of alemtuzumab in 25 patients with relapsed WM or newly diagnosed untreated WM showed an overall response rate of 76%, including 8 (32%) partial responses and 11 (44%) minor responses. Hematological toxicities were common among previously treated (but not untreated) patients and included G3/4 neutropenia (39%); thrombocytopenia (18%); anemia (7%). G3/4 nonhematological toxicity for all patients included dermatitis (11%); fatigue (7%); and infection (7%). CMV reactivation and infection was commonly seen among previously treated patients. Three patients died due to therapy-related complications (CitationHunter et al 2006).
Anti-CD70 antibody: SGN-70
Lymphoplasmacytic cells stimulate cell surface expression of TNF-family ligands through release of sCD27, which induces CD70 on mast cells. WM cells and cell lines highly express CD70 (CitationHatjiharissi et al 2006). Therefore, directly targeting CD70 using the fully humanized monoclonal antibody SGN-70 (Seattle Genetics, Inc., Bothell, WA) may represent a therapeutic option in WM. SGN-70 mediated significant dose-dependent ADCC against WM cell and mast cells at concentrations of 0.1–20μg/ml. SCID-hu mice bearing WM cells were treated with SGN-70 (1 mg/kg, i.p., qOD), and serum IgM and sCD27 levels were measured to monitor for disease progression. SGN-70 initiated 6 weeks following tumor engraftment blocked tumor growth in 12/12 treated mice, whereas all 5 untreated mice demonstrated disease progression (CitationHatjiharissi et al 2006). The results of these studies provide the framework for clinical trials to examine the therapeutic potential of the SGN-70 monoclonal antibody in WM.
Other monoclonal antibodies specifically active in MM
SGN-40
SGN-40 (Seattle Genetics, Inc.) is a humanized anti-CD40 ligand. It has been shown that CD40 induces proliferation of MM cells by activating the PI3/Akt pathway and by inducing secretion of IL-6 and VEGF from BM stromal cells (CitationTai et al 2005). Phase I study has been initiated demonstrating safety of this agent in MM with promising responses, and phase II trials are ongoing (CitationHussein et al 2006).
mAb antiCD40 receptor: HCD122
HCD122 is a a fully human, IgG1 antagonistic mAb targeting the CD40 receptor. A phase I trial has been conducted in MM patients and demonstrated that the Ab was safe, showing promising clinical activity in MM (CitationBensinger et al 2006).
Oligonucleotide antisense, Bcl-2 antisense
Bcl-2 inhibitor, G3139 (Oblimersen sodium)
Bcl-2 (Genasense, Genta Inc, Berkeley Heights, NJ) regulates apoptosis and resistance to chemotherapeutic agents; it has therefore become an attractive target for anticancer therapy in a number of malignancies including MM and WM (Chanan-Khan et al 2003). In vitro studies have shown that Bcl-2 is expressed in several B cell malignancies cells, and that downregulation of Bcl-2 and increased cytotoxicity in MM and WM cells may be achieved with G3139 (CitationBadros et al 2005). A Phase I/II clinical trial of G3139 was conducted in patients with relapsed or relapsed/refractory WM showed favorable tolerability but little activity (CitationGertz et al 2005). Similarly, a phase II study of G3139 in combination with dexamethasone and thalidomide has been initiated in relapsed MM patients: the combination is well tolerated, and the responses are promising (CitationBadros et al 2005).
Other agents
Agents with preclinical activity in WM AMD3100
Waldenström’s macroglobulinemia is characterized by widespread involvement of the BM, and lymphadenopathy in 20% of the patients, implying continuous trafficking of WM cells into and out of the BM and lymph nodes. The normal process of B-cell homing is regulated by cytokines, chemokines, and adhesion molecules (CitationLapidot et al 2005). One of the most extensively studied chemokines in migration is stromal derived factor SDF-1 and its receptor CXCR4. We recently demonstrated that WM cells and patient samples highly express CXCR4, and that SDF-1 induced migration of WM cells, with rapid activation of signaling pathways downstream of CXCR4 including pERK1/2, pAKT, and pPKC. The CXCR4 inhibitor AMD3100 (Genzyme, MA) inhibited migration of WM cells, as well as their adhesion to fibronectin. Adhesion of WM cells to stromal cells confers resistance to apoptosis and induces proliferation. The combination of AMD3100 with Bortezomib significantly enhances the cytotoxic effect of Bortezomib in the presence of stromal cells, possibly by interfering with adhesion of WM to stromal cells and thereby overcoming their protective effect (CitationNgo et al 2006). These studies provide the preclinical framework to study CXCR4 inhibitors in the regulation of homing and adhesion in WM.
Triterpenoids, CDDO, and CDDO-Im
2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) and its methyl ester derivative (CDDO-Me) and imidazolide derivative (CDDO-Im) are synthetic triterpenoids derived from oleanolic acid. In vitro studies in primary WM samples showed that CDDO-Im inhibited cell proliferation and induced apoptosis in WM cells compared to normal B cells. There was evidence of PARP cleavage in a dose-dependent manner, suggesting that CDDO-Im induced malignant cell death occurs through a caspase-dependent mechanism, and may have potential efficacy in WM patients (CitationElsawa et al 2006).
Simvastatin and resveratrol
The antineoplastic activity of simvastatin and resveratrol in WM has been reported (CitationMoreau et al 2007; CitationRoccaro et al 2008). The two compounds exert antiproliferative activity and induce apoptosis in WM. Interestingly, they target WM cells even in the presence of bone marrow microenvironment and cytokines that are known to promote WM cell growth. Moreover, they both showed synergism when used in combination with other agents widely used in WM, such as dexamethasone and Bortezomib. Those preclinical in vitro data provide the framework for clinical trials of simvastatin or resveratrol in WM.
Sildenafil citrate
Based on the clinical observation that patients receiving sildenafil citrate had a decrease in their IgM (CitationTreon et al 2004c), a phase II trial of single agent sildenafil citrate in patients with slowly progressing WM, who did not meet consensus eligibility for active therapy, was initiated. The purpose of the study was to delay time to progression in these patients. Thirty patients were treated on this study, and disease progression was suppressed in more than 50% of the patients. After 3 months of therapy, 63% showed a decrease in IgM levels and 17% showed a minor response. However, disease progression at 6 months of follow occurred in almost all the patients (CitationPatterson et al 2006).
Imatinib mesylate
Imatinib mesylate (Gleevec) targets the microenvironment of WM through inhibition of stem cell factor signaling through CD117, which is expressed on WM and mast cells. A phase II trial of single agent imatinib is ongoing in patients with relapsed or refractory WM. Imatinib is given at 400 mg daily, with dose escalation to 600 mg after one month of therapy. After 3 months of therapy, 6/13 (46.2%) of patients achieved MR. The main toxicities observed included cytopenias, edema, and hyperglycemia, leading to dose reductions in 31% patients and cessation of therapy in 23% patients (CitationTreon et al 2006d).
TACI-Ig, Atacicept
Atacicept (TACI-Ig; ZymoGenetics, Seattle, WI) contains the soluble TACI receptor that binds to the cytokines BLyS and APRIL, members of the tumor necrosis factor family that promote B-cell survival. An open-label, dose-escalation Phase 1b study enrolled 16 patients with refractory or relapsed MM or active progressive WM. Sequential cohorts received one cycle of 5 weekly subcutaneous injections of atacicept at 2, 4, 7, or 10 mg/kg. Treatment with atacicept was well tolerated, and no dose limiting toxicity was observed. A biological response was observed in this heavily treated refractory population, with disease stabilization in 75% of the patients with WM (CitationRossi et al 2006).
Conclusions
In summary, the last decade has marked a new era in the treatment of diseases characterized by monoclonal gammopaties. Indeed, a new paradigm shift has evolved utilizing novel therapeutic agents targeting the malignant clone and its bone marrow microenvironment. The combination of novel agents with chemotherapeutic drugs and/or glucocorticoids has demonstrated high response rates with complete remission rates comparable to those achieved in the stem cell transplant setting. This has been supported by in vitro and in vivo evidence showing the antitumor activity of those novel agents in MM, WM, as well as in other B cell maligancies. The future holds many more challenges for the treatment of MM and WM. These include combination of agents that achieve higher responses and longer survival, individualized therapies that are based on genetic and molecular abnormalities present in each patient, and clinical trials to test the benefit of novel agents in comparison and in addition to autologous stem cell transplantation, as well as other conventional approaches. Together, these therapies should lead to higher response rates, more durable duration of response, less toxicity and prolonged survival for patients, making plasma cell dyscrasias an increasingly chronic and treatable diseases.
Disclosure
Supported in part by Berlucchi Foundation (AMR) and Fondi 60% 2006–2007.
References
- AbdelkefiALadebSTorjmanL2008Single autologous stem cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: results of a multicentre randomized clinical trialBlood11118051017875806
- AndersonKRichardsonPChanan-KhanA2006Single-agent Bortezomib in previously untreated multiple myeloma (MM): Results of a phase II multicenter study [abstract]J Clin Oncol18S7504
- AttalMHarousseasuJLLeyvrazS2006Maintenance therapy with thalidomide improves survival in multiple myeloma patientsBlood10832899416873668
- BadrosAZGoloubevaORapoportAP2005Phase II study of G3139, a Bcl-2 antisense oligonucleotide, in combination with dexamethasone and thalidomide in relapsed multiple myeloma patientsJ Clin Oncol2340899915867202
- BensingerWJagannathABeckerP2006Phase I dose escalation study of a fully human, antagonist anti-CD40 antibody, HCD122 (Formerly CHIR-12.12) in patients with relapsed and refractory multiple myeloma [abstract]Blood1083575
- BladeJSan MiguelJNaglerA2007The prolonged time to progression with pegylated liposomal doxorubicin + Bortezomib versus Bortezomib alone in relapsed or refractory multiple myeloma is unaffected by extent of prior therapy or previous anthracycline exposure [abstract]Blood110410
- Chanan-KhanAA2004Bcl-2 antisense therapy in multiple myelomaOncology (Huntington)18214
- Chanan-KhanAARichardsonPGAlsinaM2005Phase 1 clinical trial of KOS-953 + Bortezomib (BZ) in relapsed refractory multiple myeloma (MM) [abstract]Blood10636215774616
- ChauhanDCatleyLLiG2005A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from BortezomibCancer Cell84071916286248
- ChauhanDSinghABrahmandamM2007Combination of protea-some inhibitors Bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myelomaBlood11116546418006697
- DaviesFERajeNHideshimaT2001Thalidimide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myelomaBlood982101611418482
- DaviesFEWuPSrikanthM2006The combination of cyclophosphamide, velcade and dexamethasone (CVD) induces high response rates with minimal toxicity compared to velcade alone (V) and velcade plus dexamethasone [abstract]Blood1083537
- DeocampoRRichRRyooJJ2002Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myelomaBlood1003063712384400
- DimopoulosMATsatalasCZomasA2003Treatment of Waldenström’s macroglobulinemia with single-agent thalidomide or with the combination of clarithromycin, thalidomide and dexamethasoneSemin Oncol30265912720150
- DimopoulosMASpencerAAttalM2005Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of a phase 3 study (MM010) [abstract]Blood1066
- DimopoulosMAAnagnostopoulosAKyrtsonisMC2005Treatment of relapsed or refractory Waldenström’s macroglobulinemia with BortezomibHaematologica901655816330439
- DimopoulosMAnagnostopoulosAKyrtsonisM2006Primary treatment of Waldenströms macroglobulinemia (WM) with dexamethasone, rituximab and cyclophosphamide [abstract]Blood108128
- DredgeKMarriottJBMacdonaldCD2002Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effectsBr J Cancer8711667212402158
- ElsawaSNovakAKonoplevaM2006Preferential inhibition of malignant cell growth by CDDO in Waldenströms macroglobulinemia [abstract]Blood1082528
- FaconTMaryJHarousseauJ2006Superiority of melphalan-prednisone (MP) + thalidomide (THAL) over MP and autologous stem cell transplantation in the treatment of newly diagnosed elderly patients with multiple myeloma [abstract]J Clin Oncol18S1
- FrancisLKAlsayedYLeleuX2006Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myelomaClin Cancer Res1268263517121904
- GertzMAGeyerSMBadrosA2005Early results of a phase I trial of oblimersen sodium for relapsed or refractory Waldenström’s macroglobulinemiaClin Lymphoma5282415794866
- GertzMABloodEKaminerLS2004Multicenter phase 2 trial of rituximab for Waldenström macroglobulinemia (WM): an Eastern Cooperative Oncology Group Study (E3A98)Leuk Lymphoma4520475515370249
- GhobrialIMRajkumarSV2003Management of thalidomide toxicityJ Support Oncol119420515334875
- GhobrialIFonsecaRGreippPR2004Initial immunoglobulin M ‘flare’ after rituximab therapy in patients diagnosed with Waldenström macroglobulinemia: an Eastern Cooperative Oncology Group StudyCancer1012593815493038
- HarousseauJLAttalMLeleuX2006Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II studyHaematologica91149850517043025
- HatjiharissiEHoAXuL2006Preclinical in vitro and in vivo evidence support a therapeutic role for the CD70 directed monoclonal antibody (SGN-70) in Waldenströms macroglobulinemia (WM) [abstract]Blood1082490
- HatjiharissiENgoHLeontovicAA2007Proteomic analysis of Waldenström macroglobulinemiaCancer Res6737778417440091
- HideshimaTChauhanDShimaY2000Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapyBlood9629435011049970
- HideshimaTChauhanDPodarK2001Novel therapies targeting the myeloma cell and its bone marrow microenvironmentSemin Oncol286071211740818
- HideshimaTBergsagelPLKuehlWM2004aAdvances in biology of multiple myeloma: clinical applicationsBlood1046071815090448
- HideshimaTPodarKChauhanD2004bp38 MAPK inhibition enhances PS-341 (Bortezomib)-induced cytotoxicity against multiple myeloma cellsOncogene2387667615480425
- HideshimaTCatleyLYasuiH2006Perifosine, an oral bioactive novel alkylphospholipid, inhibts Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cellsBlood10740536216418332
- HuLShiYHsuJH2003Downstream effectors of oncogenic ras in multiple myeloma cellsBlood10131263512515720
- HunterZBoxerMKahlB2006Phase II study of alemtuzumab in lymphoplasmacytic lymphoma: results of WMCTG trial 02-079 [abstract]J Clin Oncol247523
- HusseinMBerensonJNiesviskyR2006Results of a phase I trial of SGN-40 (Anti-huCd40 mAb) in patients with relapsed multiple myeloma [abstract]Blood1083576
- JagannathSBarlogieBBerensonJ2004A phase 2 study of two doses of Bortezomib in relapsed or refractory myelomaBr J Haematol1271657215461622
- JagannathSRichardsonPGBarlogieB2006Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to Bortezomib aloneHaematologica919293416818280
- KastritisEAnagnostopoulosARoussouM2007Treatment of light chain (AL) amyloidosis with the combination of Bortezomib and dexamethasoneHaematologica9213515818024372
- KnopSGereckeCToppM2006Lenalidomide (revlimid), adriamycin and dexamethasone chemotherapy (RAD) is safe and effective in treatment of relapsed multiple myeloma first results of a german multicenter Phase I/II trial [abstract]Blood108408
- KumarSRajkumarSV2006Thalidomide and lenalidomide in the treatment of multiple myelomaEur J Cancer4216122216750621
- LapidotTDarAKolletO2005How do stem cells find their way home?Blood10619011015890683
- LeleuXXiaoyingJRunnelsJ2007The Akt pathway regulates survival and homing in Waldenström macroglobulinemiaBlood11044172617761832
- MateosMVHernandezJMHernandezMT2006Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 studyBlood10821657216772605
- MitsiadesCSMitsiadesNPoulakiV2002Activation of NF-kap-paB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signalling in human multiple myeloma cells: therapeutic implicationsOncogene2156738312173037
- MitsiadesCSMitsiadesNSMcMullanCJ2006Antimyeloma activity of heat shock protein-90 inhibitionBlood107109210016234364
- MitsiadesNMitsiadesCSPoulakiV2002Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implicationsBlood9945253012036884
- MitsiadesNMcMullanCPoulakiV2004The mTOR inhibitor RAD001 (Everolimus) is active against multiple myeloma cells in vitro and in vivo [abstract]Blood1041496
- MoreauASJiaXNgoHT2007Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenström’s macroglobulinemiaBlood10949647217284528
- MoreauASJiaXLeleuX2007Simvastatin, an HMG-CoA inhibitor, induces in vitro antitumor activity in Waldenström’s macroglobulinemia [abstract]Hematologica921219
- MoreauPVoillatlBenboubkerL2006Rituximab in CD20 positive multiple myeloma: a prospective study from the IFM group [abstract]Blood1083577
- NgoHHatjiharissiELeleuX2006The CXCR4/SDF-1 axis regulates migration and adhesion in Waldenström macroglobulinemia [abstract]Blood1082418
- O’ConnorOOrlowskiRAlsinaM2006Multicenter phase I studies to evaluate the safety, tolerability, and clinical response to intensive dosing with the proteasome inhibitor PR-171 in patients with relapsed or refractory hematological malignancies [abstract]Blood1082430
- OrlowskiRZZhuangSHParekhT2006The combination of pegylated liposomal doxorubicin and Bortezomib significantly improves time to progression of patients with relapsed/refractory multiple myeloma compared with Bortezomib alone: results from a planned interim analysis of a randomized phase III study [abstract]Blood10840416790587
- PalladiniGPerfettiVObiciL2005The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL)Blood10529495115572585
- PalumboABringhenSCaravitaT2006Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trialLancet3678253116530576
- PalumboAFalcoPFalconeA2006Oral revlimid plus melphalan and prednisone (R-MP) for newly diagnosed multiple myeloma: Results of a multicenter phase I/II study [abstract]Blood108800
- PattersonCSoumeraiJHunterZ2006Sildenafil citrate suppresses disease progression in patients with Waldenström’s macroglobulinemiaJ Clin Oncol18S7556
- PodarKRaabMSZhangJ2006Targeting PKC in multiple myeloma: in vitro and in vivo effects of the novel, orally available small-molecule inhibitor Enzastaurin (LY317615.HCl)Blood10916697717023575
- RajeNKumarSHideshimaT2004Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myelomaBlood10441889315319277
- RajkumarSVFonsecaRDispenzieriA2000Thalidomide in the treatment of relapsed multiple myelomaMayo Clin Proc7589790110994824
- RajkumarSVHaymanSGertzMA2002Combination therapy with thalidomide plus dexamethasone for newly diagnosed myelomaJ Clin Oncol2043192312409330
- RajkumarSVHaymanSRLacyMQ2005Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myelomaBlood1064050316118317
- RajkumarSVBloodEVesoleD2006Eastern Cooperative Oncology Group Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology GroupJ Clin Oncol24431616365178
- RajkumarSVBloodE2006Lenalidomide and venous thrombosis in multiple myelomaN Engl J Med35420798016696148
- RajkumarVJacobusSCallanderN2007A randomized trial of lenalidomide plus high-dose dexamethasone (RD) versus lenalidomide plus low-dose dexamethasone (Rd) in newly diagnosed multiple myeloma (E4A03): A trial coordinated by the Eastern Cooperative Oncology Group [abstract]Blood1107417371947
- RenaudSFuhrPSchweikertGM2006High-dose rituximab and anti-MAG-associated polyneuropathyNeurology66742416534115
- RichardsonPGBarlogieBBerensonJ2003A phase 2 study of Bortezomib in relapsed, refractory myelomaN Engl J Med34826091712826635
- RichardsonPGSchlossmanRLWellerE2002Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myelomaBlood1003063712384400
- RichardsonPGSonneveldPSchusterMW2005aBortezomib or high-dose dexamethasone for relapsed multiple myelomaN Engl J Med35224879815958804
- RichardsonPChanan-KhanAAlsinaM2005bSafety and activity of KOS-953 in patients with relapsed refractory multiple myeloma (MM): Interim results of a phase 1 trial [abstract]Blood106361
- RichardsonPGMitsiadesCHideshimaT2006aLenalidomide in multiple myelomaExpert Rev Anticancer Ther611657316925483
- RichardsonPGBloodEMitsiadesCS2006bA randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myelomaBlood10834586416840727
- RichardsonPGMitsiadesCGhobrialI2006cBeyond single-agent Bortezomib: combination regimens in relapsed multiple myelomaCurr Opin Oncol1859860816988581
- RichardsonPLonialSJakubowiakJ2006dA multicenter phase II study of perifosine (KRX-0401) alone and in combination with dexamethasone (Dex) for patients with relapsed or relapsed/refractory multiple myeloma (MM) [abstract]Blood1083582
- RoccaroALeleuXSAccoA2008Dual targeting of the proteasome regulates survival and homing in Waldenström’s macroglobulinemiaBlood11147526318316628
- RoccaroALeleuXMoreauAS2008Resveratrol exerts antiproliferative effect and induces apoptosis in Waldesntrom’s macroglobulinemiaClin Cancer Res1418495818347188
- RossiJMoreauxJRoseM2006A Phase I/II study of atacicept (TACI-Ig) to neutralize APRIL and BLyS in patients with refractory or relapsed multiple myeloma (MM) or active previously treated Waldenström’s macroglobulinemia (WM) [abstract]Blood1083578
- SanchorawalaVWrightDGRosenzweigM2007Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trailBlood109492616960148
- San MiguelJSchlagRKhuagevaO2007MMY-3002: A phase 3 study comparing Bortezomib-melphalan-prednisone (VMP) with melphalan-prednisone (MP) in newly diagnosed multiple myeloma [abstract]Blood11076
- ShiYGeraJHuL2002Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779Cancer Res6250273412208757
- SiegelDKrishnanALonialS2006Phase II trial of SCIO-469 as monotherapy (M) or in combination with Bortezomib (MB) in relapsed refractory multiple myeloma (MM) [abstract]Blood108358016882710
- SinghalSMehtaJDesikanR1999Antitumor activity of thalidomide in refractory multiple myelomaN Engl J Med34115657110564685
- SitiaRPalladiniGMerliniG2007Bortezomib in the treatment of AL amyloidosis: targeted therapy?Haematologica921302718024367
- SpencerAPrinceHMRobertsA2007Thalidomide improve survivals when use after ASCT [abstract]Hematologica92suppl 2S7b.5
- TaiYTTongXSantosD2005Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myelomaCancer Res65589890615994968
- TamburiniJLevyVChaleteixC2005Treatment of Waldenström’s macroglobulinemia with the combination of fludarabine and cyclophosphamide: results in 49 patientsLeukemia191831416121217
- TreonSBranaganARHunterZ2004aParadoxical increases in serum IgM and viscosity levels following rituximab in Waldenström’s macroglobulinemiaAnn Oncol151481315367407
- TreonSBranaganAWasiP2004bCombination therapy with rituximab and fludarabine in Waldenström’s macroglobulinemia [abstract]Blood104753
- TreonSPTournilhacOBranaganAR2004cClinical responses to sildenafil in Waldenström’s macroglobulinemiaClin Lymphoma5205715636699
- TreonSPEmmanouilidesCKimbyE2005aExtended rituximab therapy in Waldenström’s macroglobulinemiaAnn Oncol16132815598950
- TreonSHansenMBranaganAR2005bPolymorphisms in FcγRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenström’s macroglobulinemiaJ Clin Oncol234748115659493
- TreonSPGertzMADimopoulosM2006aUpdate on treatment recommendations from the Third International Workshop on Waldenström’s macroglobulinemiaBlood1073442616410453
- TreonSPSoumeraiJPattersonC2006bBortezomib, dexamethasone and rituximab (BDR) is a highly active regimen in the primary therapy of Waldenström’s macroglobulinemia: planned interim results of WMCTG clinical trial 05–180 [abstract]Blood1082765
- TreonSPGertzMADimopoulosM2006cUpdate on treatment recommendations from the Third International Workshop on Waldenström’s macroglobulinemiaBlood1073442616410453
- TreonSPSoumeraiJPattersonC2006dImatinib mesylate (Gleevec) is active in relapsed/refractory Waldenströms macroglobulinemia: Planned interim results of WMCTG Clinical Trial 05–140 [abstract]Blood1082484
- TsaiDMaillardIDownsLH2004Use of iodine 131I-tositumomab radioimmunotherapy in a patient with Waldenström’s macroglobulinemiaLeuk Lymphoma45591515160923
- van OersMHKlasaRMarcusRE2006Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trialBlood108329530116873669
- WeberDRankinKGavinoM2003aThalidomide alone or with dexamethasone for previously untreated multiple myelomaJ Clin Oncol21161912506164
- WeberDMDimopoulosMADelasalleK2003b2-Chlorodeoxy-adenosine alone and in combination for previously untreated Waldenström’s macroglobulinemiaSemin Oncol30243712720145
- WeberDChenCNiesvizkyM2006Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): Results of a North American phase III study (MM-009) [abstract]J Clin Oncol18S7521
- ZervasKMihouDKatodritouI2006VAD-doxil vs VAD-doxil plus thalidomide as initial treatment in patients with multiple myeloma: a multicenter randomized trial of The Greek Myeloma Study Group [abstract]Blood108794
