Abstract
Objective
To systematically review the general and comparative efficacy and safety of anakinra for rheumatoid arthritis.
Methods
We searched MEDLINE®, Embase, The Cochrane Library, and the International Pharmaceutical Abstracts from 1980 to April 2009. We manually searched reference lists of pertinent review articles and explored the Center for Drug Evaluation and Research database. For efficacy we included randomized controlled trials (RCTs) comparing anakinra with placebo or other biologics. For safety both experimental and observational studies were eligible. Two persons independently reviewed abstracts and full text articles and extracted relevant data.
Results
We included data from 3 RCTs comparing anakinra with placebo for rheumatoid arthritis (RA). The pooled relative risk (RR) of an ACR50 (American College of Rheumatology) response for anakinra compared with placebo is 2.28 (95% CI 1.41 to 3.67). Adjusted indirect comparisons of ACR50 response rates of anakinra and anti-TNF agents showed a RR of 0.67 (95% CI 0.38 to 1.17) favoring the anti-TNF drugs. This result did not reach statistical significance. For safety, we included 9 experimental and observational studies of 24 weeks to 3 years duration. Up to 30% of patients withdrew from the studies due to adverse events. 67.2% (95% CI 38.7 to 95.7) of patients experienced an injection site reaction.
Conclusions
Anakinra is an effective drug for treating RA. Indirect comparisons with adalimumab, etanercept and infliximab, however, showed a trend towards greater efficacy for the anti-TNF drugs. Anakinra also seems to be associated with comparably high rates of injection site reactions. These results should be taken into account when considering biologic therapy for patients with RA.
Keywords:
Introduction
Biologic agents have been approved for the treatment of rheumatic diseases, plaque psoriasis and inflammatory bowel diseases and have tremendously changed treatment strategies for these debilitating diseases.Citation1 These agents include abatacept (Orencia®), adalimumab (Humira®), alefacept (Amevive®), anakinra (Kineret®), certolizumab pegol (Cimzia®), etanercept (Enbrel®), golimumab (Simponi®), infliximab (Remicade®), natalizumab (Tysabri®), and rituximab (Rituxan®). Evidence suggests that biologics are highly effective, although adverse events such as serious infections, lymphoma, leucopenia, malignancies, or demyelinations are of concern.Citation2,Citation9 Furthermore, they are considerably more expensive than standard treatment options.Citation10,Citation11 The cost of biologic drugs is expected to exceed US$60 billion in the US by 2010, representing a significant portion of the US drug industry.Citation12
In general, biologic agents work by selectively blocking mechanisms involved in the inflammatory and immune response. For example, the biologics adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab block tumor necrosis factor (TNF).Citation13,Citation15 In contrast, abatecept and alefacept interfere with T-lymphocyte activation.Citation16 Natalizumab binds the alpha(4) integrin, which results in partial blockade of immune-cell adhesion to vascular endothelium and subsequent tissue migration of lymphocytes.Citation17 Rituximab binds to the CD20 antigen on the surface of B lymphocytes,Citation18 which are involved in autoimmune anti-inflammatory processes, such as those involved in rheumatoid arthritis (RA). Finally, anakinra is a recombinant IL-1 (interleukin-1) receptor antagonist and has the same properties as the human IL-1 receptor agonist. Anakinra binds to the IL-1 receptor and inhibits pro-inflammatory effects by blocking signal transduction.Citation4–Citation6,Citation19
Dosing regimens and route of administration vary considerably among the various agents. Abatacept, infliximab, and rituximab are administered intravenously; adalimumab, anakinra and etanercept are administered subcutaneously. Abatacept is infused in doses of 500 to 1000 mg repeated at 2 and 4 weeks and every 4 weeks thereafter. Infliximab infusions are administered in doses of 3 mg/kg at 0, 2, and 6 weeks followed by maintenance every 8 weeks and rituximab is dosed at 1000 mg on days 1 and 15. Adalimumab is injected subcutaneously at 40 mg every other week, and etanercept is administered at 50 mg per week.Citation11 In comparison, anakinra is administered in doses of 100 mg subcutaneously every day. The burden of daily subcutaneous administration of anakinra might disadvantage it compared with the other biologic agents. presents a summary of dosing and administration of the biologics.
Table 1 Administration and dosing
Considering the high cost and differing regimes of the biologics, it is important to ascertain their comparative effectiveness. This systematic review and meta-analysis is a result of work conducted for the Oregon Drug Effectiveness Review Project (DERP) on the comparative efficacy and safety of 10 specific biologics for rheumatoid arthritis (RA), ankylosing spondylitis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and juvenile idiopathic arthritis in adult out-patients and subgroups including children. In a meta-analysis of studies up to January 2005 indirect comparisons suggested that anakinra is inferior to the anti-TNF biologics for RA.Citation20 In this report we focus on the general and comparative benefits and risks of anakinra and provide an update of this result with several newer studies included in the analysis.
Methods
Data sources
We searched MEDLINE®, Embase, Cochrane Library, and International Pharmaceutical Abstracts from 1980 up to April 2009. We used either Medical Subject Headings (MeSH) as search terms when available or key words when appropriate. In the original DERP report,Citation11 we combined terms for RA, ankylosing spondylitis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and juvenile idiopathic arthritis with a list of ten specific biologics-abatacept (Orencia®), adalimumab (Humira®), alefacept (Amevive®), anakinra (Kineret®), certolizumab (Cimzia®)*, etanercept (Enbrel®), infliximab (Remicade®), natalizumab (Tysabri®), rituximab (Rituxan®). For this review we limited results to literature identified for anakinra. For the other biologics, we present comparisons with anakinra only. We did not review data on golimumab because it was not on the market at the time of this review, and excluded efalizumamab because it was voluntarily withdrawn from the market. We limited electronic searches to “human” and “English language.”
We manually searched reference lists of pertinent review articles and letters to the editor and used the Center for Drug Evaluation and Research database to identify unpublished research submitted to the FFDA. The Scientific Resource Center of the Oregon Health and Science University invited pharmaceutical manufacturers to submit dossiers on completed research for each drug.
Study selection
Two persons independently reviewed abstracts and relevant full-text articles. To assess efficacy or effectiveness for response and quality of life we included randomized controlled trials (RCTs) and prospective controlled observational studies of at least 12 weeks duration that compared anakinra with placebo or another biologic. To assess harms (specific adverse events, rates of adverse events, and discontinuations attributable to adverse events), we also examined data from retrospective observational studies with ≥100 participants and follow-up of at least 6 months. summarizes the eligibility criteria.
Table 2 Eligibility criteria
If both reviewers agreed that the study did not meet eligibility criteria, we excluded it. We also excluded studies that met eligibility criteria but were reported only as an abstract. Investigators resolved disagreements about inclusion or exclusion by consensus or by involving a third reviewer.
Data extraction and quality assessment
We used a structured data abstraction form into which trained reviewers abstracted data from each study and assigned an initial quality rating. Abstracted data included the baseline characteristics of the included patients, health-related outcomes (such as quality of life, response, remission, pain, hospitalization and mortality), adverse events, and overall and differential attrition. A senior reviewer read each abstracted article, evaluated completeness of data abstraction, and confirmed the quality rating. Investigators resolved any disagreements by discussion and consensus or by consulting an independent party.
We assessed the internal validity (quality) of trials based on predefined criteria and applied ratings of good, fair, or poor.Citation21 Primary elements of quality assessment for RCTs included randomization and allocation concealment, similarity of compared groups at baseline, blinding, use of intention-to-treat analysis, and overall and differential loss to follow-up. To assess observational studies we used criteria involving selection of cases or cohorts and controls, adjustment for confounders, methods of outcomes assessment, length of follow-up, and statistical analysis.Citation22 Studies with a fatal flaw in one or more categories were rated “poor” quality.
To identify effectiveness studies, we used a tool that distinguishes efficacy trials from effectiveness studies based on certain elements of study design.Citation23 Such studies have a higher applicability of results than efficacy trials because they enroll less selected study populations, employ treatment modalities that mimic clinical practice, and assess health outcomes along with adverse events.
Data synthesis
Where data were insufficient to conduct meta-analyses (ie, too sparse or too heterogeneous), we synthesized the evidence on the majority of outcomes qualitatively. Where data from RCTs were sufficient, we conducted meta-analyses. The outcome of interest for efficacy was the number of patients achieving a response according to the ACR scoring system (American College of Rheumatology). We chose ACR50 as the primary outcome measure because a 50% improvement is likely to translate to a clinically significant improvement in health-related quality of life. For example, a patient with 12 swollen and 8 tender joints at baseline would need to have fewer than 6 swollen and 4 tender joints at the trial endpoint. This would be accompanied by at least a 50% improvement in at least 3 of the following 5 measures: the patient’s assessment of pain, the patient’s assessment of global disease activity, the physician’s assessment of global disease activity, the Health Assessment Questionnaire (HAQ)-Disability Index, and either a C-reactive protein (CRP) or sedimentation rate (Westergren erythrocyte sedimentation rate [WESR]). In addition, we report the results for an ACR20 (20% improvement) and ACR70 (70% improvement).
For each meta-analysis, we conducted a test of heterogeneity (I2 index) and applied both random and fixed effects models. We consider I2 greater than 60% to be too high to compare data. We report the random effects results because the results from both models were very similar in all meta-analyses. We assessed publication bias using funnel plots and Kendell’s tests. All statistical analyses used StatsDirect Statistical Software program, version 2.6.6 (StatsDirect LTD, 2008).
Because only limited head-to-head evidence on biologics was available, we conducted adjusted indirect comparisons when data was sufficient and trials were of similar design, conducted in similar settings with a comparable patient population. However, because of limited data on individual biologics as active comparators, we assessed the comparative risk of anakinra relative to anti-TNF agents as a class. We based these analyses on the method proposed by Bucher et al.Citation24 Evidence suggests that adjusted indirect comparisons agree with head-to-head trials if component studies are similar and treatment effects are expected to be consistent in patients included in different trials.Citation25,Citation26 Nevertheless, findings must be interpreted cautiously.
Results
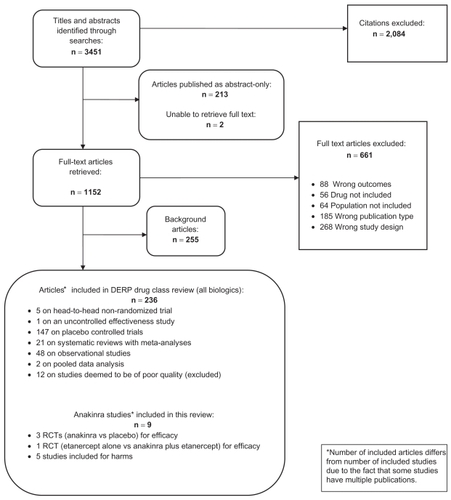
In the original DERP report on biologics, we identified 3451 citations from searches and reviews of reference lists. Of these, 236 articles met the inclusion criteria and were included. Some studies had multiple publications. Finally, for this report on anakinra, we included 4 RCTs for efficacy and 5 additional studies for saftey. represents the results of the literature search and the disposition of the literature. We did not find any study that could be classified as an effectiveness trial. All trials reported on anakinra for the treatment of rheumatoid arthritis. We did not locate any publication that met our inclusion criteria for psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn’s disease or ulcerative colitis (all other indications covered in the DERP report).
Efficacy of anakinra
We located 5 publications that met our inclusion criteria for efficacy in patients with rheumatoid arthritis. Three RCTs reported on the efficacy of anakinra compared with placebo,Citation27,Citation29 and 1 RCT compared anakinra in combination with etanacerpt with etanacerpt alone.Citation30 We found additional information on ACR response rates for 1 RCTCitation27 in the CDER database.Citation31 One RCT was published as an abstract only.Citation32 presents a summary of the included studies.
Table 3 Summary of anakinra (AKA) efficacy studies
Population and outcome measures
A total of 1625 patients were included in the trials. All patients suffered from active RA of at least 3 to 6 months duration. Mean disease duration varied between 6 and 10 years. Trials variably allowed concomitant treatment with a stable dose of MTX and/or corticosteroids. Most patients used NSAIDS in addition to the study medication. The majority of patients in the trials had active disease despite therapy with at least one disease-modifying anti-rheumatic drug (DMARD). Patients with an autoimmune disease other than RA, a history of active listeriosis or mycobacterial infection, another infection or recent antibiotic treatment were generally excluded from studies. Between 70% and 80% of trial participants were female.
All trials assessed response rates after 24 weeks of therapy as defined by the ACR. One trial also reported the European League Against Rheumatism (EULAR) response rate.Citation30 In addition, three studies evaluated functional capacity with the HAQ.Citation27,Citation29
All studies were funded by Amgen Inc., Thousand Oaks, California.Citation28,Citation30
Anakinra compared with placebo
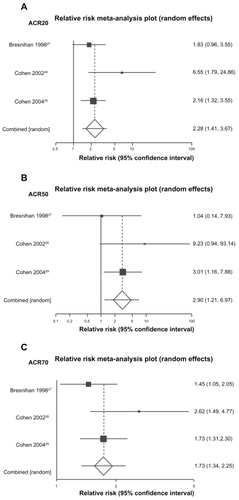
Three fair RCTs compared various doses of anakinra with placebo (between 0.04 mg/kg/day and 150 mg/day) for patients with moderate to severe RA that had not responded to DMARDs.Citation27,Citation29 Two studies allowed concomitant treatment with MTX.Citation28,Citation29 All three studies lasted 24 weeks. In total, 1392 patients were randomized to anakinra (N = 946) or placebo (N = 446). Based on the ACR criteria, significantly more patients receiving anakinra 100 mg/day, 150 mg/day, 1 mg/kg/day, or 2 mg/kg/day responded to treatment compared with placebo. Specifically, the pooled relative risk of an ACR50 response for the approved doses of anakinra compared with placebo is 2.28 (95% CI 1.41 to 3.67). The RR for an ACR20 response is 1.73 (95% CI 1.34 to 2.25) and for an ACR70 response 2.90 (95% CI 1.21 to 6.97) (see ).
In addition, in all three RCTs, patients receiving anakinra demonstrated a greater improvement in health assessment questionnaire (HAQ) than patients receiving placebo score (approximately 0.4 for anakinra compared to 0.2 for placebo; scale values 0 to 3). This result reached statistical significance for all doses except one (2 mg/kg/day).
Comparative efficacy
We did not locate any trials that directly compared anakinra to another biologic. Because of the lack of direct head-to-head evidence for anakinra compared with other biologics we conducted adjusted indirect comparisons based on meta-analyses of placebo-controlled trials to compare the treatment effects of individual biologics. We included data from published studies or from the CDER website. For all analyses we used only data derived from study arms at or near the recommended dosage. We did not perform indirect comparisons for biologics other than adalimumab, etanercept, and infliximab because the populations included in the trials were too heterogenous compared with the anakinra trials and therefore performing indirect comparisons may lead to erroneous conclusions. Appendix 1 summarizes studies included for indirect comparisons.
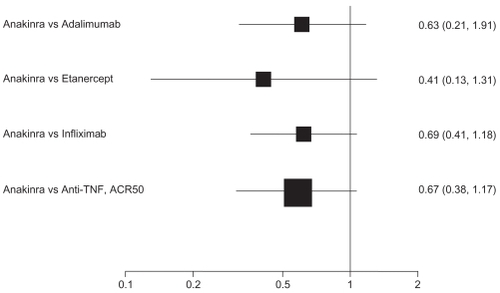
For ACR50 response, point estimates of anakinra compared with the anti-TNF biologics consistently favored the comparator: adalimumab (RR 0.64, 95% CI 0.36 to 1.14); etanercept (RR 0.41, 95% CI 0.13 to 1.31); and infliximab (RR 0.69, 95%CI 0.41 to 1.18). Compared with anti-TNF agents as a class, the RR of an ACR50 response is 0.67 (95% CI 0.38 to 1.17). That differences do not reach statistical significance is likely attributable to a lack of power. Despite this, the differences may be clinically relevant.
depicts results of adjusted indirect comparisons of anakinra with adalimumab, etanercept, infliximab, and anti-TNF drugs as a class. The evidence on abatacept, certolizumab, and rituximab was insufficient or too heterogeneous to be included for indirect comparisons.
General efficacy of anakinra for other indications
We did not locate any published studies of anakinra for psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn’s disease, or ulcerative colitis.
Safety of anakinra
General safety
For safety we included the three RCTs included for efficacy,Citation27,Citation29 1 RCT of anakinra plus etanercept compared with etaercerpt alone,Citation30 and one 6-month RCT of anakinra versus placebo with a 3-year open treatment follow-up phase.Citation4–Citation6,Citation33
In addition, we located 1 RCT that reported the safety results of 3 doses of anakinra.Citation19 (This study is an extension of Bresnihan et al. Patients who had received placebo in the initial 24 weeks were re-randomized to 30 mg/day, 75 mg/day, or 150 mg/day of anakinra and followed up for an additional 52 weeks). We also included safety data comparing patients taking anakinra and conventional DMARDs from the German RABBIT Registrar (RABBIT = Rheumatoid Arthritis – Observation of Biologic Therapy)Citation34,Citation35 and a case series of patients receiving anakinra.Citation36 presents a summary of the studies included for safety.
Table 4 Summary of studies assessing adverse events
Overall, between 6.5% and 30% of patients withdrew from anakinra therapy due to adverse events compared with 4% to 13% of patients on placebo. The most common adverse event was injection site reaction. The mean, crude incidence of injection site reactions was 67.2% (95% CI 38.7 to 95.7).Citation11
Infections occurred in 5% to 33% of patients taking anakinra compared with 12% to 26% of placebo-treated patients. Infections included influenza-like symptoms, respiratory infections, and urinary tract infections. We located 1 systematic review that reported on the safety of anakinra.Citation37 The authors included all 4 RCTs of anakinra versus placebo for rheumatoid arthritis that we also located.Citation6,Citation27–Citation29 Thirty serious infections (1.4%) occurred in the anakinra groups compared with 4 in the placebo groups (0.5%). The meta-analysis of the studies demonstrated an odds ratio for serious infections of 2.75 (95% CI 0.91 to 8.35).Citation37
Two of the 24-week RCTs reported 2 malignancies each that were “not considered to be related to the study medication”.Citation27,Citation28 The 3-year open label study of anakinraCitation33 reported a higher than expected incidence of melanoma and lymphoma (incidence compared with data from the general population). The results submitted to the CDER by Amgen reported that “21 malignancies of various types were observed in 2531 rheumatoid arthritis patients treated with anakinra for up to 50 months”. We are unable to draw any conclusions regarding malignancy due to the overall small number of participants in the studies.
Comparative harms
We did not locate any studies that directly compared the incidence of adverse events between anakinra and other biologics. We calculated a higher crude incidence of injection site reactions for anakinra (67.2%, 95% CI 38.7 to 95.7) compared with adalimumab (17.5%, 95%CI 7.1 to 27.9) and etanercept (22.4%, 95% CI 8.5 to 36.3). This is consistent with numbers reported in the respective package inserts.Citation38,Citation40
Combination strategies
Anakinra combined with etanercept compared with etanercept alone
One RCT compared anakinra 100 mg/day combined with 2 different doses of the anti-TNF agent etanercept (25 mg/once a week or 25 mg/twice a week) with etanercept alone (25 mg/twice a week) in 242 patients with moderate rheumatoid arthritis.Citation30 After 24 weeks of therapy 41% of patients in the etanercept groups had an ACR50 response, compared with 39% of those receiving once weekly etanercept and anakinra, and 31% in the groups who received the combination of twice weekly etanercept and daily anakinra (results not statistically significant). The odds ratio for an ACR50 response for the etanercept plus anakinra groups versus the etanercept alone group was 0.64 (95% CI 0.37 to 1.09).
Furthermore, 15% of patients in the twice-weekly etan-ercept plus anakinra group experienced a serious adverse event, compared with 2.5% in the etanercept alone group and 5% in the once-weekly etanercept and anakinra group. Twenty-two percent of patients in the anakinra/etanercept once per week group and 20% of the patients randomized to anakinra and etanercept twice per week did not complete the study compared with 7% of patients receiving etanercept alone. Injection-site reactions were the common reason for adverse-event related withdrawal from the study. Injection site reactions occurred in 69% of patients receiving combination therapy compared with 40% of those receiving etanercept alone.
Subgroups
We did not locate any studies that provided results on the efficacy or safety of anakinra for subgroups.
Discussion
In this systematic review, we found 3 RCTs that confirmed the efficacy of anakinra compared with placebo for adult patients with RA. Our results are consistent with several other published systematic reviews of anakinra.Citation20,Citation41–Citation43 In one RCT that used a combination of anakinra and etanercept the response rates were not better than etanercept alone, and the adverse events rates were significantly higher. There appears to be greater harm and no additional benefit in combining anakinra and the anti-TNF drugs.
No direct evidence comparing anakinra and other biologics exists, however our indirect comparision of anakinra and anti-TNF agents as a class revealed a non-significant RR of 0.67 (95% CI 0.38 to 1.17) that favors the anti-TNF drugs adalimumab, etanacerpt and infliximab. The authors of a meta-analysis with indirect comparisons calculated that anakinra is inferior to adalimumab, etanercept and infliximab.Citation20 This result was statistically significant. Our update, which includes several newer studies of the anti-TNF biologics, indicates a lesser degree of certainty.
Patients receiving anakinra experienced more injection site reactions and serious infections than patients receiving placebo. The number of patients in the included trails was too small to reach any conclusions regarding malignancies.
Our review has limitations. We did not locate any direct comparisons of anakinra compared with other biologics. Like other authors, we have relied on indirect comparisons to report the comparative efficacy of anakinra with other biologics. Although evidence suggests that adjusted indirect comparisons agree with head-to-head trials if component studies are similar and treatment effects are expected to be consistent in patients included in different trials, results have to be interpreted cautiously. Many of the underlying assumptions of indirect comparisons are not verifiable and confidence intervals are often wide leading to indeterminate results. Nevertheless, in the absence of direct evidence indirect comparisons can provide valuable information about the comparative efficacy of drugs.
Secondly, publication bias is an issue for all systematic reviews. Selective availability of studies with positive results can seriously bias conclusions of systematic reviews, particularly when the focus is on placebo controlled trials which are generally conducted for regulatory approval by the manufacturer of a specific drug. All three placebo-controlled trials included in this review were funded by Amgen, Thousand Oaks, CA, US, the makers of anakinra.
Finally, all of the studies we included for efficacy were conducted in patients with moderate to severe rheumatoid arthritis with an average disease duration of 6 to 10 years who also took anti-inflammatory drugs, corticosteroids and in 3 trials also methotrexate. The applicability of their results to the average patient with rheumatoid arthritis might be limited.
Conclusion
Anakinra is certainly effective for treating moderate to severe RA that is resistant to traditional DMARDs in comparison with placebo. Indirect comparisons with adalimumab, etanercept and infliximab, however, showed a trend towards greater efficacy for the anti-TNF drugs. Anakinra also seems to be associated with comparably high rates of injection site reactions. The frequency of administration (daily) might also disadvantage anakinra in comparison with these agents, although the subcutaneous route of administration may be preferable to intravenous. Our results and these factors should be taken into account when considering biologic therapy for patients with RA.
Acknowledgments
We wish to thank Laura Morgen from the University of North Carolina at Chapel Hill and our colleagues from the Danube University Krems Andrea Chapman and Irene Wild for help with literature searches and for administrative support.
Funding
The original comparative effectiveness report was funded through a contract from the University of North Carolina Evidence-based Practice Center with the Oregon Health and Science University under the Drug Effectiveness Review Project. We have received no funding for this update.
Disclosures
Richard Hansen has received research support from NIH, AHRQ, and Takeda Pharmaceuticals. All the other authors declare that they have no conflicts of interest.
Appendix 1: References
- FurstDEAdalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis)J Rheumatol2003301225632567114719195
- KeystoneECRadiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trialArthritis Rheum20045051400141115146409
- KimHYA randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexateAPLAR Journal of Rheumatology2007101916
- MiyasakaNClinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: The CHANGE studyMod Rheumatol200818325226218330677
- van de PutteLBEfficacy and safety of the fully human anti-tumour necrosis factor alpha monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II studyAnn Rheum Dis200362121168117714644854
- van de PutteLBEfficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failedAnn Rheum Dis200463550851615082480
- WeinblattMEAdalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trialArthritis Rheum2003481354512528101
- KlareskogLTherapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trialLancet2004363941067568115001324
- LanJLA comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12-week, double-blind, randomized, placebo-controlled studyJ Formos Med Assoc2004103861862315340661
- MorelandLWTreatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion proteinN Engl J Med199733731411479219699
- MorelandLWEtanercept therapy in rheumatoid arthritis. A randomized, controlled trialAnn Intern Med1999130647848610075615
- MathiasSDHealth-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placeboClin Ther200022112813910688396
- WeinblattMEA trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexateN Engl J Med199934042532599920948
- AbeTA multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritisJ Rheumatol2006331374416395748
- KavanaughAChimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapyJ Rheumatol200027484185010782805
- MainiRNTherapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritisArthritis Rheum1998419155215639751087
- MainiRInfliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study GroupLancet199935491941932193910622295
- WesthovensRThe safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trialArthritis Rheum20065441075108616572442
- ZhangFCInfliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A preliminary study from ChinaAPLAR Journal of Rheumatology200692127130
References
- SaketkooLAEspinozaLRImpact of biologic agents on infectious diseasesInfect Dis Clin North Am2006204931961viii17118297
- BurmesterGRMarietteXMontecuccoCAdalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trialAnn Rheum Dis200766673273917329305
- ColombelJFLoftusEVJrTremaineWJThe safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patientsGastroenterology20041261193114699483
- FleischmannRMSchechtmanJBennettRAnakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trialArthritis Rheum200348492793412687534
- TesserJFleischmannRDoreRConcomitant medication use in a large, international, multicenter, placebo controlled trial of anakinra, a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritisJ Rheumatol200431464965415088288
- SchiffMHDiVittorioGTesserJThe safety of anakinra in high-risk patients with active rheumatoid arthritis: six-month observations of patients with comorbid conditionsArthritis Rheum20045061752176015188350
- SchaibleTFLong term safety of infliximabCan J Gastroenterol200014Suppl C29C32C
- LjungTKarlenPSchmidtDInfliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm CountyGut200453684985315138212
- TakeuchiTTatsukiYNogamiYPostmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritisAnn Rheum Dis200867218919417644554
- FleurenceRSpackmanECost-effectiveness of biologic agents for treatment of autoimmune disorders: Structured review of the literatureJournal of Rheumatology2006212417086602
- GartlehnerGThiedaPMorganLCDrug Effectiveness Review Project: Targeted Immune ModulatorsPortlandOregon Health and Science UniversityIn press2009
- GrabowskiHCockburnILongGThe market for follow-on biologics: how will it evolve?Health Aff (Millwood)20062551291130116966725
- RoosJCOstorAJTumor necrosis factor inhibitors for rheumatoid arthritisN Engl J Med20063551920462047 author reply 204817093259
- KruegerGCallisKPotential of tumor necrosis factor inhibitors in psoriasis and psoriatic arthritisArch Dermatol (U S A)2004140218
- NesbittAFossatiGBerginMMechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agentsInflamm Bowel Dis200713111323133217636564
- ChitaleSMootsRAbatacept: the first T lymphocyte co-stimulation modulator, for the treatment of rheumatoid arthritisExpert Opin Biol Ther20088111512218081541
- KoHHBresslerBNatalizumab: pharmacology, clinical efficacy and safety in the treatment of patients with Crohn’s diseaseExpert Rev Gastroenterol Hepatol200711293919072431
- SchunaAARituximab for the treatment of rheumatoid arthritisPharmacotherapy200727121702171018041890
- NukiGBresnihanBBearMBMcCabeDLong-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trialArthritis Rheum200246112838284612428223
- NixonRBansbackNBrennanAThe efficacy of inhibiting tumour necrosis factor alpha and interleukin 1 in patients with rheumatoid arthritis: a meta-analysis and adjusted indirect comparisonsRheumatology20074671140114717478472
- HarrisRPHelfandMWoolfSHCurrent methods of the US Preventive Services Task Force: a review of the processAm J Prev Med2001203 Suppl213511306229
- DeeksJJDinnesJD’AmicoREvaluating non-randomised intervention studiesHealth Technol Assess2003727iiix1173
- GartlehnerGHansenRANissmanDLohrKNCareyVA simple and valid tool distinguished efficacy from effectiveness studiesJ Clin Epidemiol200659101040104816980143
- BucherHCGuyattGHGriffithLEWalterSDThe results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trialsJ Clin Epidemiol19975066836919250266
- SongFAltmanDGGlennyAMDeeksJJValidity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analysesBmj2003326738747212609941
- SauriolLLaportaMEdwardesMDMeta-analysis comparing newer antipsychotic drugs for the treatment of schizophrenia: evaluating the indirect approachClin Ther200123694295611440294
- BresnihanBAlvaro-GraciaJMCobbyMTreatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonistArthritis Rheum19984112219622049870876
- CohenSHurdECushJTreatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trialArthritis Rheum200246361462411920396
- CohenSBMorelandLWCushJJA multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexateAnn Rheum Dis20046391062106815082469
- GenoveseMCCohenSMorelandLCombination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexateArthritis Rheum20045051412141915146410
- US Food and Drug AdministrationCentre for Drug Evaluation and Research Drugs@FDA: Kineret 2009 [cited 2009 10th August]; URL: http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/index.cfm?fuseaction=Search.DrugDetails
- CohenSBMorelandLCushJJAnakinra (recombinant interleukin-1 receptor antagonist): a large, placebo controlled efficacy trial of anakinra in patients with erosive rheumatoid arthritis diseaseArthritis Rheum200144LB1
- FleischmannRMTesserJSchiffMHSafety of extended treatment with anakinra in patients with rheumatoid arthritisAnn Rheum Dis20066581006101216396977
- ListingJStrangfeldAKarySInfections in patients with rheumatoid arthritis treated with biologic agentsArthritis Rheum200552113403341216255017
- ZinkAListingJKarySTreatment continuation in patients receiving biological agents or conventional DMARD therapyAnn Rheum Dis20056491274127915708884
- LangerHEMissler-KargerBKineret: eff icacy and safety in daily clinical practice: an interim analysis of the Kineret response assessment initiative (kreative) protocolInt J Clin Pharmacol Res200323411912815224501
- SalliotCDougadosMGossecLRisk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trialsAnn Rheum Dis2009681253218203761
- Humira® (adalimumab) FDA label information URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s114lbl.pdf2008
- Kineret® (anakinra) FDA label information URL http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/103950s5039lbl.pdf2004
- FDAEnbrel® (etanercept) FDA label information URL http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103795s5359lbl.pdf2009 [cited 2009; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103795s5359lbl.pdf
- ClarkWJobanputraPBartonPBurlsAThe clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysisHealth Technol Assess2004818iiiivixx1105
- MertensMSinghJAAnakinra for rheumatoid arthritisCochrane Database Syst Rev20091CD00512119160248
- WailooABrennanABansbackNModeling the cost effectiveness of etanercept, adalimumab and anakinra compared to infliximab in the treatment of patients with rheumatoid arthritis in the Medicare programAHRQ Technology Assessment Program2006
Appendix 1
Appendix 1 Anti-TNF biologic studies included for indirect comparisons with anakinra